Significance
Strigolactones (SLs) were initially characterized as root-derived signals for parasitic and symbiotic interactions with root parasitic plants and arbuscular mycorrhizal fungi, respectively. SLs were later shown to act as endogenous hormones that regulate shoot branching. Carlactone (CL) was identified as a product of three SL biosynthetic enzymes in vitro, and therefore a putative biosynthetic precursor for SLs. However, it was neither detected from plant tissues, nor was the conversion of CL to SL demonstrated in vivo. In this paper, we show that 13C-labeled CL is converted to SLs in vivo, and that endogenous CL is successfully identified from rice and Arabidopsis. These results demonstrate that CL is a true biosynthetic precursor for SLs.
Keywords: cytochrome P450, phosphate deficiency
Abstract
Strigolactones (SLs) are a class of terpenoid plant hormones that regulate shoot branching as well as being known as root-derived signals for symbiosis and parasitism. SL has tricyclic-lactone (ABC-ring) and methyl butenolide (D-ring), and they are connected through an enol ether bridge. Recently, a putative biosynthetic intermediate called carlactone (CL), of which carbon skeleton is in part similar to those of SLs, was identified by biochemical analysis of three biosynthetic enzymes, DWARF27, CAROTENOID CLEAVAGE DIOXYGENASE 7 (CCD7), and CCD8 in vitro. However, CL has never been identified from plant tissues, and the conversion of CL to SLs has not been proven in vivo. To address these questions, we chemically synthesized 13C-labeled CL. We show that 13C-labeled CL is converted to (−)-[13C]-2′-epi-5-deoxystrigol ((−)-2′-epi-5DS) and [13C]-orobanchol, endogenous SLs in rice, in the dwarf10 mutant, which is defective in CCD8. In addition, we successfully identified endogenous CL by using liquid chromatography-quadrupole/time-of-flight tandem mass spectrometry in rice and Arabidopsis. Furthermore, we determined the absolute stereochemistry of endogenous CL to be (11R)-configuration, which is the same as that of (−)-2′-epi-5DS at the corresponding position. Feeding experiments showed that only the (11R)-isomer of CL, but not the (11S)-isomer, was converted to (−)-2′-epi-5DS in vivo. Taken together, our data provide conclusive evidence that CL is an endogenous SL precursor that is stereospecifically recognized in the biosynthesis pathway.
Strigolactones (SLs) are a group of terpenoid lactones that were originally identified as seed germination stimulants for root parasitic plants (1). They were later characterized as root-derived signals for arbuscular mycorrhizal fungi that supply inorganic nutrients to the host plants (2). In 2008, it was reported that SLs act as endogenous hormones, or their biosynthetic precursors, that regulate shoot branching (3, 4). Recent papers reported that SLs control more diverse developmental processes, such as root development, leaf senescence, secondary growth of the stem, and so on (5–7).
Until recently, little was known about the SL biosynthetic pathway. In 2005, it was reported that SL might be biosynthesized via the carotenoid pathway based on the experiment using a carotenoid biosynthesis inhibitor, fluridone, and some carotenoid metabolism mutants (8). Later studies demonstrated the contribution of two carotenoid cleavage dioxygenases (CCD7 and CCD8) to SL biosynthesis (3, 4). One cytochrome P450, MORE AXILLARY GROWTH1 (MAX1), was also shown to act in SL biosynthesis (9). Later, another SL-deficient mutant of rice, dwarf27 (d27), was characterized (10). The D27 gene was found to encode a member of a novel class of Fe-containing proteins and to localize to chloroplasts.
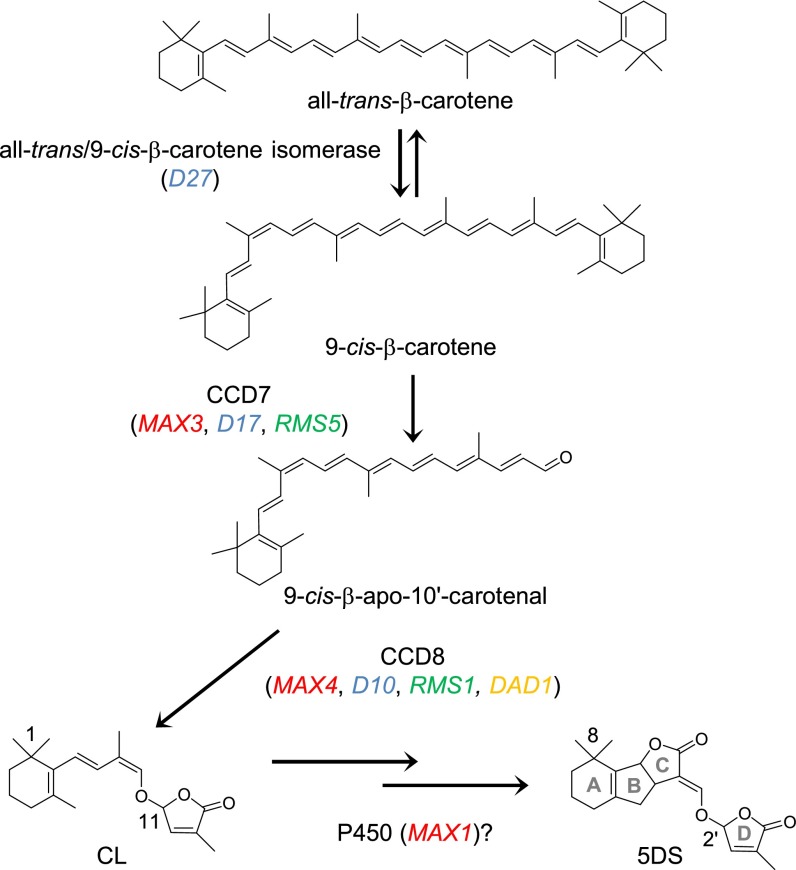
Recently, a possible SL biosynthetic intermediate called carlactone (CL), which has a SL-like carbon skeleton, was identified as a product of sequential reactions by three biosynthetic enzymes, D27, CCD7, and CCD8 in vitro (Fig. 1) (11). In this report, the authors demonstrated that D27 catalyzes the isomerization at C-9 position of all-trans-β-carotene, and the product, 9-cis-β-carotene, is further converted to CL via sequential reactions by CCD7 and CCD8. Before the discovery of CL, SLs were predicted to be biosynthesized by coupling of the carotenoid-derived ABC-ring portion with the D-ring butenolide part (8, 12). However, CL was shown to be enzymatically synthesized from 9-cis-β-apo-10′-carotenal through the intramolecular rearrangement catalyzed by CCD8, suggesting that one SL molecule is derived from one β-carotene molecule (Fig. 1). Moreover, CL was shown to inhibit shoot branching of rice SL biosynthetic mutants, such as d10 and d27, and stimulated seed germination of Striga hermonthica, suggesting that CL exerts SL activities by itself or as a biosynthetic intermediate of SLs (11). Very recently, Scaffidi et al. reported the chemical synthesis of CL and its biological activity in Arabidopsis (13). They showed that CL suppresses shoot branching of Arabidopsis via a mechanism dependent on the cytochrome P450 MAX1, indicating that MAX1 is a downstream component of CL biosynthesis (Fig. 1) (13).
Fig. 1.
Predicted biosynthetic pathway of SLs. CL is produced from all-trans-β-carotene by three enzymes, D27, CCD7, and CCD8. D27 catalyzes the isomerization reaction of all-trans-β-carotene at C-9 position. CL would be oxidized by some oxidation enzymes such as the cytochrome P450 monooxygenase MAX1 and then converted to 5DS. Red, blue, green, and orange characters indicate genes of Arabidopsis, rice, pea, and petunia, respectively. Single arrows show conversions catalyzed by a single enzyme, and double arrows denote conversions possibly catalyzed by more than two enzymes.
Although CL is a possible biosynthetic precursor for SLs, this compound has not been detected from plant tissues as an endogenous compound (11). Moreover, it has yet to be clarified whether CL is converted to SLs in vivo. To address these questions, we synthesized 13C-labeled CL and carried out feeding experiments using a putative rice CL-deficient mutant, dwarf10 (d10). We also attempted to identify endogenous CL from rice and Arabidopsis using liquid chromatography-quadrupole/time-of-flight tandem mass spectrometry (LC-MS/MS). As a result, we successfully observed the conversion of 13C-labeled CL to (−)-[13C]-2′-epi-5-deoxystrigol ((−)-2′-epi-5DS) and [13C]-orobanchol in rice. Moreover, we could detect endogenous CL in rice and Arabidopsis root extracts. In addition, we determined the absolute stereochemistry of endogenous CL to be (11R)-configuration. Furthermore, we used 13C-labeled CL as an internal standard to quantify endogenous CL in rice and Arabidopsis; we assessed whether endogenous CL levels are affected by phosphate (Pi) deficiency like endogenous SL levels.
Results
Chemical Synthesis of Stable Isotope-Labeled CL.
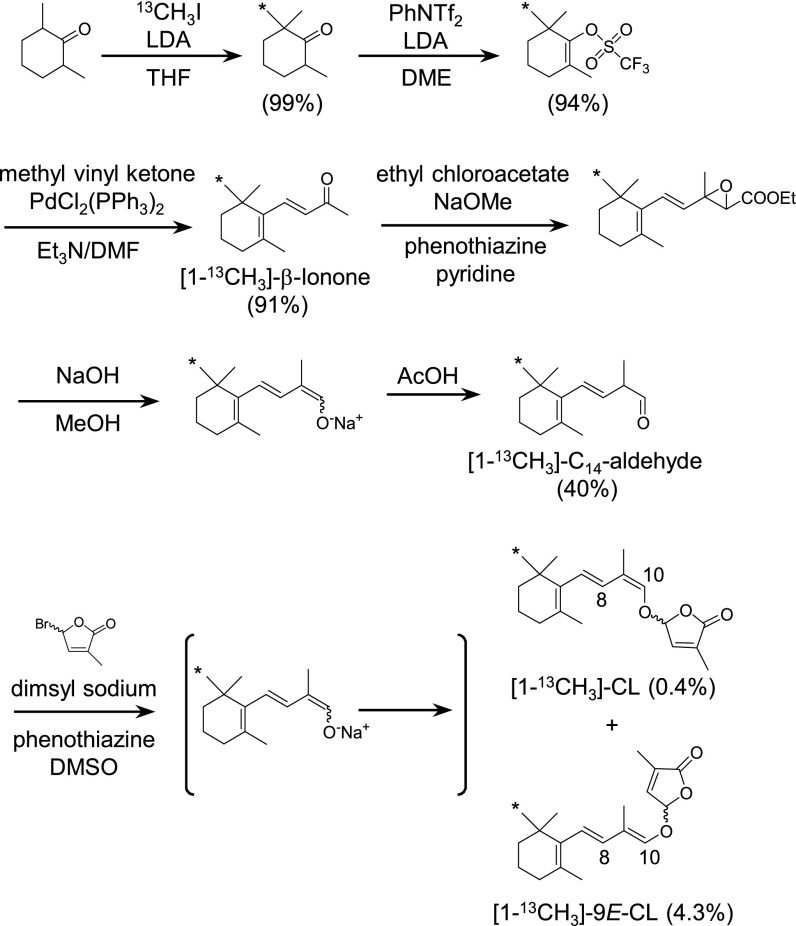
To chemically prepare [1-13CH3]-CL for feeding experiments (Fig. 2), the 13C label was incorporated at 2-CH3 of 2,2,6-trimethylcyclohexanone by methylation of 2,6-dimethylcyclohexanone with 13C-methyl iodide and lithium diisopropylamide. The 13C-labeled cyclohexanone was converted to cyclohexenyl triflate, which was subjected to the Heck reaction with methyl vinyl ketone to afford [1-13CH3]-β-ionone (14). This was converted to C14-aldehyde according to literature procedure (15). The sodium enolate was generated from the C14-aldehyde with dimsyl sodium in dimethyl sulfoxide (16), which was followed by O-alkylation with bromobutenolide to provide [1-13CH3]-CL and its (9E) geometric isomer in a ratio ∼1:10 (Fig. 2). The 1H- and 13C-NMR spectra of 13C-labeled CL were consistent with those reported previously (11), except for the splitting of signals of CH3-16 and CH3-17 protons and of C-1, C-3, and C-5 carbons due to the incorporation of a 13C label into the molecule. The 9E-geometry of the isomer was confirmed by an NOE correlation between H-8 (5.88 ppm) and H-10 (6.42 ppm) in the NOESY spectrum. Although a 4.3-fold increase was achieved in the yield of O-alkylation of C14-enolate by adding phenothiazine to the reaction mixture, the overall yield was quite low. Nevertheless, racemic [1-13CH3]-CL was synthesized and subjected to optical resolution by semipreparative chiral HPLC to afford a pair of enantiomers in sufficient quantities for feeding experiments. Unlabeled CL was synthesized from commercially available β-ionone and optically resolved as above.
Fig. 2.
Chemical synthesis of [1-13CH3]-CL. Asterisks indicate the position of 13C. Details are described in SI Materials and Methods.
Feeding Experiments of [1-13CH3]-CL to the Rice d10-1 Mutant.
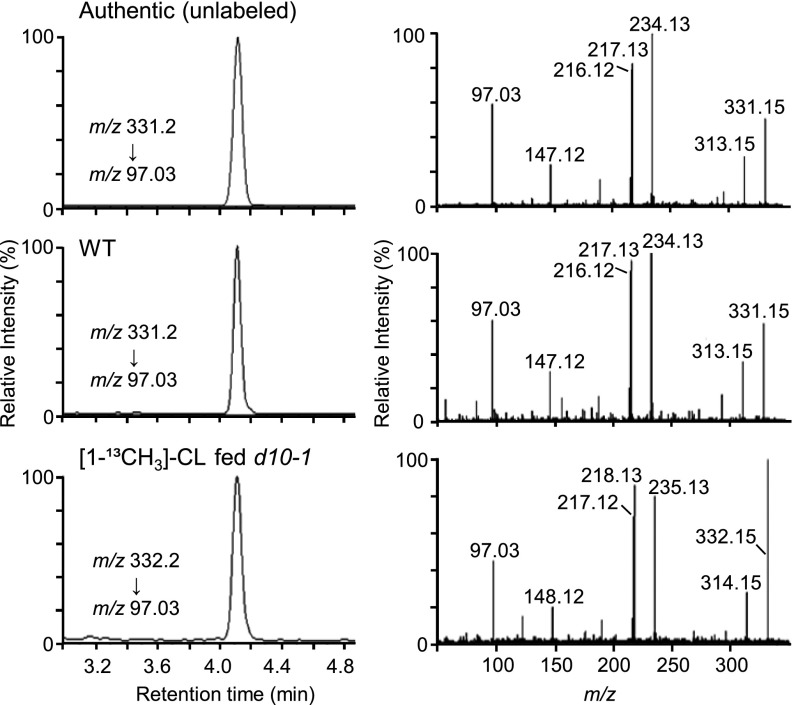
Using synthetic rac-[1-13CH3]-CL, we carried out feeding experiments to examine whether CL is converted to SLs in vivo. We used the rice d10-1 mutant, which is defective in the CCD8 gene (17). SLs were undetectable in the d10-1 mutant (4). CCD8 catalyzes the intramolecular rearrangement of 9-cis-β-apo-10′-carotenal to yield CL in vitro (11). Therefore, we can expect that the d10-1 mutant does not produce CL. The d10-1 mutant would be a suitable plant material for feeding labeled CL to see its metabolism because the labeled substrate would not be diluted by endogenous unlabeled CL. A hydroponic culture system was used for cultivating d10-1 plants, and rac-[1-13CH3]-CL was added to the hydroponic culture media at 1 μM. As a negative control, the d10-1 mutant was grown under the same condition without feeding rac-[1-13CH3]-CL. WT Shiokari cultivar without any treatment was also grown. After feeding for 2 d, we analyzed 2′-epi-5DS, which is an endogenous SL in the rice Shiokari cultivar (4). The root exudates of d10-1 were analyzed by LC-MS/MS, and we could successfully detect the peak of 2′-epi-5DS whose m/z is increased by one mass unit in rac-[1-13CH3]-CL–fed samples compared with an unlabeled authentic standard (Fig. 3). In the full-scan spectra, almost all fragment ions except for m/z 97.03, which is derived from the D-ring part, were increased by one mass unit compared with an unlabeled authentic standard or 2′-epi-5DS from WT plants. These results indicate the bioconversion of rac-[1-13CH3]-CL to [8-13CH3]-2′-epi-5DS by the d10-1 mutant. In the negative control analysis, we could not detect 2′-epi-5DS.
Fig. 3.
LC-MS/MS analysis of 2′-epi-5DS from d10-1 root exudates after feeding [1-13CH3]-CL. Selected reaction monitoring (Left) and full-scan spectra of fragment ions (Right) are shown.
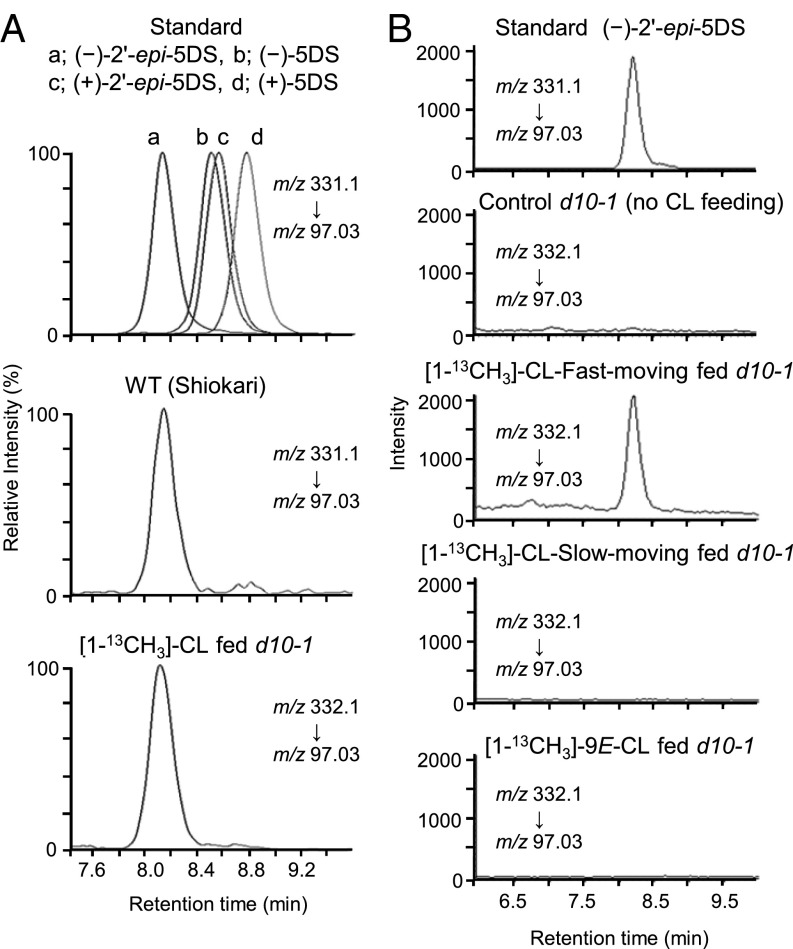
Next, we determined the absolute stereochemistry of the endogenously converted [8-13CH3]-2′-epi-5DS using chiral LC-MS/MS. For this experiment, we analyzed the root extract to examine whether the conversion occurred truly in vivo. 5DS has four stereoisomers (Fig. S1), and it was reported that the rice Nipponbare cultivar only produces (−)-2′-epi-5DS (18). Although we have previously shown that the Shiokari cultivar produces 2′-epi-5DS, its absolute stereochemistry has not been determined (4). As shown in Fig. 4A, four isomers of 5DS were eluted at different retention times in our LC condition using a reverse phase chiral column. By the comparison of its retention time with those of authentic stereoisomers, we could confirm that the Shiokari cultivar also produces only (−)-2′-epi-5DS as an endogenous isomer (Fig. 4A). Importantly, the stereoisomer of 2′-epi-5DS endogenously converted from rac-CL was determined to be (−)-2′-epi-5DS, which is the same as the endogenous one (Fig. 4A).
Fig. 4.
Determination of the stereochemistry of 5DS. (A) Chiral LC-MS/MS analysis of stereoisomers of 5DS in root extracts after feeding rac-[1-13CH3]-CL. Selected reaction monitoring of synthetic standards of 5DS stereoisomers, (−)-2′-epi-5DS from WT root extracts, and (−)-2′-epi-5DS from d10-1 root extracts after feeding rac-[1-13CH3]-CL. (B) Chiral LC-MS/MS analysis of (−)-2′-epi-5DS from root extracts after feeding each enantiomer of [1-13CH3]-CL or [1-13CH3]-9E-CL.
In the feeding experiments, we used a racemic mixture of [1-13CH3]-CL. However, only one stereoisomer of 5DS was synthesized in vivo, suggesting that the enzyme(s) involved in the conversion(s) has a stereospecificity. To examine this stereospecificity in SL biosynthesis, each enantiomer of [1-13CH3]-CL was fed to the d10-1 mutant at 1 μM. We call the isomer eluted faster from the reverse phase chiral column “CL-fast-moving” and the other isomer “CL-slow-moving.” [1-13CH3]-9E-CL, which was obtained as a by-product in the chemical synthesis of [1-13CH3]-CL, was also examined to check whether this chemical can be a precursor for (−)-2′-epi-5DS (Fig. 2). As a result, only one enantiomer, [1-13CH3]-CL-fast-moving, was converted to (−)-[8-13CH3]-2′-epi-5DS, suggesting that the CL-metabolizing enzyme recognizes only one stereoisomer of CL (Fig. 4B). To examine the [13C] incorporation from [1-13CH3]-CL into other SL molecules, we carried out a feeding experiments using the rice d10-2 mutant (17), whose background is Nipponbare, because Nipponbare is known to produce (−)-orobanchol in addition to (−)-2′-epi-5DS (18). As a result, we could detect [8-13CH3]-orobanchol in the root exudate of d10-2 seedlings after feeding [1-13CH3]-CL-fast-moving (Fig. S2). These results provide direct evidence that CL can be converted to SLs in rice in a stereospecific manner.
Identification of Endogenous CL Using LC-MS/MS.
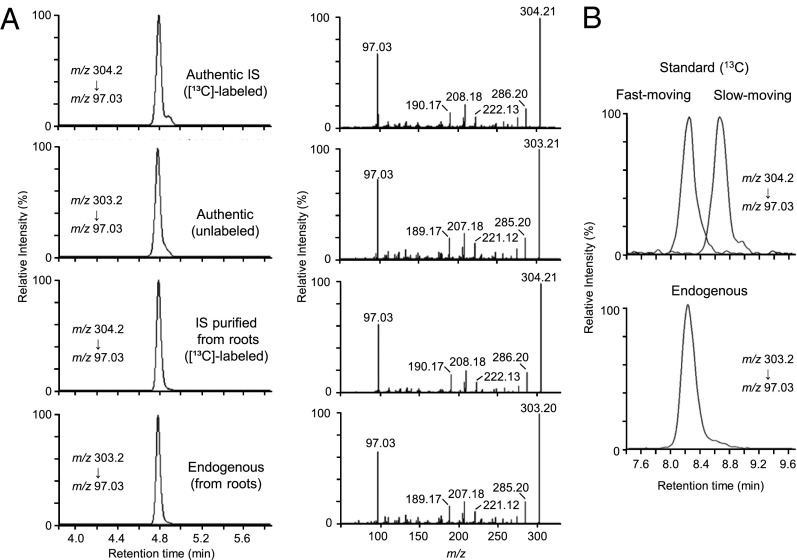
Alder et al. could not detect CL in plants (11). We attempted to detect CL from rice root extracts to assess whether CL is an endogenous precursor for SLs. We firstly selected the rice SL-insensitive mutant d14-1 (19) as a plant material, because a high level of SL is accumulated in this mutant, possibly due to feedback regulation (19). The extract of roots was purified through several column chromatography techniques and the purified sample was analyzed by LC-MS/MS using synthetic rac-[1-13CH3]-CL as an internal standard. We selected [M+H]+ (m/z 303 and m/z 304 for unlabeled and rac-[1-13CH3]-CL, respectively) as parent ions on quadrupole mass spectrometry, and detected [M+H−206]+ (m/z 97.03 for both unlabeled and rac-[1-13CH3]-CL) as a fragment ion on time-of-flight mass spectrometry after collision-induced dissociation. We could detect one peak whose retention time is identical with that of the authentic standard of rac-CL (Fig. 5A). Full-scan spectra of fragment ions (Fig. 5A) and selected reaction monitoring using major fragment ions (Fig. S3) confirmed the identity of this endogenous compound.
Fig. 5.
Identification of endogenous CL in plants. (A) LC-MS/MS analysis of CL from root extracts of rice d14-1. Selected reaction monitoring (Left) and full-scan spectra of fragment ions (Right) of authentic [1-13CH3]-CL, unlabeled authentic standard, [1-13CH3]-CL purified from root extracts, and endogenous CL. (B) Chiral LC-MS/MS analysis of CL from root extracts of rice d14-1. Selected reaction monitoring of authentic standards of two enantiomers of [1-13CH3]-CL, and endogenous CL.
Next, we analyzed stereochemistry of endogenous CL using chiral LC-MS/MS. By the comparison of the retention time of endogenous CL with that of authentic standards, we determined endogenous CL to be CL-fast-moving, which is the same one that was converted to (−)-2′-epi-5DS and orobanchol in the feeding experiment (Fig. 5B).
To examine whether CL exists in other plant species, we analyzed endogenous CL in Arabidopsis. As a plant material, we used the atd14-2 mutant, which is defective in the Arabidopsis ortholog of rice D14 (Fig. S4). Endogenous CL was successfully identified from root extracts of atd14-2 by LC-MS/MS (Fig. S5). We also found that the stereochemistry of endogenous CL in Arabidopsis is the same as that in rice (Fig. S6). These results indicate that CL-fast-moving is a common precursor for SL in rice and Arabidopsis.
Determination of the Absolute Stereochemistry of CL.
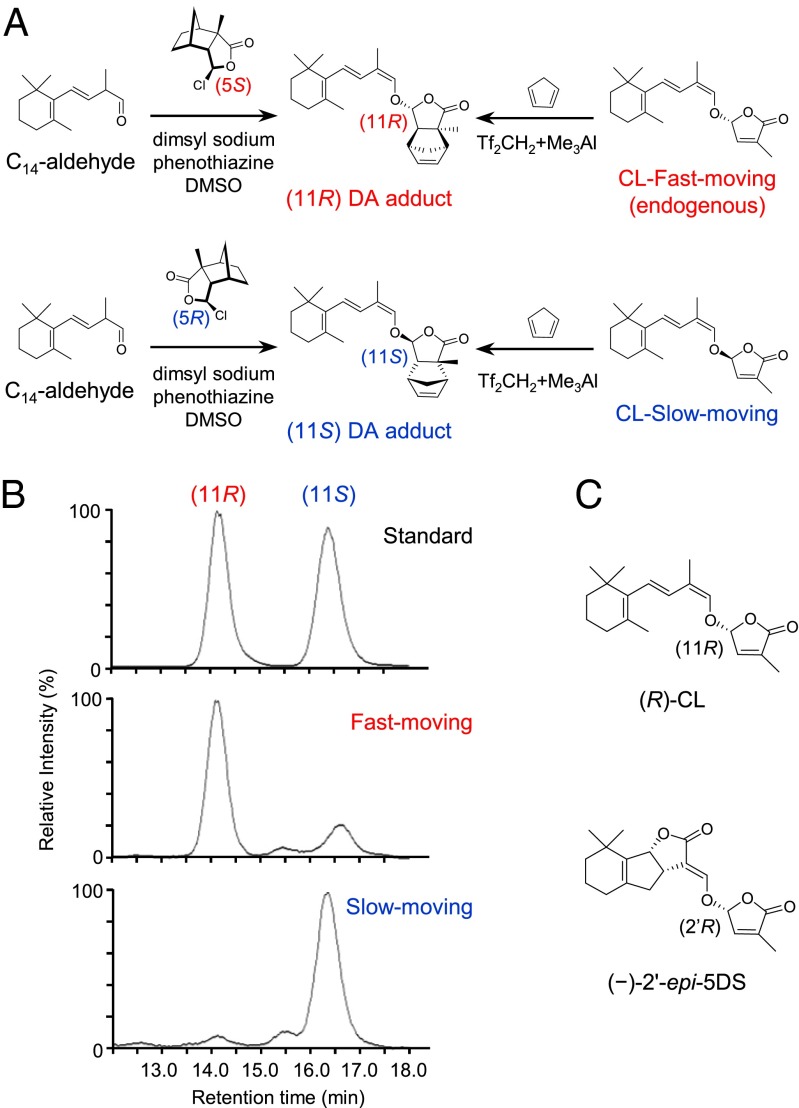
The results described so far indicate that only one enantiomer of CL is present in rice and Arabidopsis, and this isomer can be converted to (−)-2′-epi-5DS and orobanchol in rice. To clarify the absolute stereochemistry of natural CL, we determined the absolute configuration of the synthetic isomers at C-11. We first observed circular dichroism (CD) spectra of the CL-fast-moving and CL-slow-moving enantiomers. In the case of SL stereoisomers, the absolute configuration at C-2′ position in the D-ring was determined by the cotton effect around 260 nm (20, 21). However, the CD spectra of CL were not similar to those of SLs (Fig. S7). Thus, we could not use this method to determine the absolute stereochemistry of CL. We then determined the stereochemistry after derivatization of CL as a Diels-Alder (DA) adduct with cyclopentadiene. As shown in Fig. 6A, coupling of the (5S)- or (5R)-modified D-ring portion with the C14-aldehyde gave each enantiomer of DA adduct-type CL with fixed stereochemistry at C-11 (22). Then, CL-fast-moving, which is the endogenous isomer, and CL-slow-moving were separately reacted with cyclopentadiene using Lewis acid to yield DA adducts (Fig. 6A) (23). LC-MS analysis of these reaction products compared with each enantiomer of the synthetic standard allowed us to determine the configuration at the C-11 position of CL-fast-moving and CL-slow-moving to be (R) and (S), respectively (Fig. 6B). These results demonstrate that endogenous CL has (11R)-configuration, which is the same as that of the corresponding position (C-2′) of (−)-2′-epi-5DS (Fig. 6C).
Fig. 6.
Determination of the absolute stereochemistry of CL. (A) Synthetic scheme of DA adduct-type CL. As authentic standards, two enantiomers of DA adduct-type CL were prepared from C14 aldehyde by coupling with each stereoisomer (5S or 5R) of the modified butenolide portion. CL-fast-moving and CL-slow-moving were separately reacted with cyclopentadiene by DA reaction to afford the cycloadducts. (B) Chiral-LC-MS analysis of each enantiomer of DA adduct-type CL (standard) and DA adduct-type CL synthesized from CL-fast-moving or CL-slow-moving. (C) Chemical structures of (R)-CL and (−)-2′-epi-5DS.
Quantitative Analysis of Endogenous Levels of (R)-CL.
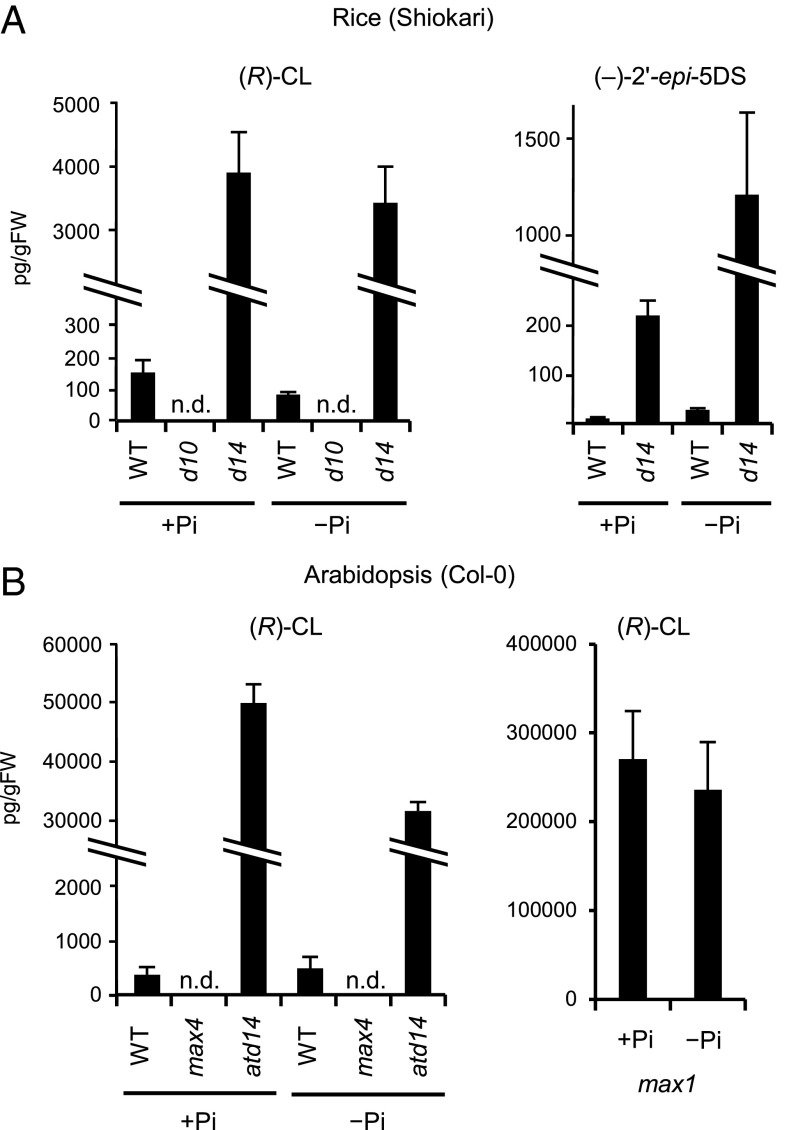
We quantified endogenous levels of (R)-CL in rice by LC-MS/MS using rac-[1-13CH3]-CL as an internal standard. Previous studies have shown that SL levels in roots and root exudates are highly elevated by phosphate (Pi) or nitrate starvation in various plant species (4, 24–27). To address whether (R)-CL levels are also affected by Pi-deficiency, we quantified endogenous (R)-CL under +Pi and −Pi conditions in WT, d10-1 and d14-1 rice seedlings. Endogenous (R)-CL was detected in WT root extracts, but not in d10-1 root extracts (Fig. 7A). (R)-CL levels were much higher in the d14-1 mutant than in WT plants, as was the case with SL levels (Fig. 7A) (19). Interestingly, Pi-deficiency did not increase the levels of (R)-CL in WT and d14-1 root extracts, unlike the case with SL ((−)-2′-epi-5DS) (Fig. 7A).
Fig. 7.
Quantitative analysis of (R)-CL and (−)-2′-epi-5DS in plants. (A) Endogenous levels of (R)-CL and (−)-2′-epi-5DS in root extracts of rice WT, d10-1, and d14-1 in the presence or absence of Pi. N.d., not detected due to low abundance. Data are the means ± SD (n = 3). (B) (R)-CL levels in root extracts of Arabidopsis WT Col-0, max4-8, atd14-2, and max1-4. N.d., not detected due to low abundance. Data are the means ± SD (n = 3).
We also quantified endogenous (R)-CL levels in Arabidopsis roots. We grew WT, max4-8 (defective in CCD8), max1-4 (defective in a cytochrome P450, Fig. S4), and atd14-2 plants, and the root extracts were analyzed by LC-MS/MS. (R)-CL was undetectable in the max4-8 mutant, whereas the atd14-2 mutant accumulated a high level of (R)-CL as did the d14-1 mutant of rice (Fig. 7B). Pi-deficiency did not affect the levels of (R)-CL so much also in Arabidopsis (Fig. 7B). MAX1 is a possible downstream component of (R)-CL biosynthesis (Fig. 1), and (R)-CL is a candidate for the MAX1 substrate (11, 13). Notably, max1-4 accumulated extremely high levels of (R)-CL; ∼5 times higher than atd14-2 and 700 times higher than WT. These results suggest that (R)-CL is a substrate for MAX1 (Fig. 7B).
To further examine the involvement of MAX1 in (R)-CL metabolism, we carried out feeding experiments using Arabidopsis. In a previous report, orobanchol and orobanchyl acetate were detected in Arabidopsis (28), whereas in our growth and analytical conditions, these known SLs were under the detection level limit. Instead, we found an SL-like molecule (SL-LIKE1) from Arabidopsis WT root extract (Fig. S8A). SL-LIKE1 showed a product ion peak characteristic of SLs at m/z 97.03, which is derived from the D-ring part (Fig. S8A). We could detect SL-LIKE1 also in the atd14-2 mutant, but not in the max1-4 mutant, suggesting the involvement of MAX1 in SL-LIKE1 biosynthesis (Fig. S8B). We fractionated atd14-2 root extracts by HPLC, and the fraction containing SL-LIKE1 stimulated the germination of S. hermonthica and Orobanche minor seeds (Fig. S8C). Then we analyzed the [13C] incorporation into this molecule from (R)-[1-13CH3]-CL. Although the max1-4 mutant produces an extremely large amount of (R)-CL, endogenous (R)-CL in the max1-4 max4-7 double mutant was undetectable (Fig. S8D). Moreover, (R)-[13C]-CL was converted into [13C]-SL-LIKE1 in the max4-7 mutant, whereas [13C]-SL-LIKE1 was not detected in the max1-4 max4-7 double mutant after feeding (R)-[1-13CH3]-CL (Fig. S8E). These results demonstrate the contribution of MAX1 to the (R)-CL metabolism pathway in Arabidopsis.
Discussion
CL was found as a putative intermediate in SL biosynthesis through in vitro experiments using recombinant enzymes. However, neither the occurrence of CL in plants has been proved nor the conversion of CL to SLs in plants has been demonstrated in a previous study (11). Here, we report the bioconversion of chemically synthesized 13C-labeled CL to (−)-[13C]- 2′-epi-5DS and [13C]-orobanchol, endogenous SLs in rice, and the detection of endogenous CL from rice and Arabidopsis. Moreover, the absolute stereochemistry of the butenolide moiety of endogenous CL was determined to be the same as that of endogenous (−)-2′-epi-5DS. These data conclusively demonstrate that (R)-CL is an endogenous biosynthetic precursor for SLs.
Interestingly, in our feeding experiments, only one stereoisomer of CL (CL-fast-moving) was converted to (−)-2′-epi-5DS as revealed by chiral LC-MS/MS analysis (Fig. 4B). In addition, only this stereoisomer was detected as endogenous CL both in rice and in Arabidopsis (Fig. 5B and Fig. S6). Chemical synthesis and chiral LC-MS analysis of DA adduct-type CL enabled us to determine the absolute stereochemistry of CL-fast-moving to be (11R)-configuration, which is the same as that of the corresponding position (C-2′) of (−)-2′-epi-5DS (Fig. 6). The stereochemistry of C-2′ position has been determined to be (R) in all natural SLs reported so far; natural 5DS is (+)-5DS or (−)-2′-epi-5DS (Fig. 6C and Fig. S1) (18, 29). Although some SLs have been detected in Arabidopsis, their absolute stereochemistry has not been determined (28). We could detect the (11R) isomer of CL from Arabidopsis, providing the indirect evidence that Arabidopsis also produces (2′R) type SLs like other plant species (Fig. S6).
Our quantitative analysis of endogenous (R)-CL revealed that (R)-CL levels in the d14-1 mutant of rice and the atd14 mutant of Arabidopsis, both of which are proposed SL perception mutants (19, 30–32), are highly elevated in comparison with WT plants, possibly due to feedback regulation (Fig. 7A). rac-CL was reported to weakly suppress hypocotyl elongation in a MAX1-independent manner (13). However, it does not inhibit shoot branching without MAX1 function, suggesting that (R)-CL is a biologically inactive precursor of SLs at least for the regulation of shoot branching (13). In the case of gid1, a mutant of a soluble gibberellin (GA) receptor, the accumulation of only the active form of GA, such as GA1, was observed (33). Therefore, the accumulation of biologically inactive (R)-CL in the d14-1 and atd14-2 mutants implies that a step(s) upstream of (R)-CL synthesis is highly elevated in these mutants through feedback regulation.
Interestingly, the levels of (R)-CL in rice and Arabidopsis were not elevated under Pi-deficient conditions, whereas the levels of (−)-2′-epi-5DS were highly elevated in rice as reported previously (Fig. 7) (4, 24–27). Rather, we could see a tendency that (R)-CL levels were slightly decreased by Pi deficiency (Fig.7). In Arabidopsis, the levels of SLs were also elevated by Pi starvation as seen in rice (28). In contrast to the slight decrease in (R)-CL levels under −Pi condition, expression levels of some SL biosynthetic genes, including the upstream components of (R)-CL synthesis, were up-regulated by Pi deficiency in rice (27). This suggests that (R)-CL synthesis might be elevated under Pi-deficient conditions. We can speculate that Pi deficiency more highly up-regulates the pathway downstream of (R)-CL synthesis. It is noteworthy that the endogenous amount of (R)-CL in the presence of Pi is about 15 times higher than that of (−)-2′-epi-5DS (Fig. 7A). Taken together, our results suggest that plants store (R)-CL or (R)-CL metabolite(s) under normal growth conditions, and when plants need SLs, such as under low nutrient conditions, SLs are synthesized from (R)-CL or (R)-CL metabolite(s) and control the plant architecture, including shoot branching. Our data imply that there is (are) key step(s) for controlling the endogenous SL levels in the pathway between (R)-CL and SL in response to nutrient conditions.
(R)-CL needs to be further oxidized to be finally converted to SLs. Grafting experiments, using Arabidopsis max1, as a rootstock, and max3 or max4 as a scion, resulted in a restored shoot branching phenotype (34). These observations suggest that MAX1 is a downstream component of MAX3 and MAX4, and that a possible substrate for MAX1 is (R)-CL (Fig. 1). As mentioned above, a recent report that rac-CL suppresses Arabidopsis shoot branching in a MAX1-dependent manner supports this hypothesis (13). Our quantitative analysis of endogenous (R)-CL revealed that the max1-4 mutant accumulates an exceptionally high level of (R)-CL. Furthermore, we demonstrated that (R)-[1-13CH3]-CL is converted into SL-LIKE1 in Arabidopsis in a MAX1-dependent manner, providing the direct evidence for the involvement of MAX1 in the (R)-CL metabolism pathway (Fig. S8).
In conclusion, our results demonstrate that (R)-CL is an endogenous biosynthetic precursor of SLs in rice and Arabidopsis. Considering the structures of CL and SLs, at least two positions of CL should be oxidized to be converted into SLs, indicating that there is one unidentified enzyme or more besides MAX1 (Fig. 1). Our discovery will assist the understanding of the whole picture of the SL biosynthetic pathway and its regulatory mechanisms.
Materials and Methods
Plant Materials and Growth Conditions.
We used rice cultivar (Oryza sativa L. cv. Shiokari or Nipponbare) as the WT, and tillering dwarf mutants, d10-1 and d14-1, in the Shiokari background, and d10-2 in the Nipponbare background in this study (17, 19). We used Arabidopsis ecotype Col-0 as the WT and max1-4 (SAIL_25A05, Fig. S4), max4-8 (SALK_02750) (4), max4-7 (SALK_082552) (4), and atd14-2 mutants. The atd14-2 mutant was obtained from a TILLING project (http://tilling.fhcrc.org/), and the mutation is shown in Fig. S4. The atd14-2 mutant was used for experiments after a backcross with Col-0. Rice and Arabidopsis plants were grown hydroponically as described previously (4). Details of growth conditions and feeding experiments are described in SI Materials and Methods.
LC-MS/MS Analysis and Chemical Synthesis.
LC-MS/MS analysis of 5DS and CL were performed as described in SI Materials and Methods. Methods of chemical synthesis are described in detail in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Prof. Koichi Yoneyama for helpful discussions, Prof. Junko Kyozuka for providing the rice d10-2 mutant, and Drs. Weiqiang Li and Noriko Takeda-Kamiya for technical assistance. This work was supported by MEXT KAKENHI Grant 24114010, the Program for Promotion of Basic and Applied Research for Innovations in Bio-Oriented Industry, and JST, CREST.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1314805111/-/DCSupplemental.
References
- 1.Cook CE, Whichard LP, Turner B, Wall ME, Egley GH. Germination of witchweed (striga lutea Lour.): Isolation and properties of a potent stimulant. Science. 1966;154(3753):1189–1190. doi: 10.1126/science.154.3753.1189. [DOI] [PubMed] [Google Scholar]
- 2.Akiyama K, Matsuzaki K, Hayashi H. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature. 2005;435(7043):824–827. doi: 10.1038/nature03608. [DOI] [PubMed] [Google Scholar]
- 3.Gomez-Roldan V, et al. Strigolactone inhibition of shoot branching. Nature. 2008;455(7210):189–194. doi: 10.1038/nature07271. [DOI] [PubMed] [Google Scholar]
- 4.Umehara M, et al. Inhibition of shoot branching by new terpenoid plant hormones. Nature. 2008;455(7210):195–200. doi: 10.1038/nature07272. [DOI] [PubMed] [Google Scholar]
- 5.Ruyter-Spira C, Al-Babili S, van der Krol S, Bouwmeester H. The biology of strigolactones. Trends Plant Sci. 2013;18(2):72–83. doi: 10.1016/j.tplants.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Brewer PB, Koltai H, Beveridge CA. Diverse roles of strigolactones in plant development. Mol Plant. 2013;6(1):18–28. doi: 10.1093/mp/sss130. [DOI] [PubMed] [Google Scholar]
- 7.Seto Y, Kameoka H, Yamaguchi S, Kyozuka J. Recent advances in strigolactone research: Chemical and biological aspects. Plant Cell Physiol. 2012;53(11):1843–1853. doi: 10.1093/pcp/pcs142. [DOI] [PubMed] [Google Scholar]
- 8.Matusova R, et al. The strigolactone germination stimulants of the plant-parasitic Striga and Orobanche spp. are derived from the carotenoid pathway. Plant Physiol. 2005;139(2):920–934. doi: 10.1104/pp.105.061382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crawford S, et al. Strigolactones enhance competition between shoot branches by dampening auxin transport. Development. 2010;137(17):2905–2913. doi: 10.1242/dev.051987. [DOI] [PubMed] [Google Scholar]
- 10.Lin H, et al. DWARF27, an iron-containing protein required for the biosynthesis of strigolactones, regulates rice tiller bud outgrowth. Plant Cell. 2009;21(5):1512–1525. doi: 10.1105/tpc.109.065987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alder A, et al. The path from β-carotene to carlactone, a strigolactone-like plant hormone. Science. 2012;335(6074):1348–1351. doi: 10.1126/science.1218094. [DOI] [PubMed] [Google Scholar]
- 12.Rani K, Zwanenburg B, Sugimoto Y, Yoneyama K, Bouwmeester HJ. Biosynthetic considerations could assist the structure elucidation of host plant produced rhizosphere signalling compounds (strigolactones) for arbuscular mycorrhizal fungi and parasitic plants. Plant Physiol Biochem. 2008;46(7):617–626. doi: 10.1016/j.plaphy.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 13.Scaffidi A, et al. Carlactone-independent seedling morphogenesis in Arabidopsis. Plant J. 2013;76(1):1–9. doi: 10.1111/tpj.12265. [DOI] [PubMed] [Google Scholar]
- 14.Breining T, Schmidt C, Polos K. A palladium-catalyzed synthesis of β-ionone from 2,2,6-trimethylcyclohexanone. Synth Commun. 1987;17(1):85–88. [Google Scholar]
- 15.Ramamurthy V, Tustin G, Yau CC, Liu RSH. Preparation of sterically hindered geometric isomers of 7-cis-β-ionyl and β-ionylidene derivatives in vitamin-A series. Tetrahedron. 1975;31(3):193–199. [Google Scholar]
- 16.Zook HD, Miller JA. Chemistry of enolates. VII. kinetics and orientation in dimethyl sulfoxide-relative nucleophilicities of enolates. J Org Chem. 1971;36(8):1112–1116. [Google Scholar]
- 17.Arite T, et al. DWARF10, an RMS1/MAX4/DAD1 ortholog, controls lateral bud outgrowth in rice. Plant J. 2007;51(6):1019–1029. doi: 10.1111/j.1365-313X.2007.03210.x. [DOI] [PubMed] [Google Scholar]
- 18.Xie X, et al. Confirming stereochemical structures of strigolactones produced by rice and tobacco. Mol Plant. 2013;6(1):153–163. doi: 10.1093/mp/sss139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arite T, et al. d14, a strigolactone-insensitive mutant of rice, shows an accelerated outgrowth of tillers. Plant Cell Physiol. 2009;50(8):1416–1424. doi: 10.1093/pcp/pcp091. [DOI] [PubMed] [Google Scholar]
- 20.Frischmuth K, et al. Routes to derivatives of strigol (the witchweed germination factor) modified in the 5-position. Tetrahedron. 1991;47(47):9793–9806. [Google Scholar]
- 21.Welzel P, Rohrig S, Milkova Z. Strigol-type germination stimulants: The C-2' configuration problem. Chem Commun (Camb) 1999;(20):2017–2022. [Google Scholar]
- 22.Willem J, Thuring JF, Nefkens GHL, Schaafstra R, Zwanenburg B. Asymmetric-synthesis of a D-ring synthon for strigol analogs and its application to the synthesis of all 4 stereoisomers of germination stimulant GR7. Tetrahedron. 1995;51(17):5047–5056. [Google Scholar]
- 23.Yanai H, Takahashi A, Taguchi T. Dimethylaluminum methide complex Tf2CHAlMe2: An effective catalyst for Diels-Alder reaction of α,β-unsaturated lactone derivatives with cyclopentadiene. Tetrahedron. 2007;63(49):12149–12159. [Google Scholar]
- 24.Yoneyama K, et al. Nitrogen deficiency as well as phosphorus deficiency in sorghum promotes the production and exudation of 5-deoxystrigol, the host recognition signal for arbuscular mycorrhizal fungi and root parasites. Planta. 2007;227(1):125–132. doi: 10.1007/s00425-007-0600-5. [DOI] [PubMed] [Google Scholar]
- 25.Yoneyama K, Yoneyama K, Takeuchi Y, Sekimoto H. Phosphorus deficiency in red clover promotes exudation of orobanchol, the signal for mycorrhizal symbionts and germination stimulant for root parasites. Planta. 2007;225(4):1031–1038. doi: 10.1007/s00425-006-0410-1. [DOI] [PubMed] [Google Scholar]
- 26.Yoneyama K, et al. Strigolactones, host recognition signals for root parasitic plants and arbuscular mycorrhizal fungi, from Fabaceae plants. New Phytol. 2008;179(2):484–494. doi: 10.1111/j.1469-8137.2008.02462.x. [DOI] [PubMed] [Google Scholar]
- 27.Umehara M, Hanada A, Magome H, Takeda-Kamiya N, Yamaguchi S. Contribution of strigolactones to the inhibition of tiller bud outgrowth under phosphate deficiency in rice. Plant Cell Physiol. 2010;51(7):1118–1126. doi: 10.1093/pcp/pcq084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kohlen W, et al. Strigolactones are transported through the xylem and play a key role in shoot architectural response to phosphate deficiency in nonarbuscular mycorrhizal host Arabidopsis. Plant Physiol. 2011;155(2):974–987. doi: 10.1104/pp.110.164640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ueno K, et al. Ent-2′-epi-Orobanchol and its acetate, as germination stimulants for Striga gesnerioides seeds isolated from cowpea and red clover. J Agric Food Chem. 2011;59(19):10485–10490. doi: 10.1021/jf2024193. [DOI] [PubMed] [Google Scholar]
- 30.Zhao LH, et al. Crystal structures of two phytohormone signal-transducing α/β hydrolases: Karrikin-signaling KAI2 and strigolactone-signaling DWARF14. Cell Res. 2013;23(3):436–439. doi: 10.1038/cr.2013.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamiaux C, et al. DAD2 is an α/β hydrolase likely to be involved in the perception of the plant branching hormone, strigolactone. Curr Biol. 2012;22(21):2032–2036. doi: 10.1016/j.cub.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 32.Kagiyama M, et al. Structures of D14 and D14L in the strigolactone and karrikin signaling pathways. Genes Cells. 2013;18(2):147–160. doi: 10.1111/gtc.12025. [DOI] [PubMed] [Google Scholar]
- 33.Ueguchi-Tanaka M, et al. GIBBERELLIN INSENSITIVE DWARF1 encodes a soluble receptor for gibberellin. Nature. 2005;437(7059):693–698. doi: 10.1038/nature04028. [DOI] [PubMed] [Google Scholar]
- 34.Booker J, et al. MAX1 encodes a cytochrome P450 family member that acts downstream of MAX3/4 to produce a carotenoid-derived branch-inhibiting hormone. Dev Cell. 2005;8(3):443–449. doi: 10.1016/j.devcel.2005.01.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.