Abstract
Importin α is well known as an adaptor that functions with Importin β in the nuclear import of proteins containing specific nuclear localization signals (NLSs). We show here that either an excess or a lack of Importin α blocks nuclear envelope (NE) assembly in vitro, and our data suggest that soluble Importin α functions in NE assembly in conjunction with NLS-containing partner proteins. Surprisingly, a significant proportion of Importin α is found to fractionate with Xenopus egg membranes. We demonstrate that membrane association of Importin α is regulated by phosphorylation. Using mutant forms of Importin α that either do not bind membranes or are not released from them by phosphorylation, we provide evidence that membrane-associated Importin α is required for NE formation. Unlike other functions of Importin α, this membrane-associated activity does not require interaction with NLS proteins.
Keywords: Importin α, nuclear assembly, nuclear envelope, protein phosphorylation
Introduction
In eukaryotes, nuclear and cytoplasmic structures and components are kept separate during interphase. The nucleus is delimited by the nuclear envelope (NE), consisting of the inner (INM) and outer (ONM) nuclear membranes separated by a lumen and penetrated by nuclear pore complexes (NPCs). The INM harbours a unique set of proteins, which interact either with the underlying nuclear lamina and/or with chromatin (Foisner, 2001; Holmer and Worman, 2001). The ONM is continuous with the endoplasmic reticulum (ER) and no ONM-specific proteins have yet been described. The INM and ONM are joined at NPCs, large proteinaceous structures that form aqueous channels through which both active and passive macromolecular movement occurs.
In metazoa, the NE disassembles during mitosis. The NE membranes and INM proteins are apparently dispersed throughout the endoplasmic reticulum (Ellenberg et al, 1997; Yang et al, 1997; Mattaj, 2004). Late in mitosis, the NE reassembles around the decondensing chromosomes. The process of NE re-assembly can be reconstituted in vitro using cell-free extracts such as those obtained from Xenopus laevis eggs (Marshall and Wilson, 1997). In vitro NE assembly can be divided into several steps: membrane vesicle docking to the decondensing chromatin surface, fusion of those membranes to form a tubular network on the chromatin surface that already contains NPC components, further fusion leading to closed nuclear envelope (CNE) formation and, finally, nuclear growth after NPC insertion (Wiese et al, 1997; Hetzer et al, 2001, 2002; Mattaj, 2004).
The GTPase Ran has been shown to play a crucial role in NE assembly in vitro and in vivo (Hetzer et al, 2000; Zhang and Clarke, 2000; Askjaer et al, 2002; Bamba et al, 2002). Other functions of Ran in nucleocytoplasmic transport and mitotic spindle assembly are mediated by the Importin β (or Karyopherin) family of importins and exportins (Görlich and Kutay, 1999; Nachury et al, 2001; Hetzer et al, 2002; Quimby and Dasso, 2003). Indeed, in vivo data obtained in both Drosophila melanogaster and Caenorhabditis elegans suggest a role for Importin β in postmitotic NE assembly (Askjaer et al, 2002; Timinszky et al, 2002; Tirian et al, 2003), and in vitro studies indicate that Importin β might be involved in two separate steps of NE formation, membrane fusion and NPC insertion (Harel et al, 2003; Walther et al, 2003).
In nucleocytoplasmic transport, Importin β can either act alone, by binding directly to cargo molecules, or in conjunction with other transport mediators. The best studied of the latter is the adaptor molecule Importin α. Importin α binds to Importin β via an N-terminal IBB domain and functions in the import of cargoes via several classes of nuclear localization signals (NLSs), including the so-called classical and bipartite basic NLSs (Görlich and Kutay, 1999). Genetic data have suggested that Importin α might have roles independent of its function in nuclear import. For example, a study in Schizosaccharomyces pombe indicated a function for Importin α in mitotic chromosome condensation (Matsusaka et al, 1998). More recently, one of the Importin α isoforms in D. melanogaster has been shown to play a role in the formation of ovarian ring canals, plasma membrane structures that bridge between germline cells in the follicle. Although mechanistically related to nuclear import, this function of Importin α is apparently entirely distinct from its transport role (Gorjanacz et al, 2002) and may instead relate to the proposed general role of nuclear transport mediators as protein chaperones (Jäkel et al, 2002).
Here we investigate the role of Importin α in NE assembly in vitro. Recent in vivo studies have provided evidence of such a role for the C. elegans IMA-2 gene product, one of the Importin αs produced in this organism (Askjaer et al, 2002; Geles et al, 2002). Our data demonstrate a requirement for Importin α in NE assembly in vitro, and suggest two distinct functions for Importin α in this process. One function seems to require interaction with NLS-containing proteins. The second is unconventional, and involves phosphorylation state-dependent association of Importin α with membranes, but does not depend on Importin α-NLS protein binding.
Results
NE formation is inhibited both by excess Importin α and BSA-NLS
To obtain the first indication of whether Importin α might have a role in NE formation, we performed in vitro nuclear assembly reactions in interphase Xenopus egg extracts in the presence of exogenous Importin α. Importin α, when added to 10 μM over its endogenous concentration of 3 μM (Görlich et al, 1994), had a strong inhibitory effect on CNE formation (Figure 1A, middle and bottom panels). Although membrane vesicles (green) were able to bind to chromatin (blue) in the presence of excess Importin α, CNE formation was greatly reduced. Importin α at this concentration did not completely block membrane fusion, as a membrane network was observed on the surface of some chromatin substrates (Figure 1A, bottom panel). In the buffer control, most of the chromatin substrates were assembled into a CNE and nuclear growth occurred normally. Upon addition of exogenous Importin α, only 30% of the substrates formed a CNE (Figure 1B).
Figure 1.
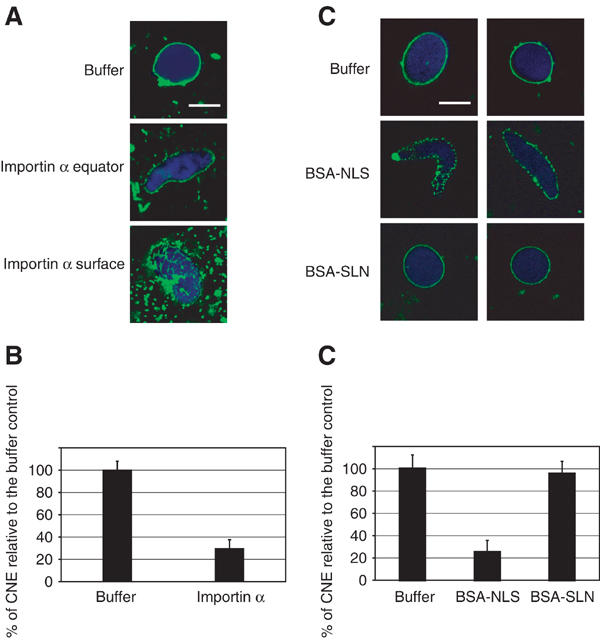
Excess of exogenous Importin α and BSA-NLS inhibits CNE formation. (A) Recombinant Importin α (10 μM) was pre-incubated with Xenopus laevis interphase egg extracts for 10 min on ice before addition of sperm chromatin and membranes. Reactions were then incubated at 20°C for 90 min. In the control reaction (buffer), CNEs formed, with high efficiency. Upon addition of exogenous Importin α, CNE formation was much less efficient. However, membrane vesicles still docked to chromatin and a membrane network was observed on the surface of the chromatin. DNA was stained with DAPI (blue), membranes were post-stained with DiIC6 (green). Scale bar, 10 μm. (B) The formation of a CNE was scored for at least 80 randomly chosen chromatin substrates and normalized to the buffer control (data from three independent experiments). (C) Recombinant BSA-NLS or BSA-SLN (20 μM) were pre-incubated with Xenopus laevis interphase egg extracts for 10 min on ice before addition of sperm chromatin and membranes. Addition of BSA-NLS strongly inhibited CNE formation, although membrane vesicles docked to chromatin. BSA-SLN did not inhibit CNE formation and nuclear growth occurred normally. Scale bar, 10 μm. (D) The formation of a CNE was scored for at least 80 randomly chosen chromatin substrates and normalized to the buffer control (data from three independent experiments).
Another way to address the potential role of Importin α in NE formation was to test whether saturation of Importin α with an exogenous NLS-containing substrate would affect CNE formation. We therefore tested the ability of BSA conjugated either to NLS peptides (BSA-NLS) or control peptides of identical amino-acid composition, but with the reverse sequence (BSA-SLN) to inhibit CNE formation. When BSA-NLS was added to the reaction, a strong inhibition of CNE formation was observed (Figure 1C). The chromatin stayed elongated and membrane fusion was inhibited (middle panels). BSA-SLN did not inhibit CNE formation (bottom panels). Quantitation of three independent experiments (Figure 1D) showed that no significant difference was observed between the buffer control and BSA-SLN, with around 95% of chromatin substrates assembled into a CNE. Upon addition of BSA-NLS, only 25% of the substrates formed a CNE. Perturbing Importin α by addition of BSA-NLS thus prevents NE formation. This, together with the effect of excess Importin α described above, suggested a direct or indirect role for Importin α in NE assembly.
Importin α depletion prevents NE formation
To further examine the role of Importin α in NE formation, the effect of adding an affinity-purified anti-Importin α antibody was tested. Xenopus interphase egg cytosol was pre-incubated for 20 min on ice with anti-Importin α antibodies or control antibody, prior to the addition of membranes and sperm chromatin. Reactions were further incubated and CNE formation was monitored. Addition of anti-Importin α antibodies strongly blocked NE formation (Figure 2A, middle panels), while control antibodies added under the same conditions did not show any inhibitory effect (lower panels). No quantitative difference was observed between the addition of buffer or of this control antibody, whereas addition of antibodies directed against Importin α strongly inhibited the formation of a CNE around chromatin (Figure 2B).
Figure 2.
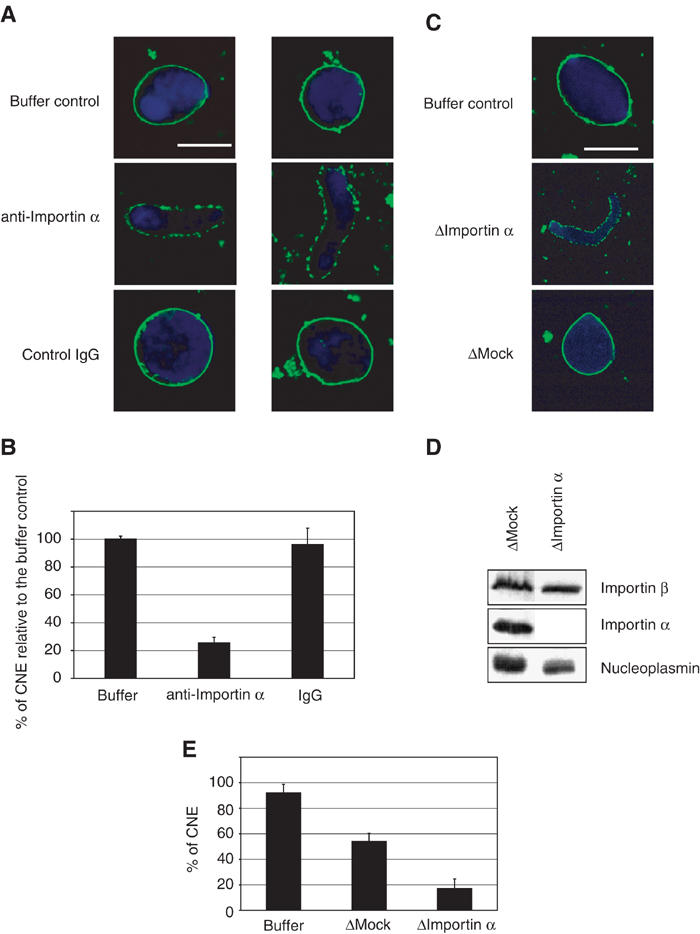
Depletion of endogenous Importin α prevents NE formation. (A) Affinity-purified antibodies directed against Importin α were pre-incubated with Xenopus interphase egg extracts for 20 min on ice before addition of sperm chromatin and membranes. This resulted in a strong reduction in CNE formation (middle panels) compared to addition of either buffer or a control antibody under the same conditions (lower and upper panels). DNA was stained with DAPI (blue), membranes were post-stained with DiIC6 (green). Scale bar, 10 μm. (B) Quantitation of CNE formation as a fraction of chromatin substrates normalized to the buffer control. In all, 100 randomly chosen chromatin substrates were counted in each of three independent experiments. (C) Depletion of Importin α from Xenopus interphase egg extracts inhibits CNE formation. NE assembly reactions were carried out in the presence of untreated extract (buffer control) and mock- or Importin α-depleted extracts. Formation of CNEs occurred in mock-depleted extracts, although not as efficiently as in the untreated extracts (55% as compared to 85% CNE formation). In Importin α-depleted extracts, a strong additional inhibition of CNE formation was observed (15% CNE formation). (D) Equal quantities of either mock- or Importin α-depleted extracts, as indicated, were analysed by Western blotting with anti-Importin β, anti-Importin α or anti-nucleoplasmin antibodies. Nucleoplasmin is a marker for NLS-containing proteins. (E) Quantitation of CNE formation as a fraction of chromatin substrates (data from three independent experiments).
Next, Importin α was depleted from the cytosolic fraction using affinity-purified anti-Importin α antibody. Efficient depletion of Importin α from cytosol was seen (Figure 2D). While Importin β was not co-depleted, a significant reduction in the levels of nucleoplasmin was observed (Figure 2D), suggesting that, not unexpectedly, some NLS-containing proteins were partially co-depleted with Importin α.
The effect of Importin α depletion on NE assembly was assayed. CNE formation was somewhat reduced by mock depletion as compared to the buffer control (Figures 2C and E). Importin α depletion, however, resulted in a much greater reduction in CNE assembly as compared to the controls (Figure 2C, middle panel, 2E). Re-addition of recombinant Importin α to the Importin α-depleted extracts only led to a minor recovery in the rate of CNE formation (data not shown). This lack of complementation is possibly due to the co-depletion of NLS-containing cargo proteins with Importin α. In summary, the data thus far suggest that soluble Importin α, via interaction with NLS-containing proteins, functions in NE assembly. The data further suggest that the correct balance of Importin α and NLS proteins is critical.
A fraction of Importin α is membrane-associated
Importin α is well known as a soluble adaptor molecule involved in the import of NLS-bearing proteins and as a regulated inhibitor of mitotic spindle formation (Görlich and Kutay, 1999; Gruss et al, 2001). Unexpectedly, however, when quantities of cytosolic (C) and membrane (M) fractions of egg extracts containing an equal protein concentration were analysed using anti-Importin α antibodies, we found that Importin α was present on the membranes as well as in the cytosol (Figure 3A). In contrast, Importin β, CAS and Ran, all direct or indirect partners of Importin α in nucleocytoplasmic transport and spindle formation, were not detectable in the membrane fractions.
Figure 3.
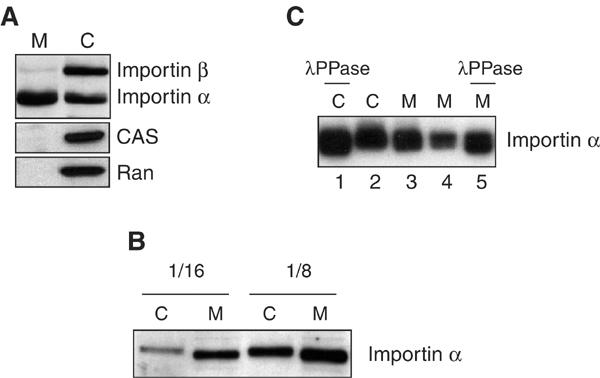
Importin α is associated with egg extract membranes. (A) Interphase egg extract was fractionated into cytosol (C) and membranes (M). Equal quantities of protein from these fractions were analysed by Western blotting with anti-Importin β, anti-Importin α , anti-CAS or anti-Ran antibodies. (B) Cytosolic and membrane-associated Importin α exhibit different mobilities on SDS–PAGE. Cytosolic (C) and membrane (M) fractions of interphase egg extracts were diluted 1:16 or 1:8 in buffer, separated by 10% SDS–PAGE and anti-Importin α Western blotting was carried out. (C) Cytosolic and membrane-associated Importin α are differentially phosphorylated. Cytosol (C) and membrane (M) fractions were treated with lambda phosphatase (λPPase) for 30 min at 30°C. Two different amounts of the membrane fraction (M) were analysed. Aliquots of the reactions were loaded on a 12% SDS–PAGE gel and analysed by Western blotting with anti-Importin α antibodies.
Closer examination of the Importin α in the membrane or cytosolic fractions revealed a difference in SDS–PAGE mobility between the two (Figure 3B). The cytosolic form of Importin α displayed a slightly lower mobility than the membrane-bound form of Importin α. To test whether this could be due to phosphorylation, cytosolic and membrane fractions were incubated with lambda protein phosphatase, and the mobility of Importin α was analysed. Phosphatase treatment increased the mobility of the Importin α in both fractions (Figure 3C, compare lane 1 with 2 and lane 5 with 3 and 4; note that the electrophoresis conditions are different in 3B and 3C, and that Importin α runs as a doublet in 3C). The cytosolic fraction of Importin α was more affected in mobility by phosphatase treatment than the membrane-associated fraction. This suggests that both cytosolic and membrane-associated Importin α are phosphorylated, but that the cytosolic protein is more highly phosphorylated.
In vitro phosphorylation of Importin α
Importin α has been reported to be phosphorylated in yeast by casein kinase II (CKII) in an NLS-dependent manner (Azuma et al, 1995, 1997). To test whether this would also be the case for Xenopus Importin α, bacterially expressed Importin α was incubated with CKII in the presence or absence of BSA-NLS. A mobility shift of Importin α was only seen when Importin α was incubated in the presence of both BSA-NLS and CKII (Figure 4A). Analysis of [32P]-γ-phosphate transfer from ATP to recombinant Importin α by purified CKII revealed that Importin α can be phosphorylated under those conditions both in the presence and absence of NLS peptides (data not shown), but that the event that changes Importin α mobility only occurs in the presence of BSA-NLS. A mutant form of Importin α that does not bind efficiently to NLS proteins, the ED mutant (Gruss et al, 2001), did not change mobility in the presence of CKII and NLS peptides (Supplementary Figure 1A). This suggests that one or more specific amino-acid residues in Importin α are phosphorylated on NLS binding, whereas other residues are phosphorylated in an NLS-independent manner.
Figure 4.
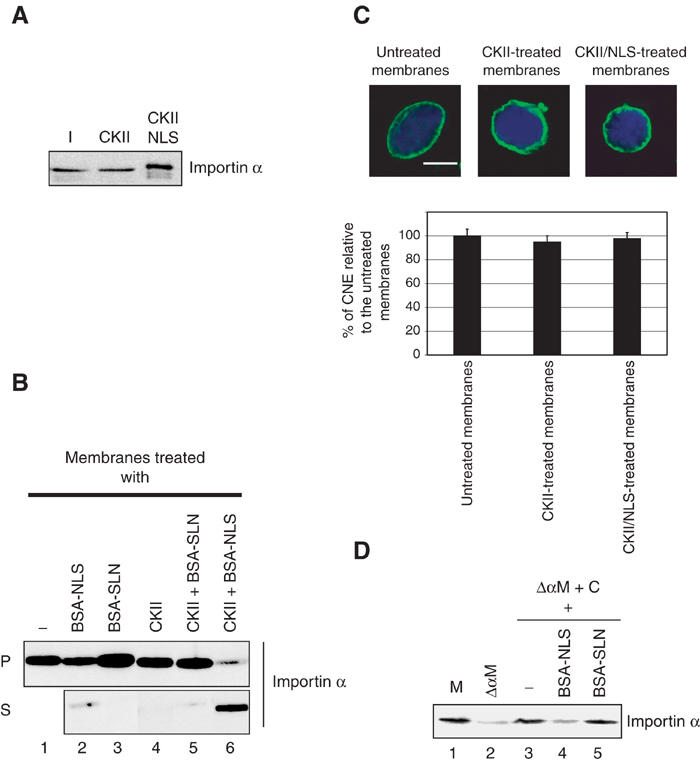
Phosphorylation of membrane-bound Importin α causes its specific release from membranes. (A) In vitro phosphorylation of recombinant Importin α in the presence or absence of NLS proteins. Importin α (700 nM) was incubated alone (lane 1) or in the presence or absence of BSA-NLS and CKII for 45 min at 30°C as indicated. Samples were analysed by Western blotting with anti-Importin α antibodies. (B) Phosphorylation affects the membrane association of Importin α. Membranes were incubated with CKII and either BSA-NLS or BSA-SLN. Membrane pellets and supernatants were separated by centrifugation. Pellets (P) and supernatant (S) were loaded on a 12% SDS–PAGE gel and analysed by Western blotting with anti-Importin α antibodies. (C) Importin α-free membranes are able to assemble a CNE. Importin α-depleted membranes were generated by CKII phosphorylation in the presence of BSA-NLS. Untreated or treated membranes were used in nuclear assembly reactions and CNE formation was assayed. Quantitation of CNE formation as a fraction of chromatin substrates normalized to the untreated membrane control (data from three independent experiments). (D) Cytosolic Importin α is recruited to Importin α-depleted membranes. Importin α-depleted membranes were incubated with either extracts or extracts pre-incubated with BSA-NLS or BSA-SLN (both at 20 μM) for 60 min at 20°C, then were washed and re-isolated. The presence of Importin α on the membranes was analysed by Western blotting. Incubating cytosol with Importin α-depleted membranes resulted in a very efficient recruitment of Importin α to membranes (compare lane 3 to lanes 1 and 2). Endogenous levels of Importin α on the membranes were restored. This recruitment of cytosolic Importin α to depleted membranes was blocked by pre-incubating cytosol with BSA-NLS, but not BSA-SLN (lanes 4 and 5).
Phosphorylation affects the membrane association of Importin α
In order to investigate the role of phosphorylation in Importin α localization to either membranes or cytosol, in vitro phosphorylation of membrane-associated Importin α was performed (Figure 4B). Membranes were either untreated (lane 1), treated with BSA-NLS alone (lane 2) or BSA-SLN alone (lane 3), CKII alone (lane 4), CKII and BSA-SLN (lane 5), or CKII and BSA-NLS (lane 6). Membrane pellets (P) were recovered by centrifugation. Supernatants (S) that contain the proteins released from membranes were analysed together with the membrane pellets by Western blotting using anti-Importin α antibodies. When membranes were incubated in the presence of BSA-NLS, a minor fraction of membrane-bound Importin α was released from the membranes (lane 2). This effect was not observed when the membranes were incubated with the BSA-SLN control (lane 3). When membranes were treated with CKII alone or in the presence of BSA-SLN, no release of Importin α from membranes was observed (lanes 4, 5). However, when membranes were incubated under the conditions in which a mobility shift in importin α was induced, that is, with CKII and BSA-NLS, most of the membrane-bound Importin α was released from the membranes and found in the supernatant (lane 6). Thus, increased phosphorylation of Importin α causes it to dissociate from the membranes.
Identification of phosphorylation sites within Importin α
In order to generate mutant forms of Importin α with which to test the effects of membrane association, we mapped the phosphorylation sites in Importin α. For this purpose, cytosolic and membrane fractions were loaded on 2D gels (Hartinger et al, 1996), and the spots corresponding to Importin α were identified by Western blotting. Importin α from both the cytosolic and membrane fractions was excised and digested with trypsin (Shevchenko et al, 1996). The peptide mixture was analysed with nanoelectrospray-based precursor ion scanning for the phosphate-derived marker ion m/z −79 (Köcher et al, 2003). The measured peptide masses were assigned to the calculated tryptic peptide masses of the Importin α sequence by addition of multiples of 80 Da. We detected a peptide with a mass of 1720.9 Da in both forms of Importin α. Tandem mass spectrometry in positive ion mode confirmed its identity as the phosphorylated peptide NVCLPEELIL(pS)PEK. In the sample obtained from the cytosolic protein, we detected two additional phosphorylated peptides, which were assigned to the triply and quadruply phosphorylated peptide (GDFKAQKEAVWAVTNYTSGGTVEQVVQL VQCGVLEPLLNLLTIK) with masses of 5058 and 5138 Da, and the triply phosphorylated peptide (LIQLMYSPELSIVTPSLRTVGNIVTGTDKQTQAAIDAGVL SVLPQLLR) with a mass of 5378 Da.
These two latter regions of Importin α comprise the residues 291–341 and 382–429. The peptides were too large to identify the sites of phosphorylation by direct sequence analysis. Therefore, a clustal alignment (Clustal X 1.81) was generated to identify the phosphorylatable residues that are highly conserved between species (Figure 5A). Of the nine potential phosphoresidues in the first region (291–341), three serine/threonine residues were highly conserved between Xenopus, human, mouse, Drosophila and yeast. Of the six potential phosphoresidues in the second region (382–429), four serine/threonine residues were highly conserved. Most of the potential phosphoresidues are located on the protein surface (E Conti, personal communication).
Figure 5.
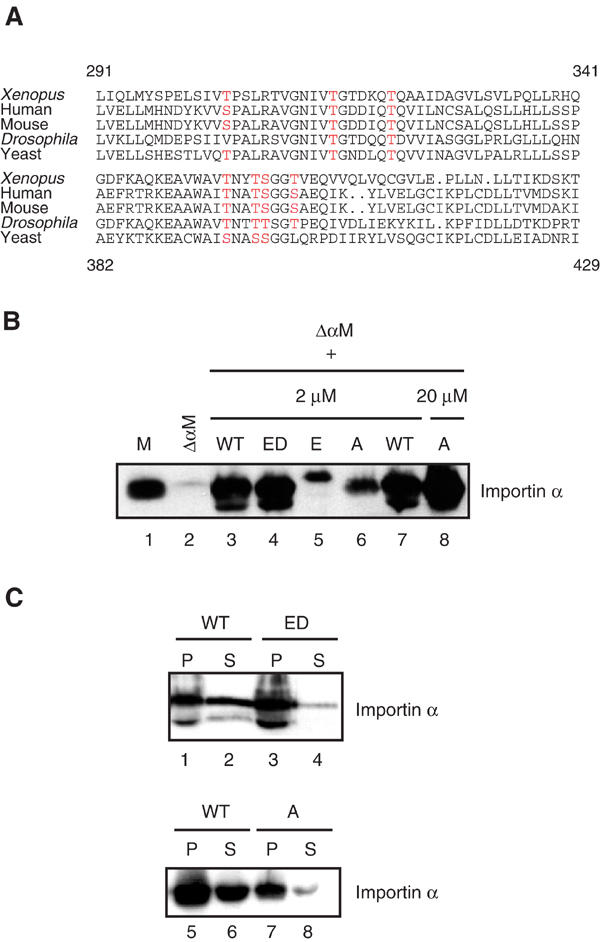
Two regions within Importin α contain clustered phosphorylation sites. (A) Clustal alignment of two regions of Importin α from different species comprising residues 291–341 and 382–429 (Xenopus numbering). Phosphorylation sites predicted by mass spectrometry, which are conserved between species, are depicted in red. (B) Exogenous Importin α can bind to stripped membranes. Membranes (M) were washed with 1 M NaCl (ΔαM) to remove peripheral membrane-associated proteins and then re-purified over a sucrose cushion. Recombinant Importin α WT, the ED and E mutants (2 μM) or the A mutant (2 or 20 μM), as indicated, were incubated in the presence of stripped membranes for 10 min on ice, then membranes were recovered by centrifugation after washing. The presence of Importin α was assayed by Western blotting. (C) Exogenous Importin α pre-bound to stripped membranes can be re-released upon phosphorylation. Recombinant Importin α WT, ED or A (2 μM) was bound to stripped membranes as indicated. The membranes were re-purified by centrifugation and subjected to phosphorylation by addition of CKII kinase and BSA-NLS. Membrane pellets (P) and supernatants (S) were analysed by Western blotting with anti-Importin α antibodies.
Membrane association of Importin α phosphorylation site mutants
To determine whether those sites are important for Importin α localization to membranes, we generated mutations in the two regions by replacing the conserved potential phosphoresidues either by alanine (A mutant) to inhibit their phosphorylation, or by glutamate (E mutant) to mimic phosphorylation. The Importin α A and E mutants, together with wild-type Importin α (WT) and the ED mutant, which is defective in binding to NLS proteins (Gruss et al, 2001), were expressed in E. coli and purified. Their ability to bind to stripped membranes (ΔαM) was then assayed (Figure 5B). Membranes (M) were washed with 1 M NaCl in order to completely remove endogenous Importin α (ΔαM), then re-purified by centrifugation over a sucrose cushion. We used salt-stripped membranes rather than CKII plus NLS-stripped membranes in most subsequent experiments, as they were more easily prepared, could be frozen, and rebound Importin α on addition to cytosolic egg extract (Supplementary Figure 1B). The stripped membranes were incubated with the Importin α variants on ice, then washed to remove excess unbound protein and re-purified by centrifugation. As shown in Figure 5B, stripped membranes (ΔαM) were efficiently depleted of Importin α as compared to control membranes (M) (lanes 1 and 2). Importin α wild type (WT) was efficiently rebound to stripped membranes (lanes 3 and 7), suggesting that the Importin α-binding sites on the membranes are still available after the high-salt wash. The Importin α ED mutant also bound well to Importin α-free membranes (lane 4). However, the phosphorylation mutants of Importin α, the alanine (A) and glutamate (E) mutants, bound less efficiently (Figure 5B, lanes 5 and 6). The alanine mutant bound better than the glutamate mutant, and in order to further study its behaviour, 10-fold more of the mutant protein was added to the rebinding assay. In this excess, the alanine mutant bound to stripped membranes to roughly the same final extent as the WT protein at 2 μM (lanes 7 and 8). The E mutant, when added at 20 μM, still bound poorly to stripped membranes (data not shown).
The release of the newly bound Importin α variants upon phosphorylation was next analysed. After binding of exogenous Importin α protein to stripped membranes, the membranes were washed and re-purified before incubation with CKII and BSA-NLS. Membrane pellets (P) and supernatants (S) were analysed by Western blotting. The WT Importin α protein was partially released from the membranes upon phosphorylation (Figure 5C, lanes 2 and 6). However, although the ED mutant could bind to stripped membranes (lane 3), it was not released from them (lane 4), presumably because of its lack of interaction with BSA-NLS and resultant reduced phosphorylation by CKII. To investigate the role of the Importin α phosphorylation sites identified above on Importin α association with membranes, the Importin α alanine mutant was bound to the stripped membranes (Figure 5C, lane 7). Almost no release of the mutant protein was observed after treatment with CKII and NLS (lane 8). This lack of release was also seen when the Importin α alanine mutant was bound to stripped membranes at a higher concentration (data not shown). The amounts of WT, ED and A mutants released from the membrane were quantified, and were 57, 4 and 20%, respectively (Supplementary Figure 1C). To rule out the trivial explanation that the A mutant does not interact with NLS proteins, we analysed NLS-protein binding in vitro and found that the A mutant did bind to NLS-containing proteins (data not shown). Thus, at least some of the phosphoamino acids identified by mass spectrometric analysis of the membrane-bound fraction of Importin α, and mutated in the A and E mutants, are indeed critical for the reversible binding to and release from membranes.
Importin α is required on membranes for CNE formation
The fact that we could deplete Importin α from membranes (Figure 4B) prompted us to address the functional significance of this association in NE assembly. We first examined the effect on membrane-bound Importin α of the treatments that had blocked CNE formation, that is, the addition of BSA-NLS or of excess recombinant Importin α (Figure 1). Neither of these treatments significantly affected membrane-associated Importin α (Supplementary Figure 1D). We conclude that these treatments likely affect the function of soluble Importin α.
To examine the role of membrane-bound Importin α, we performed NE assembly reactions using either untreated membranes, CKII-treated membranes or Importin α-depleted membranes (i.e. CKII/NLS-treated membranes). In all cases, no inhibition of CNE formation was observed and the treated membranes could assemble a CNE around more than 95% of the chromatin substrates (Figure 4C). This suggested either that Importin α-membrane interaction had no role in NE assembly or that Importin α could be restored to the membrane fraction on addition of cytosol.
To test the latter possibility, the Importin α-depleted membranes were incubated with cytosol, washed and re-purified to remove cytosolic contamination. Analysis of Importin α by Western blotting revealed that cytosolic Importin α could indeed be recruited efficiently to Importin α-free membranes (Figure 4D, lanes 1–3). This recruitment could be partially inhibited if the cytosol was incubated with BSA-NLS, but not BSA-SLN prior to incubation with Importin α-free membranes (lanes 4 and 5), showing the specificity of this recruitment.
The mutant Importin α proteins provided tools to assess the role of Importin α-membrane interaction in NE assembly. NE formation reactions were performed using either Importin α-stripped membranes (ΔαM) or stripped membranes to which WT Importin α or the mutant derivatives were re-bound (ΔαM/WT or A or ED or E, see Figure 6B). To ensure equal quantities of bound WT, ED and A protein, the latter was initially added in 10-fold excess. After binding, removal of the nonbound fraction left comparable amounts of membrane-bound protein (Figure 6B).
Figure 6.
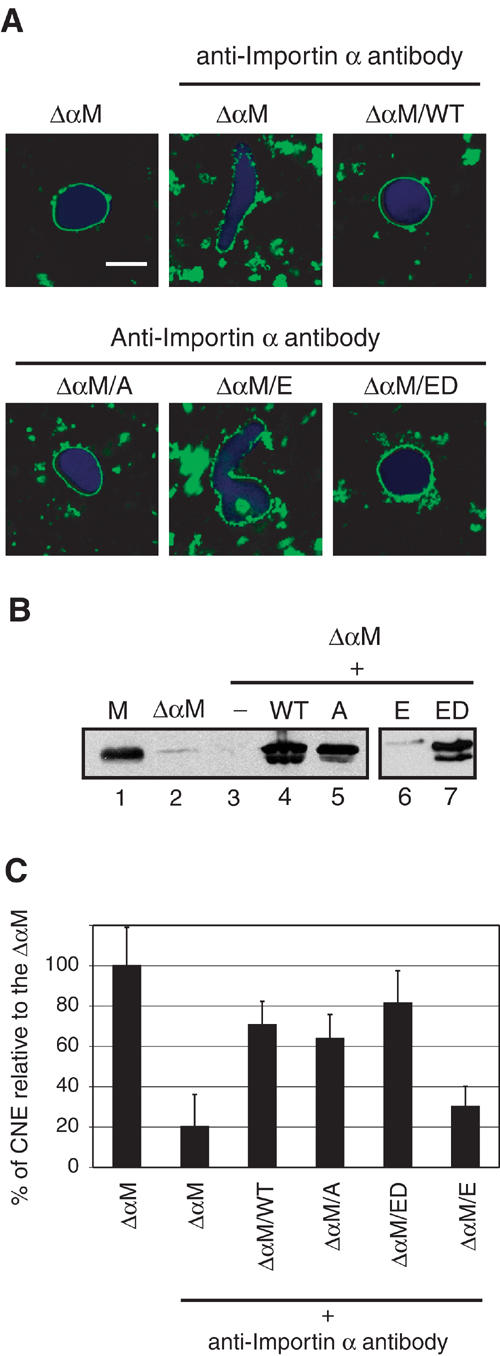
Membrane-bound Importin α is involved in CNE formation. (A) Importin α-free membranes (ΔαM) generated by 1 M NaCl washes of the egg membrane fraction were pre-incubated with either buffer alone, Importin α WT, ED or E mutants at 2 μM or the A mutant at 20 μM. The membranes were then washed to remove unbound Importin α and re-purified over a sucrose cushion. These membranes were then incubated in cytosolic extracts blocked by addition of affinity-purified Importin α antibodies for 90 min at 20°C. The Importin α-stripped membranes (ΔαM) were able to form CNEs around chromatin substrates when supplemented with untreated cytosol (top left). Addition of antibodies against Importin α to the cytosolic fraction resulted in a strong inhibition of CNE formation (top centre). When stripped membranes prebound to either WT Importin α or to the ED or A mutants were added to antibody-treated cytosol, CNE formation was restored (bottom right, left panels). However, the E mutant that does not bind to stripped membranes did not rescue the inhibitory phenotype (bottom centre). (B) Western blot analysis of membrane fractions using anti-Importin α antibodies. Membrane fractions (M) and Importin α-stripped fractions (ΔαM) were analysed together with Importin α-stripped membranes that had been re-incubated in the absence of Importin α (−) or with WT Importin α (WT) or the A, E or ED mutants, as indicated, prior to washing and re-purification, SDS–PAGE and Western blotting. (C) Quantitation of CNE formation on 80 chromatin substrates normalized to the ΔαM (mock-depleted) reaction (data from four independent experiments).
While less active than control membranes, membrane fractions depleted of Importin α in this way were still able to assemble a CNE around chromatin substrates (Figure 6A, top left panel). Presumably, this activity was due to recruitment of Importin α from the cytosol (Supplementary Figure 1B). We therefore reduced the soluble pool of Importin α in the cytosol by adding affinity-purified anti-Importin α antibodies to the reaction. This resulted in a strong inhibition of CNE formation (Figure 6A, top middle panel, 6C). Adding the E mutant, which does not bind membranes (Figure 6B, lane 6) and is a negative control, did not affect CNE formation (Figure 6A, bottom middle panel, 6C). In contrast, when Importin α WT was re-bound to stripped membranes to endogenous concentrations (Figure 6A, top right panel, 6B, lane 4) prior to addition into the NE assembly reaction, the assembly inhibition was reversed.
Western blot analysis confirmed that the inactive membranes did not contain significant amounts of Importin α (Figure 6B, lanes 2–3) and that WT Importin α associated efficiently to the stripped membranes when added (Figure 6B, lane 4). Efficient CNE formation was also observed when the Importin α alanine mutant (A) was bound to the depleted membranes (Figure 6A, bottom left, 6C, 6B, lane 5). Addition of the ED mutant to endogenous concentration to stripped membranes also rescued the inhibition (Figure 6A, bottom right, 6C, 6B, lane 7). Thus, association of Importin α with membranes is required for the assembly of the NE in vitro, but the regulated, phosphorylation-dependent, release of Importin α from the membranes does not seem to be required.
Discussion
At the end of mitosis in metazoa, the NE has to reassemble around chromatin as part of the process of reforming a functional nucleus. The molecular mechanisms of NE assembly are incompletely understood, but several studies have pointed to a role for the Ran GTPase in the process (Hetzer et al, 2000; Zhang and Clarke, 2000; Askjaer et al, 2002; Bamba et al, 2002). In this paper, we provide evidence that Importin α is involved in NE formation, and that it probably has at least two distinct roles. The role that we have examined in some detail is unusual in relation to previously described functions of Importin α in that it does not depend on binding either NLS proteins or Importin β, but does involve regulated association of Importin α with membranes. The second Importin α function in NE formation involves interaction with NLS-containing proteins and, since the membrane-associated function does not require NLS binding, we conclude that the second function is carried out by soluble Importin α.
Importin α blocks NE formation
Previous in vivo work, involving RNAi-based depletion of IMA-2, one of the C. elegans Importin α isoforms, had suggested a role for the protein in postmitotic NE assembly (Askjaer et al, 2002; Geles et al, 2002). However, given the pleiotropic effects caused by Importin α depletion or mutation, we wished to examine the role of Importin α in NE assembly in vitro, where indirect effects can be reduced or eliminated. We could show that excess Importin α blocks in vitro NE formation by inhibiting the fusion of membranes around chromatin. This effect seems to act on later steps of membrane fusion, as a membrane network can be observed on the chromatin substrate even in the presence of high levels of Importin α, suggesting that vesicle docking and primary vesicle–vesicle fusion events are not prevented. Excess Importin α may negatively affect NE formation either by binding to and affecting NLS-bearing proteins involved in NE formation or by binding to Importin β, which has been implicated in CNE formation by several studies (Zhang et al, 2002; Harel et al, 2003; Walther et al, 2003). Somewhat paradoxically, the addition of BSA-NLS or preincubation of the soluble cytoplasmic egg fraction with Importin α antibodies also severely affects CNE formation. Thus, either by saturating Importin α, and thereby displacing bound NLS proteins, or by removing Importin α via antibodies, the formation of a proper nuclear membrane rim around chromatin is inhibited. The importance of Importin α for in vitro NE formation is also supported by the inhibitory effect of immuno-depletion of Importin α from extracts. All of these treatments lead to a strong inhibition of CNE formation. Thus, both too much or too little Importin α blocks proper NE formation, suggesting that Importin α plays a role in this process and that its levels need to be tightly regulated and kept in the correct balance with its binding partners. The combination of the data just discussed also suggests that Importin α's role in the cytoplasm is different from that in the membrane fraction, as the latter does not require NLS binding. After depletion of Importin α from extracts, adding back recombinant Importin α only led to a rather weak restoration of NE assembly, possibly because of co-depletion of NLS-bearing proteins together with Importin α. It is also possible that the tight requirement for a specific concentration of Importin α prevents successful complementation with exogenous protein.
Membrane association of Importin α
An unexpected finding of our study was the discovery of a function for membrane-associated Importin α. The association of Importin α with membranes in interphase does not depend on any of the previously characterized major binding partners of Importin α. Indeed, Importin β, CAS and Ran are all absent from membranes and an Importin α mutant that does not bind to NLS proteins, the ED mutant (Gruss et al, 2001), associates with membranes and apparently functions in NE assembly. Membrane-bound Importin α can be washed off by high-salt treatment and exogenous Importin α can specifically reassociate to those depleted membranes. As the ED mutant was able to associate to stripped membranes, the mode of interaction of Importin α with the membranes appears to be NLS-independent. This raises the interesting question of how Importin α associates with the membranes and what its binding partners are in this compartment, a question that we intend to pursue in the future.
A function for Importin α phosphorylation
The localization of Importin α to either the membrane or cytosolic fractions correlated with post-translational modification of the protein. The soluble form of Importin α present in the cytosolic fraction was more highly phosphorylated than the membrane-bound form, as determined both by mobility change on SDS–PAGE and by phosphopeptide mapping. Furthermore, in vitro phosphorylation of Importin α by CKII in the presence of NLS proteins displaced Importin α from membranes. Although the two populations of Importin α are differentially modified, we identified one common phosphorylation site in both. This site corresponds to serine 67 of yeast Srp1p (Importin α) that is phosphorylated by CKII both in vitro and in vivo (Azuma et al, 1997). Mutations of Ser67 to valine or aspartic acid in yeast did not affect cell growth (Azuma et al, 1997), but the conservation of the site suggests that it might have some role in Importin α function. An NLS requirement for Importin α phosphorylation was first shown for yeast Srp1p (Azuma et al, 1997). It might be that the presence of NLS cargoes changes the conformation of Importin α and allows access of the kinase to phosphorylation sites.
Our study also identified two regions that were heavily phosphorylated in the cytosolic form of Importin α, comprising residues 291–341 and 382–429. Although the size of the modified peptides precluded direct sequence analysis of the phosphorylated sites, mutagenesis of seven potential phosphorylation sites in these two regions affected Importin α membrane association, and mutation of subsets of the sites resulted in intermediate phenotypes (our unpublished data). Moreover, the results obtained with the NLS-binding defective ED mutant further supports the hypothesis that the presence of Importin α on membranes is regulated by phosphorylation. ED is not released from the membranes and, since it cannot interact with NLS proteins, it is not hyperphosphorylated by CKII.
The effect of phosphorylation on Importin α association with membranes in principle allows regulation of the presence of the protein on the membranes through the action of phosphatases and kinases. Indeed, we have shown that cytosolic Importin α can be recruited to Importin α-depleted membranes and conversely that membrane-bound Importin α can be released by the combined action of CKII and NLS proteins. We conclude that Importin α association with membranes is regulated by phosphorylation and that at least some of the relevant sites of phosphorylation have been identified.
The apparent requirement for at least two Importin α functions, a cytosolic and a membrane-based one, made it extremely challenging to demonstrate experimentally a function for the membrane-bound protein. Nevertheless, by combined use of mutant forms of Importin α that either bound very poorly to membranes or were not released by phosphorylation, and the use of small amounts of affinity-purified anti-Importin α antibodies to reduce the concentration of Importin α in the cytosol, it was possible to demonstrate that while membrane-bound Importin α is indeed required for the formation of a CNE, the release of Importin α from the membranes is not. Our future plans are to investigate in detail this new and unexpected function for the import adaptor. Our data suggest that Importin α may be functioning in quite a different way when membrane-associated, than in its better understood soluble form.
Materials and methods
Cloning of Importin α mutants expression constructs
The Xenopus laevis Importin α clone was that of Görlich et al (1994). In the Importin α alanine mutant construct, the following amino acids were changed to alanine: T304, T315, T317, T321, T395, T398, S399 and T402, using the QuickChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA, USA). In the Importin α glutamate mutant construct, the following amino acids were changed to glutamate: T304, T315, T395, T398, S399 and T402, using the same procedure.
Expression and purification of proteins
Recombinant His-tagged Importin α WT or the Importin α mutants ED, E and A were expressed in Escherichia coli and purified on TALON beads as described previously (Görlich et al, 1994; Gruss et al, 2001).
Xenopus laevis egg extract preparation
Fractionated egg extracts, sperm chromatin and nuclear assembly were prepared and assayed as described (Hetzer et al, 2000). Membranes were resuspended in 1/10 volume of cytosol. Stripped membranes (ΔαM) were prepared by diluting them 20-fold in 1 M NaCl instead of S250 (50 mM KCl, 2.5 mM MgCl2, 20 mM HEPES KOH (pH 7.5)) and incubating them for 10 min on ice, before centrifugation through a cushion of S250 containing 0.5 M sucrose (S500) at 15 000 g for 15 min at 4°C. The stripped membranes were carefully resuspended in S500, divided into aliquots of 10 μl, frozen, and stored in liquid nitrogen. Importin α-depleted membranes were also generated via phosphorylation as described below. In this case, pelleted membranes were resuspended in S500 and used immediately.
Depletion of Importin α from Xenopus egg extracts
Polyclonal rabbit antibodies were raised against full-length, his-tagged Xenopus Importin α, affinity-purified, and covalently linked to Affigel 10 (Bio-Rad). For mock depletion, a column was prepared using nonimmune rabbit IgG. The columns were subsequently blocked with 50 mg/ml BSA in S250 buffer for 30 min, washed and incubated twice with freshly prepared extracts for 30 min.
Dephosphorylation assays
In all, 30 μl cytosol or 3 μl membranes were incubated with purified λ protein phosphatase (New England BioLabs) for 30 min at 30°C in a 25 μl reaction containing 50 mM Tris–HCl (pH 7.5), 0.1 mM Na2EDTA, 5 mM dithiothreitol, 0.01% Brij 35 and supplemented with 2 mM MgCl2. Reactions were loaded on a 12% SDS–PAGE gel, subjected to electrophoresis and analysed by Western blotting with anti-Importin α antibodies.
In vitro phosphorylation of recombinant Importin α
Phosphorylation of Importin α was carried out by incubating bacterially expressed his-Importin α with CKII (New England BioLabs) at 30°C for 45 min in the presence or absence of 20 μM BSA-NLS in a 25 μl reaction containing CKII buffer (20 mM Tris–HCl, 50 mM KCl, 10 mM MgCl2) supplemented with 400 μM ATP. The reaction mixtures were analysed by SDS–PAGE, followed by Western blotting with anti-Importin α antibodies.
In vitro phosphorylation of membrane-bound Importin α
Membranes (3 μl) were incubated with CKII at 30°C for 30 min in the presence or absence of 20 μM BSA-NLS or BSA-SLN in a 25 μl reaction containing CKII buffer supplemented with 400 μM ATP. The reaction mixtures were centrifuged at 45 000 rpm in a TLA-100 rotor in a tabletop ultracentrifuge (Beckmann) for 20 min at 4°C. The supernatant proteins were precipitated using TCA. Membrane pellets were resuspended in SDS-loading buffer. The membrane pellets and precipitated supernatant were analysed by SDS—PAGE, followed by Western blotting with anti-Importin α antibodies. The NLS peptide and the control peptide with a reverse sequence, SLN, were: CGGGPKKKRKVED and CGGGDEVKRKKKP, respectively (reversed sequences underlined).
Recruitment of cytosolic Importin α to depleted membranes
Importin α-free membranes were generated by phosphorylation. In parallel, cytosol was pre-incubated with either buffer or 20 μM BSA-NLS or BSA-SLN. Then, cytosol and depleted membranes were incubated together for 30 min at 20°C. The reaction mixtures were washed with S250 and centrifuged through a cushion of S500 for 10 min at 4°C at maximum speed in a tabletop centrifuge. The membrane pellets were analysed by SDS–PAGE, followed by Western blotting with anti-Importin α antibodies.
Identification of phosphoresidues within Importin α
Cytosolic and membrane fractions were analysed by 2D gels (16-BAC for the first dimension (Hartinger et al, 1996) and SDS–PAGE for the second dimension). Western blot analysis identified Importin α spots that were analysed by mass spectrometry. Coomassie-stained gel spots were excised, washed, reduced and alkylated, followed by digestion with trypsin as previously described (Shevchenko et al, 1996). Sample preparations and detection of phosphopeptides was performed as described (Köcher et al, 2003). The peptide mixture was extracted from the gel matrix and dried in a vacuum centrifuge. The peptides were resolved and subsequently desalted on reversed-phase material using a double-column system consisting of an R2 column and an OligoR3 column. Each column was stepwise eluted under neutral and basic conditions directly into the spraying needle and analysed separately. Phosphopeptides were detected with precursor ion scanning for the reporter ion m/z −79 using an API III triple quadrupole instrument (PE-Sciex, Ontario, Canada) with a nanoelectrospray source installed.
Binding exogenous Importin α to stripped membranes and release by phosphorylation
Purified recombinant Importin α protein (2 or 20 μM) was incubated with stripped membranes in S250/0.3% BSA buffer in a 25 μl reaction volume for 10 min at 4°C; membranes were washed with S250 and recovered by centrifugation over an S500 cushion to remove unbound Importin α protein. Phosphorylation was performed as described above.
SDS–PAGE and Western blotting
SDS–PAGE was performed as described (Laemmli, 1970) on 12% gels. For Western blotting, proteins were transferred to Nitrocellulose after SDS–PAGE and decorated with rabbit polyclonal antibodies generated against Xenopus importin α, Ran, Importin β, CAS, nucleoplasmin, followed by anti-rabbit IgG secondary antibodies HRP-labeled from donkey (Amersham Bioscience). For quantitative Western blot, we used an Alexa-680 secondary antibody and for quantitation we used an Li-cor fluoroimager with Odyssey software.
Supplementary Material
Supplemental figure
Acknowledgments
We would like to thank Wolfram Antonin, Peter Askjaer, Kevin Czaplinski, Elena Conti, Matyas Gorjanacz, Martin Hetzer, Olivier Hachet, Elisa Izaurralde, Melpomeni Platani, Katharina Ribbeck, Sebastian Ulbert, Masami Yamada, Cerstin Franz and Ulrike Bauer for critical comments on the manuscript. We owe special thanks to Vincent Galy and Maria Koffa. This work was funded by EMBL and the French ministry of Research.
References
- Askjaer P, Galy V, Hannak E, Mattaj IW (2002) Ran GTPase cycle and importins alpha and beta are essential for spindle formation and nuclear envelope assembly in living Caenorhabditis elegans embryos. Mol Biol Cell 13: 4355–4370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma Y, Tabb MM, Vu L, Nomura M (1995) Isolation of a yeast protein kinase that is activated by the protein encoded by SRP1 (Srp1p) and phosphorylates Srp1p complexed with nuclear localization signal peptides. Proc Natl Acad Sci USA 92: 5159–5163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma Y, Takio K, Tabb MM, Vu L, Nomura M (1997) Phosphorylation of Srp1p, the yeast nuclear localization signal receptor, in vitro and in vivo. Biochimie 79: 247–259 [DOI] [PubMed] [Google Scholar]
- Bamba C, Bobinnec Y, Fukuda M, Nishida E (2002) The GTPase Ran regulates chromosome positioning and nuclear envelope assembly in vivo. Curr Biol 12: 503–507 [DOI] [PubMed] [Google Scholar]
- Ellenberg J, Siggia ED, Moreira JE, Smith CL, Presley JF, Worman HJ, Lippincott-Schwartz J (1997) Nuclear membrane dynamics and reassembly in living cells: targeting of an inner nuclear membrane protein in interphase and mitosis. J Cell Biol 138: 1193–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foisner R (2001) Inner nuclear membrane proteins and the nuclear lamina. J Cell Sci 114: 3791–3792 [DOI] [PubMed] [Google Scholar]
- Geles KG, Johnson JJ, Jong S, Adam SA (2002) A role for Caenorhabditis elegans importin IMA-2 in germ line and embryonic mitosis. Mol Biol Cell 13: 3138–3147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorjanacz M, Adam G, Torok I, Mechler BM, Szlanka T, Kiss I (2002) Importin-alpha 2 is critically required for the assembly of ring canals during Drosophila oogenesis. Dev Biol 251: 271–282 [DOI] [PubMed] [Google Scholar]
- Görlich D, Prehn S, Laskey RA, Hartmann E (1994) Isolation of a protein that is essential for the first step of nuclear protein import. Cell 79: 767–778 [DOI] [PubMed] [Google Scholar]
- Görlich D, Kutay U (1999) Transport between the cell nucleus and the cytoplasm. Annu Rev Cell Dev Biol 15: 607–660 [DOI] [PubMed] [Google Scholar]
- Gruss OJ, Carazo-Salas RE, Schatz CA, Guarguaglini G, Kast J, Wilm M, Le Bot N, Vernos I, Karsenti E, Mattaj IW (2001) Ran induces spindle assembly by reversing the inhibitory effect of importin alpha on TPX2 activity. Cell 104: 83–93 [DOI] [PubMed] [Google Scholar]
- Harel A, Chan RC, Lachish-Zalait A, Zimmerman E, Elbaum M, Forbes DJ (2003) Importin β negatively regulates nuclear membrane fusion and nuclear pore complex assembly. Mol Biol Cell 14: 4387–4396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartinger J, Stenius K, Hogemann D, Jahn R (1996) 16-BAC/SDS–PAGE: a two-dimensional gel electrophoresis system suitable for the separation of integral membrane proteins. Anal Biochem 240: 126–133 [DOI] [PubMed] [Google Scholar]
- Hetzer M, Bilbao-Cortes D, Walther TC, Gruss OJ, Mattaj IW (2000) GTP hydrolysis by Ran is required for nuclear envelope assembly. Mol Cell 5: 1013–1024 [DOI] [PubMed] [Google Scholar]
- Hetzer M, Gruss OJ, Mattaj IW (2002) The Ran GTPase as a marker of chromosome position in spindle formation and nuclear envelope assembly. Nat Cell Biol 4: 177–184 [DOI] [PubMed] [Google Scholar]
- Hetzer M, Meyer HH, Walther TC, Bilbao-Cortes D, Warren G, Mattaj IW (2001) Distinct AAA-ATPase p97 complexes function in discrete steps of nuclear assembly. Nat Cell Biol 3: 1086–1091 [DOI] [PubMed] [Google Scholar]
- Holmer L, Worman HJ (2001) Inner nuclear membrane proteins: functions and targeting. Cell Mol Life Sci 58: 1741–1747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jäkel S, Mingot JM, Schwarzmaier P, Hartmann E, Görlich D (2002) Importins fulfil a dual function as nuclear import receptors and cytoplasmic chaperones for exposed basic domains. EMBO J 21: 377–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köcher T, Allmaier G, Wilm M (2003) Nanoelectrospray-based detection and sequencing of substoichiometric amounts of phosphopeptides in complex mixtures. J Mass Spectrom 38: 131–137 [DOI] [PubMed] [Google Scholar]
- Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685 [DOI] [PubMed] [Google Scholar]
- Marshall ICB, Wilson KL (1997) Nuclear envelope assembly after mitosis. Trends Cell Biol 7: 69–74 [DOI] [PubMed] [Google Scholar]
- Matsusaka T, Imamoto N, Yoneda Y, Yanagida M (1998) Mutations in fission yeast Cut15, an importin alpha homolog, lead to mitotic progression without chromosome condensation. Curr Biol 8: 1031–1034 [DOI] [PubMed] [Google Scholar]
- Mattaj IW (2004) Sorting out the nuclear envelope from the endoplasmic reticulum. Nat Rev Mol Cell Biol 5: 65–69 [DOI] [PubMed] [Google Scholar]
- Nachury MV, Maresca TJ, Salmon WC, Waterman-Storer CM, Heald R, Weis K (2001) Importin beta is a mitotic target of the small GTPase Ran in spindle assembly. Cell 104: 95–106 [DOI] [PubMed] [Google Scholar]
- Quimby BB, Dasso M (2003) The small GTPase Ran: interpreting the signs. Curr Opin Cell Biol 15: 338–344 [DOI] [PubMed] [Google Scholar]
- Shevchenko A, Wilm M, Vorm O, Mann M (1996) Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal Chem 68: 850–858 [DOI] [PubMed] [Google Scholar]
- Timinszky G, Tirian L, Nagy FT, Toth G, Perczel A, Kiss-Laszlo Z, Boros I, Clarke PR, Szabad J (2002) The importin-beta P446L dominant-negative mutant protein loses RanGTP binding ability and blocks the formation of intact nuclear envelope. J Cell Sci 115: 1675–1687 [DOI] [PubMed] [Google Scholar]
- Tirian L, Timinszky G, Szabad J (2003) P446L-importin-beta inhibits nuclear envelope assembly by sequestering nuclear envelope assembly factors to the microtubules. Eur J Cell Biol 82: 351–359 [DOI] [PubMed] [Google Scholar]
- Walther TC, Askjaer P, Gentzel M, Habermann A, Griffiths G, Wilm M, Mattaj IW, Hetzer M (2003) RanGTP mediates nuclear pore complex assembly. Nature 42: 4689–4694 [DOI] [PubMed] [Google Scholar]
- Wiese C, Goldberg MW, Allen TD, Wilson KL (1997) Nuclear envelope assembly in Xenopus extracts visualized by scanning EM reveals a transport-dependent ‘envelope smoothing' event. J Cell Sci 110: 1489–1502 [DOI] [PubMed] [Google Scholar]
- Yang L, Guan T, Gerace L (1997) Integral membrane proteins of the nuclear envelope are dispersed throughout the endoplasmic reticulum during mitosis. J Cell Biol 137: 1199–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Clarke PR (2000) Chromatin-independent nuclear envelope assembly induced by Ran GTPase in Xenopus egg extracts. Science 288: 1429–1432 [DOI] [PubMed] [Google Scholar]
- Zhang C, Hutchins JR, Muhlhausser P, Kutay U, Clarke PR (2002) Role of importin β in the control of nuclear envelope assembly by Ran. Curr Biol 12: 498–502 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental figure


