Significance
Science-based polices are needed to inform sustainable bioenergy landscape design. Our key finding is that the linkage between biodiversity and ecosystem services is dependent not only on the choice of bioenergy crop but also on its location relative to other habitats. The implication is that careful design of bioenergy landscapes has the potential to enhance multiple services in food and energy crops, leading to important synergies that have not yet informed the ongoing bioenergy debate. This study is especially timely as high commodity prices are driving conversion of marginal lands to annual crop production, reducing future flexibility.
Keywords: energy policy, greenhouse gas mitigation
Abstract
Agriculture is being challenged to provide food, and increasingly fuel, for an expanding global population. Producing bioenergy crops on marginal lands—farmland suboptimal for food crops—could help meet energy goals while minimizing competition with food production. However, the ecological costs and benefits of growing bioenergy feedstocks—primarily annual grain crops—on marginal lands have been questioned. Here we show that perennial bioenergy crops provide an alternative to annual grains that increases biodiversity of multiple taxa and sustain a variety of ecosystem functions, promoting the creation of multifunctional agricultural landscapes. We found that switchgrass and prairie plantings harbored significantly greater plant, methanotrophic bacteria, arthropod, and bird diversity than maize. Although biomass production was greater in maize, all other ecosystem services, including methane consumption, pest suppression, pollination, and conservation of grassland birds, were higher in perennial grasslands. Moreover, we found that the linkage between biodiversity and ecosystem services is dependent not only on the choice of bioenergy crop but also on its location relative to other habitats, with local landscape context as important as crop choice in determining provision of some services. Our study suggests that bioenergy policy that supports coordinated land use can diversify agricultural landscapes and sustain multiple critical ecosystem services.
In agricultural landscapes, balancing the provisioning of food and energy with maintenance of biodiversity and ecosystem functions is a global challenge. To avoid impacts on food production, attention is increasingly being focused on the potential for marginal lands to support bioenergy production (1). Marginal lands, those suboptimal for food production, may consist of relatively small areas within generally productive landscapes or larger regions where conditions generally limit crop productivity. However, there is increasing recognition that these lands are already performing a variety of useful functions, and their conversion to bioenergy cropping could reduce these services. For example, in the north central United States, rising commodity prices are predicted to bring marginal croplands—including Conservation Reserve Program lands—into annual crop production with negative impacts on wildlife habitat and water quality (2, 3). With 2013 corn plantings at recent record highs (4) and new reports of grassland and wetland conversion to cropland (5, 6), this may be occurring already.
An alternative to annual cropping is conversion of marginal croplands to perennial, cellulosic crops for bioenergy. Although current US biofuel production centers on grain ethanol derived from annual monocultures of maize (Zea mays), this situation could change with full implementation of the 2007 US Energy Independence and Security Act (7), which calls for increased production of cellulosic biofuels. In the Midwest United States, perennial grasses and forbs grown on marginal lands could provide up to 25% of national targets for cellulosic biofuel, with substantial greenhouse gas (GHG) benefits (8). Moreover, increasing the area of perennial cover on the landscape is predicted to positively affect a diverse array of organisms and ecological functions (9–11), leading to important synergies that have not yet informed the ongoing bioenergy debate. Here we provide the most comprehensive empirical evaluation of this hypothesis to date, reporting data that elucidate the impacts of different bioenergy cropping systems on a wide variety of organisms and the ecosystem functions they perform.
Previous studies have examined the ability of select bioenergy crops to support specific taxa (12) or individual services such as energy production (13) or GHG mitigation (14), without consideration of the tradeoffs or synergies that can arise when considering entire suites of organisms and ecosystem functions. We report on a unique multidisciplinary study of matched sets of organisms and ecosystem services and show that perennial grass energy crops (switchgrass, Panicum virgatum, and mixed prairie plantings) synergistically enhance diversity of a variety of organisms and levels of the services they provide. We further quantify the importance of landscape context on service provisioning, suggesting that policy supporting intentional design of bioenergy landscapes could increase sustainability of both food and energy production.
Results
We compared biodiversity and key ecological processes among maize, switchgrass, and prairie, three plant communities representing distinct alternatives for use of marginal lands that vary in management intensity, perenniality, and sown diversity (Fig. S1). To accomplish this, we identified and sampled 115 maize, switchgrass, and prairie fields across the major agricultural production regions of Michigan and Wisconsin (Fig. S1 and Tables S1–S3). Maize was grown as an annual monoculture and managed for high yields using herbicides and fertilizers (Table S2). Switchgrass and prairie sites were planted as perennial monocultures and polycultures, respectively, and managed using prescribed burns or mowing on 2–5 y cycles. Supplemental analyses found no evidence that recent burning or mowing negatively affected response variables (Text S2, Figs. S2 and S3, and Table S4).
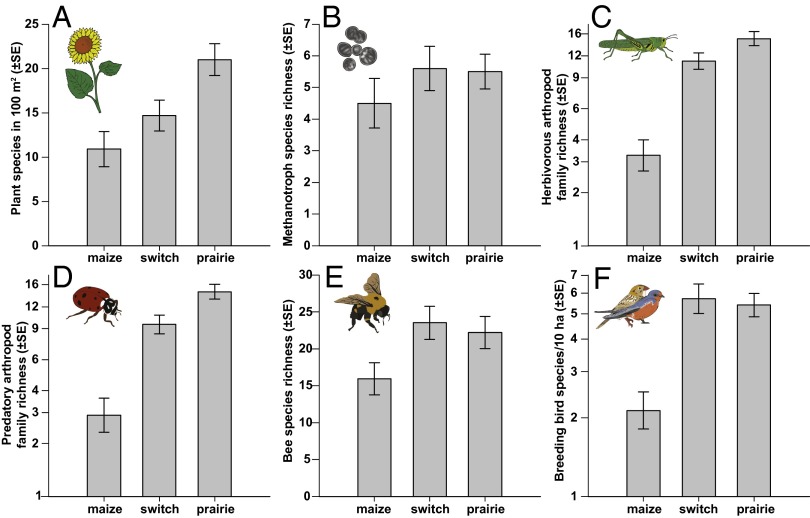
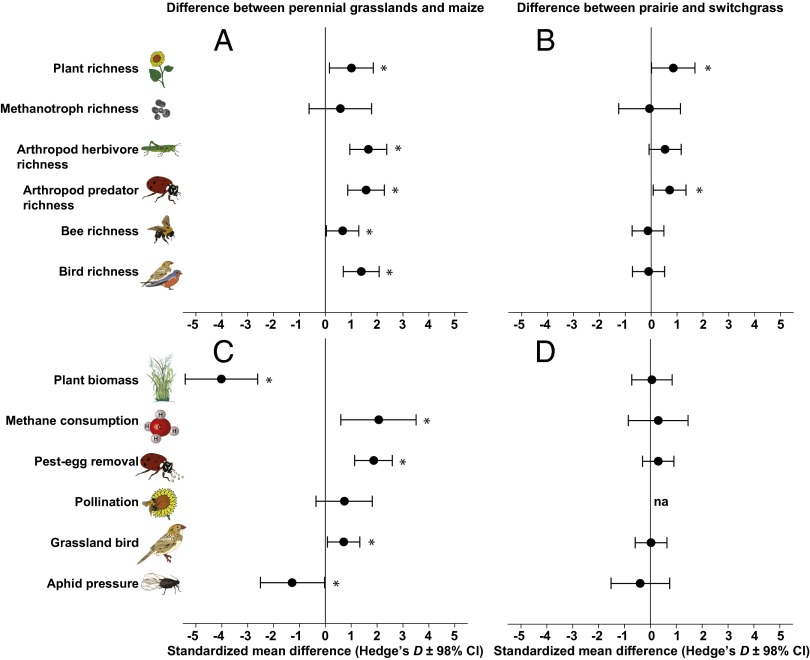
Biodiversity was quantified by measuring the taxonomic richness of plants, methane-consuming soil bacteria (methanotrophs), predatory and herbivorous arthropods, bees, and breeding birds using sampling methods and measures of richness appropriate for each group. As expected, maize fields contained a low-diversity plant community dominated by the crop itself (99% of biomass; Figs. 1A and 2A). Although planted as monocultures, switchgrass stands were more diverse, with biomass typically composed of 20% opportunistic forbs and grasses. Prairies were most diverse in both plant species and biomass composition. Diversity of herbivorous and predatory arthropods showed a stairstep increase from maize to prairie that mirrored trends in plant diversity (Fig. 1 C and D), whereas methanotroph, bee, and breeding-bird diversity was equally high in the two perennial grasslands compared with maize (Fig. 1 B, E, and F). Effect statistics (Hedge’s D) were used to standardize differences and test for statistical significance (15). Compared with maize, perennial grass plantings had positive effects on diversity of all taxa, which were statistically significant for all but methanotrophs (Fig. 3A). Differences in richness between the two perennial grass systems were either smaller (plant and predatory arthropod richness) or near zero (all other organisms; Fig. 3B).
Fig. 1.
Compared with maize, perennial grasslands supported a greater diversity of organisms ranging from plants to vertebrates. Graphs show variation between maize, switchgrass, and prairie in richness of plants (A), methanotrophic soil bacteria (B), herbivorous arthropods (C), predatory and parasitic arthropods (D), bees (E), and breeding birds (F). Error bars are ±1 SE. Note logarithmic axes in C, D, and F.
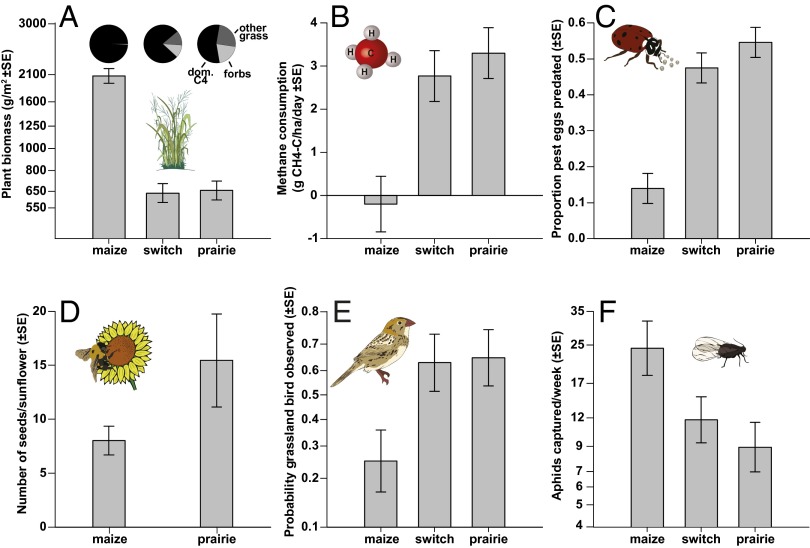
Fig. 2.
Key ecological processes varied between maize and perennial grasslands. Variation between maize, switchgrass, and prairie in production of aboveground plant biomass (A), methane consumption by soil methanotrophs (B), predation of pest eggs by beneficial insects (C), pollination of sentinel sunflower plants (maize and prairie only) (D), occurrence of obligate grassland birds (E), and pest aphid pressure (F). Pie graphs in A show the percentage of biomass composed of the dominant C4 grass species (Z. mays in maize, P. virgatum in switchgrass, and A. gerardii in prairie), other grasses, and forbs. Error bars are ±1 SE. Note logarithmic (A and F) and logit-scale axes (E).
Fig. 3.
Differences in richness and ecological processes were larger between the two perennial grasslands and maize than between prairie and switchgrass. Standardized effect sizes (Hedge’s D) are shown for differences in richness and key ecological processes between grasslands and maize (A and C) (effect is difference between average of the two grasslands and maize) and prairie compared with switchgrass (B and D). Error bars show 98% confidence intervals. Asterisks indicate statistical significance at α = 0.02.
We also quantified ecological processes involving each focal group, including plant primary productivity, consumption of methane by soil bacteria, consumption of insect pest eggs by arthropod natural enemies, pollination, colonization by pest aphids, and habitat use of grassland birds. Although maize fields produced an order of magnitude more aboveground biomass than the two perennial grass systems, all other beneficial processes measured were greater in grasslands (Fig. 2). In grasslands, rates of methane consumption were an order of magnitude higher, predation of pest eggs by beneficial insects increased by a factor of two, and grassland birds—a nationally imperiled group (16)—were observed twice as frequently (Fig. 2 A–D). In addition, pollination of sentinel sunflowers almost doubled adjacent to prairie, and pressure of pest aphids was ∼50% lower in prairie compared with maize (Fig. 2 E and F). Effect statistics likewise identified substantial differences between maize and perennial grass plantings, which were statistically significant for all processes except pollination (Fig. 3C). In contrast, differences between prairie and switchgrass were near zero (Fig. 3D).
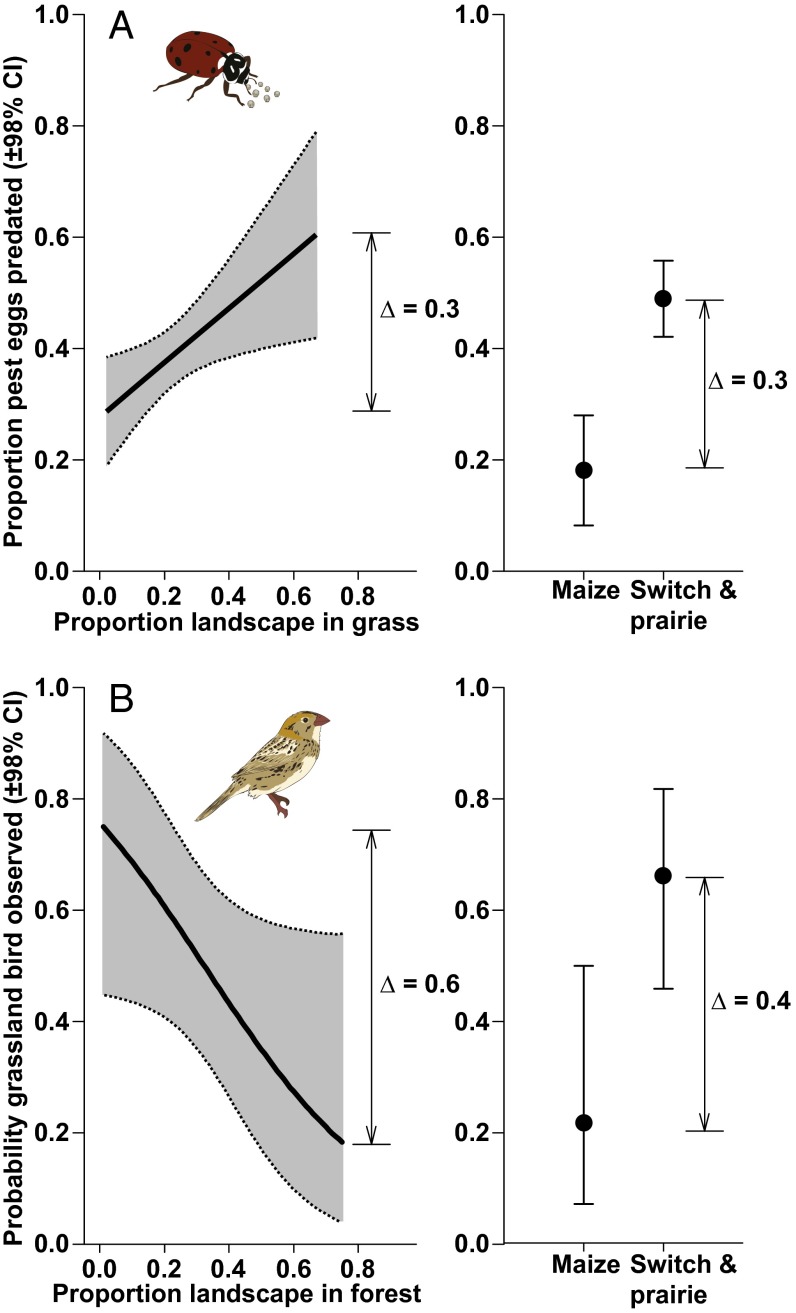
Multiple regression models that incorporated landscape influences on pest suppression (17) and bird prevalence (16) showed that landscape composition—the identity and extent of nearby vegetation types—can have as strong an impact on service provisioning as the nature of the planting itself (Fig. 4). After accounting for differences in crop types, our model predicted that rates of predation of pest eggs by beneficial insects increased by 30% as the extent of grassland within 1.5 km of a focal field increased to maximum observed values (Fig. 4A). This was comparable in size to the difference in within-field service provisioning between maize and perennial grass plantings. Likewise, our model of landscape influence on grassland birds predicted a 60% increase in occupancy of patches as surrounding landscapes became less forested at the 1.5-km scale (Fig. 4B), an increase that was again comparable to differences between planting types. Land use patterns at these scales are typically influenced by multiple landowners, suggesting a role for regional planning to maximize provisioning of ecosystem services in agricultural landscapes.
Fig. 4.
Multiple-regression models indicated that habitats surrounding bioenergy crops can have as large an effect on ecosystem services as the crop itself. Predation of pest eggs (A) increased by 30% as the proportional area of grasslands within 1.5 km of sites increased, an effect as large as differences between maize and the two grasslands. The probability of observing a grassland-dependent bird species (B) declined by 60% as forest land cover increased within 1.5 km of sites, an effect larger than differences between maize and grasslands.
Discussion
Considering a broad suite of ecosystem services could alter conclusions about the relative value of different bioenergy crops. Relatively high aboveground productivity (Fig. 2A) along with current price and policy incentives cause maize to outcompete switchgrass and prairie when farmer income is the only consideration (18). However, our data show that perennial grasses support greater biodiversity and higher rates of a variety of other ecosystem services (e.g., pest suppression and pollination) valuable to society as a whole (Fig. 3). Planting perennial energy crops on marginal lands could maintain or enhance these functions, complementing and even feeding back to benefit commodity production on prime agricultural land (10). In this respect, these perennial systems provide a means to support bioenergy goals, broaden the portfolio of services supported by agricultural landscapes, and support their long-term functioning (19).
Site-level management decisions are likely to alter ecosystem services provided by bioenergy crops. For example, incorporating features of perennial systems into maize, through practices like cover cropping, could enhance services such as greenhouse gas mitigation (20). Alternatively, intensifying grassland management by fertilization may increase biomass production but reduce other services. For example, fertilization will likely reduce plant diversity (21), particularly of forbs, and so likely also reduce pollination and pest suppression services (17). Our landscape analyses—in which grasslands were broadly defined to include lands such as pastures and hayfields which are managed for agricultural production—have detected positive effects of grasslands on services (Fig. 4A) (9, 10). This suggests that it is possible to actively manage grasslands for biomass while maintaining their positive contribution to other ecosystem services.
For agricultural landscapes to be sustainable, production of food, energy, and biodiversity need to coexist (22). Using policy to encourage thoughtful placement of energy crops in the landscape could allow agriculture to take advantage of ecosystem service synergies (23). Highly intensified annual crop landscapes might be strategically diversified with perennial grassland bioenergy crops, increasing biodiversity (9) and pest suppression in annual crops (10) while reducing water pollution (11), GHG fluxes (14), and reliance on pesticides (24, 25). Our ability to design sustainable agroenergy landscapes that producers will implement requires additional understanding of the costs of potential ecosystem service tradeoffs (25), elucidation of “bundles” of ecosystem services that could be jointly produced (26), and understanding society’s perception of the value of these services (27). Finally, realizing the benefits of such landscape design will require policies that encourage landowners to make informed and coordinated decisions at the landscape scale. Prior work on biodiversity conservation at landscape scales suggests that the benefits of such coordinated land use decisions are substantial (28) and that spatially explicit incentives show promise as a voluntary tool for achieving desired landscape configurations (29, 30). Applying such lessons to the development of agricultural landscapes for sustainable bioenergy production is a logical next step.
Materials and Methods
Sample Sites.
We collaborated with farmers, private landowners, and state land managers to locate 115 maize, switchgrass, and prairie plantings across the major crop production regions of Michigan and Wisconsin (Fig. S1 and Table S3). Management of these plantings is described in Table S2. Sampling at each site was conducted in four plots spaced 50 m apart at the corners of a 50 × 50 m square, with two plots located 50 m from the habitat edge and two located 100 m away. For more narrow sites, plots were arranged in a linear transect down the center of each patch with the first plot 50 m from the edge and remaining stations spaced 50 m apart. Departures from this design are noted below.
Taxonomic Richness.
We measured the taxonomic richness of plants, microbes, and animals present in each habitat, sampling different subsets of our total pool of sites for each experiment (Text S1 and Table S3). Plant species richness was measured by recording all species (planted and naturally colonizing) present in four 100-m2 circular plots at 10 maize, 13 switchgrass, and 12 prairie sites between 2008 and 2010. Methanotroph richness was measured by taking soil samples (10 cm deep × 2.5 cm diameter) from four maize, five switchgrass, and eight prairie plantings during 2009–2011 and quantifying the total number of genetically distinct strains [operational taxonomic units (OTUs)] present at each site. Two cores were taken at each of the four plots and aggregated at the site level and transported to the laboratory on ice, where they were sieved to 4 mm and then stored at −80 °C for genetic analysis (Text S1). For insects and spiders (“arthropods”), we separately measured richness of herbivores, predators, and bees. Richness of herbivorous and predatory arthropods was measured by taking 100 sweeps with a sweep net at 19 maize, 20 switchgrass, and 20 prairie sites in June and July of 2008 and 2009 and determining the number of families in each of these two groups; methods are detailed elsewhere (31). Bee species richness was measured by trapping bees in arrays of white, yellow, and blue 29-mL soufflé cups filled with soapy water. Traps were deployed for 48 h in June, July, and August on platforms (Text S1) at four plots in 20 maize, 20 switchgrass, and 20 prairie sites in 2009. Before analysis, one observation was removed from both the predatory arthropod and bee richness datasets; these observations were sixfold and fivefold greater than median values of predatory arthropod and bee richness, respectively, and were the largest outliers across all datasets. Finally, breeding bird richness was measured by visually or aurally identifying all species perching, feeding, or singing during whole-field searches at 20 sites of each habitat during 2008 and 2009; see published methods (16). All data are available in Dataset S1.
Ecological Processes.
We measured key ecological processes supported by the plant, microbe, and animal communities inhabiting maize, switchgrass, and prairie plantings (Table S1). For plants, we measured the current year’s production of aboveground biomass by collecting, drying (65 °C for ≥72 h), and weighing standing vegetation from four 0.5 × 2 m quadrats at 16 maize, 10 switchgrass, and 10 prairie sites during 2008–2010 (Text S1). We sorted vegetation into the dominant C4 grasses (Andropogon gerardii, Sorghastrum nutans, and P. virgatum), other grasses, and forbs to estimate functional group composition. We measured consumption of methane by soil microbes by sinking seven cylindrical chambers (28 cm diameter and 26 cm height) 5 cm into the ground at five maize, six switchgrass, and six prairie sites in 2011. Chambers were equipped with a removable lid and septum, allowing multiple samples to be taken on July 15, August 18, and October 5, 2011 (Text S1). For arthropods, we measured the attractiveness of habitats to aphids (Hemiptera: Aphididae), which is related to the incidence of plant viruses that these insect herbivores vector. We sampled aphids using yellow bowl traps (horizontal surface area ∼145 cm2) filled with 25% (vol/vol) propylene glycol and water. Four traps spaced 40 m apart were deployed at five maize, seven switchgrass, and six prairie sites. Traps were 0.5 m above vegetation and were raised as canopies grew taller. Samples were taken weekly between June 8 and 29, 2009. We also quantified the ability of predatory arthropods to suppress pests by measuring predation of pest eggs placed out at four plots in 20 maize, 20 switchgrass, and 20 prairie sites in June and July 2009. This technique, detailed in published work (17), provides information on the activity of a wide range of invertebrate predators important in suppressing crop pests. For bees, we measured pollination of sentinel dwarf sunflowers (Helianthus annuus L., “Sunspot”) placed out at 10 maize and 10 prairie sites in 2010; switchgrass sites were not sampled for pollination. Sunflowers were grown in the greenhouse; after developing two to eight florets, two sets of four plants were placed in the grassy margins adjacent to each maize and prairie field and exposed to pollination for 1 wk. Cages were used to exclude or allow pollinator access to flowers to estimate the effect of bee pollination on seed set (Text S1). Finally, we recorded the presence or absence of obligate grassland birds during area searches at 20 maize, 19 switchgrass, and 20 prairie sites in 2008 and 2009 using published methods (16). All data are available in Dataset S1.
Data Analysis.
Either samples were lumped for each site (richness of methanotrophic bacteria) or data were averaged across subsamples to obtain a single site average. Datasets used for plant richness and biomass, herbivorous and predatory arthropods, methanotrophs, and birds contained a mixture of single-year and multiyear observations (Text S1). Preliminary analyses suggested that there was no systematic variation in these variables between years (Text S1) (16). Consequently, data from sites visited in multiple seasons were averaged across years to obtain a single observation for analysis.
We estimated taxonomic richness using approaches appropriate for each organism. For plants, we used the mean number of species per 100 m2 as a measure of species density that was directly comparable among sites. For methanotroph bacteria, we used raw data on richness of OTUs because past work indicated that the level of sampling conducted here was sufficient to sample the majority of species in the community. However, for arthropods, it was unlikely that all taxa were detected. In this case, raw values of richness would have been affected by the total number of individuals captured. This could confound differences in richness with differences in abundance or sampling efficiency between habitats. To account for this, a Chao1 estimator was used to estimate asymptotic richness for families of herbivorous and predatory arthropods and bee species at each site (32).
We applied a common set of statistical analyses to compare richness and rates of key ecological processes between maize, switchgrass, and prairie (Dataset S1). Generalized linear models were used to calculate means and confidence intervals by specifying habitat type (maize, switchgrass, or prairie) as a categorical variable. For birds, we also included log10(x)-transformed patch area as a covariate because grassland bird richness is known to increase with patch area (16). Models were fit using Gaussian (plant, methanotroph and bee richness, methane consumption, and log10(x)-transformed captures of aphids), Poisson (breeding bird richness), quasi-Poisson (arthropod predators and herbivores), gamma (plant biomass), or binomial distributions (occurrence of grassland birds) as indicated by residual diagnostic plots (33). For pollination, we fit a generalized least squares model with separate variances for maize and prairie because neither data transformation nor use of nonnormal distributions accounted for overdispersion. Models were implemented using the “glm” and “gls” functions of R version 2.15.1 (34); means and SEs were calculated using the “effects” package of R. For birds, mean richness was calculated for each habitat at a common area of 10 ha.
We next used preplanned contrasts in combination with standardized effect statistics to test hypotheses and display differences on a common scale. A first group of contrasts was used to compare response variables between maize vs. switchgrass and prairie, to test the hypothesis that taxonomic richness and rates of key processes differ between lightly managed perennial grasslands compared with a highly managed annual crop. A second group of contrasts was used to compare variables between switchgrass and prairie and test the hypothesis that within lightly managed grasslands, communities differ between habitats with low and high levels of planted diversity. We then calculated Hedge’s D and 98% confidence intervals using the approach of Nakagawa and Cuthill (15) and R code therein. Setting α = 0.02 for each contrast maintained an overall error rate of α = 0.04 for the full set of two contrasts calculated for each variable. Contrasts were calculated using the “contrast” package of R version 2.15.1 (34). For pollination we were able to estimate differences between only maize and prairie because pollination was not measured in switchgrass. Statistical code is available in Dataset S2.
Highlighting Landscape Dependencies.
Earlier work has shown that rates of ecological processes in maize, switchgrass, and prairie depend not only on local plant communities but also on the composition of surrounding landscapes (16, 17). Specifically, predation of pests (17) and the occurrence of grassland birds (16) varied with the extent of grassland and forest cover in landscapes. Here we combine multiple regression models with effect displays (35) to directly compare the magnitude of these landscape effects to differences between plant communities. To focus on the major differences documented between maize and grassland habitats (Figs. 1 and 2), we first changed the three-level habitat variable (maize, switchgrass, or prairie) used in initial analyses into a binary one that indicated whether a patch was maize vs. one of the two perennial grasslands. Likelihood ratio tests suggested that reducing habitat type to a binary variable did not result in a significant loss of explanatory power (predation: χ2 = 1.5, df = 1, P = 0.2; bird occurrence: χ2 = 0.01, df = 1, P = 0.9).
To directly compare habitat and landscape effects, we next incorporated landscape covariates identified as important in earlier work. For predation of pest eggs, we included variables describing the areal extent of grasslands and forests within 1.5 km of sites (17). The effect of forest cover was only modeled to ensure accurate estimation of the effect of grassland extent; forest effects were weak and are not reported here (17). For grassland birds, earlier analyses used principal component analysis (PCA) to describe a gradient ranging from crop- to forest-dominated landscapes. Here we described this gradient using the proportion of forest in the landscape within 1.5 km of sites, which was strongly correlated with the original PCA variable (Pearson’s r = 0.96) and is more interpretable. Calculation of landscape variables is described in refs. 16 and 17. As expected, likelihood ratio tests showed that incorporating landscape variables significantly improved model fit for predation of pest eggs (χ2 = 10.4, df = 2, P = 0.006) and birds (χ2 = 5.6, df = 1, P = 0.02).
Effect displays (35) were constructed from these regression models to isolate and compare landscape effects to differences between plant communities. Briefly, landscape effects were visualized by plotting variation in the across-habitat mean of responses as a function of landscape composition, whereas differences between plant communities were isolated by calculating means for maize and grasslands at average levels of landscape covariates. Further details of this approach are described in ref. 35. Regression lines, means, and confidence intervals were calculated using the “effects” package of R version 2.15.1 (34).
Supplementary Material
Acknowledgments
We thank C. Baker, N. Batora, and numerous undergraduate students for help with data collection; S. Nakagawa for advice on effect statistics; D. Schemske and J. Tiedje for critical reviews; cooperating landowners; and Brett Blaauw and the US Department of Energy (DOE) Genomic Science program (http://genomicscience.energy.gov) for illustrations. This work was funded in part by the DOE Great Lakes Bioenergy Research Center DOE Biological and Environmental Research Office of Science (Grant DE-FC02-07ER64494), the DOE Office of the Biomass Program Office of Energy Efficiency and Renewable Energy (Grant DE-AC0576RL01830), the US National Science Foundation Long-Term Ecological Research program Division of Environmental Biology (Grant 1027253), US Department of Agriculture National Institute of Food and Agriculture (Grant 2011-67009-30137), and Michigan State University AgBioResearch.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1309492111/-/DCSupplemental.
References
- 1.Dauber J, et al. Bioenergy from “surplus” land: Environmental and socio-economic implications. BioRisk. 2012;7:5–50. [Google Scholar]
- 2.Langpap C, Wu J. Potential environmental impacts of increased reliance on corn-based bioenergy. Environ Resour Econ. 2011;49(2):147–171. [Google Scholar]
- 3.Secchi S, Gassman PW, Williams JR, Babcock BA. Corn-based ethanol production and environmental quality: A case of Iowa and the conservation reserve program. Environ Manage. 2009;44(4):732–744. doi: 10.1007/s00267-009-9365-x. [DOI] [PubMed] [Google Scholar]
- 4. USDA-NASS (2012) Acreage (USDA-NASS, Washington, DC). Available at www.usda.gov/nass/PUBS/TODAYRPT/acrg0613.pdf. Accessed October 30, 2013.
- 5. Faber S, Rundquist S, Male T (2012) Plowed Under: How Crop Subsidies Contribute to Massive Habitat Losses (Environmental Working Group, Washington, DC). Available at http://static.ewg.org/pdf/plowed_under.pdf. Accessed April 21, 2013.
- 6.Wright CK, Wimberly MC. Recent land use change in the Western Corn Belt threatens grasslands and wetlands. Proc Natl Acad Sci USA. 2013;110(10):4134–4139. doi: 10.1073/pnas.1215404110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Energy Independence and Security Act of 2007 (2007) H.R.6. 110th Congress of the United States of America, 1st Session.
- 8.Gelfand I, et al. Sustainable bioenergy production from marginal lands in the US Midwest. Nature. 2013;493(7433):514–517. doi: 10.1038/nature11811. [DOI] [PubMed] [Google Scholar]
- 9.Meehan TD, Hurlbert AH, Gratton C. Bird communities in future bioenergy landscapes of the Upper Midwest. Proc Natl Acad Sci USA. 2010;107(43):18533–18538. doi: 10.1073/pnas.1008475107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meehan TD, Werling BP, Landis DA, Gratton C. Pest-suppression potential of midwestern landscapes under contrasting bioenergy scenarios. PLoS ONE. 2012;7(7):e41728. doi: 10.1371/journal.pone.0041728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parish ES, et al. Multimetric spatial optimization of switchgrass plantings across a watershed. Biofuels Bioprod Biorefin. 2012;6(1):58–72. [Google Scholar]
- 12.Dauber J, Jones MB, Stout JC. The impact of biomass crop cultivation on temperate biodiversity. GCB Bioenergy. 2010;2(6):289–309. [Google Scholar]
- 13.Gelfand I, Snapp SS, Robertson GP. Energy efficiency of conventional, organic, and alternative cropping systems for food and fuel at a site in the U.S. Midwest. Environ Sci Technol. 2010;44(10):4006–4011. doi: 10.1021/es903385g. [DOI] [PubMed] [Google Scholar]
- 14.Robertson GP, Hamilton SK, Del Grosso SJ, Parton WJ. The biogeochemistry of bioenergy landscapes: Carbon, nitrogen, and water considerations. Ecol Appl. 2011;21(4):1055–1067. doi: 10.1890/09-0456.1. [DOI] [PubMed] [Google Scholar]
- 15.Nakagawa S, Cuthill IC. Effect size, confidence interval and statistical significance: A practical guide for biologists. Biol Rev Camb Philos Soc. 2007;82(4):591–605. doi: 10.1111/j.1469-185X.2007.00027.x. [DOI] [PubMed] [Google Scholar]
- 16.Robertson BA, Doran PJ, Loomis LR, Robertson JR, Schemske DW. Perennial biomass feedstocks enhance avian diversity. GCB Bioenergy. 2011;3(3):235–246. doi: 10.1371/journal.pone.0016941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Werling BP, Meehan TD, Robertson BA, Gratton C, Landis DA. Biocontrol potential varies with changes in biofuel-crop plant communities and landscape perenniality. GCB Bioenergy. 2011;3(5):347–359. [Google Scholar]
- 18.James LK, Swinton SM, Thelen KD. Profitability analysis of cellulosic energy crops compared with corn. Agron J. 2010;102(2):675–687. [Google Scholar]
- 19.Atwell RC, Schulte LA, Westphal LM. How to build multifunctional agricultural landscapes in the US Corn Belt: Add perennials and partnerships. Land Use Policy. 2010;27(4):1082–1090. [Google Scholar]
- 20.Robertson GP, Paul EA, Harwood RR. Greenhouse gases in intensive agriculture: Contributions of individual gases to the radiative forcing of the atmosphere. Science. 2000;289(5486):1922–1925. doi: 10.1126/science.289.5486.1922. [DOI] [PubMed] [Google Scholar]
- 21.DiTommaso A, Aarssen LW. Resource manipulations in natural vegetation: A review. Plant Ecol. 1989;84(1):9–29. [Google Scholar]
- 22.Tilman D, et al. Energy. Beneficial biofuels—The food, energy, and environment trilemma. Science. 2009;325(5938):270–271. doi: 10.1126/science.1177970. [DOI] [PubMed] [Google Scholar]
- 23.Power AG. Ecosystem services and agriculture: Tradeoffs and synergies. Philos Trans R Soc Lond B Biol Sci. 2010;365(1554):2959–2971. doi: 10.1098/rstb.2010.0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meehan TD, Werling BP, Landis DA, Gratton C. Agricultural landscape simplification and insecticide use in the Midwestern United States. Proc Natl Acad Sci USA. 2011;108(28):11500–11505. doi: 10.1073/pnas.1100751108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang W, Ricketts TH, Kremen C, Carney K, Swinton SM. Ecosystem services and dis-services to agriculture. Ecol Econ. 2007;64(2):253–260. [Google Scholar]
- 26.Raudsepp-Hearne C, Peterson GD, Bennett EM. Ecosystem service bundles for analyzing tradeoffs in diverse landscapes. Proc Natl Acad Sci USA. 2010;107(11):5242–5247. doi: 10.1073/pnas.0907284107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martín-López B, et al. Uncovering ecosystem service bundles through social preferences. PLoS ONE. 2012;7(6):e38970. doi: 10.1371/journal.pone.0038970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Polasky S, et al. Where to put things? Spatial land management to sustain biodiversity and economic returns. Biol Conserv. 2008;141(6):1505–1524. [Google Scholar]
- 29.Parkhurst GM, Shogren JF. Spatial incentives to coordinate contiguous habitat. Ecol Econ. 2007;64(2):344–355. [Google Scholar]
- 30.Parkhurst GM, et al. Agglomeration bonus: An incentive mechanism to reunite fragmented habitat for biodiversity conservation. Ecol Econ. 2002;41(2):305–328. [Google Scholar]
- 31.Robertson BA, Porter C, Landis DA, Schemske DW. Agroenergy crops influence the diversity, biomass, and guild structure of terrestrial arthropod communities. BioEnergy Res. 2012;5(1):179–188. [Google Scholar]
- 32.Chao A. Non-parametric estimation of the number of classes in a population. Scand J Stat. 1984;11(4):265–270. [Google Scholar]
- 33.Zuur AF, Ieno EN, Walker N, Saveliev AA, Smith GM. Mixed Effects Models and Extensions in Ecology with R. New York: Springer; 2009. [Google Scholar]
- 34. R Core Development Team (2006) R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna). Available at www.R-project.org. Accessed April 21, 2013.
- 35.Fox J. Effect displays in R for generalised linear models. J Stat Software. 2003;8(15):1–27. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.