Significance
In this series of studies, we provide a rare illustration of how a chromosomal polymorphism has affected overt social behavior in a vertebrate. White-throated sparrows occur in two alternative phenotypes, or morphs, distinguished by a chromosomal rearrangement. That the morphs differ in territorial and parental behavior has been known for decades, but how the rearrangement affects behavior is not understood. Here we show that genetic differentiation between the morphs affects the transcription of a gene well known to be involved in social behavior. We then show that in a free-living population, the neural expression of this gene predicts both territorial and parental behavior. We hypothesize that this mechanism has played a causal role in the evolution of alternative life-history strategies.
Keywords: estradiol, testosterone, morph, reproductive tactics
Abstract
The evolution of behavior relies on changes at the level of the genome; yet the ability to attribute a behavioral change to a specific, naturally occurring genetic change is rare in vertebrates. In the white-throated sparrow (Zonotrichia albicollis), a chromosomal polymorphism (ZAL2/2m) is known to segregate with a behavioral phenotype. Individuals with the ZAL2m haplotype engage in more territorial aggression and less parental behavior than individuals without it. These behaviors are thought to be mediated by sensitivity to sex steroids, and the chromosomal rearrangement underlying the polymorphism has captured a prime candidate gene: estrogen receptor 1 (ESR1), which encodes estrogen receptor α (ERα). We therefore hypothesized that the behavioral effects of the ZAL2m rearrangement are mediated by polymorphism in ESR1. We report here that (i) the ESR1 promoter region contains fixed polymorphisms distinguishing the ZAL2m and ZAL2 alleles; (ii); those polymorphisms regulate transcription efficiency in vitro and therefore potentially do the same in vivo (iii); the local expression of ERα in the brain depends strongly on genotype in a free-living population; and (iv) ERα expression in the medial amygdala and medial preoptic area may fully mediate the effects of genotype on territorial aggression and parenting, respectively. Thus, our study provides a rare glimpse of how a chromosomal polymorphism has affected the brain and social behavior in a vertebrate. Our results suggest that in this species, differentiation of ESR1 has played a causal role in the evolution of phenotypes with alternative life-history strategies.
Because the genetic basis of social behavior is complex and involves many genes throughout the genome, it has been difficult to link behavior with specific genes—particularly in vertebrates. The white-throated sparrow (Zonotrichia albicollis), a common North American songbird, represents a unique model for connecting genetic sequence with behavior (1). This species occurs in two plumage morphs (Fig. 1) that segregate absolutely with a rearranged chromosome 2 called ZAL2m (2). ZAL2m consists of at least two pericentric inversions (3) and is present in all white-striped (WS) individuals but never in tan-striped (TS) individuals. The rearrangement occurs equally in males and females, and almost all breeding pairs consist of one individual with and one without it (4). This disassortative mating system is the mechanism by which balancing selection acts to maintain ZAL2/2m in the population, and consequently, homozygosity for ZAL2m is rare (4, 5). Recombination between ZAL2 and ZAL2m is profoundly suppressed (3), and this genetic isolation has resulted in the differentiation of alternative chromosome types (6).
Fig. 1.

Plumage polymorphism in white-throated sparrows. Birds of the TS morph (Left) have alternating brown and tan stripes on the crown, whereas WS birds (Right) have black and white stripes.
Genetic differentiation of ZAL2/2m is associated with a behavioral phenotype (1, 4); WS males engage in more territorial aggression and mate-finding than do TS males. In contrast, TS males show more nestling provisioning and mate guarding. In songbirds, territorial and parental behaviors typically depend on sex steroids; during the breeding season, WS birds have higher plasma testosterone (T) than do their same-sex TS counterparts (7, 8). Morph differences in behavior cannot be entirely explained by these hormones, however, because the differences persist even when plasma levels are experimentally equalized (9). Individual variation in steroid-dependent behavior may be better explained by neural sensitivity to the hormones, for example by variation in the distribution and abundance of steroid receptors (10, 11).
The gene encoding estrogen receptor α (ERα), estrogen receptor 1 (ESR1), has been captured by the ZAL2m rearrangement (3) and has therefore likely differentiated between the haplotypes. We hypothesized that this differentiation has affected cis-acting regulation of ERα expression, which could explain morph differences in behavior in this species. To test this hypothesis we first identified fixed polymorphisms in the ESR1 allele and tested whether they affect transcription efficiency in vitro. We next compared the level of ERα expression between the morphs in vivo and tested whether that expression predicts territorial song or provisioning of nestlings. We found that polymorphisms in the ZAL2/2m ESR1 promoter have the potential to affect expression of ERα mRNA. Further, in a free-living population, ERα expression depends strongly on morph yet predicts both song and parental behavior independently of morph. Thus, our results illustrate a detailed chain of events linking a chromosomal rearrangement to changes in overt social behavior.
Results
Differentiation Between ZAL2 and ZAL2m Has Led to Cis-Acting Polymorphisms.
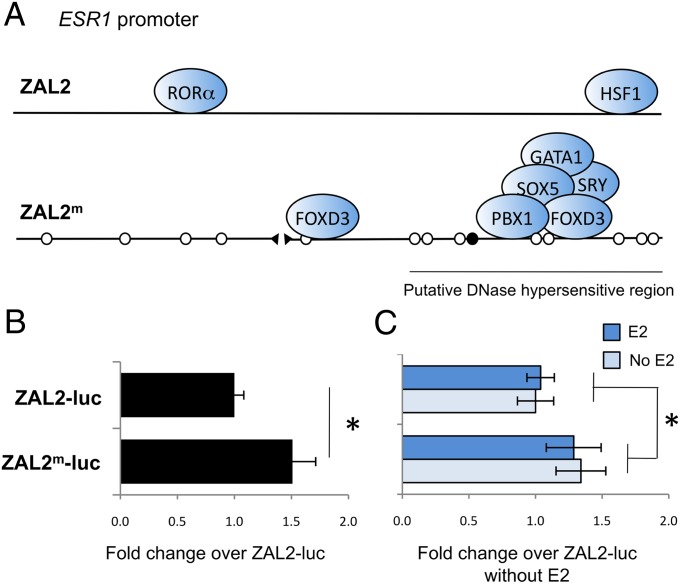
To investigate whether fixed polymorphisms between the ZAL2 and ZAL2m haplotypes could affect ESR1 expression, we compared predicted transcription factor binding to the ESR1 promoter between the haplotypes. TFSEARCH, a software program that locates potential transcription factor binding sites (12), predicted the binding of several transcription factors known to regulate ER expression, such as GATA-3 (13) and Oct-1 (14). Notably, differences between the ZAL2 and ZAL2m ESR1 promoter sequences cause variation in predicted transcription factor binding to these elements (Fig. 2A). Predicted binding sites for HSF1 and retinoid related orphan receptor α are present in the ZAL2 ESR1 promoter but are eliminated by SNPs within the ZAL2m promoter. Conversely, several sites are predicted exclusively within the ZAL2m ESR1 promoter, most of which were gained within a region corresponding to the DNase hypersensitive region of the mammalian ESR1 promoter (Fig. 2A). At least one of these transcription factors, PBX1, is thought to affect ER expression (15), indicating that differentiation of the ESR1 promoter sequences may drive differences in ERα expression.
Fig. 2.
Differentiation of the ESR1 promoter sequence has led to changes in transcription efficiency. (A) Predicted loss or gain of transcription factor binding sites. SNPs are indicated by white circles, insertions by black circles, and deletions by gaps, compared with the ZAL2 haplotype. The sequence alteration that potentially confers binding of PBX1 and several other transcription factors is located within a region corresponding to the mammalian DNase hypersensitive region of the ESR1 promoter, indicating that these changes have likely occurred within an active region for transcription factor binding. (B) In HeLa cells, the ZAL2m construct inserted upstream of luciferase (luc) resulted in significantly more expression compared with the ZAL2 construct (P = 0.007). (C) E2 added to the cell culture (1 µM) did not alter transcription efficiency for either construct (E2 × construct interaction, P = 0.941).
Cis-Acting Polymorphisms Have the Potential to Regulate ERα Gene Expression.
To assess whether the cis-acting polymorphisms we identified have the potential to modify ERα gene expression, we prepared constructs with the ESR1 promoter sequence from the ZAL2 or ZAL2m haplotypes upstream of firefly luciferase, and transfected them into HeLa cells (SI Materials and Methods). The constructs with the ZAL2m allele produced ∼50% more luciferase activity than the construct with the ZAL2 allele [F(1,191) = 7.505; P = 0.007; Fig. 2B]. Experiments were run at four different concentrations of the construct, but the concentration had no effect, nor did it interact with the main effect of construct. Overall, this assay demonstrated that the differences in promoter sequence between the ZAL2 and ZAL2m alleles of ESR1 have the potential to modify gene expression in vivo.
Many of the brain regions containing ERα mRNA are rich in aromatase (16), which converts T to estradiol (E2). We therefore tested whether the local E2 concentration alters ERα promoter efficiency and, if so, whether that change depends on haplotype. In a second series of experiments, although we replicated the effect of construct [F(1,143) = 4.033; P = 0.047], adding E2 to the cells had no effect [F(1,143) = 0.311; P = 0.578; Fig. 2C]. Further, the effect of construct did not depend on the presence or absence of E2 [F(1,191) = 0.006; P = 0.941]. Thus, we found no evidence that E2 regulates its own receptor via the cis-acting elements in the promoter region we analyzed, or that such regulation depends on haplotype. E2 may nonetheless regulate ERα expression via other mechanisms, such as alternative promoters or transcription factors not present in our preparation, which themselves may be expressed in a morph-dependent way.
As is the case for most mRNAs, the transcription of ERα mRNA depends on many factors both cis- and transacting. The ZAL2m rearrangement is likely to have captured elements that may interact with ESR1 and differentially regulate transcription, none of which were available in our in vitro preparation. Thus, we cannot draw conclusions about the direction of the effect or the details of in vivo regulation, only that the promoter sequence we analyzed contains enough variation to affect transcription even without those elements.
ERα Gene Expression Depends on Morph in a Free-Living Population.
The results above demonstrate that under in vitro conditions, ERα mRNA expression is regulated in an allele-specific way. To test for such regulation in vivo, we quantified ERα mRNA expression in the brains of 79 free-living, behaviorally characterized white-throated sparrows during the breeding season near Argyle, Maine. We focused our analysis on nine distinct brain regions, several of which make up an E2-sensitive social behavior network (17, 18). To control for type I error due to multiple tests, we first conducted an omnibus multiple analysis of covariance (MANCOVA) on all of the ERα values for each sex. These tests showed a significant effect of morph [males: F(9, 28) = 24.914; P < 0.001; females: F(9, 17) = 14.835; P < 0.001], even when plasma concentrations of T and E2 were controlled as covariates. There was no effect of in situ hybridization (ISH) run, plasma T, or plasma E2 on ERα mRNA expression in either sex, nor were there any interactions between ISH run and morph.
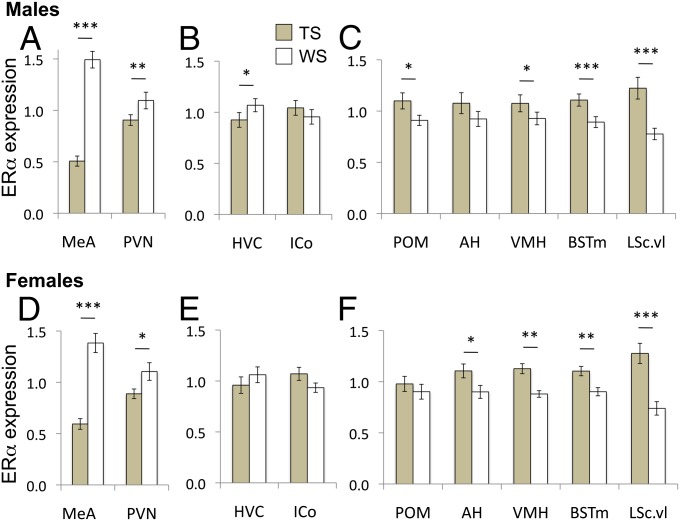
Because the overall MANCOVAs showed significant effects of morph, we then conducted univariate ANCOVAs on the ERα values for each region of interest in each sex. The results are plotted in Fig. 3. In both sexes, ERα mRNA was expressed in the medial amygdala (MeA) at much higher levels in WS than TS birds. ERα mRNA expression was higher in WS birds also in the paraventricular nucleus (PVN) in both sexes, and in HVC of males. In contrast with those regions, ERα expression was higher in TS than WS birds in the ventromedial hypothalamus, medial bed nucleus of the stria terminalis (BSTm), and the ventrolateral portion of the caudal lateral septum (LSc.vl). The effect was particularly striking in LSc.vl, a region in which Fos and Egr-1 expression is negatively correlated with territorial aggression in song sparrows (Melospiza melodia) and in which we previously demonstrated a morph difference in both vasotocin and vasoactive intestinal peptide innervation (reviewed in ref. 19). There were morph differences in the same direction in both the medial preoptic area (POM) and the anterior hypothalamus, but these were significant only in the males and the females, respectively.
Fig. 3.
Morph differences in ERα mRNA expression in the brains of male (A–C) and female (D–F) white-throated sparrows. Values for each region were normalized to the series mean, and means and SEMs for the normalized values are plotted. Relative ERα expression was higher in WS than TS birds in MeA and PVN in both sexes (A and D) and in HVC in males (B). In the hypothalamus, BSTm, and LSc.vl, however, ERα expression was higher in TS than WS birds (C and F). AH, anterior hypothalamus; HVC, used as a proper name; ICo, intercollicular nucleus; VMH, ventromedial hypothalamus. *P < 0.05; **P < 0.01; ***P < 0.001. n = 46 males (23 WS, 23 TS), 33 females (17 WS, 16 TS). These samples included all of the behaviorally characterized birds in the study (peak territorial and parental), plus three birds (two WS males and one TS female) for whom no reliable behavioral data were collected.
Overall, in most of the hypothalamic and dorsal diencephalic regions, ERα expression was significantly higher in the TS than in the WS birds. These results suggest that in these regions, ERα mRNA expression may be under similar transcriptional control. In contrast, WS birds had higher levels than TS in MeA, PVN and, in males, HVC, suggesting that the local environment interacts with the cis-acting mechanisms we have identified (Fig. 2) to regulate transcription of this gene in a region-dependent way. The pattern of morph differences was remarkably similar in both sexes, with no meaningful variation between males and females. The transcriptional mechanisms that mediate morph differences in ERα expression therefore do not seem to vary according to sex.
ERα Gene Expression Predicts Territorial Song and Parental Behavior.
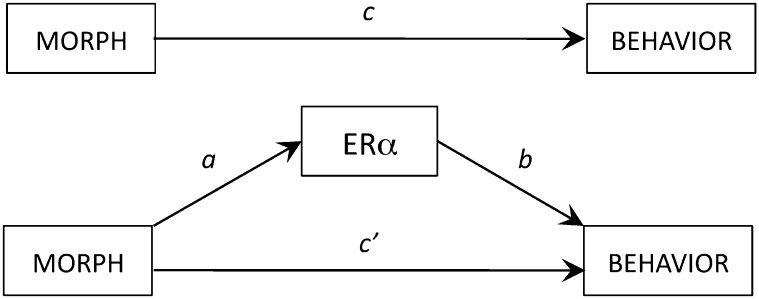
We next asked whether ERα expression could explain morph differences in behavior. Our model is shown in Fig. 4. Using mediation analysis with bootstrapping (20, 21), we tested whether the predictive power of the indirect effect of morph on behavior via ERα expression (ab path) was greater than that of the direct path (not via ERα expression, c’ path). Males showed robust morph differences in singing induced by simulated territorial intrusion [STI; t(21) = 2.479; P = 0.022] and nestling provisioning [t(10) = 2.319; P = 0.043; SI Materials and Methods]. Thus, the c path, which is the relationship between morph and behavior, was satisfied for both behaviors in males. In females the c path was satisfied for singing [t(8) = 2.501; P = 0.037]. As in previous studies (22), however, we did not detect a morph difference in female provisioning rate [t(18) = 0.616; P = 0.546]. Thus, the c path was satisfied in females only for song.
Fig. 4.
Hypothesized model in which ERα expression mediates the effect of morph on behavior. To demonstrate that the relationship between morph and behavior (c path; Upper) may be mediated by ERα expression, we aimed to show a significant relationship between morph and ERα expression (a path) and between ERα expression and behavior (b path). Mediation analysis with bootstrapping (20, 21) was then used to test whether the inclusion of ERα expression reduces or eliminates the direct relationship between morph and behavior (c’ path).
To reduce the number of regions in which we tested for mediation effects, and thus minimize type I error, we performed the analysis only for regions for which the a and b paths were satisfied when controlling for multiple tests (SI Materials and Methods and Table S1). The results are described below and in Table 1. The independent contributions to behavior made by plasma T, morph, and ERα expression in the regions we considered are plotted in Fig. 5.
Table 1.
Results of mediation analysis
|
a |
b |
c |
c’ |
CI | df | ||||||
| Variable | Coefficient | P | Coefficient | P | Coefficient | P | Coefficient | P | |||
| Song | MeA | 4.351 | <0.001 | 5.630 | 0.017 | 10.417 | 0.022 | −14.639 | 0.156 | 2.389, 45.956 | 22 |
| PVN | 6.282 | 0.049 | 0.666 | 0.041 | 10.417 | 0.022 | 4.92 | 0.304 | 0.691, 12.117 | 22 | |
| Trips | POM | −16.680 | 0.037 | 0.093 | 0.003 | −1.741 | 0.042 | 0.023 | 0.973 | −3.503, −0.510 | 11 |
The effect of morph on singing in response to STI (c path, Fig. 4) was reduced and no longer significant (c’ path) when the ab path including ERα expression in the MeA or the PVN, which were both significant, was considered. Similarly, the effect of morph on provisioning trips was reduced and no longer significant when the ab path including ERα expression in the POM was considered. These results are consistent with the hypothesis that ERα expression in MeA, PVN, and POM fully mediates morph differences in behavior.
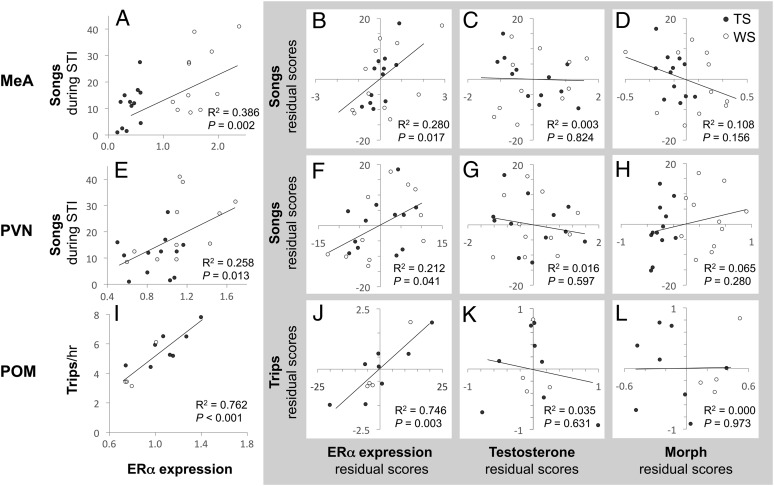
Fig. 5.
ERα expression explains individual variation in territorial song and parental behavior in male white-throated sparrows. (Left) Zero-order Pearson correlations showing the relationships between (A) the number of songs given during STI and ERα expression in the medial amygdala (MeA), (E) the number of songs during STI and ERα expression in the PVN, and (I) the number of provisioning trips per hour (first nest of the season) and ERα expression in POM. The partial correlations, shown in the shaded area, depict how well behavior was predicted by ERα expression, plasma T, or morph when all three variables were included in the same model (models also included plasma E2). ERα expression in MeA (B) and PVN (F) was positively related to singing, and in POM (J) was related to provisioning trips, both in the uncorrected zero-order correlation and in the models that controlled for T, E2, and morph. Neither plasma T (C, G, and K) nor E2 (MeA R2 = 0.020; PVN R2 = 0.095; POM R2 = 0.192) significantly predicted song or trips when ERα expression was controlled. Similarly, morph did not significantly predict singing behavior after ERα expression was controlled (D, H, and L). Thus, morph differences in song and in parental behavior can be explained by ERα expression in MeA and PVN or in POM, respectively, independently of morph.
Male song.
In males, ERα expression was related to both morph and song (ab paths) only in MeA and PVN (Fig. 3A and Table S1). Mediation analysis (Table 1) confirmed that paths a and b were significant and that the c’ path (direct effect of morph not mediated by ERα expression) was not. The confidence intervals (CIs) did not span zero for either region. This result shows that ERα expression in MeA or PVN are possible mediators of the effect of morph on singing behavior. Further, neither plasma T nor E2 could be mediators: in both cases, the a and b paths for both hormones were nonsignificant, and all CIs spanned zero. Overall, our results suggest that morph differences in the vocal response to STI can be explained by variation in ERα expression in MeA and PVN, and that plasma sex steroids do not explain vocal responses.
Examination of the individual contributions of ERα expression, plasma T, E2, and morph to variation in male singing behavior (Fig. 5 B–D and F–H) revealed that when the sex steroids and morph were held constant, there was still a significant correlation between song and ERα expression in both MeA and PVN. Finally, when ERα expression and sex steroids were controlled, the morph difference in singing behavior disappeared. These results confirm our findings in the mediation analysis, suggesting that variation in ERα expression in MeA and PVN, not plasma sex steroids, can completely explain morph differences in singing behavior.
Female song.
Females do not respond as vigorously to STI and are more difficult to capture; thus our sample size was smaller for females than for males. We were unable to detect significant correlations between singing and ERα expression in any region of interest after correcting for multiple comparisons (Table S1). There were trends, however, for singing to correlate positively with ERα expression in PVN and MeA, suggesting that the mechanisms underlying morph differences in territorial vocal responses may be similar in both sexes.
Provisioning in males.
The indirect paths for provisioning trips were satisfied by ERα expression in POM only (Fig. 3A and Table S1). The a and b paths were significant for POM, and the significant effect of morph on trips was eliminated when the mediation effect of ERα expression in POM was considered (compare c and c’ in Table 1). The CI for POM did not span zero, suggesting that ERα expression acts as a true mediator. As was the case for the song analysis above, neither plasma T nor E2 showed significant a or b paths, and the CIs spanned zero in all cases. These results indicated that ERα expression in POM is a possible mediator of the morph difference in nestling provisioning trips and that sex steroids are not potential mediators.
Examination of the individual contributions of ERα expression, plasma T, E2, and morph to variation in male provisioning behavior (Fig. 5 J–L) revealed that when plasma sex steroids and morph were held constant, a significant correlation remained between ERα expression in POM and provisioning trips. When ERα expression in POM was controlled, the morph difference in provisioning behavior disappeared. These results confirm our findings in the mediation analysis, suggesting that variation in ERα expression in POM, and not plasma sex steroids, can completely explain morph differences in parental behavior. Although our sample sizes were small, the relationship between ERα expression in POM and provisioning trips was quite strong. This relationship should be explored in future studies.
Alternative Promoter Use Does Not Vary by Haplotype.
To determine whether ERα mRNA is processed differently in the two morphs, we looked for evidence of alternative splice isoforms from each haplotype. Two alternative first exons were noted (Fig. S1), but they were each transcribed from both haplotypes (SI Results).
The ZAL2 and ZAL2m ERα Alleles Encode Alternative Protein Isoforms.
We observed two fixed differences driving a Val73Ile and Ala552Thr polymorphism in ZAL2m allele of the ERα protein (Fig. S2). On the basis of their locations and the ability of ERα to tolerate heterogeneity at these sites, we do not expect either polymorphism to impact ligand binding or interaction with transcriptional coactivators (SI Results).
Discussion
In this series of studies, we have assembled a vertically integrated model of how a change in genetic sequence has affected overt social behavior in a vertebrate. In white-throated sparrows, because of a lack of recombination between ZAL2 and ZAL2m (3), fixed polymorphisms have accumulated and driven the evolution of alternative phenotypes that differ in plumage (Fig. 1), territorial behavior, and parental behavior. Here we showed that the promoter sequence of ESR1, which encodes ERα, has been altered by the rearrangement (Fig. 2A). We then showed that this differentiation affects transcription efficiency in vitro (Fig. 2B) and therefore could do so in vivo. We demonstrated further that in a free-living population, the neural expression of ERα mRNA differs between individuals with and without the rearrangement (Fig. 3). Finally, we showed that in the brain regions MeA and PVN, both of which are associated with aggression in birds (19, 23–25), ERα expression predicts territorial singing. Similarly, nestling provisioning is predicted by ERα expression in POM, a region associated with avian parental behavior (26). In both cases, ERα expression predicts behavior even when genotype is controlled (Fig. 5), and genotype has no further predictive value for behavior when ERα expression in these regions is controlled (Fig. 5 and Table 1). These results are consistent with the hypothesis that differentiation of ESR1 has played a causal role in the evolution of phenotypes with alternative life-history strategies.
WS birds invest more in mating effort, whereas TS birds invest more in parental effort. This life-history tradeoff is believed to be mediated in part by T, which increases singing and mate-finding and decreases parental behavior in many seasonally breeding sparrows (27–30). In free-living populations of white-throated sparrows, plasma T levels are higher in WS birds (7, 8), which have the ZAL2m rearrangement, than in TS birds, which do not (2, 31). Therefore, we might expect morph differences in social behavior to be driven by T alone. Exogenous administration of sex steroids, however, stimulates more singing in WS than TS birds of both sexes, despite producing identical plasma levels (9). This result strongly suggests that sensitivity to plasma steroid manipulations differs between the morphs. Other authors have suggested that steroid-sensitive behaviors are often better correlated with steroid receptors than with plasma steroid levels (10, 11, 32–34). In dark-eyed juncos (Junco hyemalis), a relative of white-throated sparrows, aggressive responses to STI were positively correlated with ERα mRNA expression in the ventral telencephalon, which contains MeA, and negatively correlated with it in the hypothalamus (11). It is thus interesting that in our study, the more aggressive morph showed higher ERα expression in MeA but lower expression in most of the hypothalamic regions we looked at (Fig. 3). We did not find simple correlations between territorial singing and ERα expression in regions other than MeA and PVN, but we suspect that any such relationship may be masked by the strong effect of genotype on expression.
By far the most striking morph difference in ERα expression occurs in MeA (Fig. 3 A and D). In both sexes, expression levels in this region were nonoverlapping between the morphs; in other words, individuals could be assigned to a morph simply by the level of ERα expression in MeA. Moreover, expression was related to territorial behavior even within morph (Fig. 5B). MeA is a gateway for sensory information to enter the social behavior network; in rodents it receives massive projections from the olfactory bulb and is critical for initiating social responses to pheromonal signals (35). In birds, MeA receives projections from both the auditory system and the song system (36), thus serving as an important hub for the integration of communication signals and the social behavior network. MeA lesions disrupt behavioral responses to social signals in ring doves and Japanese quail (37, 38) and inhibit male-directed song in zebra finches (25), suggesting an important role in the processing of social stimuli.
We are not the first to suggest a role for ERα expression in the MeA in the evolution of life-history tradeoffs. Cushing et al. (32) worked with two populations of prairie voles (Microtus ochrogaster), one from a monogamous, biparental Illinois population and another from a Kansas population with less male parental care and more promiscuity. Compared with the Illinois males, the Kansas males had higher levels of ERα mRNA expression in MeA. Importantly, experimental overexpression of ERα in MeA profoundly inhibited paternal behavior and increased interest in novel females (38). Together with these and other studies in rodents (e.g., ref. 39), our results suggest that ERα in MeA may underlie the evolution of a suite of complex correlated traits that constitute a “personality” (40) or “behavioral syndrome” (41) that maximizes territoriality and mate-seeking while minimizing prosocial behaviors, such as monogamy and parenting. Direct manipulation of ERα expression in white-throated sparrows will be necessary to explore this hypothesis further.
The ZAL2m chromosome is in several ways strikingly similar to the mammalian Y. First, nearly all breeding pairs of white-throated sparrows consist of one individual that lacks the chromosome and one heterozygous for it (4). Second, profound suppression of recombination has caused it to differentiate from its counterpart (3, 6). Most interestingly, many behaviors that segregate with ZAL2m—higher territorial aggression, higher levels of mate-seeking, and lower parental investment—are the same behaviors that segregate with the mammalian Y. These traits are typically selected along dimensions defined by life-history tradeoffs, which favors their sequestration into alternative phenotypes—WS or TS, male or female (1). In white-throated sparrows, these behaviors differ by sex as well as by morph; males sing more, and females provision young more (4). The evolutionary forces linking these behaviors with ZAL2m are not strong enough to mask sex differences—in fact the opposite; morph differences in female provisioning rates are difficult to detect (22; cf. ref. 42). Morph thus interacts with sex, and we should expect that the neural mechanisms meditating the behavioral effects of morph also depend on sex. In the present study, we induced territorial song by presentation of a male decoy, thus the social context of the behavioral test differed between male and female residents. Future studies should compare the neural mechanisms underlying morph-dependent behavior more directly between males and females.
Materials and Methods
Details are provided in SI Materials and Methods.
Sequence Analysis.
To analyze differentiation of the ESR1 promoter region, we aligned a 2-kb sequence upstream of the most proximal exon 1 and confirmed all polymorphisms by sequencing the region in five WS and five TS individuals. Sequences were then submitted to TFSEARCH for prediction of transcription factor binding sites (12). To assess which regions of the ESR1 promoter may be readily available for transcription factor binding, we used DNase hypersensitivity data from the ENCODE project (43), accessed through the University of California, Santa Cruz genome browser (44).
Luciferase Reporter Assays.
A 2-kb sequence in the ESR1 promoter (of the most proximal exon 1; Fig. S1) was isolated from a ZAL2/2m heterozygote and cloned upstream of firefly luciferase in the pGL3 basic vector. Constructs were prepared from both the ZAL2 and ZAL2m alleles, which were then transfected with Renilla luciferase into culture HeLa cells. After 18 h, firefly and Renilla luciferase activities were measured with the Dual-Glo assay system (Promega) on a Biotek Synergy plate reader. In a second experiment, cells were cultured for 24 h, then 1 μM 17-β estradiol (Calbiochem) or media was added. After an additional 24 h, firefly and Renilla luciferase activities were measured as above.
Field Study.
Free-living white-throated sparrows were behaviorally characterized and collected near Argyle, Maine, under appropriate state and federal permits. We quantified territorial aggression using STI (45) during the early stages of breeding to coincide with the peak of territorial behavior. During the nestling stage, at a different location on the site, we quantified the rate at which the parents provisioned the nestlings (number of trips per hour made by the parents to the nest to feed nestlings) by placing cameras near nests. The methods for each type of behavioral test were as previously described (22, 45, 46). Within 1 d of the last observation, birds were captured in mist nets and a blood sample taken. Brains were harvested, rapidly frozen on dry ice and shipped to Atlanta. Plasma T and E2 were measured in blood samples by RIA (47).
In Situ Hybridization.
35S-labeled riboprobes were prepared from a 501-bp sequence spanning exons 4–8, which contained no ZAL2/2m polymorphisms (Fig. S1). ISH was performed on 20-µm brain sections according to a standard protocol (48). Tissue from each sex and behavioral study was run separately. After hybridization, autoradiographic film was placed against the slides, exposed for 3 d protected from light, developed, and scanned at 2,400 dpi on a digital scanner. Using ImageJ (National Institutes of Health), the gray value of the ERα mRNA signal was obtained in each region of interest in two to four consecutive sections, which in this series were 140 µm apart. Background measurements were taken in nearby regions containing no obvious signal, and the difference between the signal and the background was used in the analysis. To test for effects of morph, we first entered the corrected gray values for each region of interest in all birds into a single MANCOVA for each sex, with morph and ISH run as between-subjects variables and plasma T and E2 as covariates. We found highly significant effects of morph for both sexes (Results) and no interactions between ISH run and morph. These tests were thus followed by univariate tests for effects of morph on ERα expression within each region of interest.
To test whether ERα expression may mediate the effects of morph on behavior, we performed mediation analysis with bootstrapping and bias-corrected confidence estimates (20, 21). This analysis was performed for the regions for which there was a significant effect of morph on ERα expression and a correlation between that expression and behavior (two regions for the peak territorial males and one for the parental males). In each model we included plasma T and plasma E2 as possible alternative mediators. We obtained 95% CIs for each indirect effect with 5,000 bootstrap samples.
Supplementary Material
Acknowledgments
We thank D. Abebe, G. Bhat, J. Davis, C. Horoszko, O. Laur (Emory University Custom Cloning Core Facility), C. Leung, J. Liang, and C. MacDowell for technical assistance in Atlanta; A. Annis, E. Burns, J. Cava, A. Cornell, C. Gurguis, J. Michaud, and C. McKee for field assistance in Maine; C. Henry for access to freezer space and other resources at the University of Maine; the Forest Society of Maine and J. Metzler for permission to conduct our field study at the Hemlock Stream Forest; P. Wolff and I. Waldman for advice on statistical methods; I. Moore for the use of radioimmunoassay facilities; and the Departments of Biology at Emory University and University of Maine for the use of shared resources. This research was funded by National Institutes of Health (NIH) Grant 1R01MH082833 and National Science Foundation (NSF) Grant IOS-0723805 (to D.L.M.), NIH Grant 1R01DK095750 (to E.A.O.), NIH Grant R21MH090418 (to J.W.T.), NIH Grant 5T32GM008602 and American Heart Association Grant 13PRE16920012 (to W.H.H.), and NSF Grant SMA-1306132 (to W.M.Z.-K.). J.W.T. was supported by the National Human Genome Research Institute Intramural Research program at the NIH.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. E.D.K. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1317165111/-/DCSupplemental.
References
- 1.Maney DL. Endocrine and genomic architecture of life history trade-offs in an avian model of social behavior. Gen Comp Endocrinol. 2008;157(3):275–282. doi: 10.1016/j.ygcen.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 2.Thorneycroft HB. A cytogenetic study of the white-throated sparrow, Zonotrichia albicollis. Evolution. 1975;29:611–621. doi: 10.1111/j.1558-5646.1975.tb00855.x. [DOI] [PubMed] [Google Scholar]
- 3.Thomas JW, et al. The chromosomal polymorphism linked to variation in social behavior in the white-throated sparrow (Zonotrichia albicollis) is a complex rearrangement and suppressor of recombination. Genetics. 2008;179(3):1455–1468. doi: 10.1534/genetics.108.088229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Falls JB, Kopachena JG (2010) White-throated sparrow (Zonotrichia albicollis). The Birds of North America, ed Poole A (Cornell Laboratory of Ornithology, Ithaca, NY)
- 5.Horton BM, et al. Behavioral characterization of a white-throated sparrow homozygous for the ZAL2(m) chromosomal rearrangement. Behav Genet. 2013;43(1):60–70. doi: 10.1007/s10519-012-9574-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis JK, et al. NISC Comparative Sequencing Program Haplotype-based genomic sequencing of a chromosomal polymorphism in the white-throated sparrow (Zonotrichia albicollis) J Hered. 2011;102(4):380–390. doi: 10.1093/jhered/esr043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spinney LH, Bentley GE, Hau M. Endocrine correlates of alternative phenotypes in the white-throated sparrow (Zonotrichia albicollis) Horm Behav. 2006;50(5):762–771. doi: 10.1016/j.yhbeh.2006.06.034. [DOI] [PubMed] [Google Scholar]
- 8.Swett MB, Breuner CW. Plasma testosterone correlates with morph type across breeding substages in male white-throated sparrows. Physiol Biochem Zool. 2009;82(5):572–579. doi: 10.1086/605392. [DOI] [PubMed] [Google Scholar]
- 9.Maney DL, Lange HS, Raees MQ, Reid AE, Sanford SE. Behavioral phenotypes persist after gonadal steroid manipulation in white-throated sparrows. Horm Behav. 2009;55(1):113–120. doi: 10.1016/j.yhbeh.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 10.Ball GF, Balthazart J. Individual variation and the endocrine regulation of behaviour and physiology in birds: A cellular/molecular perspective. Philos Trans R Soc Lond B Biol Sci. 2008;363(1497):1699–1710. doi: 10.1098/rstb.2007.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosvall KA, et al. Neural sensitivity to sex steroids predicts individual differences in aggression: Implications for behavioural evolution. Proc Biol Sci. 2012;279(1742):3547–3555. doi: 10.1098/rspb.2012.0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heinemeyer T, et al. Databases on transcriptional regulation: TRANSFAC, TRRD and COMPEL. Nucleic Acids Res. 1998;26(1):362–367. doi: 10.1093/nar/26.1.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eeckhoute J, et al. Positive cross-regulatory loop ties GATA-3 to estrogen receptor alpha expression in breast cancer. Cancer Res. 2007;67(13):6477–6483. doi: 10.1158/0008-5472.CAN-07-0746. [DOI] [PubMed] [Google Scholar]
- 14.Hosey AM, et al. Molecular basis for estrogen receptor alpha deficiency in BRCA1-linked breast cancer. J Natl Cancer Inst. 2007;99(22):1683–1694. doi: 10.1093/jnci/djm207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheung CL, et al. Pre-B-cell leukemia homeobox 1 (PBX1) shows functional and possible genetic association with bone mineral density variation. Hum Mol Genet. 2009;18(4):679–687. doi: 10.1093/hmg/ddn397. [DOI] [PubMed] [Google Scholar]
- 16.Balthazart J, et al. Distribution of aromatase-immunoreactive cells in the forebrain of zebra finches (Taeniopygia guttata): Implications for the neural action of steroids and nuclear definition in the avian hypothalamus. J Neurobiol. 1996;31(2):129–148. doi: 10.1002/(SICI)1097-4695(199610)31:2<129::AID-NEU1>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 17.Newman SW. The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Ann N Y Acad Sci. 1999;877(1):242–257. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- 18.Maney DL, Goode CT, Lange HS, Sanford SE, Solomon BL. Estradiol modulates neural responses to song in a seasonal songbird. J Comp Neurol. 2008;511(2):173–186. doi: 10.1002/cne.21830. [DOI] [PubMed] [Google Scholar]
- 19.Maney DL, Goodson JL. Neurogenomic mechanisms of aggression in songbirds. Adv Genet. 2011;75:83–119. doi: 10.1016/B978-0-12-380858-5.00002-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mackinnon DP, Lockwood CM, Williams J. Confidence limits for the indirect effect: Distribution of the product and resampling methods. Multivariate Behav Res. 2004;39(1):99–128. doi: 10.1207/s15327906mbr3901_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods Instrum Comput. 2004;36(4):717–731. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- 22.Horton BM, Holberton RL. Variation in baseline corticosterone and the adrenocortical response in breeding white-throated sparrows. Auk. 2010;127(3):540–548. [Google Scholar]
- 23.Maley MJ. Electrical stimulation of agonistic behavior in the mallard. Behaviour. 1969;34(3):138–160. [Google Scholar]
- 24.Phillips RE, Youngren OM. Brain stimulation and species-typical behaviour: Activities evoked by electrical stimulation of the brains of chickens (Gallus gallus) Anim Behav. 1971;19(4):757–779. doi: 10.1016/s0003-3472(71)80180-x. [DOI] [PubMed] [Google Scholar]
- 25.Ikebuchi M, Hasegawa T, Bischof HJ. Amygdala and socio-sexual behavior in male zebra finches. Brain Behav Evol. 2009;74(4):250–257. doi: 10.1159/000264660. [DOI] [PubMed] [Google Scholar]
- 26.Slawski BA, Buntin JD. Preoptic area lesions disrupt prolactin-induced parental feeding behavior in ring doves. Horm Behav. 1995;29(2):248–266. doi: 10.1006/hbeh.1995.1018. [DOI] [PubMed] [Google Scholar]
- 27.Wingfield JC. Androgens and mating systems: Testosterone-induced polygyny in normally monogamous birds. Auk. 1984;101(4):665–671. [Google Scholar]
- 28.Ketterson ED, Nolan V, Wolf L, Ziegenfus C. Testosterone and avian life histories—effects of experimentally elevated testosterone on behavior and correlates of fitness in the dark-eyed junco (Junco hyemalis) Am Nat. 1992;140(6):980–999. [Google Scholar]
- 29.Lynn SE, Prince LE, Schook DM, Moore IT. Supplementary testosterone inhibits paternal care in a tropically breeding sparrow, Zonotrichia capensis. Physiol Biochem Zool. 2009;82(6):699–708. doi: 10.1086/605915. [DOI] [PubMed] [Google Scholar]
- 30.Rosvall KA. Life history trade-offs and behavioral sensitivity to testosterone: An experimental test when female aggression and maternal care co-occur. PLoS ONE. 2013;8(1):e54120. doi: 10.1371/journal.pone.0054120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Michopoulos V, Maney DL, Morehouse CB, Thomas JW. A genotyping assay to determine plumage morph in the white-throated sparrow, Zonotrichia albicollis. Auk. 2007;124(4):1330–1335. [Google Scholar]
- 32.Cushing BS, et al. Intraspecific variation in estrogen receptor alpha and the expression of male sociosexual behavior in two populations of prairie voles. Brain Res. 2004;1016(2):247–254. doi: 10.1016/j.brainres.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 33.Trainor BC, Greiwe KM, Nelson RJ. Individual differences in estrogen receptor alpha in select brain nuclei are associated with individual differences in aggression. Horm Behav. 2006;50(2):338–345. doi: 10.1016/j.yhbeh.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ketterson ED, Atwell JW, McGlothlin JW. Phenotypic integration and independence: Hormones, performance, and response to environmental change. Integr Comp Biol. 2009;49(4):365–379. doi: 10.1093/icb/icp057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wood RI, Coolen LM. Integration of chemosensory and hormonal cues is essential for sexual behaviour in the male Syrian hamster: Role of the medial amygdaloid nucleus. Neuroscience. 1997;78(4):1027–1035. doi: 10.1016/s0306-4522(96)00629-x. [DOI] [PubMed] [Google Scholar]
- 36.Cheng M, Chaiken M, Zuo M, Miller H. Nucleus taenia of the amygdala of birds: Anatomical and functional studies in ring doves (Streptopelia risoria) and European starlings (Sturnus vulgaris) Brain Behav Evol. 1999;53(5-6):243–270. doi: 10.1159/000006597. [DOI] [PubMed] [Google Scholar]
- 37.Thompson RR, Goodson JL, Ruscio MG, Adkins-Regan E. Role of the archistriatal nucleus taeniae in the sexual behavior of male Japanese quail (Coturnix japonica): A comparison of function with the medial nucleus of the amygdala in mammals. Brain Behav Evol. 1998;51(4):215–229. doi: 10.1159/000006539. [DOI] [PubMed] [Google Scholar]
- 38.Cushing BS, Perry A, Musatov S, Ogawa S, Papademetriou E. Estrogen receptors in the medial amygdala inhibit the expression of male prosocial behavior. J Neurosci. 2008;28(41):10399–10403. doi: 10.1523/JNEUROSCI.1928-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murakami G, Hunter RG, Fontaine C, Ribeiro A, Pfaff D. Relationships among estrogen receptor, oxytocin and vasopressin gene expression and social interaction in male mice. Eur J Neurosci. 2011;34(3):469–477. doi: 10.1111/j.1460-9568.2011.07761.x. [DOI] [PubMed] [Google Scholar]
- 40.Wolf M, van Doorn GS, Leimar O, Weissing FJ. Life-history trade-offs favour the evolution of animal personalities. Nature. 2007;447(7144):581–584. doi: 10.1038/nature05835. [DOI] [PubMed] [Google Scholar]
- 41.Bell AM. Future directions in behavioural syndromes research. Proc R Soc B Biol Sci. 2007;274(1611):755–761. doi: 10.1098/rspb.2006.0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kopachena JG, Falls JB. Re-evaluation of morph-specific variations in parental behavior of the white-throated sparrow. Wilson Bull. 1993;105(1):48–59. [Google Scholar]
- 43.ENCODE Project Consortium A user’s guide to the encyclopedia of DNA elements (ENCODE) PLoS Biol. 2011;9(4):e1001046. doi: 10.1371/journal.pbio.1001046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meyer LR, et al. The UCSC Genome Browser database: Extensions and updates 2013. Nucleic Acids Res. 2013;41(Database issue):D64–D69. doi: 10.1093/nar/gks1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Horton BM, Hauber ME, Maney DL. Morph matters: Aggression bias depends on self-morph in a polymorphic sparrow. PLoS ONE. 2012;7(10):e48705. doi: 10.1371/journal.pone.0048705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Horton BM, Holberton RL. Corticosterone manipulations alter morph-specific nestling provisioning behavior in male white-throated sparrows, Zonotrichia albicollis. Horm Behav. 2009;56(5):510–518. doi: 10.1016/j.yhbeh.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 47.Stevenson TJ, Small TW, Ball GF, Moore IT. Variation in the gonadotrophin-releasing hormone-1 and the song control system in the tropical breeding rufous-collared sparrow (Zonotrichia capensis) is dependent on sex and reproductive state. Gen Comp Endocrinol. 2012;178(1):1–7. doi: 10.1016/j.ygcen.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leung CH, et al. Neural distribution of vasotocin receptor mRNA in two species of songbird. Endocrinology. 2011;152(12):4865–4881. doi: 10.1210/en.2011-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.