Abstract
ARTS is an unusual septin-like mitochondrial protein that was originally shown to mediate TGF-beta-induced apoptosis. Recently, we found that ARTS is also important for cell killing by other pro-apoptotic factors, such as arabinoside, etoposide, staurosporine and Fas. In Drosophila, the IAP antagonists Reaper, Hid and Grim are essential for the induction of virtually all apoptotic cell death. We found that mutations in peanut, which encodes a Drosophila homologue of ARTS, can dominantly suppress cell killing by Reaper, Hid and Grim, indicating that peanut acts downstream or in parallel to these. In mammalian cells, ARTS is released from mitochondria upon pro-apoptotic stimuli and then binds to XIAP. Binding of ARTS to XIAP is direct, as recombinant ARTS and XIAP proteins can bind to each other in vitro. ARTS binding to XIAP is specific and related to its pro-apoptotic function, as mutant forms of ARTS (or related septins) that fail to bind XIAP failed to induce apoptosis. ARTS leads to decreased XIAP protein levels and caspase activation. Our data suggest that ARTS induces apoptosis by antagonizing IAPs.
Keywords: apoptosis, ARTS, mitochondria, XIAP
Introduction
Programmed cell death by apoptosis is a major mechanism for regulating cell number and tissue homeostasis (Thompson, 1995; Meier et al, 2000). Apoptosis is tightly controlled through the action of both activators and inhibitors of caspases (Song and Steller, 1999; Shi, 2002). The best studied family of caspase inhibitors are the Inhibitors of Apoptosis Proteins (IAPs) (Salvesen and Duckett, 2002). All IAPs contain between one to three baculoviral IAP repeat (BIR) domains. BIR domains can directly interact with caspases and inhibit their apoptotic activity (Deveraux and Reed, 1999; Shi, 2002). Some of the IAP proteins also contain a RING domain and are thought to function as E3-ubiquitin ligases (Yang et al, 2000; Hu and Yang, 2003). Among these, XIAP has been the best studied. XIAP contains three BIR domains and can directly inhibit caspases 3, 7 and 9 (Deveraux et al, 1997; Sun et al, 1999, 2000).
In cells that are doomed to die, inhibition of apoptosis has to be overcome. In Drosophila, Reaper, Hid and Grim are essential for the induction of virtually all apoptotic cell death (White et al, 1994; Bergmann et al, 1998). Reaper, Hid and Grim induce apoptosis by binding to and inhibiting Drosophila IAP-1 (Diap1) (Wang et al, 1999; Goyal et al, 2000). Additional IAP antagonists have been identified both in insects and mammals (Du et al, 2000; Verhagen et al, 2000, 2002; Martins et al, 2002; Salvesen and Duckett, 2002). Like in Drosophila, these proteins use a short, conserved N-terminal sequence termed IBM (IAP-Binding Motif) for IAP binding and inhibition (Shi, 2002). Binding of Reaper/Hid/Grim to DIAP1 has two consequences: release of caspases bound to the IAP BIR domains causes de-repression of caspases and stimulation of auto-ubiquitination, leading to IAP protein degradation (Martin, 2002). In addition, Diap1 is also the target for caspase cleavage and degradation by the ‘N-end rule' pathway (Ditzel et al, 2003). Although auto-ubiquitination and degradation of IAPs is also seen in mammalian apoptosis (Yang et al, 2000), a role of mammalian IBM proteins in these processes remains to be established.
ARTS is a pro-apoptotic protein derived by differential splicing from the human septin H5/PNUTL2/CDCrel-2a (Sept4) gene (Larisch et al, 2000a, 2000b). ARTS contains a P-loop GTP-binding motif conserved in this family. Yet, unlike most other family members, it is localized to the mitochondria and promotes apoptosis. Although ARTS was initially found to mediate TGF-beta-induced apoptosis, it also promotes apoptosis induced by many other pro-apoptotic stimuli, such as etoposide, arabinoside (ara-C), staurosporine (STS) and Fas. Here we demonstrate that ARTS promotes apoptosis through direct binding and inhibition of XIAP. Upon induction of apoptosis, ARTS is released from the mitochondria and forms a stable complex with XIAP. Binding of ARTS to XIAP causes a significant reduction in XIAP levels and leads to caspase activation and cell death.
Results
We previously reported that ARTS induces caspase-3 activity in response to TGF-β treatment in NRP154 and COS-7 cells (Larisch et al, 2000b). To examine the effect of ARTS in a wider range of cells and with several different apoptotic inducers, we used two leukemic cells lines (HL-60 and K562) and a lung carcinoma cell line (A549) in addition to NRP154 and COS-7 cells in the current study. All cell lines were transiently transfected with ARTS and a control vector. HL-60 and K562 cells were treated with ara-C, A549 with TGF-β and COS-7 with etoposide. In all these cases, we observed that overexpression of ARTS led to increased caspase-3 activity in response to pro-apoptotic stimuli (P-values (ANOVA) of K562 and HL-60 cells were 0.02 and 0.001, respectively; A549 P-value (ANOVA) was 0.09, Figure 1A). The expression of ARTS alone had little or no effect on caspase-3 activity in A549, K562 and COS-7 cells, but it increased caspase-3 activity more than three-fold in HL-60 cells. Although the basis for this differential response is not clear, it should be noted that HL-60 cells are completely devoid of endogenous ARTS, whereas all the other cell lines express various levels of endogenous ARTS protein. We found that variations in expression levels of transfected ARTS are probably responsible for the observed differential response of cells. Higher expression levels of ARTS lead to increased apoptosis (Rotem, Lotan and Larisch, unpublished results).
Figure 1.
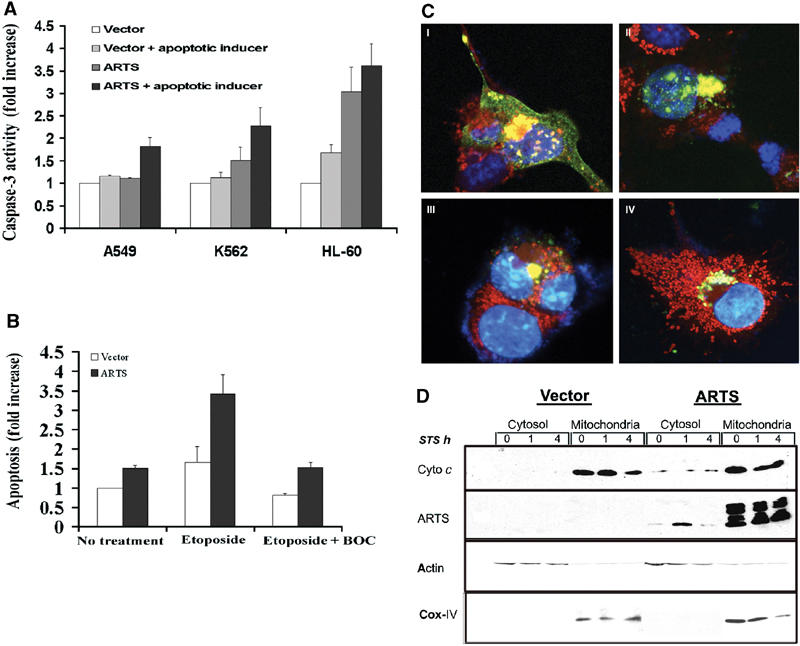
ARTS induces caspase-3 activity in response to a variety of apoptotic inducers, and caspase activity affects ARTS localization. Cells were transiently transfected with either control vector or the AU5-ARTS construct and treated with various apoptotic inducers. (A) ARTS induces caspase-3 activity in response to different apoptotic inducers. A549 cells were treated with TGF-β, K562 and HL-60 with ara-C. Caspase activity is presented as fold increase compared to cells transfected with control vector without apoptotic treatment. (B) COS-7 cells were treated with 50 μM etoposide for 16 h with and without BOC. (C) Immunofluorescence assay of COS-7 cells with MitoTracker® Red CMXRos and anti-ARTS antibodies. The cells were treated with 50 μM of etoposide for 16 h without (C I, II) and with BOC (III, IV). Cells treated with etoposide show typical morphological changes associated with apoptosis and accumulated ARTS in the nucleus (C I, II). Upon addition of caspase inhibitors nuclear translocation of ARTS is blocked, although perinuclear clustering of ARTS-positive mitochondria is still observed (C III, IV). (D) COS-7 cells transfected with AU5-ARTS or AU5-empty vector were treated with 1 μM STS for 0, 1 and 4 h prior to cell fractionation. Western blot analysis using anti-cytochrome c antibodies (upper panel) show cytochrome c restricted to the heavy membrane/mitochondrial fraction. Reduced cytochrome c levels were seen after 4 h of treatment with STS. Upon overexpression of ARTS, some cytochrome c was found in the cytosol. This is consistent with the increased levels of apoptosis seen under these conditions. The middle panel shows the distribution of ARTS in different cell fractions, using an anti-ARTS antibody (Sigma). In nonapoptotic cells, the vast majority of ARTS was found in the mitochondria. However, low amounts of ARTS protein were also detected in the cytosol of cells overexpressing ARTS. Upon apoptotic induction (1 h with STS), ARTS was released from the mitochondria and elevated levels were found in the cytosol. An antibody against the OxPhosComplex IV subunit IV (COX IV) was used as a mitochondrial marker.
Next, we wanted to examine the role of caspase activity on the subcellular distribution of ARTS protein. ARTS translocates from mitochondria to the nucleus during apoptosis (Larisch et al, 2000b), but nothing is known about the underlying molecular mechanism. Caspase activity promotes the release of several other mitochondrial proteins, including cytochrome c and Smac/Diablo, from mitochondria into the cytosol (Li et al, 1998; Bossy-Wetzel and Green, 1999; Adrain et al, 2001; Guo et al, 2002). Therefore, we tested whether caspase inhibitors affected the mitochondrial release of ARTS. Inhibition of caspases in COS-7 cells transfected with ARTS blocked etoposide-induced apoptosis (P-value (ANOVA) 0.0026, Figure 1B). Using immunofluorescence staining, we examined the effect of caspase inhibitors on ARTS protein localization in COS-7 cells (Figure 1C). Transiently transfected cells were treated with etoposide and incubated with or without caspase inhibitors. In the absence of caspase inhibitors, etoposide-treated cells underwent the typical morphological changes associated with apoptosis and accumulated ARTS in the nucleus (Figure 1C I and II). Interestingly, we saw clusters of ARTS-positive mitochondria in the immediate proximity of the nucleus preceding the nuclear entry of ARTS (Figure 1C I, II and data not shown). Similar clusters of mitochondria were described in Bid-induced apoptosis (Li et al, 1998). The addition of caspase inhibitors blocked the nuclear translocation of ARTS, although we still observed the peri-nuclear clustering of ARTS-positive mitochondria (Figure 1C III and IV). Therefore, although ARTS promotes caspase activation, its distribution is also affected by caspase activity. To further confirm the release of ARTS from mitochondria upon pro-apoptotic stimuli, ARTS levels were measured in subcellular fractions from both ARTS-transfected and nontransfected cells. Under nonapoptotic conditions, ARTS was mainly detected in the mitochondria, although low levels of ARTS were also found in the cytosol (Figure 1D). This suggests that some ARTS protein constantly leaks from mitochondria into the cytoplasm. However, because ARTS is a very short-lived protein (Lotan, Rotem and Larisch, unpublished data), it apparently cannot accumulate to levels sufficient for the induction of apoptosis under these conditions. When treated with STS for 1 h, ARTS levels in the cytosol were strongly increased (Figure 1D), and this occurred prior to any detectable release of cytochrome c (Figure 1D, upper panel). ARTS levels decrease following longer exposure to STS, presumably due to the degradation of ARTS in this compartment.
In order to gain an insight into where in the apoptotic pathway ARTS may act in the apoptotic pathway, we took advantage of the existence of a Drosophila homologue of the Sept4 locus. The peanut gene of Drosophila is highly homologous to the mammalian Sept4 locus, which encodes both ARTS and H5 (Neufeld and Rubin, 1994; Larisch et al, 2000b). Although it has been previously shown that peanut is required for cytokinesis, a role of this locus in apoptosis has not yet been reported (Neufeld and Rubin, 1994). Interestingly, in addition to the previously described cell-surface distribution characteristic for septins, some Peanut protein was also detected in the mitochondria (Neufeld and Rubin, 1994; data not shown), suggesting that this gene may also have an ARTS-like pro-apoptotic function. In order to examine this possibility, we tested the effect of loss-of-function peanut mutations on cell killing induced by Drosophila Reaper, Hid and Grim. Expression of Reaper, Hid and Grim in the developing Drosophila eye induces apoptosis that is sensitive to the dosage of other genes affecting cell death (McCall and Steller, 1997). In animals heterozygous for peanut (pnut1/+ and pnutXP/+), the eye ablation phenotypes induced by Reaper, Hid and Grim were significantly reduced (Figure 2). The extent of suppression by peanut was greater than the consequence of reducing the dosage of the Drosophila Apaf1 homologue (Figure 2, rightmost panel), suggesting that peanut works downstream of Reaper, Hid and Grim, but proximal to the conversion with the Apaf-1 activated caspase pathway (Song and Steller, 1999; Zhou et al, 1999). One possible target for the pro-apoptotic action of Peanut/ARTS are the IAPs, which act immediately downstream of Reaper, Hid and Grim to inhibit caspases (Goyal, 2001).
Figure 2.
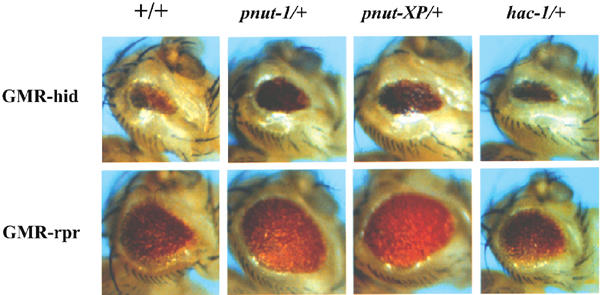
Mutations in Drosophila peanut dominantly suppress cell killing induced by Reaper and Hid. Ectopic expression of reaper, hid and grim under the control of the eye-specific GMR promoter induces apoptosis in cells of the developing retina, resulting in rough and reduced compound eyes. This provides a sensitive assay for mutations in other cell death genes (Bergmann et al, 1998). Reducing the amount of peanut by 50% (in pnut1/+ or pnutXP/+ heterozygous animals) significantly increased the eye size of both GMR-hid and GMR-reaper flies compared to a control background (+/+). Similar results were obtained for GMR-grim (data not shown). Under the same conditions, reducing the dosage of the Drosophila Apaf-1 homolog (hac-1/+) had very little effect.
In order to explore this possibility, we tested whether ARTS can physically interact with IAPs. For this purpose, we performed GST pull-down assays with GST–ARTS from COS-7 cells that were transiently transfected with pcDNA3 mycXIAP. Western blot analysis with anti-XIAP and anti-Myc shows that XIAP can indeed bind to ARTS in vitro (Figure 3A, B). To confirm these results, we also performed the opposite type of pull-down experiment by using GST-XIAP to precipitate ARTS from COS-7 cells transfected with AU5-ARTS. As a control, we used COS-7 cells transfected with AU5-ARTSΔC or AU5-H5. The AU5-ARTSΔC construct lacks 68aa at the ARTS C′-terminus unique sequence. The H5 septin protein is derived by differential splicing from the same locus as ARTS and both proteins share most exons, but H5 is a considerably larger protein that lacks pro-apoptotic activity (Larisch et al, 2000b). Significantly, only ARTS, but not ARTSΔC or H5, were able to bind to XIAP (Figure 3C).
Figure 3.
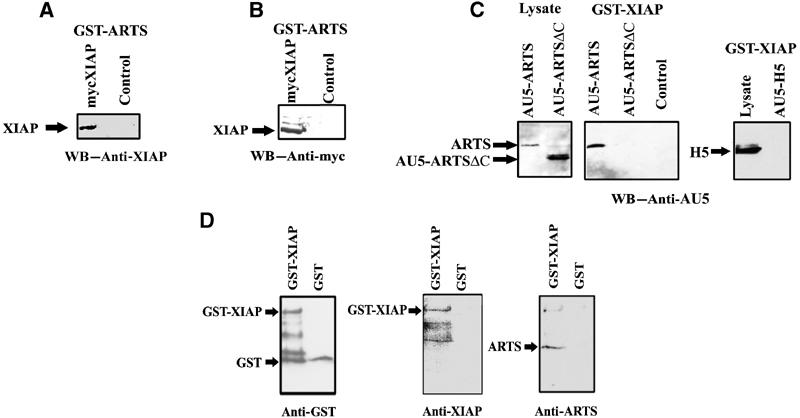
ARTS specifically binds XIAP in vitro. In vitro binding was tested using the GST pull-down assay. (A) COS-7 cells were transient transfected with pcDNA myc XIAP construct. GST pull-down was performed using GST–ARTS and Western blot was caried out with anti-XIAP and (B) with anti-myc. (C) COS-7 cells were transient transfected with AU5-ARTS, AU5-ARTSΔC or AU5-H5. GST pull-down was performed using GST–XIAP and Western blot was carried out with anti-AU5. Binding to glutathione beads alone served as a negative control. (D) Direct binding of ARTS to XIAP is shown in an in vitro assay using GST–XIAP and purified recombinant ARTS protein. GST protein served as a negative control.
In order to test whether ARTS can bind directly to XIAP, we performed GST pull-down assays with GST-XIAP and purified recombinant ARTS protein. As a control, we incubated GST protein with the purified recombinant ARTS protein. Western blot analyses showed that ARTS can indeed bind to XIAP directly (Figure 3D).
Next, we tested whether endogenous ARTS and XIAP interact in vivo. For this purpose, we induced apoptosis in COS-7 cells with STS and used an anti-ARTS antibody coupled to protein G/A sepharose beads to recover ARTS–protein complexes. Western blot analysis of these complexes showed a band of the predicted size for XIAP at 57 kDa (Figure 4A). This suggests that in apoptotic cells at least some of the endogenous ARTS and XIAP proteins bind to each other.
Figure 4.
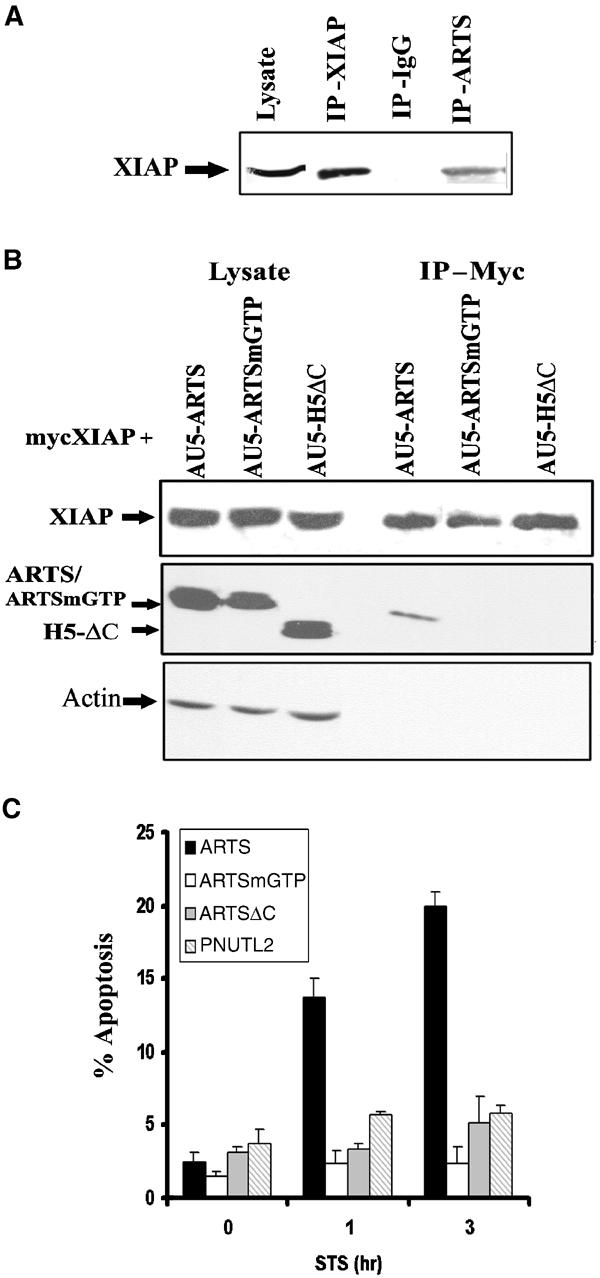
ARTS binding to XIAP is specific and related to its apoptotic function. (A) In vivo binding of ARTS to XIAP was tested using co-immunoprecipitation using anti-ARTS, anti-XIAP or mouse IgG as a negative control. Western blot analysis was carried out using anti-XIAP antibodies. (B) Co-transfected COS-7 cells with pcDNA-mycXIAP and AU5-ARTS, AU5-ARTSmGTP or H5 ΔC were co-immunoprecipitated using agarose-anti-myc beads. Western blot analysis was carried out using rabbit anti-myc and anti-ARTS (NT) antibodies directed against the common N′ terminus of ARTS and H5. Mutant forms of ARTS as well as H5 failed to bind to XIAP. (C) COS-7 cells were transient transfected with AU5-ARTS, AU5-ARTSmGTP, AU5-ARTSΔC or AU5-PNUTL2. Percent apoptosis was determined with the TMR kit. Unlike ARTS, mutant forms of ARTS as well as related septin do not promote apoptosis in STS-treated cells. The P-values (ANOVA) were 0.03 for non-treated cells, compared to 0.000004 for 1 h and 0.00001 for 3 h STS treatment.
Previously, we have shown that ARTS but not H5 or ARTSmGTP induce apoptosis in response to TGF-β (Larisch et al, 2000b). We found that mutant ARTS (ARTSmGTP) and H5ΔC did not bind to XIAP in a co-IP assay (Figure 4B). In addition, unlike ARTS, mutant derivatives (ARTSmGTP, ARTSΔC) and related but another nonapoptotic septin (PNUTL2) failed to induce apoptosis (Figure 4C). Therefore, binding of ARTS to XIAP is specific and highly correlated with its apoptotic function.
If significant levels of ARTS protein bind to XIAP in response to pro-apoptotic stimuli, we would expect to see co-localization of these proteins in apoptotic cells. To test this, we performed immunofluorescence double-labeling of COS-7 cells transfected with AU5-ARTS and pcDNA3-Myc-XIAP (Figure 5). Apoptosis was induced with etoposide and cells were stained cells after 2 and 16 h. In nonapoptotic cells, ARTS and XIAP had overall distinct, nonoverlapping distributions (Figure 5, top panel). ARTS was primarily localized to the mitochondria as previously described (Larisch et al, 2000b), whereas XIAP staining was cytoplasmic, with some perinuclear concentration. In contrast, 2 h after the induction of apoptosis, there was an extensive overlap between ARTS and XIAP staining (Figure 5, mid panel). Both proteins co-localized as aggregates that were most prominent in the vicinity of the nucleus. After 16 h of induction, most of the staining for both proteins was confined to the nucleus, consistent with previous reports on the localization of these proteins in apoptotic cells (Larisch et al, 2000b; Liston et al, 2001). However, our data reveal a striking level of co-localization of ARTS and XIAP in cells that are doomed to die, which was previously not recognized. These observations suggest that once ARTS is released from mitochondria in response to apoptotic stimuli, it binds rapidly and efficiently to XIAP, and both proteins appear to remain in a complex throughout much of the apoptosis.
Figure 5.
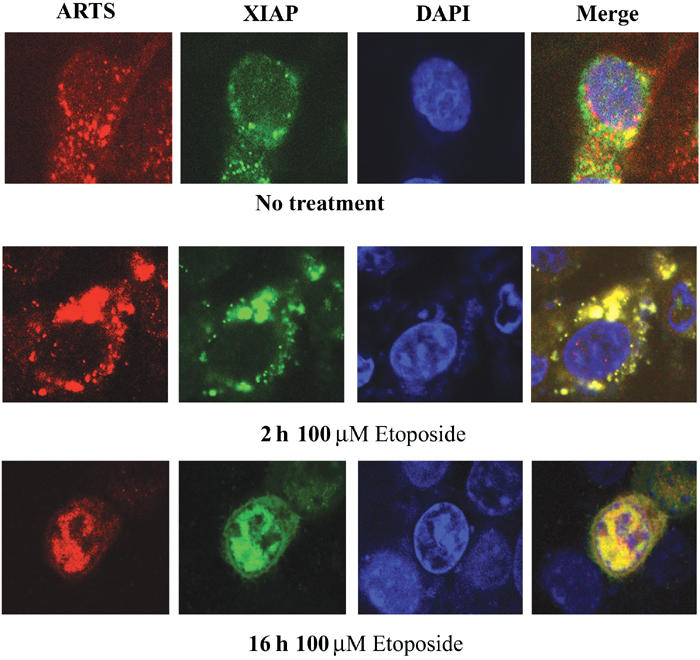
ARTS and XIAP co-localize during apoptosis. Immunofluorescence assay of COS-7 cells with anti-ARTS antibodies (Red), and anti-XIAP antibodies (green). The nucleus is stained with Dapi (blue). Cells were treated with 100 μM etoposide for 2 h (mid panel) and for 16 h (lower panel), as compared to non-treated cells (upper panel). The cells were analyzed using confocal microscopy (× 60). The merged images (right panel) are shown in yellow. Nonapoptotic cells had very little overlap between ARTS and XIAP staining (top panel). In nonapoptotic cells, ARTS was primarily localized to mitochondria, whereas XIAP staining was cytoplasmic, with some perinuclear concentration. At 2 h after the induction of apoptosis, co-localization of ARTS and XIAP occurred mainly in the vicinity of the nucleus (mid panel). After 16 h of apoptotic induction, both proteins perfectly co-localized in the nucleus.
We also used co-IP experiments to examine interactions between endogenous ARTS and XIAP in response to apoptotic stimuli. For this purpose, we initially used cells that express high levels of endogenous ARTS. We induced apoptosis with etoposide for 3 and 6 h and compared binding to non-induced controls (Figure 6A). In living cells, we detected only a small amount of XIAP bound to ARTS, presumably due to a small amount of cells undergoing apoptosis without induction. In contrast, after 3 h of etoposide treatment, a large amount of XIAP was immunoprecipitated together with ARTS, and this amount even increased further after 6 h of apoptotic induction.
Figure 6.
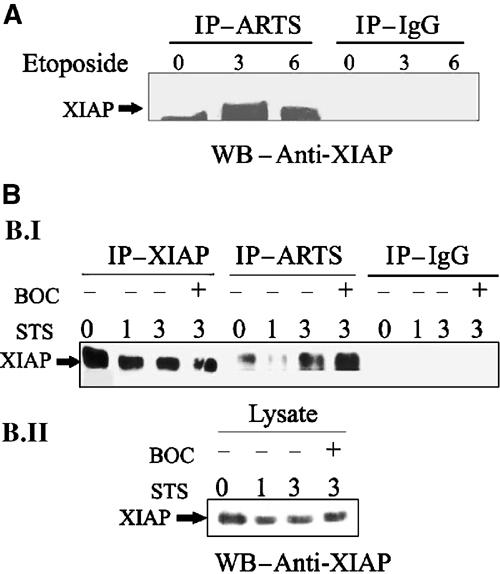
Upon apoptotic induction, ARTS binds higher levels of XIAP in a caspase-independent manner. (A) NRP-154 cells without apoptotic treatment, or treated with 100 μM etoposide for 3 and 6 h were used for co-immunoprecipitation using anti-ARTS antibodies. Western blot analysis was carried out with anti-XIAP antibodies. (B.I) COS-7 cells without apoptotic treatment, or treated with 1 μM STS for 1 and 3 h were used for co-immunoprecipitation using anti-ARTS antibodies. COS-7 cells were also treated with BOC (caspase inhibitor). (B.II) XIAP levels in the same lysates used for IP (B. I) are shown. During apoptotic induction, both NRP-154 cells and COS-7 cells show a significant increase of ARTS binding to XIAP. ARTS–XIAP interactions do not depend on caspase activity and do not occur as a consequence of apoptosis.
As many of our previous studies were performed in COS-7 cells, we repeated co-IP experiments with ARTS and XIAP in this cell type, even though COS-7 cells express very low levels of endogenous ARTS. COS-7 cells were treated with STS for 1 and 3 h to induce apoptosis. Thereafter, lysates were prepared for co-IP/Western blot analysis using a monoclonal antibody to detect XIAP (Figure 6B). As in cells, we observed very little interaction between ARTS and XIAP without apoptotic induction. After 3 h of incubation with STS, there was a significant increase in ARTS–XIAP binding. As ARTS and XIAP reside in different cellular compartments in living cells, we considered the possibility that caspase activity and execution of apoptosis may be required to release ARTS from mitochondria and allow interaction with XIAP. To test this possibility, we repeated co-IP experiments in the presence of the broad-spectrum caspase inhibitor BOC (Figure 6B). Treatment of BOC did not inhibit the binding of ARTS to XIAP in response to apoptotic stimuli. If anything, the amount of XIAP immunoprecipitated by ARTS under these conditions was even greater. Therefore, ARTS–XIAP interactions appear not to depend on caspase activity and do not occur as a consequence of apoptosis. We also noted a decrease in XIAP protein levels (compare ‘IP–XIAP' 0 h with 1 and 3 h induction) that was readily detectable after 1 h. As this reduction of XIAP protein was not sensitive to the caspase inhibitor BOC, it cannot be simply the consequence of caspase activation and apoptosis. Taken together, these results demonstrate that the induction of apoptosis promotes interactions between ARTS and XIAP, presumably due to the release of ARTS from mitochondria. Moreover, cytochrome c and Smac/Diablo release from mitochondria is reported to occur in a caspase-dependent manner (Adrain et al, 2001). Our data suggest that the release of ARTS from mitochondria is a caspase-independent event. Thus, it seems that the release of ARTS may precede the release of both cytochrome c and Smac/Diablo from mitochondria, and ARTS may therefore act upstream of both Smac/Diablo and cytochrome c.
It was previously shown that XIAP is degraded upon the induction of apoptosis (Yang et al, 2000). As overexpression of ARTS promotes apoptosis and was associated with downregulation of XIAP, we further investigated the consequence of increased levels of ARTS on XIAP protein. COS-7 cells were transiently transfected with pcDNA3-myc-XIAP and AU5-ARTS, and XIAP levels were assessed both with anti-Myc and anti-XIAP antibodies. The amount of XIAP was significantly decreased in cells overexpressing ARTS compared to cells transfected with XIAP alone (Figure 7, see also Figure 6B for endogenous levels of ARTS). Similar results were obtained in COS-7 cells that had been stably transfected with ARTS expression construct (Figure 7B). These cells, which overexpress high levels of ARTS, show high levels of the apoptotic marker H2A.X that is detected only in apoptotic cells (Figure 7B; Paull et al, 2000). In addition, we found that COS-7 cells transiently transfected with ARTS and treated with STS had reduced XIAP protein levels, while cells transfected with a nonapoptotic-related septin (PNUTL2) showed no effect (Figure 7C). Furthermore, treatment of cells with the proteasome inhibitor MG132 blocked ARTS-induced reduction of XIAP levels, indicating that proteasome-mediated protein degradation is involved in ARTS-mediated downregulation of XIAP (Supplementary Figure 1). We therefore suggest that ARTS promotes apoptosis through binding to and inhibiting the antiapoptotic function of XIAP. At least part of this inhibition of XIAP appears to involve a reduction of XIAP protein levels via proteasome-mediated protein degradation (Figure 8, see Discussion).
Figure 7.
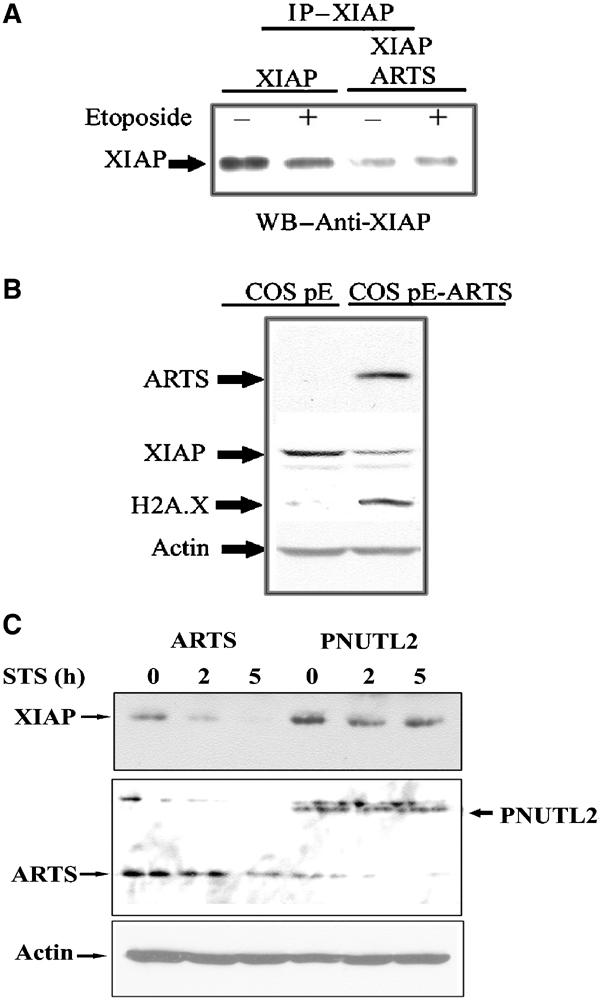
ARTS causes downregulation of XIAP levels during apoptosis. (A) COS-7 cells were transient transfected with either pcDNA3 myc XIAP alone, or co-transfected with pcDNA3 myc XIAP and AU5-ARTS. Cells with or without treatment with 100 μM etoposide are shown. Immunoprecipitation with anti-myc antibodies was carried out, followed by Western blot analysis with anti-XIAP antibodies. (B) COS pE cells and COS pE stably transfected with AU5-ARTS. Analysis was carried out with anti-XIAP, anti-AU5, anti-H2A.X and anti-actin antibodies. COS pE-ARTS cells expressing high levels of ARTS exhibited significantly reduced levels of XIAP, which probably leads to the observed increase in apoptosis. The phospho-histone H2A.X, detected only under apoptotic conditions, was used to identify cells undergoing apoptosis. (C) COS-7 cells were transient transfected with AU5-ARTS or AU5-PNUTL2. Cells were treated with 1 μM STS for 0, 2 and 5 h. Western blot analysis was carried out using anti-XIAP and anti-ARTS (NT) antibodies, which recognize the common N′ terminus of ARTS and H5. XIAP levels show a significant reduction only in lysates from ARTS-transfected cells. ARTS, but not the nonapoptotic-related septin (PNUTL2), causes downregulation of XIAP levels during apoptosis.
Figure 8.
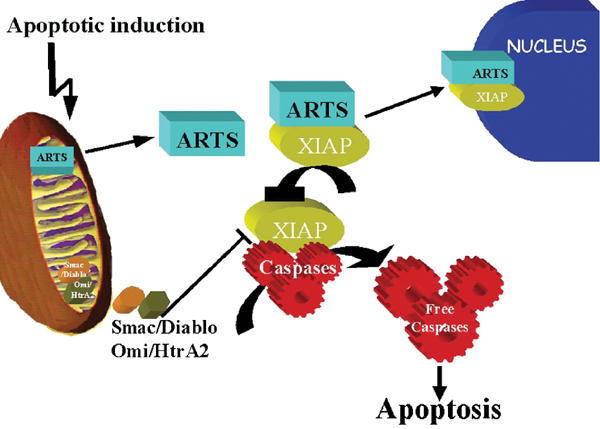
Schematic model of ARTS mechanism of action in apoptosis. In living cells, ARTS is localized to the mitochondria. Upon pro-apoptotic stimuli, ARTS is released from mitochondria in a caspase-independent manner and binds to XIAP. Eventually, the ARTS–XIAP complex translocates to the nucleus. As the release of Smac/Diablo from mitochondria is thought to require caspase activity, it appears that ARTS acts at an earlier stage during apoptosis. ARTS binding to XIAP downregulates XIAP levels, which releases caspase activation, leading to apoptotic cell death.
Discussion
IAPs are a conserved family of proteins that can inhibit caspases (Salvesen and Duckett, 2002). In cells that are doomed to die, this inhibition of death has to be overcome (Song and Steller, 1999; Shi, 2002). Recent studies in both Drosophila and mammalian systems showed that decreasing IAP levels by ubiquitination increases caspase activity and leads to apoptosis (Martin, 2002). In the absence of the Drosophila IAP antagonists Reaper, Hid and Grim, virtually no apoptosis can occur (White et al, 1994; McCall and Steller, 1997), demonstrating the importance of IAP inactivation for the induction or cell death. In this study, we show that ARTS also induces apoptosis, at least in part, by targeting IAPs.
Several lines of evidence show that ARTS interacts with XIAP upon induction of apoptosis. In living cells, the localization of ARTS and XIAP is distinct, but both proteins co-localize extensively in apoptotic cells. Likewise, GST pull-down and co-IP assays demonstrate that ARTS and XIAP are in a complex under apoptotic conditions. As recombinant ARTS protein can bind XIAP in vitro, this interaction appears to be direct. This interaction appears to be highly specific, since it was not seen with nonapoptotic proteins closely related to ARTS.
The mitochondrial localization of ARTS and its ability to bind XIAP are reminiscent of Smac/Diablo and Omi/HtrA2, but unlike these proteins ARTS does not contain a recognizable IBM (Shi, 2002; Vaux and Silke, 2003). Possibly, binding of ARTS to XIAP requires the unique C-terminus of ARTS, a stretch of 27 amino acids not found in H5 (Larisch et al, 2000b). Consistent with this idea, we show that a C-terminal deletion of ARTS lacking this region did not bind to XIAP in vitro and did not induce apoptosis (Figures 3C and 4C, respectively). These results suggest that the unique C′ terminus of ARTS is important for its binding to XIAP. Interestingly, a GTP-binding domain mutant of ARTS also did not bind to XIAP and did not induce apoptosis. Therefore, this domain seems to be required for XIAP binding in combination with the unique C′ terminus of ARTS. One consequence of ARTS binding to XIAP appears to be a reduction in XIAP protein levels. As this reduction of XIAP protein was also seen in cells treated with the caspase inhibitor BOC, it cannot simply be the consequence of caspase activation and apoptosis. Collectively, these results suggest that ARTS promotes apoptosis by binding to IAPs and decreasing their protein levels, which in turn de-represses caspases. Presumably, ARTS can interact with multiple IAPs, since inactivation of XIAP alone cannot account for the induction of apoptosis (Harlin et al, 2001).
ARTS and XIAP co-localize during most, if not all, of apoptosis and eventually both accumulate in the nucleus. The role of this nuclear complex is not clear at this time. As both proteins are subject to ubiquitin-mediated degradation (Yang et al, 2000; Lotan, Roten and Larisch, unpublished results), accumulation in the nucleus may simply be the result of differential protein stability in different cellular compartments. Alternatively, both proteins may play a role in the nucleus. Importantly, in apoptotic cells, which do not overexpress ARTS, only low levels of diffuse XIAP staining were seen (Supplementary Figure 2). In contrast, in cells overexpressing ARTS, XIAP was found at significantly higher levels in the nucleus. Thus, it appears that ARTS may be responsible for XIAP translocation to the nucleus. Interestingly, it has been reported that another XIAP-associated protein, XAF1, triggers the redistribution of XIAP from the cytosol to the nucleus when overexpressed (Liston et al, 2001). Therefore, it is possible that nuclear translocation of XIAP–antagonist complexes is a common feature in apoptosis.
Several studies have shown feedback and feed-forward loops in the control of caspases (Slee et al, 1999). For example, IAPs are both inhibitors of and targets for caspases: mammalian XIAP and Drosophila Diap1 are cleaved by caspases during apoptosis, and this cleavage appears to be functionally significant (Deveraux et al, 1999; Ditzel et al, 2003). In addition, the release of cytochrome c promotes caspase activation, and caspase activity can promote the release of cytochrome c via cleavage of Bid (Li et al, 1998; Bossy-Wetzel and Green, 1999). Interestingly, binding of ARTS to XIAP does not require caspase activity and the execution of cell death. On the other hand, caspase inhibitors blocked the nuclear translocation of ARTS. Taken together, our results suggest the following role of ARTS for the induction of apoptosis (Figure 8). Upon apoptotic stimuli, ARTS is released from mitochondria by a caspase-independent mechanism that remains to be determined. The exit of ARTS from mitochondria enables ARTS to bind XIAP and reduce its protein levels, presumably by proteosome-mediated degradation. As a result, caspases become de-repressed and apoptosis is facilitated. ARTS shares its mitochondrial localization and IAP-binding properties with Smac/Diablo, although there is no resemblance in the sequence of both proteins. Also, a reduction of IAP protein levels by Smac/Diablo has not yet been reported. In this respect, the function of ARTS is similar to that of the Drosophila IAP antagonists Reaper and Grim, even though again there is no detectable amino-acid similarity between ARTS and these proteins. As septins are thought to serve as scaffold proteins (Field and Kellogg, 1999; Kartmann and Roth, 2001; Kinoshita et al, 2002), it is possible that ARTS promotes the assembly of multi-protein complexes between IAPs and other cell death regulators, including IAP antagonists and ubiquitin pathway proteins. Alternatively, ARTS may employ novel protein motifs to carry out its IAP-inhibiting activities. In either case, our results demonstrate that ARTS is an important mitochondrial protein that promotes apoptosis through regulation of IAPs.
Materials and methods
Mammalian cell culture and plasmids
K562 and HL-60 cells were grown in RPMI 1640 medium. A549, COS-7 cells were grown in Dulbecco's modified Eagle medium (DMED) with 4.5 g/l D-glucose. All cells were grown at 37°C in 5% CO2 atmosphere. All media were supplemented with 10% fetal calf serum (FCS), penicillin (100 U/ml), streptomycin (100 μg/ml) and glutamine (2 mM), medium (Biological Industries, Israel). Treatment of cells with proteosome inhibitor (MG132, Calbiochem) at 20 μM for 2 h was carried out prior to the addition of the apoptotic inducer. COS-7, K562 and HL-60 were transient transfected using electroporation (Easyject plus—Equibio). A549 was transient transfected using lipofectamine™ (Invitrogen), according to the manufacturer's protocol.
pEF1-AU5 and pEF1-AU5-ARTS constructs were used for all ARTS transient transfection experiments. AU5 tag was attached to the N′ terminus of ARTS (Larisch et al, 2000b). The AU5-ARTSmGTP construct was generated by site-directed mutagenesis (QuickChange™, Stratagene) with the primer ggagagtctggcctggagaatcccacacttgtcaatagcc replacing three amino acids (G K S with E N P) at the GTP-binding site.
The AU5-ARTS ΔC construct lacks 68aa at the ARTS C′-terminus unique sequence and was generated using PCR with the primers: L1: BamHI—atcaagcgtttcctggaggacaccacgg and S-207: EcoRI—ctatgccacaggcttccagcactc.
pEF1-AU5-H5, pEF1-AU5-PNTUL2 isoform 3, AU5-ARTSmGTP, AU5-H5 ΔC and AU5-ARTS ΔC were obtained from Dr Seong-Jin Kim, NCI/NIH.
Stably transfected COS pE-ARTS cells were generated using the pEF1-IRES vector (Hobbs et al, 1998). Briefly, the construct contains EF-1 promoter followed by a multi-cloning site, EMC (Encephalomyocarditis virus) internal ribosome entry sites (IRES) and puromycin resistance gene. AU5-tagged ARTS was inserted into the XhoI site downstream of the EF-1 promoter. The construct was stably transfected into COS-7 cells. COS pE cells stably transfected with pEF1-AU5 empty vector served as control. The cells were maintained in medium containing 4 μg/ml puromycin.
The mammalian expression construct encoding Myc epitope-tagged wild-type XIAP in pcDNA3 and the pGEX-XIAP were obtained from Dr Colin S Duckett.
For the GST-pull-down assays, we cloned full-length ARTS into the pGEX 4T (Pharmacia Biotech) construct; GST–ARTS fusion protein was generated using the PCR method with the following primers: BamHI—5′-TCGAGGATCCATCAAGCGTTTCCTGGAGGACACCACGG-3′ and EcoRI—5′-CTAGTGGCAGCCCTGCCCCTGGTGC-3′, and cloned into BamHI and EcoRI sites in pGEX 4T.
Apoptosis assays
In order to induce apoptosis, cells were treated with different apoptotic agents 40 h after transient transfections. The following apoptotic agents were used: TGF-beta (10 ng/ml) for 24 h in medium containing 1% FCS, 100 μg/ml etoposide for 2 and 16 h (Sigma), STS (1 μM) for 0–3 h (Sigma) and arabinoside-c (ara-C, cytosar) 100 μM for 4 h (Pharmacia). For caspase inhibition, we added 40 μM of BOC-Asp(Ome)CH2F (Enzyme Systems Products) 1 h prior to addition of the apoptotic agents. Apoptotic cells were detected using the anti-H2A.X antibody (Upstate; Paull et al, 2000) or TUNEL. For TUNEL assays, COS-7 cells were transiently transfected with AU5-ARTS, AU5-ARTS ΔC, AU5-ARTSmGTP and AU5-H5. At 24 h after transfection, the cells were treated with STS (1 μM) for 1 and 3 h. The cells were fixed and permeabilized. Apoptosis levels were determined using the TUNEL In situ cell death detection TMR kit (Roche) according to the manufacturer's protocol. All slides were coded and the experiments were carried out in a blind manner.
Caspase-3 activity assay
Caspase-3 activity was tested in K562, HL-60 and A549 cells using caspase-3 activity assay kit (Roche) according to the manufacturer's protocol. Caspase-3 activity was tested in COS-7 cells by immunofluorescence staining with anti-active caspase-3 antibodies 1:4000 (R&D systems). Results are presented as fold increase relative to results in cells transfected with control vector without treatment with apoptotic inducers.
GST pull-down experiments
For in vitro binding studies, recombinant GST–ARTS or GST–XIAP fusion proteins were purified from bacteria. After sonication, 0.1% Triton X-100 and protease inhibitors (Mini-Complete™, Roche) were added to the bacterial extract, followed by 10 000 rpm centrifugation. Supernatants of bacterial extracts were collected and incubated in the presence of glutathione-sepharose 4B beads (Amersham Biosciences) for 30 min at 4°C. The beads were washed three times. COS-7 cells were transient transfected with pcDNA3-mycXIAP construct, pEF1-AU5-ARTS construct or pEF1-AU5-H5 construct as a control. The cells were lysed in RIPA buffer (150 mM NaCl, 50 mM Tris–HCl (pH 8), 1% NP-40, 0.1% SDS, 0.5% deoxycholate acid containing protease inhibitors (mini Complete, Roche)). Each sample was divided into two tubes; one was rotated for 4 h at 4°C with GST–ARTS fusion protein coupled with the glutathione beads, or GST–XIAP fusion protein. The second tube served as control and was incubated with glutathione beads alone. Samples were centrifuged at 4000 rpm at 4°C for 4 min and washed five times in lysis buffer. Elution of proteins from beads was carried out by 5 min boiling in sample buffer. Proteins were separated on 12.5% SDS–PAGE gel, followed by Western blot analysis using monoclonal anti-ARTS antibodies (Sigma) or monoclonal anti-XIAP antibodies (BD Transduction Laboratories) or monoclonal anti-myc antibodies.
In vitro binding assay
Recombinant ARTS protein was generated with the TNT-Quick Coupled Transcription/Translation System (Promega) and incubated overnight at 4°C with either recombinant GST-XIAP bound to glutathione beads, or with GST alone bound to glutathione beads (beads were washed and treated as described in the above paragraph). SDS–PAGE analysis and Western blot was performed using monoclonal anti-ARTS antibodies (Sigma) and anti-GST antibodies (Gibco).
Co-immunoprecipitation
Cells were grown in 100 mm culture dishes and treated with or without apoptotic agents. Protein extracts were prepared with lysis buffer containing 150 mM NaCl, 50 mM Tris–HCl (pH 8), 1% NP-40, 0.5% deoxycholate acid with protease inhibitors (mini complete, Roche). Protein levels were determined and equal amounts were used for each sample. Lysates were pre-cleared with 1 mg mouse IgG (Sigma) coupled with protein A/G sepharose mix (Amersham Biosciences). Complexes were incubated overnight at 4°C, followed by low-speed centrifugation. Supernatants were immunoprecipitated using 5 μl of monoclonal anti-ARTS antibodies (Sigma) for 4 h or monoclonal anti-XIAP antibodies (BD Transduction Laboratories) or monoclonal anti-myc antibodies (Clontech). As a control, we used mouse IgG (Sigma). Protein A/G sepharose beads were added to immunoprecipitate complex for 1 h, collected and washed four times with PBS.
For Western blot analysis, antibodies against XIAP (BD Transduction Laboratories), Myc (Santa Cruz), ARTS/ARTSmGTP/H5 (ProSci Incorporated) were used.
Immunofluorescence assay
Cells were fixed with 4% paraformaldehyde in PBS for 20 min at room temperature, washed with PBS and permeabilized with 0.5% Triton-X in PBS for 5 min, and incubated with primary antibodies for 2 h at RT (rabbit polyclonal anti-ARTS 1:20 000 (Sigma), monoclonal anti-ARTS (Sigma) and monoclonal anti-XIAP 1:500 (BD Transduction Laboratories). Cells were washed (× 3) with PBS/0.1% Triton-X and incubated with FITC conjugated anti-mouse and RhodaminTX conjugated anti-rabbit secondary antibodies (Jackson). Image analysis was carried out using confocal laser microscopy (Zeiss LSM 510).
Cell fractionation
Cell fractionation was carried out as described (Chandra et al, 2002). Briefly, cells were homogenized in 20 mM HEPES-KOH, pH 7.5, 10 mM KCl, 1.5 mM MgCl2, 1 mM sodium EDTA, 1 mM sodium EGTA and 1 mM dithiothreitol in the presence of 250 mM sucrose and protease inhibitors (Mini-Complete™, Roche). Homogenates were centrifuged at 500 g for 5 min at 4°C, and the supernatant was centrifuged at 10 000 g for 20 min to obtain mitochondria. The mitochondrial pellet was washed and solubilized in TNC buffer (10 mM Tris acetate, pH 8.0, 0.5% Nonidet P-40, 5 mM CaCl2) containing protease inhibitors. Protein concentration was determined by Micro-BCA kit (Pierce).
Anti-OxPhosComplex IV subunit IV antibodies (COX IV, Molecular Probes) were used to identify specifically the mitochondrial subcellular fraction.
Supplementary Material
Supplementary Figure legends:
Acknowledgments
We thank Drs Colin Duckett for XIAP constructs, Seong-Jin Kim for the AU5-tagged constructs and S Hobbs for pEF1-IRES. We also thank Dr Zvi Ben-Ishai from Rambam Medical Center, Haifa, Israel for his continuous support. HS is an Investigator with the Howard Hughes Medical Institute. Part of this work was supported by an FIRCA grant from the National Institute of Health to HS and SL.
References
- Adrain C, Creagh EM, Martin SJ (2001) Apoptosis-associated release of Smac/DIABLO from mitochondria requires active caspases and is blocked by Bcl-2. EMBO J 20: 6627–6636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann A, Agapite J, Steller H (1998) Mechanisms and control of programmed cell death in invertebrates. Oncogene 17: 3215–3223 [DOI] [PubMed] [Google Scholar]
- Bossy-Wetzel E, Green DR (1999) Caspases induce cytochrome c release from mitochondria by activating cytosolic factors. J Biol Chem 274: 17484–17490 [DOI] [PubMed] [Google Scholar]
- Chandra D, Liu JW, Tang DG (2002) Early mitochondrial activation and cytochrome c up-regulation during apoptosis. J Biol Chem 277: 50842–50854 [DOI] [PubMed] [Google Scholar]
- Deveraux QL, Leo E, Stennicke HR, Welsh K, Salvesen GS, Reed JC (1999) Cleavage of human inhibitor of apoptosis protein XIAP results in fragments with distinct specificities for caspases. EMBO J 18: 5242–5251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deveraux QL, Reed JC (1999) IAP family proteins—suppressors of apoptosis. Genes Dev 13: 239–252 [DOI] [PubMed] [Google Scholar]
- Deveraux QL, Takahashi R, Salvesen GS, Reed JC (1997) X-linked IAP is a direct inhibitor of cell-death proteases. Nature 388: 300–304 [DOI] [PubMed] [Google Scholar]
- Ditzel M, Wilson R, Tenev T, Zachariou A, Paul A, Deas E, Meier P (2003) Degradation of DIAP1 by the N-end rule pathway is essential for regulating apoptosis. Nat Cell Biol 5: 467–473 [DOI] [PubMed] [Google Scholar]
- Du C, Fang M, Li Y, Li, Wang X (2000) Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell 102: 33–42 [DOI] [PubMed] [Google Scholar]
- Field CM, Kellogg D (1999) Septins: cytoskeletal polymers or signaling GTPases? Trends Cell Biol 10: 387–394 [DOI] [PubMed] [Google Scholar]
- Goyal L (2001) Cell death inhibition: keeping caspases in check. Cell 104: 805–808 [DOI] [PubMed] [Google Scholar]
- Goyal L, McCall K, Agapite J, Hartwieg E, Steller H (2000) Induction of apoptosis by Drosophila reaper, hid and grim through inhibition of IAP function. EMBO J 19: 589–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Srinivasula SM, Druilhe A, Fernandes-Alnemri T, Alnemri ES (2002) Caspase-2 induces apoptosis by releasing proapoptotic proteins from mitochondria. J Biol Chem 277: 13430–13437 [DOI] [PubMed] [Google Scholar]
- Harlin H, Reffey SB, Duckett CS, Lindsten T, Thompson CB (2001) Characterization of XIAP-deficient mice. Mol Cell Biol 21: 3604–360811313486 [Google Scholar]
- Hobbs S, Jitrapakdee S, Wallace JC (1998) Development of a bicistronic vector driven by the human polypeptide chain elongation factor 1alpha promoter for creation of stable mammalian cell lines that express very high levels of recombinant proteins. Biochem Biophys Res Commun 252: 368–372 [DOI] [PubMed] [Google Scholar]
- Hu S, Yang X (2003) Cellular inhibitor of apoptosis 1 and 2 are ubiquitin ligases for the apoptosis inducer Smac/DIABLO. J Biol Chem 278: 10055–10060 [DOI] [PubMed] [Google Scholar]
- Kartmann B, Roth D (2001) Novel roles for mammalian septins: from vesicle trafficking to oncogenesis. J Cell Sci 1114: 839–844 [DOI] [PubMed] [Google Scholar]
- Kinoshita M, Field CM, Coughlin ML, Straight AF, Mitchison TJ (2002) Self- and actin-templated assembly of mammalian septins. Dev Cell 6: 791–802 [DOI] [PubMed] [Google Scholar]
- Larisch S, Danielpour D, Roche NS, Lotan R, Hsing AY, Kerner H, Hajouj T, Lechleider RJ, Roberts AB (2000a) Selective loss of the transforming growth factor-beta apoptotic signaling pathway in mutant NRP-154 rat prostatic epithelial cells. Cell Growth Differ 11: 1–10 [PubMed] [Google Scholar]
- Larisch S, Yi Y, Lotan R, Kerner H, Eimerl S, Tony Parks W, Gottfried Y, Birkey Reffey S, de Caestecker MP, Danielpour D, Book-Melamed N, Timberg R, Duckett CS, Lechleider RJ, Steller H, Orly J, Kim SJ, Roberts AB (2000b) A novel mitochondrial septin-like protein, ARTS, mediates apoptosis dependent on its P-loop motif. Nat Cell Biol 2: 915–921 [DOI] [PubMed] [Google Scholar]
- Li H, Zhu H, Xu CJ, Yuan J (1998) Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 94: 491–501 [DOI] [PubMed] [Google Scholar]
- Liston P, Fong WG, Kelly NL, Toji S, Miyazaki T, Conte D, Tamai K, Craig CG, McBurney MW, Korneluk RG (2001) Identification of XAF1 as an antagonist of XIAP anti-caspase activity. Nat Cell Biol 3: 128–133 [DOI] [PubMed] [Google Scholar]
- Martins LM, Iaccarino I, Tenev T, Gschmeissner S, Totty NF, Lemoine NR, Savopoulos J, Gray CW, Creasy CL, Dingwall C, Downward J (2002) The serine protease Omi/HtrA2 regulates apoptosis by binding XIAP through a reaper-like motif. J Biol Chem 277: 439–444 [DOI] [PubMed] [Google Scholar]
- Martin SJ (2002) Destabilizing influences in apoptosis: sowing the seeds of IAP destruction. Cell 109: 793–796 [DOI] [PubMed] [Google Scholar]
- McCall K, Steller H (1997) Facing death in the fly: genetic analysis of apoptosis in Drosophila. Trends Genet 13: 222–226 [DOI] [PubMed] [Google Scholar]
- Meier P, Finch A, Evan G (2000) Apoptosis in development. Nature 407: 796–801 [DOI] [PubMed] [Google Scholar]
- Neufeld TP, Rubin GM (1994) The Drosophila peanut gene is required for cytokinesis and encodes a protein similar to yeast putative bud neck filament proteins. Cell 77: 371–379 [DOI] [PubMed] [Google Scholar]
- Paull TT, Rogakou EP, Yamazaki V, Kirchgessner CU, Gellert M, Bonner WM (2000) A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr Biol 10: 886–895 [DOI] [PubMed] [Google Scholar]
- Salvesen GS, Duckett CS (2002) IAP proteins: blocking the road to death's door. Nat Rev Mol Cell Biol 3: 401–410 [DOI] [PubMed] [Google Scholar]
- Shi Y (2002) A conserved tetrapeptide motif: potentiating apoptosis through IAP-binding. Cell Death Differ 9: 93–95 [DOI] [PubMed] [Google Scholar]
- Slee EA, Adrain C, Martin SJ (1999) Serial killers: ordering caspase activation events in apoptosis. Cell Death Differ 6: 1067–1074 [DOI] [PubMed] [Google Scholar]
- Song Z, Steller H (1999) Death by design: mechanism and control of apoptosis. Trends Cell Biol 9: M49–M52 [PubMed] [Google Scholar]
- Sun C, Cai M, Gunasekera AH, Meadows RP, Wang H, Chen J, Zhang H, Wu W, Xu N, Ng SC, Fesik SW (1999) NMR structure and mutagenesis of the inhibitor-of-apoptosis protein XIAP. Nature 401: 818–822 [DOI] [PubMed] [Google Scholar]
- Sun C, Cai M, Meadows RP, Xu N, Gunasekera AH, Herrmann J, Wu JC, Fesik SW (2000) NMR structure and mutagenesis of the third Bir domain of the inhibitor of apoptosis protein XIAP. J Biol Chem 275: 33777–33781 [DOI] [PubMed] [Google Scholar]
- Thompson CB (1995) Apoptosis in the pathogenesis and treatment of disease. Science 267: 1456–1462 [DOI] [PubMed] [Google Scholar]
- Vaux DL, Silke J (2003) Mammalian mitochondrial IAP binding proteins. Biochem Biophys Res Commun 304: 499–504 [DOI] [PubMed] [Google Scholar]
- Verhagen AM, Ekert PG, Pakusch M, Silke J, Connolly LM, Reid GE, Moritz RL, Simpson RJ, Vaux DL (2000) Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins. Cell 102: 43–53 [DOI] [PubMed] [Google Scholar]
- Verhagen AM, Silke J, Ekert PG, Pakusch M, Kaufmann H, Connolly LM, Day CL, Tikoo A, Burke R, Wrobel C, Moritz RL, Simpson RJ, Vaux DL (2002) HtrA2 promotes cell death through its serine protease activity and its ability to antagonize inhibitor of apoptosis proteins. J Biol Chem 277: 445–454 [DOI] [PubMed] [Google Scholar]
- Wang SL, Hawkins CJ, Yoo SJ, Muller HA, Hay BA (1999) The Drosophila caspase inhibitor DIAP1 is essential for cell survival and is negatively regulated by HID. Cell 98: 453–463 [DOI] [PubMed] [Google Scholar]
- White K, Grether ME, Abrams JM, Young L, Farrell K, Steller H (1994) Genetic control of programmed cell death in Drosophila. Science 264: 677–683 [DOI] [PubMed] [Google Scholar]
- Yang Y, Fang S, Jensen JP, Weissman AM, Ashwell JD (2000) Ubiquitin protein ligase activity of IAPs and their degradation in proteasomes in response to apoptotic stimuli. Science 288: 874–877 [DOI] [PubMed] [Google Scholar]
- Zhou L, Song Z, Tittel J, Steller H (1999) HAC-1, a Drosophila homolog of APAF-1 and CED-4 functions in developmental and radiation-induced apoptosis. Mol Cell 4: 745–755 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure legends:


