Significance
Thymus-derived regulatory T cells (tTregs) are vital to maintaining immune homeostasis, and as such the signals driving their development have been extensively studied. Despite this, a cohesive model describing tTreg generation and aligning all conflicting data has been elusive. Here we outline a comprehensive model controlling the generation of tTregs and show that tTreg generation is tied to thymic apoptosis. We show that the presence of apoptotic cells in the thymus drives the production of TGFβ intrathymically and that this cytokine then acts to specify the tTreg fate. Thus, our results reveal an apoptosis–TGFβ–Foxp3 axis that mediates the development of tTregs.
Keywords: thymic Treg, phagocytes, TCR affinity
Abstract
Maintenance of immune tolerance critically depends upon regulatory T cells that express the transcription factor forkhead box P3 (Foxp3). These CD4+ T cells can be generated in the thymus, termed thymus-derived regulatory T cells (tTregs), but their developmental pathway remains incompletely understood. tTreg development has been shown to be delayed compared with that of CD4+ single positive (SP) thymocytes, with tTregs being detected only in neonatal thymi by day 3 after birth. Here, we outline the reasons for this delayed emergence of Foxp3+ tTregs and demonstrate that thymocyte apoptosis is intrinsically tied to tTreg development. We show that thymic apoptosis leads to the production of TGFβ intrathymically from thymic macrophages, dendritic cells, and epithelial cells. This TGFβ then induces foxp3 expression and drives tTreg generation. Thymocyte apoptosis has previously been shown to accelerate after birth, which drives increases in TGFβ in the neonatal thymus. We highlight a paucity of TGFβ in the neonatal thymus, accounting for the delayed development of tTregs compared with CD4+ SP thymocytes. Importantly, we show that enhanced levels of apoptosis in the thymus result in an augmented tTreg population and, moreover, that decreasing thymic apoptosis results in reduced tTregs. In addition to this, we also show that T-cell receptor (TCR) signals of different affinity were all capable of driving tTreg development; however, to achieve this TGFβ signals must also be received concomitant with the TCR signal. Collectively, our results indicate that thymic apoptosis is a key event in tTreg generation and reveal a previously unrecognized apoptosis–TGFβ–Foxp3 axis that mediates the development of tTregs.
The thymus houses and controls the development of committed T-cell precursors to thymocytes to T cells. Within the thymus, checkpoints ensure that all T cells are capable of seeing antigen and can therefore contribute to an immune response (positive selection). In addition, carefully orchestrated mechanisms limit the pathogenicity that would be mediated by self-reactive T cells. First, those thymocytes expressing a TCR with high affinity for self are purged from the repertoire and undergo apoptosis (negative selection). Second, the thymus generates regulatory T cells (thymus-derived Tregs; tTregs), a CD4+ T-cell population characterized by their expression of the transcription factor Foxp3 (1, 2), which play vital roles in suppressing autoimmunity and in maintaining immune homeostasis.
Placing tTregs as a central player maintaining a balanced immune system means that the factors governing the expression of Foxp3 and tTreg development have received much attention. tTreg development is known to be delayed compared with CD4+ single positive (SP) thymocytes, with tTregs being detected only by day 3 after birth (3). One major hypothesis that has emerged is that differentiation of tTregs is initiated upon recognition of high-affinity self-antigens in the thymus (4–6). These data suggest that tTreg generation is TCR-instructive, yet other data have countered this, demonstrating that the higher frequencies of tTregs associated with cognate antigen interactions are due to reduced populations of non-Tregs (7). Thus, whether tTreg generation is a TCR-instructive process with a specific “quality” of TCR stimulus specifying the fate remains debatable. Notably, an exclusively TCR-instructive process cannot explain the lack of tTregs in the neonatal thymus until day 3 after birth. Indeed, delayed tTreg generation suggests the neonatal thymus lacks a Foxp3-inducing factor(s).
In concert with TCR, other signals have been shown to be important in thymic tTreg specification, including CD28 costimulation (8) and the transcription factors NFAT, AP-1, NF-κB, and Foxo1/3 (9–12). Cytokines also function in tTreg generation, with the common γ-chain cytokines, importantly IL-2 and TGFβ, both involved. A two-step model of tTreg differentiation was proposed, which suggested that high-affinity TCR interactions permit CD25 expression on CD4+SP thymocytes and that IL-2–signaling then promotes Foxp3 induction (13, 14). However, it now seems likely that IL-2 instead functions to promote both the survival and the proliferation of thymocytes differentiating into tTregs (15, 16). TGFβ signals are vital for the induction of Foxp3 in naive CD4+ T cells (17), but whether TGFβ plays a role in tTreg specification remains debated with studies showing that it is both vital (15) and redundant (18).
One process overlooked in tTreg generation is apoptosis; all developmental processes in the thymus occur under a blanket of thymocyte apoptosis as negative and positive selection occur. Despite these high levels of apoptosis occurring in the thymus, apoptotic thymocytes are not easily found due to the extremely efficient uptake of apoptotic cells by thymic phagocytes. Monitoring apoptosis in the neonatal thymus, Surh and Sprent showed that thymocyte apoptosis suddenly accelerates after birth (19), with few apoptotic thymocytes present in fetal thymus yet significant populations of apoptotic cells being present by day 2 after birth. One would envision that the presence of apoptotic cells, phagocytes, and the factors produced by phagocytes in response to apoptotic cells may well influence thymocyte developmental pathways. Indeed, here we show that thymocyte apoptosis is intrinsically tied to tTreg generation. It is well established that sensing and uptake of apoptotic cells results in TGFβ secretion (20, 21), and we show the activity of this pathway in the thymus. We demonstrate that TGFβ is vital for tTreg development, and, importantly, we demonstrate that tTreg generation occurs more readily in thymi with higher levels of thymocyte apoptosis. Our data reveal that thymic apoptosis induces TGFβ production intrathymically, driving tTreg generation. Acceleration of thymocyte apoptosis after birth (19) occurs on a time course preceding the emergence of tTregs and coincides with increasing levels of intrathymic TGFβ. Thus, thymocyte apoptosis leads to the production of TGFβ in the thymus, which, along with TCR engagement, induces foxp3 expression and ultimately drives tTreg generation. We therefore propose an apoptosis–TGFβ–Foxp3 axis that is responsible for the development of tTregs.
Results
TGFβ Is Required for tTreg Generation Not to Selectively Protect tTregs from Apoptosis.
A role for TGFβ signaling in tTreg generation remains contentious. Previous data have indicated that TGFβ signaling is essential for tTreg generation (15); however, another study reported that TGFβ was instead required to specifically keep tTregs alive (18). To better explore this issue, we generated Tgfbr1f/fFoxp3-cre+ mice to delete TGFβ Receptor (TβR) I expression following tTreg generation (Fig. S1 A and B). If the tTreg deficiencies that result from the absence of TGFβ signaling were exclusively due to increased tTreg death, then Tgfbr1f/fFoxp3-cre+ mice should exhibit the same tTreg deficiencies. We examined tTregs in neonate and adult thymi of Tgfbr1f/fFoxp3-cre+ mice and importantly found no decrease in tTregs; instead, Tgfbr1f/fFoxp3-cre+ mice had similar frequencies of tTregs compared with controls (Fig. S1C). These data exclude a simple anti-apoptotic role for TGFβ signaling in tTregs, indicating that TGFβ signaling does not function to specifically keep Foxp3+ thymocytes alive and instead plays other roles in tTreg generation.
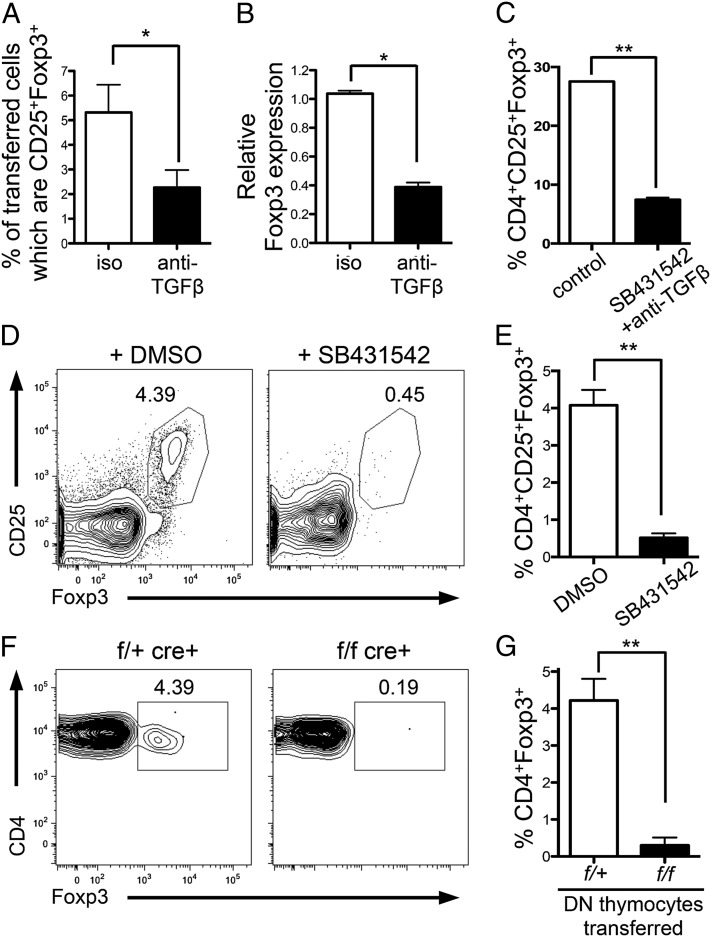
Next we determined whether TGFβ signaling was required to specify tTreg fate. As Foxp3+ thymocytes arise from CD4+SP cells (22), we examined whether TGFβ played a role in Foxp3 induction in CD4+ SP thymocytes. We transferred CD4+CD25− thymocytes from DO11.10xRag−/− mice (Foxp3− thymocytes) intrathymically into BALB/c mice, along with cognate antigen (ovalbumin peptide 323-339; pOVA). Consistent with previous reports (23), Foxp3+DO11.10xRag−/− SP thymocytes were detected after 5 d (Fig. 1A). Inhibition of TGFβ signaling by intrathymic injection of anti-TGFβ led to a significant decrease in the frequency (Fig. 1A) and total number (control: 5,821 ± 1,253; plus anti-TGFβ: 2,807 ± 461; P = 0.0332) of Foxp3+DO11.10xRag−/− SP thymocytes. DO11.10xRag−/− thymocytes were also sorted from recipient thymi 18 h after intrathymic transfer, and foxp3 mRNA expression was examined. TGFβ inhibition led to decreased foxp3 mRNA (Fig. 1B), indicating that TGFβ is key in switching on foxp3 gene expression.
Fig. 1.
TGF-β drives tTreg generation. (A and B) CD4+CD25− DO11.10xRag−/− thymocytes were i.t.-transferred to BALB/c mice with 1 μg pOVA plus 150 μg anti-TGFβ or isotype control. (A) Thymi were harvested after 5 d, and Foxp3 expression in the transferred population was examined by FACS or (B) after 18 h when transferred cells were FACS-sorted and foxp3 expression examined by quantitative PCR (qPCR). Data represent three to five experiments. (C) CD4+CD25−GFP− SP thymocytes were FACS-sorted and cultured with anti-CD3+anti-CD28 for 18 h followed by 36 h rest in the presence or absence of anti-TGFβ and TGFβ inhibitor (SB431542) or isotype control and DMSO. Bar graph shows frequency of CD25+Foxp3+ cells in cultures. Data represent two experiments. (D and E) FTOC cultures were established with E16.5 fetal lobes and cultured for 1 wk with TGFβ inhibitor (SB431542) or DMSO control. (D) Representative FACS plots gated on CD4+SP thymocytes. (E) Bar graph showing frequency of CD25+Foxp3+ cells. Data represent three experiments. (F and G) FACS-sorted DN thymocytes from Tgfbr1+/fCD4-cre+ (control; f/+) or Tgfbr1f/fCD4-cre+ (KO; f/f) mice were transferred i.t. into congenic C57BL/6 mice. Thymi were harvested after 12 d. (F) Representative FACS plots gated on transferred CD4+SP thymocytes. (G) Bar graph showing frequency of Foxp3+ cells in transferred CD4+SP thymocytes. Data represent five experiments. Error bars represent mean ± SEM. *P < 0.05, **P < 0.005 (unpaired two-tailed Student t test).
To further support a role for TGFβ in tTreg generation, we performed similar experiments using CD4+SP thymocytes from OT-IIxRag−/− mice crossed with Tgfbr1f/fCD4-cre+ mice (here called OT-II-TβR1KO and OT-II-TβR1WT). OT-II-TβR1KO or OT-II-TβR1WT CD4+SP thymocytes were intrathymically transferred into congenic hosts along with pOVA. Examination of thymocytes 5 d later showed few Foxp3+ OT-II-TβR1KO thymocytes, whereas Foxp3+ OT-II-TβR1WT developed as expected (Fig. S1 D–F), again indicating that TGFβ is vital for the generation of Foxp3+ thymocytes.
Early withdrawal of TCR stimulation has been shown to promote Foxp3 expression (24). Culture of CD4+SP thymocytes with TCR stimulation for 18 h followed by withdrawal of stimulation for 36 h promotes development of Foxp3+ thymocytes. Of note, generation of Foxp3+ cells in these cultures was significantly reduced when TGFβ signals were inhibited (Fig. 1C), suggesting that TGFβ plays a critical role in the generation of Foxp3+ thymocytes in this setting.
Examining tTreg generation in more physiological settings, we established fetal thymic organ cultures (FTOC) with thymi obtained from wild-type embryonic day 16.5 (E16.5) embryos. When TGFβ signaling was impaired by addition of the TGFβ inhibitor SB431542, the development of Foxp3+CD4+SP thymocytes was significantly reduced (Fig. 1 D and E).
To examine this in vivo, we intrathymically injected sorted double-negative (DN) thymocytes from Tgfbr1f/fCD4-cre+ (KO; f/f) or Tgfbr1+/fCD4-cre+ (control; f/+) mice into congenic wild-type thymi and allowed them to develop within the thymus for 12 d (a time point by which SP populations had developed). As DN thymocytes were transferred into wild-type thymi, this experimental approach provided a normal, noninflammatory environment in which transferred polyclonal thymocytes could develop. Twelve days following transfer of control DN thymocytes, Foxp3+CD4+SP thymocytes were present at frequencies normally found in wild-type thymi (3–5%) (Fig. 1 F and G). Transferred KO DN thymocytes generated a similar size population of CD4+SP thymocytes (Fig. S1G), yet these cells exhibited a dramatically reduced ability to become Foxp3+, as determined by both frequency (Fig. 1 F and G) and total number (control DN transfer: 307 ± 59.9; KO DN transfer: 12.98 ± 3.16; P < 0.0001). Collectively, these data establish that TGFβ signaling is vital for the generation of tTregs.
Thymic TGFβ Expression Increases After Birth.
Thymic tTreg generation is temporally restricted after birth (3, 15), with few tTregs detected until day 3 after birth. As we have demonstrated that TGFβ is vital for tTreg development, we next asked whether this delayed emergence of Foxp3+ thymocytes in the neonatal thymus was due to a paucity of TGFβ at early time points after birth.
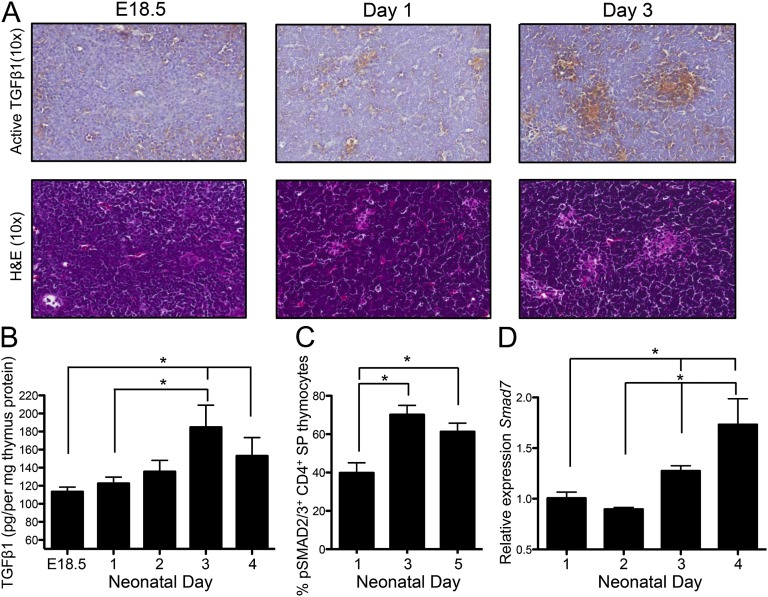
We examined levels of TGFβ activity within the neonatal thymus by staining thymus sections with antibody against active TGFβ1 [LC(1–30)] (25). There was an increase in active TGFβ1 in the thymus over the time period from birth (E18.5) through the neonatal period (Fig. 2A). Although the thymic medulla regions are only beginning to coalesce and form over the neonatal period, from H&E staining (in particular by day 3) there was an indication that the areas rich in TGFβ1 activity were medullary regions. Indeed, this was confirmed by immunohistochemical (IHC) examination of adult thymus sections (Fig. S2A). Enriched TGFβ1 activity in the thymic medulla is a vital observation considering that this is the thymic region in which the precursors of tTregs (CD4+ SP thymocytes) (22) are found, and which has also been shown to support tTreg development (26, 27). Thus, our IHC staining indicates that the thymic medulla is an environment enriched for TGFβ1 activity and, more importantly, that intrathymic TGFβ1 activity gradually increases after birth over the neonatal time frame.
Fig. 2.
TGFβ levels increase in the thymus after birth. (A) Representative IHC sections of thymus from E18.5, day 1, and day 3 neonates stained (Upper) with antibody against active TGFβ1 (positive brown stain with counterstain in blue) and (Lower) H&E. Magnification is 10×. Sections are within five serial sections and are representative of sections from three to four separate mice. (B) Intrathymic amounts of active TGFβ1 were determined by ELISA in E18.5 fetal thymi and day 1, 2, 3, and 4 neonate thymi. Data are representative of two independent experiments with three to seven thymi per time point. (C) Flow cytometric analysis of Smad2/3 phosphorylation in CD4+SP thymocytes from day 1, 3, and 5 neonatal thymus (n = 6–7). (D) CD4+SP thymocytes were FACS-sorted from day 1–4 neonate thymi, and levels of Smad7 expression were examined by qPCR. Graph shows data from two separate experiments; results are presented relative to day 1. Error bars represent mean ± SEM. *P < 0.05 (unpaired two-tailed Student t test).
We also examined TGFβ protein levels by ELISA, and, in line with our IHC data, intrathymic TGFβ1 increased significantly over the neonatal period (Fig. 2B). Furthermore, we examined the protein levels of TGFβ2 and TGFβ3 over the neonate period and found these also increased over this time frame (Fig. S2 B and C).
As CD4+ SP thymocytes are the immediate precursors of tTregs, we next examined TGFβ-signaling events in these thymocytes after birth. We examined levels of phosphorylated Smad2/3 (pSmad) in CD4+SP thymocytes from 1-, 3-, and 5-d-old pups. The frequency of pSmad2/3+ CD4+SP thymocytes increased over this neonate period (Fig. 2C). To further confirm that TGFβ signaling increased in CD4+SP thymocytes after birth, we FACS-sorted this population from the thymi of day 1, 2, 3, and 4 neonates. We examined the level of Smad7 expression in these sorted thymocytes, as Smad7 is induced in response to TGFβ signaling (28). After birth there was a gradual increase in Smad7 message, which was significantly enhanced by days 3 and 4 post birth in CD4+SP thymocytes (Fig. 2D). Thus, we saw increases in TGFβ in the thymus over the short period after birth and enhanced TGFβ signaling in tTreg precursors over this same time frame. Collectively, our data show that there are reduced levels of intrathymic TGFβ at early time points after birth, and highlight a gradual increase in TGFβ levels in the neonatal thymus after birth, which precedes the emergence of tTregs.
Thymocyte Apoptosis Increases Levels of Intrathymic TGFβ.
Having demonstrated a vital role for TGFβ in tTreg generation and a paucity of TGFβ in neonatal thymus, we next wanted to identify the stimulus for TGFβ production in the neonatal thymus. We examined the role of thymocyte apoptosis in this process. Thymocyte apoptosis has been shown to accelerate after birth; at fetal day E18.5 few apoptotic cells were seen in the thymus, but by day 2 after birth populations of apoptotic cells were identified (19). This timing in the induction of thymic apoptosis follows the same time course as the increase in TGFβ in the neonate thymus after birth (Fig. 2). As it is well established that uptake of apoptotic cells by phagocytes induces TGFβ secretion from phagocytes (20, 21), we hypothesized that thymocyte apoptosis, and consequent TGFβ production, might be the initiation steps in tTreg generation.
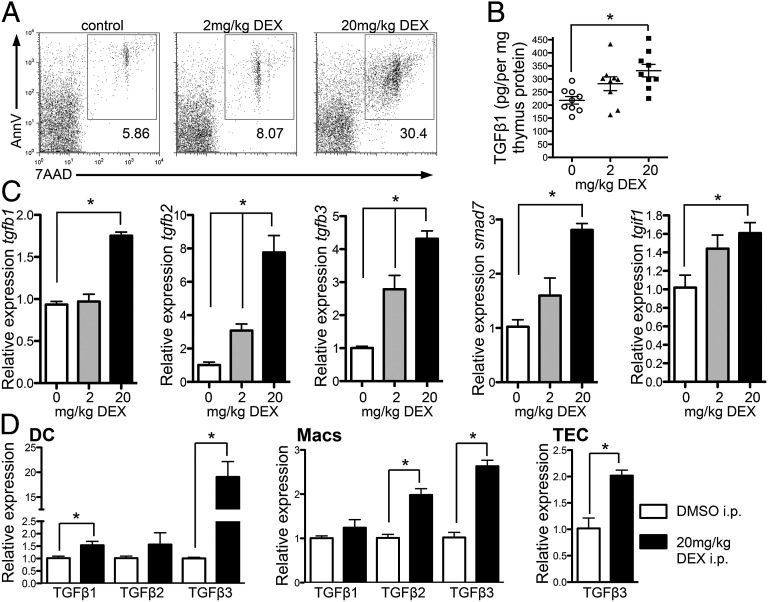
We tested this in adult thymus, asking whether induction of apoptosis could cause elevated levels of intrathymic TGFβ. We examined thymic TGFβ levels after administration of dexamethasone (DEX), which induces thymocyte apoptosis (Fig. 3A), to wild-type mice and examined intrathymic TGFβ 18 h later. We saw increased intrathymic TGFβ1 protein following induction of thymocyte apoptosis (Fig. 3B), as well as increases in message for TGFβ1, -2, and -3 (Fig. 3C). Indeed, there was a positive correlation between mRNA expression of TGFβ1–3 and the frequency of apoptotic cells in the thymus (Fig. S3). In addition, TGFβ signaling was also enhanced in thymi of mice with enhanced levels of apoptosis, as we saw increased mRNA for Smad7 and TGIF1 [genes induced in response to TGFβ signaling (29)] (Fig. 3C). Importantly, we FACS-sorted dendritic cells (DC) and macrophages from thymus following DEX treatment and saw increased message for TGFβ1, -2, and/or -3 in these phagocytes following enhancement of apoptosis (Fig. 3D). We also FACS-sorted thymic epithelial cells (TECs) and saw increased levels of TGFβ3 mRNA in TECs from DEX-treated compared with DMSO-treated control mice (Fig. 3D). These data suggest that apoptotic thymocytes trigger thymic phagocytes to produce TGFβ.
Fig. 3.
Increased thymocyte apoptosis leads to increased intrathymic TGFβ. (A–D) C57BL/6 mice were injected i.p. with 2 or 20 mg/kg DEX or DMSO control, and thymi were examined 18 h later. (A) FACS plots show AnnexinV versus 7AAD staining. (B) Intrathymic amounts of total TGFβ1 in whole thymi determined by ELISA. (C) mRNA expression of tgfb1, tgfb2, tgfb3, Smad7, and tgif1 in whole thymi; results are presented relative to DMSO control. (D) DC, macrophages (Macs), and TECs were FACS-sorted from thymi of mice treated with DMSO (white bars) or 20 mg/kg DEX (black bars) 18 h after treatment and TGFβ expression examined by qPCR; results are presented relative to DMSO control. Data represent two to three experiments. (B and D) Data from two to three independent experiments combined. Error bars represent mean ± SEM. *P < 0.05 (unpaired two-tailed Student t test).
As IL-2 is another cytokine shown to be important in supporting tTreg generation, we also examined levels of IL-2 intrathymically following increases in apoptosis. However, unlike TGFβ, intrathymic levels of IL-2 did not correlate with levels of apoptosis nor increase over the neonate period (Fig. S4).
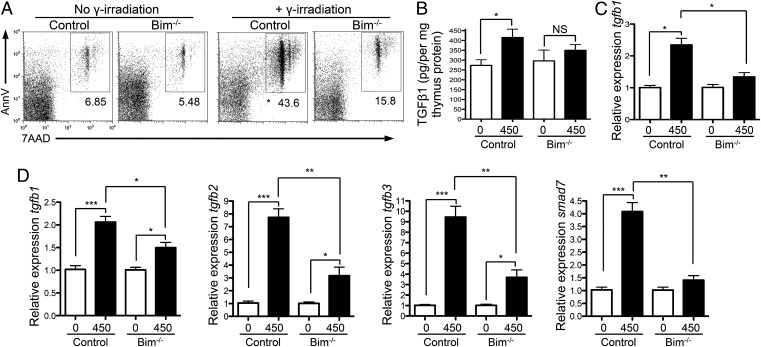
Next we used other methods to induce thymic apoptosis to determine the generality of thymic apoptosis in driving TGFβ production intrathymically. We exposed mice to γ-irradiation (Fig. 4A), or administered anti-CD3 (Fig. S5A), both of which induce apoptosis in the thymus. In both cases we saw increased intrathymic TGFβ levels in mice that exhibited enhanced levels of thymic apoptosis (γ-irradiation, Fig. 4 B–D; anti-CD3, Fig. S5 B and C). Thus, these data suggest that enhanced thymic apoptosis leads to higher levels of intrathymic TGFβ.
Fig. 4.
Thymocyte apoptosis increases intrathymic TGFβ. (A–D) Bim−/− and Bim+/− mice received 0 or 450 rad of γ-irradiation, and thymi were removed 18 h later. (A) FACS examination of AnnexinV and 7AAD staining of thymocytes. (B) Intrathymic amounts of total TGFβ1 in whole thymi determined by ELISA. (C) tgfb1 expression in sorted thymic macrophages determined by qPCR; results are presented relative to unirradiated control of the same genotype. (D) mRNA expression of tgfb1, tgfb2, tgfb3, and Smad7 in whole thymi; results are presented relative to unirradiated control of same genotype. Data represent three experiments, apart from in C, which represents two separate experiments. Error bars represent mean ± SEM. *P < 0.05, **P < 0.005, ***P < 0.0001 (unpaired two-tailed Student t test).
To directly assess whether thymic apoptosis was responsible for this enhanced intrathymic TGFβ, we examined TGFβ levels in Bim−/− thymi following induction of apoptosis. Thymocytes in Bim−/− mice are more resistant to apoptosis as Bim is a proapoptotic factor (30) (Fig. 4A). We found that the increased levels of thymic TGFβ seen following γ-irradiation were indeed due to increased apoptosis; TGFβ1 protein levels did not increase as much in Bim−/− thymi compared with control thymi following γ-irradiation (Fig. 4B). This was also true for mRNA levels of tgfb1, -2, -3, and Smad7 in whole thymus and tgfb1 mRNA in sorted thymic macrophages (Fig. 4 C and D). Therefore, in response to apoptotic stimuli, intrathymic TGFβ was reduced in Bim−/− thymi compared with control thymi (γ-irradiation, Fig. 4; anti-CD3, Fig. S5). Collectively, our data indicate that thymic apoptosis stimulates production of TGFβ in the thymus.
Thymic Apoptosis Promotes tTreg Generation.
Having demonstrated that increased thymic apoptosis caused increased levels of intrathymic TGFβ, we next examined whether alterations in thymic apoptosis could affect tTreg generation. To do this, we experimentally altered levels of thymic apoptosis to determine whether apoptosis, and subsequent TGFβ production, influenced tTreg generation. First, we performed these experiments in the adult thymus, asking if increased levels of thymic apoptosis could promote tTreg generation (below). Second, we altered thymic apoptosis in day 1 neonates, which contain no tTregs (Alterations in Thymic Apoptosis Affect tTreg Generation in Neonatal Mice).
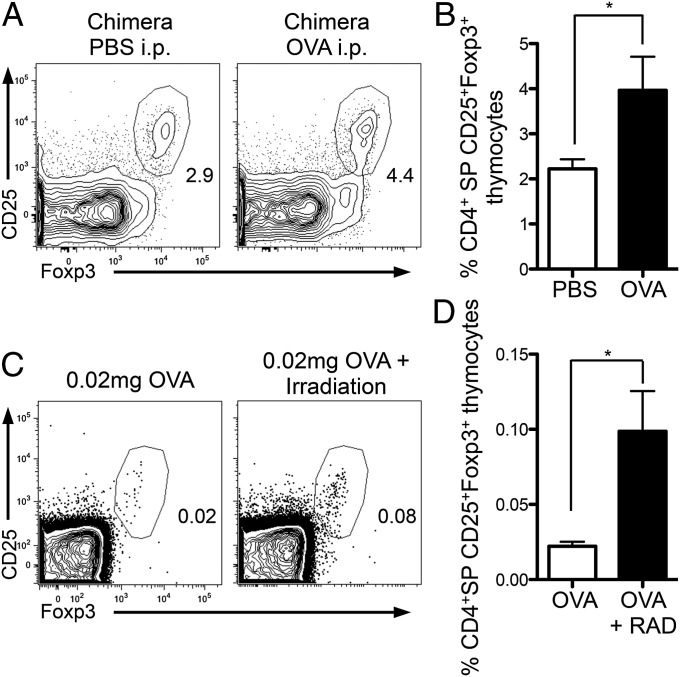
We generated mixed bone marrow chimeras in DO11.10xRag−/− mice reconstituted with 75% DO11.10xRag−/− and 25% BALB/c bone marrow. Sixteen days after reconstitution chimeric mice received either 2 mg ovalbumin (OVA) or PBS intraperitoneally. Foxp3+CD4+SP thymocytes were examined 5 d later, a total of 3 wk after reconstitution (Fig. S6A); tTregs were assessed 3 wk after generation of chimeras to examine the Foxp3+ cells in a setting most similar to that in neonates. OVA administration induces apoptosis in only DO11.10xRag−/− thymocytes. We wanted to determine whether increased apoptosis of DO11.10xRag−/− thymocytes would influence generation of Foxp3+BALB/c polyclonal thymocytes, our hypothesis being that there would be increased Foxp3+BALB/c thymocytes in chimeras receiving OVA. Significantly more BALB/c Foxp3+CD4+SP tTregs were seen in the thymi of chimeric mice that had received OVA i.p. compared with controls (Fig. 5 A and B). Thus, we demonstrate that a thymic environment with higher levels of apoptosis promotes the generation of a larger population of tTregs.
Fig. 5.
Enhanced levels of thymic apoptosis result in larger tTreg populations in adult thymus. (A and B) Mixed bone marrow chimeras were made in DO11.10xRag−/− with 75% DO11.10xRag−/− and 25% BALB/c bone marrow. Sixteen days later chimeras received either PBS or 2 mg OVA i.p. At 3 wk thymi were harvested from mice and tTreg frequencies were examined in CD4+ SP thymocytes of the BALB/c compartment. (A) Plots showing frequency of CD25+Foxp3+ thymocytes in CD4+SP BALB/c polyclonal thymocytes. (B) Bar graph showing frequency of CD25+Foxp3+ cells in CD4+SP BALB/c thymocytes. Data represent two experiments with four to five mice per group. (C and D) DO11.10xRag−/− mice received 0.02 mg OVA i.p. ± low dose γ-irradiation (50 rad), and CD25+Foxp3+ thymocytes were examined 5 d later. (C) Representative FACS plots gated on CD4+ SP thymocytes. (D) Bar graph showing frequency of CD4+CD25+Foxp3+ thymocytes. Data represent three experiments. Error bars represent mean ± SEM. *P < 0.05 (unpaired two-tailed Student t test).
Next, we used another experimental system to examine the positive effect of thymic apoptosis on tTreg development. Administration of cognate antigen to TCR transgenic mice on the Rag−/− background induces tTreg generation; indeed, administration of titrated doses of OVA to DO11.10xRag−/− mice led to a corresponding graded increase in tTregs (Fig. S6B). To test whether increases in thymic apoptosis could influence tTreg generation, we used a dose of OVA that induced few tTregs: 0.02 mg OVA. We examined the frequency of tTregs generated following administration of 0.02 mg OVA versus 0.02 mg OVA plus an apoptotic stimulus; we used low-dose γ-irradiation. Generation of tTregs was enhanced following OVA plus γ-irradiation compared with OVA alone, both in terms of frequency (Fig. 5 C and D) and total number (OVA alone: 100.4 ± 44; OVA+RAD: 629.3 ± 165; P = 0.0196). Therefore, we demonstrate that, by enhancing thymic apoptosis, increased populations of tTregs can be generated. Collectively, our data indicate that an enhanced frequency of apoptotic thymocytes augments tTreg generation.
Alterations in Thymic Apoptosis Affect tTreg Generation in Neonatal Mice.
Having examined the link between apoptosis and tTreg generation in the adult, we next returned our studies to the neonatal thymus, an environment initially containing no tTregs. We determined whether manipulation of thymic apoptosis in neonates could affect tTreg generation. As few tTregs exist in the thymus before day 3, neither intra-tTreg competition nor increased tTreg survival would account for any differences seen. Instead, differences observed would be attributed to tTreg induction, and we could test our hypothesis that thymocyte apoptosis, through induction of TGFβ, drives tTreg generation.
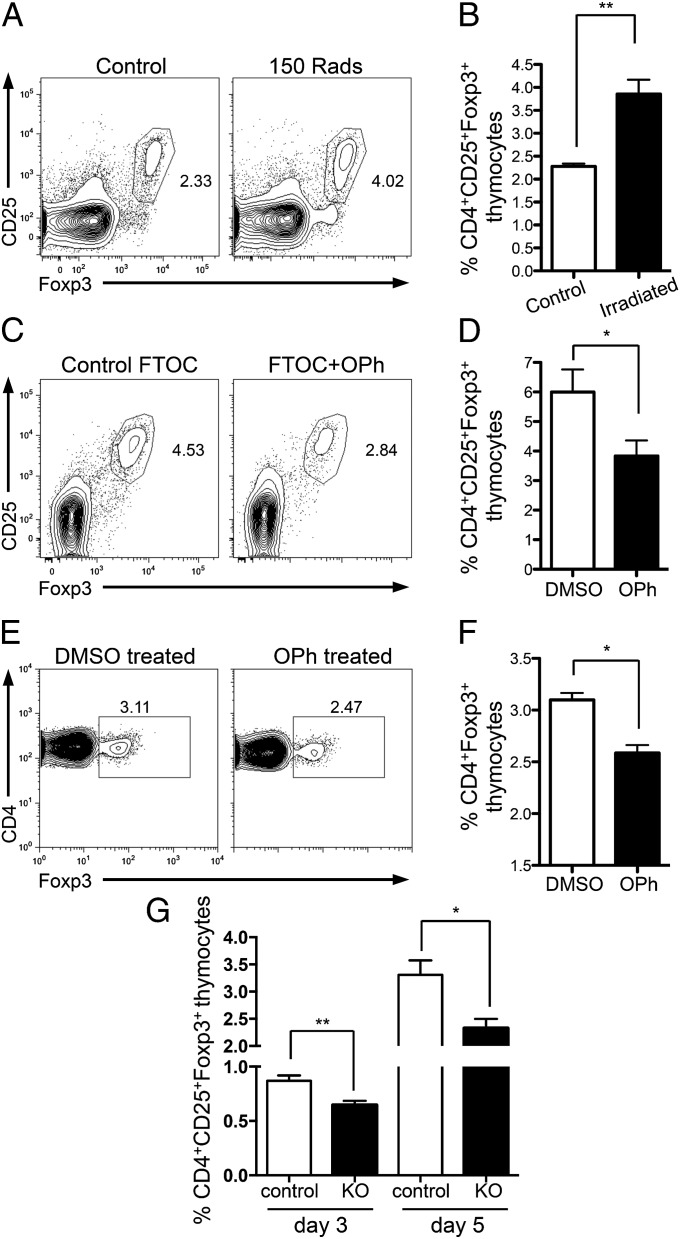
Neonatal mice received 150 Rad of γ-irradiation on the day that they were born. After 24 h, increased frequencies of apoptotic thymocytes and enhanced levels of TGFβ were seen in the thymi of irradiated neonates (Fig. S7 A and B). Notably, 4 d later, there was a significant increase in tTreg frequency (Fig. 6 A and B) and total number (Fig. S7C) in the thymi of neonates that had received γ-irradiation compared with littermate controls. Thus, by increasing the frequency of apoptotic thymocytes early after birth, a larger population of tTregs was generated.
Fig. 6.
Alterations in thymic apoptosis affect tTreg development in neonatal mice. (A and B) Day 1 wild-type neonates received 0 or 150 rad of γ-irradiation. tTregs were examined at day 5. (A) Representative FACS plots gated on CD4+SP thymocytes. (B) Bar graph showing frequency of CD4+CD25+Foxp3+SP thymocytes. Data represent four separate experiments. (C and D) FTOC cultures were established with E16.5 fetal lobes and cultured for 1 wk with 5 μM Q-VD-OPh (OPh) or DMSO control. (C) Representative FACS plots gated on CD4+SP thymocytes. (D) Bar graph showing frequency of CD25+Foxp3+ cells. Data represent five experiments. (E and F) Day 1 neonates received control DMSO or 2.5 μg OPh i.p. for 4 d before Foxp3+ thymocytes were examined at day 5. (E) Representative FACS plots gated on CD4+ SP thymocytes. (F) Bar graph showing frequency of CD4+Foxp3+ thymocytes. Data represent three separate experiments. (G) CD4+SP CD25+Foxp3+ thymocytes were examined in the thymi of day 3 (n = 8–10) and day 5/6 (n = 10–15) Bim+/− (control; white bars) and Bim−/− (KO; black bars) neonates. Error bars represent mean ± SEM. *P < 0.05, **P < 0.005 (unpaired two-tailed Student t test).
We next ascertained whether decreased levels of apoptosis in the neonatal thymus would result in a reduced tTreg population. We first examined tTreg generation in a simplified in vitro FTOC system by culturing FTOC in the presence or absence of the caspase inhibitor Q-VD-OPh (OPh) (31), which inhibits apoptosis. In this in vitro setting, addition of OPh, and inhibition of apoptosis in these cultures, led to a reduced tTreg population (Fig. 6 C and D).
Next, we used the caspase inhibitor OPh in vivo and administered it to neonatal mice daily from the day they were born until day 5. Strikingly, inhibition of apoptosis over the neonatal period led to a decreased tTreg frequency (Fig. 6 E and F) and total number (Fig. S7D).
To further examine the effect of thymocyte apoptosis on neonatal tTreg generation, we examined tTreg generation in neonatal Bim−/− mice. As Bim−/− mice exhibit reduced thymocyte apoptosis (30), we queried whether, at early time points after birth, these mice would have a reduced tTreg population. Indeed, the Foxp3+ tTreg population in 3- and 5/6-d-old neonatal Bim−/− mice was significantly reduced compared with that of littermate controls, both in terms of percentage (Fig. 6G) and absolute number (Fig. S7E). These data show that in the neonatal thymus during the time period in which tTregs first start to develop, alterations in thymic apoptosis influence the resultant size of the emerging tTreg population. Collectively, we have shown that by altering apoptosis in the neonate thymus, and consequently intrathymic levels of TGFβ, generation of tTregs can either be augmented (more apoptosis) or reduced (less apoptosis). These data support our hypothesis and suggest that an intrathymic apoptosis–TGFβ–Foxp3 axis mediates tTreg generation.
tTreg Generation Following Encounter with High- and Low-Affinity Antigens Is TGFβ-Dependent.
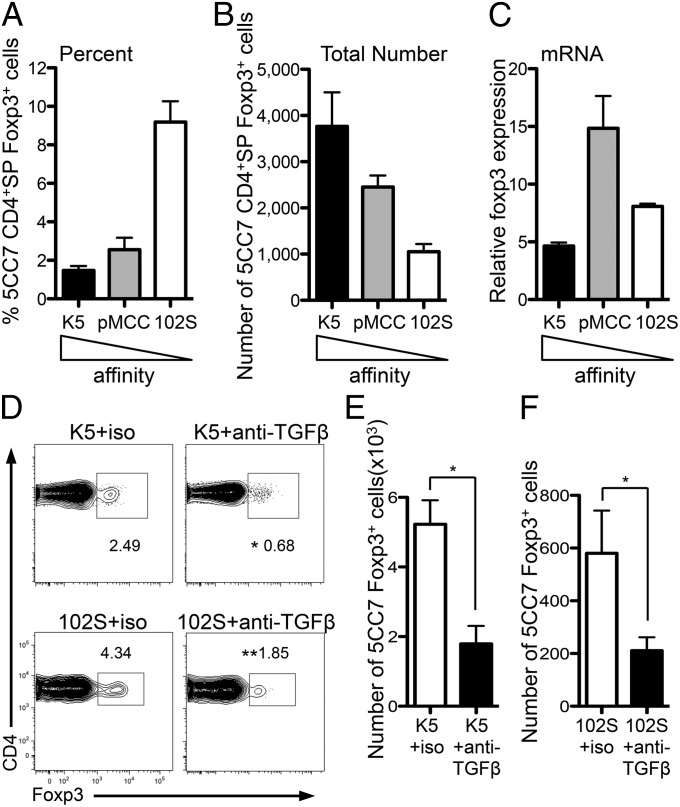
Our data could align previous disagreements concerning the role of TCR affinity in tTreg generation. They suggest that high-affinity TCR interactions could promote tTreg generation as a result of the fact that these signals cause negative selection and thymocyte apoptosis. This would allow for the generation of tTregs via the apoptosis–TGFβ–Foxp3 axis. We tested the ability of peptides that have different affinity for TCR to drive tTreg generation by using the 5CC7xRag−/− TCR transgenic. When TCR transgenic cells were intrathymically injected to wild-type thymi along with peptides of increasing affinity, all of the TCR signals induced Foxp3+ 5CC7 thymocytes (Fig. 7 A–C; K5 = high, pMCC = medium, 102S = low affinity). Moreover, when TCR transgenic CD4+SP thymocytes that have a higher affinity for cognate ligand (SMARTAxRag−/−) were cotransferred with CD4+SP thymocytes that have a lower affinity for cognate ligand (OT-IIxRag−/−) (32), both were equally capable of differentiating into tTregs (Fig. S8). Thus, in line with previous reports (33), TCR signals of different affinity can drive tTreg generation, yet TCR signals certainly play important roles in tTreg differentiation, as it should be noted that, although peptides of different affinity drive tTreg generation, they differed in their abilities to do this (Fig. 7 A–C). Importantly for the data presented here, inhibition of TGFβ signaling led to significantly decreased populations of Foxp3+ thymocytes irrespective of TCR affinity (Fig. 7 D–F). Thus, although TCR affinity is important in tTreg generation, the resultant TCR signaling must occur in a TGFβ-rich environment for the tTreg fate to be specified.
Fig. 7.
Peptides with a broad range of affinity for TCR drive tTreg generation in a TGFβ-dependent manner. (A–C) CD4+CD25− 5CC7xRag−/− thymocytes were i.t.-injected into B10.A mice with 1 μg of K5 (high affinity: black bars), pMCC (medium affinity: gray bars), or 102S (low affinity: white bars) peptide. Thymi were harvested after 5 d. Bar graphs show (A) frequency and (B) total number of Foxp3+ 5CC7 thymocytes. (C) Transferred CD4+SP 5CC7 thymocytes were sorted from thymi 18 h after transfer and foxp3 mRNA was examined by qPCR; results are presented relative to 5CC7 CD4+ SP thymocytes pretransfer. Data are from two to four separate experiments combined. (D–F) CD4+CD25− 5CC7xRag−/− thymocytes were i.t.-injected into B10.A mice with 1 μg of the high-affinity (K5) or low-affinity (102S) peptide plus isotype control (white bars) or anti-TGFβ (black bars), and thymi were harvested after 5 d. (D) Representative FACS plots showing frequency of 5CC7 Foxp3+ cells gated on 5CC7 thymocytes. (E and F) Bar graph showing total number of Foxp3+-transferred cells. Data represent two to three experiments. Error bars represent mean ± SEM. *P < 0.05, **P < 0.005 (unpaired two-tailed Student t test).
Collectively, our data show that within the thymus the presence of apoptotic cells drives TGFβ production, which then specifies the tTreg fate. Reduced levels of apoptosis in the neonatal thymus result in reduced levels of intrathymic TGFβ, which are insufficient to drive tTreg generation. Thus, we outline an apoptosis–TGFβ–Foxp3 axis that governs tTreg generation.
Discussion
During thymocyte development, differences in TCR signaling drive cell-fate decisions. TCR signaling is resolutely required for tTreg generation, and although there is much data suggesting that tTreg generation is a TCR-instructive process, it is clear that TCR instructions are not the only ones driving tTreg fate. Here we propose that TGFβ, a cytokine whose role in tTreg development has been debated, is a dominant factor driving tTreg differentiation. We show that, in the absence of TGFβ signals, Foxp3 expression in CD4+SP thymocytes does not occur, resulting in failure to generate tTregs. This conclusion is most strikingly demonstrated by our experiments using FTOC and intrathymic transfer of DN thymocytes. Here, Foxp3+ thymocytes failed to emerge in the absence of TGFβ signals, confirming that TGFβ is indispensable for tTreg generation. Previously, it had been reported that TGFβ plays no role in tTreg specification but was instead important for keeping tTregs alive (18). Although TGFβ plays vital anti-apoptotic roles, this role is not limited to tTregs, and most thymocyte populations show increased apoptosis in the absence of TGFβ (34). Indeed, our data from mice in which tTregs lack TβRI expression would preclude a simple anti-apoptotic role. Additional data that argued against a role for TGFβ-Smad signaling in tTreg generation came from mice lacking an enhancer of foxp3 gene expression [conserved noncoding sequence 1 (CNS1)]. The CNS1 contains the only consensus Smad-binding sequence in the foxp3 gene, CNS, or promoter regions, yet CNS1−/− mice exhibited normal tTreg frequencies (35, 36). These data suggest that Smad proteins do not directly drive foxp3 expression, but do not exclude a role for TGFβ signals; other factors downstream of TGFβ drive foxp3 expression including Tak1 (37), Runx1/3 (38), TRAF6 (39), and E2A (40). In addition, Smad proteins may interact with other transcription regulators to cause transcriptional changes, occurring in the absence of Smad DNA binding. Indeed, the precise molecular events downstream of TGFβ required for tTreg generation await further elucidation.
Our data provide a model to explain why tTreg generation is delayed compared with CD4+ SP thymocytes, as we show that there is a paucity of TGFβ in the neonatal thymus. Our data indicate that the key stimulus for intrathymic TGFβ production is thymocyte apoptosis. Thymic apoptosis increases after birth over the neonate period (19). Our data show increases in intrathymic TGFβ levels over this neonatal period. Importantly, we show increases in intrathymic TGFβ in response to thymic apoptosis, something not seen for IL-2 [a cytokine important for tTreg survival (16)], highlighting the specific capability of thymic apoptosis to trigger TGFβ secretion intrathymically. We confirmed a causal link between thymocyte apoptosis and tTreg generation by manipulating levels of thymic apoptosis and examining the resultant Foxp3+ population. Both in the adult and, most importantly, in the neonatal thymus, we show that increases in apoptosis result in larger populations of tTregs. Conversely, lower thymic apoptosis results in reduced tTregs. Importantly, we show that neonatal Bim−/− mice, which have reduced thymocyte apoptosis, have reduced frequencies and total numbers of tTregs compared with littermate controls. Interestingly, this decrease in initial tTreg specification is masked in later life as adult Bim−/− mice have more tTregs due to enhanced tTreg survival (41). We suggest that induction of thymic apoptosis and subsequent production of TGFβ drive differentiation of tTregs. This model of tTreg generation accounts for the delayed development of tTregs compared with CD4+SP thymocytes, something that current models of tTreg generation cannot do.
The apoptosis–TGFβ–Foxp3 axis could also explain the link between high-affinity TCR signals and tTreg induction; high-affinity TCR signals initiate negative selection and apoptosis. Induction of thymocyte apoptosis by high-affinity TCR ligands would create a TGFβ-rich environment that would promote tTreg generation. This raises questions of whether high-affinity TCR interactions directly drive tTreg development or whether they result in increased levels of apoptosis and, subsequently, in increases in intrathymic TGFβ. Recent studies have shown that a broad range of TCR affinities can induce tTreg differentiation (33), suggesting that TCR affinity itself may not be the primary determinant for tTreg generation. In addition, this same study showed that the efficiency of tTreg generation was positively correlated with affinity, as other TCR-dependent processes are. So tTreg induction could be a readout of the ability of peptide–MHC complexes to activate TCR sufficiently. Yet, there are much data showing that induction of tTreg fate is favored by high-affinity TCR signals (42). What our findings importantly demonstrate is that tTreg generation, similar to that of other agonist-selected T-cell populations [invariant natural killer T-cells and TCRαβ+CD8αα+ intestinal intraepithelial lymphocytes (34, 43)] is also exquisitely dependent on TGFβ.
Interestingly, our data indicate that TGFβ activity is not diffuse throughout the thymus but enriched in the medulla. Our data therefore localize factors vital for tTreg generation to the same thymic environment; the medulla is the location of tTreg precursors (CD4+ SP thymocytes) (22), medullary TECs (27), apoptotic thymocytes (44), and we show that it is also enriched for TGFβ1 activity. Our data suggest that the medulla possesses a heightened ability to activate TGFβ and raise interesting questions about the mechanisms controlling TGFβ activation in this region. As such, future work should address whether activation of thymic TGFβ is mediated by enzymes, integrins, or by certain antigen presenting cells in the medulla.
Most importantly, our data show that thymic apoptosis and generation of tTregs are connected. By limiting the generation of self-reactive thymocytes through negative selection and subsequent apoptosis, the thymus acquires the stimulus necessary for tTreg generation—TGFβ. Thus, our data intrinsically link induction of thymocyte apoptosis and differentiation of tTregs. Phagocytes modulate their function when encountering apoptotic cells, creating an anti-inflammatory milieu, chiefly through the secretion of TGFβ (20, 21, 45). Here we describe the activity of this process in the thymus and show elevated message for TGFβ1, -2 and/or -3 in DC, macrophages, and TECs following increased thymic apoptosis. Thus, we propose a model for tTreg generation in which thymic apoptosis, through the induction of TGFβ production, leads to the development of tTregs (Fig. S9). We suggest that tTregs are not found in the neonate thymus before day 3 due to a paucity of TGFβ. Increased thymocyte apoptosis after birth accounts for the increasing levels of intrathymic TGFβ over the neonate period and emergence of tTregs at day 3. Thus, we uncover a previously unrecognized intrathymic apoptosis–TGFβ–Foxp3 axis that is responsible for the development of tTregs.
Materials and Methods
Mice.
C57BL/6, CD45.1 (C57BL/6), BALB/c, and Bim−/− mice were purchased from the Jackson Laboratory. DO11.10xRag−/−, OT-IIxRag−/−, 5CC7xRag−/−, and B10.A mice were purchased from Taconic. tgfbr1f/fCD4-cre+ mice were previously described (15). Tgfbr1f/fFoxp3-cre+ mice were generated in house by crossing Tgfbr1f/f with Foxp3-cre (46) mice obtained from Y. Wan (University of North Carolina, Chapel Hill, NC) and OT-II-TβR1KO mice generated by crossing OT-IIxRag−/− and tgfbr1f/fCD4-cre+ mice. All mouse experiments were approved by the Institutional Animal Care and Use Committee of the National Institute of Dental and Craniofacial Research, National Institutes of Health.
Intrathymic Injections.
Anesthetized mice were intrathymically (i.t.) injected using a Hamilton syringe. Cells and antibodies to be injected were resuspended in 20 μL per mouse. Either 0.5–1 × 106 CD4+CD25− TCR transgenic thymocytes with 1 μg peptide (Invitrogen) or 3–5 × 105 DN thymocytes were injected i.t. For some experiments, 150 μg anti-TGFβ (1D11; BioXCell) or isotype control (MOPC1; BioXCell) were coinjected.
FTOC.
Organ cultures were established from individual thymic lobes of C57BL/6 fetuses at E16.5. FTOCs were cultured for 7 d in the presence of 5 μM SB431542 (TGFβ inhibitor), 5 μM Q-VD-OPh, or DMSO control.
Flow Cytometry.
Cell were stained with antibodies from eBioscience and Invitrogen. Foxp3 expression was examined using the eBioscience Foxp3 Treg kit. Intracellular pSmad staining was examined immediately after mechanical disruption of thymi by immediately fixing cells in eBioscience Fix/Perm overnight. The next day cells were stained with pSMAD2/3 (Cell Signaling) followed by secondary fluorophore-conjugated antibody and surface-stained. Samples were analyzed using an LSRII or FACSCalibur flow cytometer (BD Biosciences) and analyzed using FlowJo software (Treestar).
FACS Sorting.
Thymocyte populations were sorted following staining with CD4, CD8α, 7AAD, CD25 (7D4 only), and KJ1-26. For cell sorting of macrophage and dendritic cells, thymi were first digested in Liberase TL (Roche). TECs were isolated as previously described (26). Cells were sorted on a FACSAria II or III (BD Biosciences).
In Vitro Generation of tTregs.
CD4+SP thymocytes (CD4+CD8−CD25−GFP−) were FACS-sorted from Foxp3GFP mice and cultured as previously described (24). TGFβ inhibitor SB431542 (5 μM; Selleck Chemicals) or DMSO and anti-TGFβ (50 μg/mL; 1D11) or isotype control (50 μg/mL; MOPC21) were added for the entire culture period.
Real-Time PCR.
RNA was extracted from thymus or cells and reversed-transcribed. Quantitative PCR was done according to the protocol of TaqMan gene expression assay kits (Applied Biosystems). For Tgfbr1 detection in cells sorted from Tgfbr1f/fFoxp3-cre+ mice, previously described primers were used (15).
ELISA.
Thymus samples were lysed in defined volumes of buffer and protein concentration of the supernatant determined. Quantification of TGFβ1, -2, and -3 was determined using TGFβ ELISA kits (TGFβ1: Promega; TGFβ2 and -3: R&D Systems) by loading equal amounts of protein.
Generation of Bone-Marrow Chimeras.
Bone marrow was isolated from mice and depleted of T cells using anti-CD90 beads (Miltenyi Biotech). Cells (∼5 × 106) were then injected i.v. at the indicated ratios into irradiated hosts.
Immunohistochemistry.
Thymi were formalin-fixed and embedded in paraffin. Sections were cut and antigen retrieval performed. Staining for active TGFβ1 was done using the antibody LC(1–30) (25), and detection was performed using the immPRESS anti-rabbit reagent kit and ImmPACT DAB kit (both Vector Labs). Slides were scanned by an Aperio ScanScope (Aperio Technologies).
Statistical Analysis.
Unless otherwise noted, statistical analysis was performed using the unpaired two-tailed Student t test in Prism (GraphPad).
Supplementary Material
Acknowledgments
We thank Dr. N. Moutsopoulos and D. Zhang for technical expertise; Drs. J. J. O’Shea and E. Wohlfert for critical reading of the manuscript; Dr. K. Flanders for the LC(1-30) antibody and staining protocols; the National Heart, Lung, and Blood Institute and National Institute of Dental and Craniofacial Research (NIDCR) FACS cores for cell sorting; Dr. B. J. Fowlkes for FTOC advice; and Dr. K. Tarbell for use of equipment. This work was supported by the Intramural Research Programs of the NIDCR and of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1320319111/-/DCSupplemental.
References
- 1.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299(5609):1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 2.Fontenot JD, et al. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22(3):329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 3.Fontenot JD, Dooley JL, Farr AG, Rudensky AY. Developmental regulation of Foxp3 expression during ontogeny. J Exp Med. 2005;202(7):901–906. doi: 10.1084/jem.20050784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jordan MS, et al. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol. 2001;2(4):301–306. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 5.Apostolou I, Sarukhan A, Klein L, von Boehmer H. Origin of regulatory T cells with known specificity for antigen. Nat Immunol. 2002;3(8):756–763. doi: 10.1038/ni816. [DOI] [PubMed] [Google Scholar]
- 6.Moran AE, et al. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J Exp Med. 2011;208(6):1279–1289. doi: 10.1084/jem.20110308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Santen HM, Benoist C, Mathis D. Number of T reg cells that differentiate does not increase upon encounter of agonist ligand on thymic epithelial cells. J Exp Med. 2004;200(10):1221–1230. doi: 10.1084/jem.20041022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tai X, Cowan M, Feigenbaum L, Singer A. CD28 costimulation of developing thymocytes induces Foxp3 expression and regulatory T cell differentiation independently of interleukin 2. Nat Immunol. 2005;6(2):152–162. doi: 10.1038/ni1160. [DOI] [PubMed] [Google Scholar]
- 9.Mantel PY, et al. Molecular mechanisms underlying FOXP3 induction in human T cells. J Immunol. 2006;176(6):3593–3602. doi: 10.4049/jimmunol.176.6.3593. [DOI] [PubMed] [Google Scholar]
- 10.Kerdiles YM, et al. Foxo transcription factors control regulatory T cell development and function. Immunity. 2010;33(6):890–904. doi: 10.1016/j.immuni.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Long M, Park SG, Strickland I, Hayden MS, Ghosh S. Nuclear factor-kappaB modulates regulatory T cell development by directly regulating expression of Foxp3 transcription factor. Immunity. 2009;31(6):921–931. doi: 10.1016/j.immuni.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 12.Ruan Q, et al. Development of Foxp3(+) regulatory t cells is driven by the c-Rel enhanceosome. Immunity. 2009;31(6):932–940. doi: 10.1016/j.immuni.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burchill MA, et al. Linked T cell receptor and cytokine signaling govern the development of the regulatory T cell repertoire. Immunity. 2008;28(1):112–121. doi: 10.1016/j.immuni.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lio CW, Hsieh CS. A two-step process for thymic regulatory T cell development. Immunity. 2008;28(1):100–111. doi: 10.1016/j.immuni.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Y, et al. A critical function for TGF-beta signaling in the development of natural CD4+CD25+Foxp3+ regulatory T cells. Nat Immunol. 2008;9(6):632–640. doi: 10.1038/ni.1607. [DOI] [PubMed] [Google Scholar]
- 16.Tai X, et al. Foxp3 transcription factor is proapoptotic and lethal to developing regulatory T cells unless counterbalanced by cytokine survival signals. Immunity. 2013;38(6):1116–1128. doi: 10.1016/j.immuni.2013.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen W, et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198(12):1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ouyang W, Beckett O, Ma Q, Li MO. Transforming growth factor-beta signaling curbs thymic negative selection promoting regulatory T cell development. Immunity. 2010;32(5):642–653. doi: 10.1016/j.immuni.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Surh CD, Sprent J. T-cell apoptosis detected in situ during positive and negative selection in the thymus. Nature. 1994;372(6501):100–103. doi: 10.1038/372100a0. [DOI] [PubMed] [Google Scholar]
- 20.Fadok VA, et al. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101(4):890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiao YQ, et al. Transcriptional and translational regulation of TGF-beta production in response to apoptotic cells. J Immunol. 2008;181(5):3575–3585. doi: 10.4049/jimmunol.181.5.3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee HM, Hsieh CS. Rare development of Foxp3+ thymocytes in the CD4+CD8+ subset. J Immunol. 2009;183(4):2261–2266. doi: 10.4049/jimmunol.0901304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wirnsberger G, Mair F, Klein L. Regulatory T cell differentiation of thymocytes does not require a dedicated antigen-presenting cell but is under T cell-intrinsic developmental control. Proc Natl Acad Sci USA. 2009;106(25):10278–10283. doi: 10.1073/pnas.0901877106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sauer S, et al. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc Natl Acad Sci USA. 2008;105(22):7797–7802. doi: 10.1073/pnas.0800928105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flanders KC, et al. Transforming growth factor-beta 1: Histochemical localization with antibodies to different epitopes. J Cell Biol. 1989;108(2):653–660. doi: 10.1083/jcb.108.2.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aschenbrenner K, et al. Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire+ medullary thymic epithelial cells. Nat Immunol. 2007;8(4):351–358. doi: 10.1038/ni1444. [DOI] [PubMed] [Google Scholar]
- 27.Cowan JE, et al. The thymic medulla is required for Foxp3+ regulatory but not conventional CD4+ thymocyte development. J Exp Med. 2013;210(4):675–681. doi: 10.1084/jem.20122070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakao A, et al. Identification of Smad7, a TGFbeta-inducible antagonist of TGF-beta signalling. Nature. 1997;389(6651):631–635. doi: 10.1038/39369. [DOI] [PubMed] [Google Scholar]
- 29.Chen F, et al. Regulation of TG-interacting factor by transforming growth factor-beta. Biochem J. 2003;371(Pt 2):257–263. doi: 10.1042/BJ20030095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bouillet P, et al. BH3-only Bcl-2 family member Bim is required for apoptosis of autoreactive thymocytes. Nature. 2002;415(6874):922–926. doi: 10.1038/415922a. [DOI] [PubMed] [Google Scholar]
- 31.Caserta TM, Smith AN, Gultice AD, Reedy MA, Brown TL. Q-VD-OPh, a broad spectrum caspase inhibitor with potent antiapoptotic properties. Apoptosis. 2003;8(4):345–352. doi: 10.1023/a:1024116916932. [DOI] [PubMed] [Google Scholar]
- 32.Mandl JN, Monteiro JP, Vrisekoop N, Germain RN. T cell-positive selection uses self-ligand binding strength to optimize repertoire recognition of foreign antigens. Immunity. 2013;38(2):263–274. doi: 10.1016/j.immuni.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee HM, Bautista JL, Scott-Browne J, Mohan JF, Hsieh CS. A broad range of self-reactivity drives thymic regulatory T cell selection to limit responses to self. Immunity. 2012;37(3):475–486. doi: 10.1016/j.immuni.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Konkel JE, et al. Control of the development of CD8αα+ intestinal intraepithelial lymphocytes by TGF-β. Nat Immunol. 2011;12(4):312–319. doi: 10.1038/ni.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng Y, et al. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature. 2010;463(7282):808–812. doi: 10.1038/nature08750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schlenner SM, Weigmann B, Ruan Q, Chen Y, von Boehmer H. Smad3 binding to the foxp3 enhancer is dispensable for the development of regulatory T cells with the exception of the gut. J Exp Med. 2012;209(9):1529–1535. doi: 10.1084/jem.20112646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wan YY, Chi H, Xie M, Schneider MD, Flavell RA. The kinase TAK1 integrates antigen and cytokine receptor signaling for T cell development, survival and function. Nat Immunol. 2006;7(8):851–858. doi: 10.1038/ni1355. [DOI] [PubMed] [Google Scholar]
- 38.Bruno L, et al. Runx proteins regulate Foxp3 expression. J Exp Med. 2009;206(11):2329–2337. doi: 10.1084/jem.20090226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shimo Y, et al. TRAF6 directs commitment to regulatory T cells in thymocytes. Genes Cells. 2011;16(4):437–447. doi: 10.1111/j.1365-2443.2011.01500.x. [DOI] [PubMed] [Google Scholar]
- 40.Maruyama T, et al. Control of the differentiation of regulatory T cells and T(H)17 cells by the DNA-binding inhibitor Id3. Nat Immunol. 2011;12(1):86–95. doi: 10.1038/ni.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhan Y, et al. Defects in the Bcl-2-regulated apoptotic pathway lead to preferential increase of CD25 low Foxp3+ anergic CD4+ T cells. J Immunol. 2011;187(4):1566–1577. doi: 10.4049/jimmunol.1100027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hsieh CS, Lee HM, Lio CW. Selection of regulatory T cells in the thymus. Nat Rev Immunol. 2012;12(3):157–167. doi: 10.1038/nri3155. [DOI] [PubMed] [Google Scholar]
- 43.Doisne JM, et al. iNKT cell development is orchestrated by different branches of TGF-beta signaling. J Exp Med. 2009;206(6):1365–1378. doi: 10.1084/jem.20090127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stritesky GL, et al. Murine thymic selection quantified using a unique method to capture deleted T cells. Proc Natl Acad Sci USA. 2013;110(12):4679–4684. doi: 10.1073/pnas.1217532110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perruche S, et al. CD3-specific antibody-induced immune tolerance involves transforming growth factor-beta from phagocytes digesting apoptotic T cells. Nat Med. 2008;14(5):528–535. doi: 10.1038/nm1749. [DOI] [PubMed] [Google Scholar]
- 46.Zhou X, et al. Selective miRNA disruption in T reg cells leads to uncontrolled autoimmunity. J Exp Med. 2008;205(9):1983–1991. doi: 10.1084/jem.20080707. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.