Abstract
Significance: Hydrogen sulfide (H2S) has been recognized as a physiological mediator with a variety of functions. It regulates synaptic transmission, vascular tone, inflammation, transcription, and angiogenesis; protects cells from oxidative stress and ischemia-reperfusion injury; and promotes healing of ulcers. Recent Advances: In addition to cystathionine β-synthase and cystathionine γ-lyase, 3-mercaptopyruvate sulfurtransferase along with cysteine aminotransferase was recently demonstrated to produce H2S. Even in bacteria, H2S produced by these enzymes functions as a defense against antibiotics, suggesting that the cytoprotective effect of H2S is a universal defense mechanism in organisms from bacteria to mammals. Critical Issues: The functional form of H2S—undissociated H2S gas, dissociated HS ion, or some other form of sulfur—has not been identified. Future Directions: The regulation of H2S production by three enzymes may lead to the identification of the physiological signals that are required to release H2S. The identification of the physiological functions of other forms of sulfur may also help understand the biological significance of H2S. Antioxid. Redox Signal. 20, 783–793.
Introduction
The study of hydrogen sulfide (H2S) as a physiological mediator began with the discovery of endogenous sulfide in the brain (28, 64, 77). Although the levels were re-evaluated to be much lower than initially reported, this discovery served to confirm the existence of H2S in tissues (24, 32). Survivors of sulfide poisonings commonly suffered from memory loss, suggesting that H2S may be involved in memory formation under physiological conditions. We found that H2S facilitates the induction of hippocampal long-term potentiation, a synaptic model of memory formation, by enhancing the activity of N-methyl-d-aspartate (NMDA) receptors (1). Based on this, as well as the additional finding that cystathionine β-synthase (CBS) is expressed in the brain, we proposed that H2S is a neuromodulator. Nitric oxide (NO) was discovered as an endothelium-derived vascular smooth muscle relaxation factor (23), and it was later found to also function in the brain (27). This finding led to the identification of NO synthase in the brain (7). It was also possible that H2S might function in other tissues to which the producing enzymes are localized. Based on the observations that H2S relaxed vascular smooth muscle, the ileum, and the portal vein, and that either cystathionine γ-lyase (CSE) or CBS or both were expressed in these tissues (31), we proposed that H2S functions as a smooth muscle relaxant. H2S was later found to activate the adenosine triphosphate (ATP)-sensitive, intermediate conductance, and small conductance-potassium channels (53, 83).
We and others discovered the cytoprotective effect of H2S; it protects neurons from oxidative stress by recovering glutathione levels decreased by oxidative stress (40). Although scavenging of reactive oxygen species (ROS) by H2S is less efficient than the glutathione cascade induced by H2S, the scavenging effect may also contribute to its neuroprotective effect (39, 78). These findings led to the identification of the cardioprotective effect of H2S, which preserves mitochondrial function against ischemia-reperfusion injury (21). The cytoprotective effect of H2S has also been observed in bacteria. H2S produced by the bacterial counterparts of CBS, CSE, and 3-mercaptopyruvate sulfurtransferase (3MST) was discovered to be a key molecule involved in bacterial resistance to antibiotics (66). The cytoprotective effect of H2S is a universal defense mechanism from bacteria to mammals.
Various other functions of H2S have been demonstrated: (i) H2S modulates inflammation; it suppresses leukocyte adherence and infiltration as well as edema formation (81). The proinflammatory activity of H2S has been observed in endotoxic shock (45). (ii) H2S suppresses the release of insulin by stimulating ATP-sensitive K+ channels or by suppressing glucose-induced oscillations in intracellular concentrations of Ca2+ in pancreatic beta cells (36, 80). It was later found that H2S protects pancreatic beta cells from apoptotic cell death due to high glucose (35). (iii) H2S induces angiogenesis, as observed in endothelial cell proliferation, adhesion, and the formation of tube-like structures by Akt phosphorylation in vitro, and neovascularization in mice in vivo (8). Vascular endothelium growth factor-induced angiogenesis is also mediated by H2S (60). (iv) H2S functions as an oxygen sensor; this was initially demonstrated by Olson and colleagues and was recently confirmed by other researchers (59, 62).
H2S dissolves well in water and dissociates to H+, HS−, and S2−. The undissociated form (H2S) also dissolves well in lipids. However, it is not well understood which form—H2S, HS−, or S2−—is physiologically relevant. H2S is a reducing substance and can reduce the cysteine disulfide bond, thereby changing the conformation and activity of enzymes, channels, and receptors. It can also modify cysteine residues in proteins by sulfhydration or the production of trisulfide bridge and modulate their function. Since other forms of sulfur, which are produced from H2S, are more stable than H2S, they have been proposed to be able to elicit physiological functions.
Production of H2S
CBS, CSE, and 3MST, along with cysteine aminotransferase (CAT), were found to have a capacity to produce H2S in vitro in1950s. However, H2S was simply considered a by-product of metabolic pathways or a marker for the evaluation of enzyme activity, rather than being recognized as a physiologically active molecule. We demonstrated the expression of CBS in the brain, and of CSE in smooth muscle, including in the vascular system (1, 31). Since then, both enzymes have been studied as major H2S producing enzymes in a variety of tissues. We recently demonstrated that 3MST, along with CAT, is a third H2S-producing enzyme in neurons, vascular endothelium, and the retina (49, 50, 68, 69). 3MST requires a reducing substance such as dithiothreitol (DTT) for the production of H2S, but the endogenous reducing substance has not been identified. Optimal activity of 3MST is achieved under alkaline conditions. For these reasons, this pathway was not recognized to be functional. We recently found that thioredoxin and dihydrolipoic acid (DHLA) are endogenous reducing substances that cause 3MST to release H2S (49).
H2S Production by CBS and CSE
CBS produces H2S by a β-replacement reaction with cysteine. CBS also catalyzes a β-replacement of cysteine by homocysteine to produce H2S (12, 34).
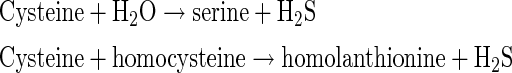 |
CSE produces H2S by the α, β-elimination reaction with cysteine. In the presence of high concentrations of homocysteine as in hyperhomocysteinemia, the γ-replacement reaction between two molecules of homocysteine becomes dominant in the production of H2S (34).
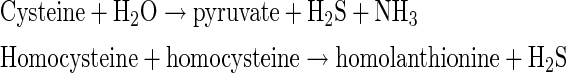 |
Banerjee and colleagues intensively studied the contributions of both enzymes to the production of H2S in the liver, kidney, and brain. CSE is more abundant than CBS in the liver and kidney; whereas in the brain, CBS is abundant but CSE has much lower levels. CBS and CSE have equal capacity for H2S production in the liver, while CBS is a major contributor to H2S production in the brain and kidney (34). H2S is metabolized by sulfur dioxygenase, which is present in higher levels in the liver and kidney than in the brain. Even small deviations in the rates of H2S production and clearance may lead to rapid and dramatic changes in the levels of H2S, providing an essential feature for a signaling molecule (76).
H2S Production by 3MST and CAT
3MST produces H2S from 3-mercaptopyruvate (3MP), which is produced by CAT from cysteine and α-ketoglutarate (16, 48, 68, 69). CAT is identical to aspartate aminotransferase (75).
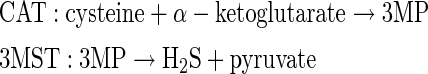 |
3MST localizes to mitochondria and the cytosol
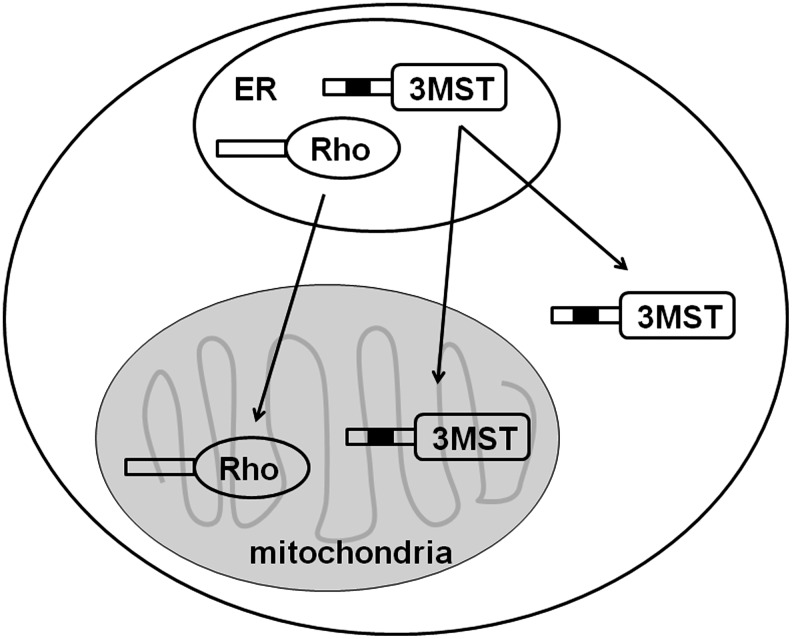
CBS and CSE are localized in the cytosol, while 3MST is localized in mitochondria as well as the cytosol (54, 69). Although the total activity of 3MST is greater in the cytosol compared with that in mitochondria, the specific activity is several-fold greater in mitochondria than in the cytosol. 3MST is highly homologous (60% amino acid identity) to rhodanese, which is predominantly localized in mitochondria and has a similar functional structure (4, 54, 56). The majority of mitochondrial proteins have a signal sequence at their amino terminus that targets these proteins to the mitochondria. After proteins are imported into the matrix, the signal sequence is usually removed. However, a group of mitochondrial proteins that have signal sequences lack the sequence which is essential for processing. These proteins, including rhodanese, remain in mitochondria without their signal sequence being processed. The amino-terminal residues 11–22 of rhodanese form an α–helix, which is a putative signal for mitochondrial transport. Two positively charged amino acids, Lys13 and Arg23, positioned on one side of the α-helix, are considered important for the function of the signal sequence to allow targeting to mitochondria. Arg23 is conserved in both 3MST and rhodanese, while Lys13 is replaced with Gln13 in 3MST. Therefore, the lack of Lys13 may cause 3MST to localize to both mitochondria and the cytosol (Fig. 1). Although rat, hamster, mouse, bovine, and chicken rhodanese have the conserved Lys13, human rhodanese has Gln13, which is similar to 3MST (55). A repetitive sequence (Gly-Lys-X)2 at the carboxyl terminal of rhodanese is also proposed to be a signal for mitochondrial retention. The sequence is modified in 3MST, suggesting that the signal may determine the differences in mitochondrial retention seen between rhodanese and 3MST (51).
FIG. 1.
Localization of 3MST and rhodanese. The signal sequence of 3MST for targeting mitochondria is homologous to that of rhodanese. One of the two positively charged amino acids in the sequence is replaced with a neutral amino acid in 3MST. Rhodanese is localized to mitochondria, while 3MST is localized to both mitochondria and cytosol. 3MST, 3-mercaptopyruvate sulfurtransferase; ER, endoplasmic reticulum.
In contrast to 3MST, there are two forms of CAT, that is, mitochondrial and cytosolic. These two CATs share 48% identity in their amino acid sequences (3, 20, 75), and produce 3MP, which is provided to 3MST for H2S production.
3MST requires thioredoxin or DHLA for H2S production
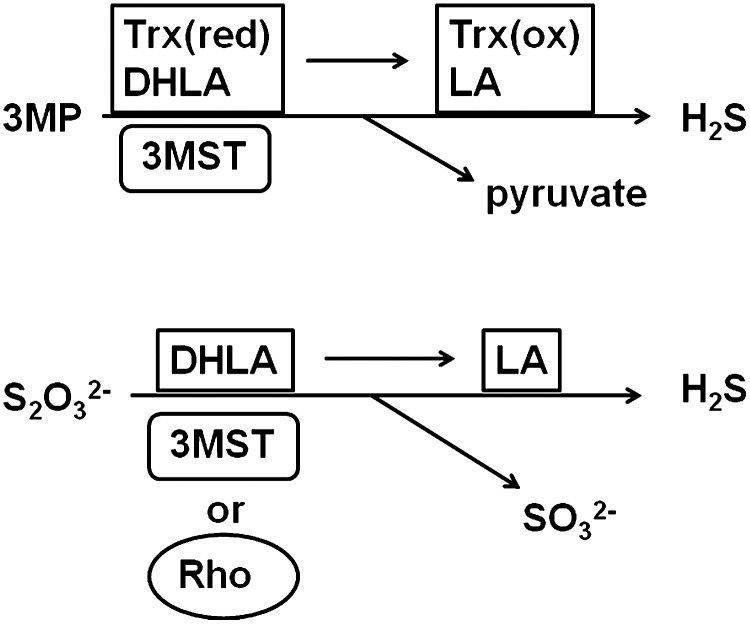
3MST has not been recognized as a H2S-producing enzyme because of its requirement for a reducing substance such as DTT, the endogenous counterpart of which has not been identified. We recently found that 3MST produced H2S from 3MP in the presence of thioredoxin (30, 49) (Fig. 2). The endogenous level of thioredoxin (20 μM) is four times as potent as DTT in releasing H2S from 3MST (49).
FIG. 2.
H2S production by 3MST from 3MP and thiosulfate. 3MST produces H2S from 3MP in the presence of thioredoxin or DHLA as cofactors. 3MST and rhodanese produce H2S from thiosulfate in the presence of DHLA. Rhodanese is more specific to thiosulfate than is 3MST. H2S, hydrogen sulfide; 3MP, 3-mercaptopyruvate; DHLA, dihydrolipoic acid.
There are two forms of thioredoxin in mammals. Thioredoxin 1 is localized in the cytosol, while thioredoxin 2 is localized in mitochondria (70). The CysXXCys motif, which contains cysteine residues at the active site, is highly conserved among different species, and similar to bacterial thioredoxin, thioredoxin 2 is resistant to oxidative stress. For these reasons, bacterial thioredoxin is widely used as a replacement of mammalian thioredoxin 2. Since thioredoxin is readily oxidized, it has to be reduced using thioredoxin reductase before it is used for experiments. Mammalian thioredoxin reductase is a selenoprotein, but the bacterial protein synthetic machinery does not appropriately incorporate selenium into the product (5). Therefore, A549 human lung adenocarcinoma cells, which contain abundant thioredoxin reductase, are often used as a source of the enzyme (22).
Another reducing substance, DHLA, exists ∼40 μM in brain mitochondria (37, 63). DHLA is as potent as DTT in causing 3MST to release H2S (Fig. 2). Other reducing substances such as cysteine, glutathione, nicotinamide adenine dinucleotide phosphate (NADPH), nicotinamide adenine dinucleotide (NADH), and CoA do not have any effect on the production of H2S by 3MST, even at high concentrations (1 mM) (49).
Thioredoxin, DTT, and DHLA have redox potentials in the range of −0.26 to −0.33 V. The redox potentials of monothiols such as glutathione, cysteine, and CoA are between −0.22 and −0.35 V, and those of NADH and NADPH are between −0.320 and −0.324 V. There is no correlation between the reducing potential and the ability to produce H2S. DTT and DHLA are dithiols, and thioredoxin has two cysteine residues at its active site, which may function as a dithiol. Therefore, the presence of a dithiol is a critical factor for these reducing substances to induce the release of H2S by 3MST (49). A possible mechanism for the production of H2S by 3MST in the presence of dithiols is that 3MST receives sulfide from 3MP to produce 3MST persulfide, which is then transferred to one of the thiol residues in thioredoxin or DHLA to produce thioredoxin persulfide or DHLA persulfide. The remaining thiol in thioredoxin or DHLA attacks the persulfide to release sulfide.
H2S production from thiosulfate
Thiosulfate is an intermediate of sulfur metabolism from cysteine, which results in the production of sulfite; sulfite is further oxidized to sulfate by sulfite oxidase (74). It has been proposed that rhodanese metabolizes thiosulfate to produce H2S and sulfite (41). As predicted from the structural similarity to rhodanese and from the fact that 3MST interacts with thiosulfate, 3MST was found to have the ability to produce H2S from thiosulfate in the presence of a high concentration (1 mM) of DHLA (49). 3MST produces H2S from both 3MP and thiosulfate, while rhodanese is more specific to thiosulfate than to 3MP (Fig. 2). However, neither the endogenous concentration (20 μM) of thioredoxin nor the same concentration of DTT was seen to support H2S production from thiosulfate by 3MST. Considering the requirement for high concentrations of DHLA, the production of H2S from thiosulfate under physiological conditions remains to be elucidated.
Ca2+ regulation on 3MST/CAT pathway
We found that H2S production by the 3MST/CAT pathway is regulated by Ca2+. In the absence of Ca2+, the production of H2S is maximal, and the production is suppressed by Ca2+ in a concentration-dependent manner (50). Based on the observation that the application of calmodulin or a calmodulin-specific inhibitor, W-7, did not change the activity, we concluded that calmodulin is not involved in the regulation of the H2S-producing activity of the 3MST/CAT pathway by Ca2+. Based on the following observations, we determined that the enzyme regulated by Ca2+ is CAT and not 3MST. First, the production of H2S from 3MP in the presence of 3MST was not affected by Ca2+. Second, H2S production from cysteine and α-ketoglutarate in the presence of 3MST and CAT was decreased by Ca2+ in a concentration-dependent manner. The best way to demonstrate the regulation of CAT by Ca2+ is to measure the levels of 3MP, the product of CAT, but 3MP is unstable and difficult to measure. The development of a method that can accurately measure 3MP levels is awaited.
The regulation of enzyme activities by Ca2+ without the involvement of calmodulin or any Ca2+-binding domain has been demonstrated for three mitochondrial dehydrogenases: pyruvate dehydrogenase, NAD-isocitrate dehydrogenase, and oxoglutarate dehydrogenase (18). The other example is serine racemase, a pyridoxal 5′-phosphate (PLP)-dependent enzyme similar to CAT, which has glutamate and aspartate residues bound to Ca2+; however, CAT does not have such a Ca2+-binding site (15). In contrast to these enzymes being activated by Ca2+, CAT is suppressed by Ca2+. CAT is regulated by Ca2+ via a novel but unknown mechanism.
Active Forms of H2S
H2S gas, HS−, and sulfane sulfur
H2S dissolves well in water and dissociates into H+, HS−, and S2−.
 |
Under physiological extracellular conditions at pH 7.4, ∼1/5 of the total H2S exists in an undissociated form (H2S) and the remaining 4/5 as HS- with a trace amount of S2−. The basal intracellular pH is different among organelles. The pH of the cytosol, mitochondria, Golgi apparatus, and lysosomes is 7.0–7.2, 8.0, 6.0–6.7, and 4.7, respectively (10). The ratio of H2S/HS− differs depending on their pH. For example, HS− is more dominant in mitochondria than in the cytosol.
H2S also dissolves well in lipids. H2S readily passes through the lipid bilayer of the plasma membrane, which contains diffusion-resistant substances such as cholesterol and sphingomyelin; while HS− does not pass through either the lipid bilayer or negative ion channels such as Cl− channels (47). The distribution of H2S and HS− on the two sides of the plasma membrane depends on the pH of the cytosol and the extracellular fluid. Since the extracellular environment (pH 7.4) is slightly more alkaline than the cytosol (pH 7.0–7.2), H2S may preferentially flow from the cytosol to the extracellular compartment.
The situation is different in bacteria. A HS− channel was recently discovered in bacteria (17). Since the extracellular environment of bacteria is acidic (pH 6.0) while the intracellular pH is 7.4, the HS− concentration is much greater in the interior of the cells than in the extracellular environment where H2S gas is dominant. Therefore, extracellular H2S passes through the bacterial membrane into the interior of the cells. Once H2S enters into cells, the equilibrium between H2S and HS− shifts toward HS−. The increased intracellular HS−, in turn, passes through HS− channels to the extracellular environment.
The products of the sulfur compounds in garlic, such as diallyl disulfide and diallyl trisulfide, contain sulfane sulfur. However, whether similar substances are produced in mammals is not well understood. The identification of such substances in mammals and the clarification of their functions may help understand the physiological roles of sulfur compounds, including H2S.
Regulation of Ca2+ Influx by H2S
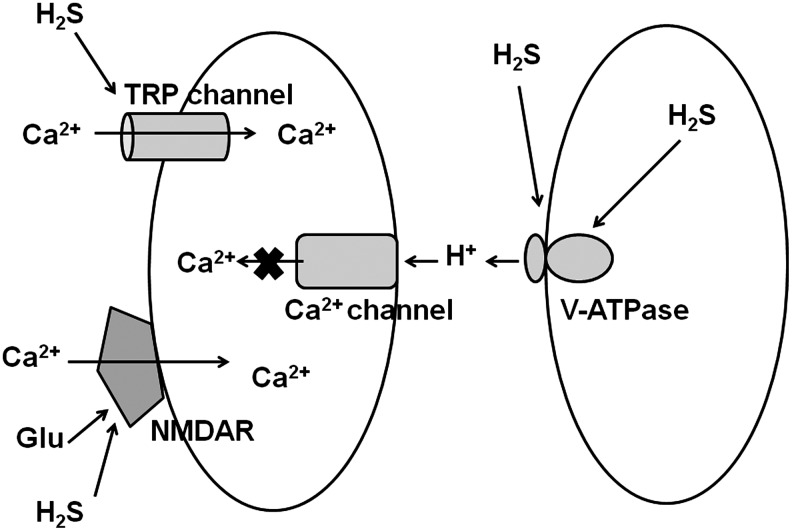
H2S induces Ca2+ influx in neurons and astrocytes in the brain, whereas it suppresses Ca2+ influx in the retinal photoreceptor cells. These contrasting effects are caused by differences in the receptors or channels that are involved in responses to H2S (Fig. 3).
FIG. 3.
Regulation of Ca2+ influx by H2S. H2S enhances the activity of NMDA receptors activated by the neurotransmitter glutamate and thereby increases Ca2+ influx in neurons. H2S activates TRP channels to increase Ca2+ influx in astrocytes. In contrast, in retinal neurons, H2S suppresses Ca2+ influx by activating V-ATPase, which releases H+ to suppress Ca2+ channels. NMDA, N-methyl-d-aspartate; TRP, transient receptor potential; V-ATPase, vacuolar-type proton ATPase.
Induction of Ca2+ influx in neurons and glia
H2S enhances the activity of NMDA receptors. H2S alone has no effect on NMDA receptors; however, the responses of NMDA receptors to their ligands, the neurotransmitter glutamate, or its synthetic agonist NMDA are augmented by H2S (1). The reduction or oxidation of disulfide bonds in proteins, including NMDA receptors, regulates their function (2). DTT enhances the activity of NMDA receptors by reducing cysteine disulfide bonds in these receptors. In spite of having lower negative reducing potential compared with DTT, 10-fold lower concentrations of H2S further enhance the activity of NMDA receptors even after enhancement by DTT. Similar to DTT, H2S reduces cysteine disulfide bonds in NMDA receptors to activate them. The difference between DTT and H2S is that H2S can further sulfhydrate the receptors or make a trisulfide bridge in them (25, 73). Sulfhydration, which has been proposed to modify the function of proteins, including ATP-activated K+ channels, may be a possible mechanism for the enhancement of NMDA receptor activity (25). Alternatively, the trisulfide bridge formation may be involved in the modification of the receptor activity (69).
The repetitive application of 100 to 300 μM H2S to cultures of cerebellar granule neurons increases the intracellular Ca2+ concentrations to the toxic range. Nimodipine and nifedipine, L-type Ca2+ channel blockers, and MK-801 and 2-amino-5-phosphonovalerate, inhibitors of NMDA receptors, attenuate the effect of H2S, suggesting the involvement of L-type Ca2+ channels and NMDA receptors (26). Since H2S evaporates from culture medium, the concentrations of H2S in the medium return to the basal level immediately after the repetitive application. However, the repetitive application of H2S causes the accumulation of bound sulfane sulfur in the cells that may influence the cellular reponses (32). It is necessary to re-evaluate the effect of H2S.
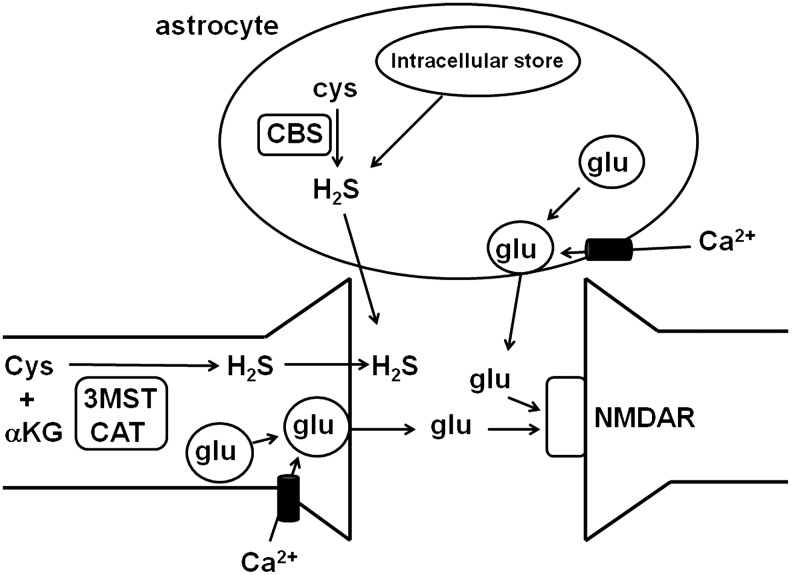
Astrocytes, a type of glia, were thought to be quiescent cells that surround and support neurons. Recently, however, astrocytes have been found to have receptors for neurotransmitters, such as glutamate, acetylcholine, noradrenaline, dopamine, γ-aminobutyric acid (GABA), and ATP (6). The transmitters released during synaptic activity spill out of the cleft at concentrations sufficient to activate receptors on the astrocytes surrounding the synapse. Stimulation of astrocytes induces increases in intracellular Ca2+ concentrations that propagate into neighboring astrocytes as intercellular Ca2+ waves. The mechanisms for increasing intracellular Ca2+ concentrations are different among the various neurotransmitters involved. For example, ATP activates purinergic receptors to induce Ca2+ release from the intracellular Ca2+ stores; whereas glutamate depolarizes the membrane, which, in turn, activates voltage-gated Ca2+ channels to induce Ca2+ influx. When the intracellular concentrations of Ca2+ reach sufficient levels in astrocytes, the fusion of vesicles containing gliotransmitters such as glutamate and ATP is induced, and the gliotransmitters are released by exocytosis (84) (Fig. 4). These gliotransmitters, in turn, activate pre- or post-synaptic receptors in neurons and modulate synaptic activity.
FIG. 4.
Release of H2S and gliotransmitters from astrocytes. Gliotransmitters such as glutamate and ATP are released from gliotransmitter vesicles to the extracellular space when Ca2+ enters into cells. The release of gliotransmitters from astrocytes is similar to the release of neurotransmitters from neurons. In contrast, H2S, which is produced by enzymes and intracellular stores, readily passes through the plasma membrane without the need for vesicle formation. ATP, adenosine triphosphate; CAT, cysteine aminotransferase; CBS, cystathionine β-synthase.
H2S increases intracellular Ca2+ concentrations in astrocytes that propagate to the surrounding astrocytes as Ca2+ waves (57) (Fig. 3). This increase in intracellular Ca2+ concentrations is greatly suppressed in the absence of extracellular Ca2+ and weakly suppressed by thapsigargin, which depletes intracellular Ca2+ stores, suggesting that H2S mainly increases Ca2+ influx, similar to glutamate, and partially increases Ca2+ release from intracellular stores. The responses to H2S are strongly suppressed by La3+, Gd3+, and ruthenium red, which are known as broad-spectrum inhibitors of transient receptor potential (TRP) channels; these channels are categorized as nonselective cation channels, and some of them are highly selective for Ca2+ (14) (Fig. 3). TRP channels are activated by several physiological stimuli such as osmolarity, pH, mechanical force, and ligand interaction. The involvement of TRP channels in response to H2S has been demonstrated. For example, the contraction of the detrusor muscle by H2S in the urinary bladder is regulated by TRP channels, the contraction of airway muscle by H2S is induced by activating TRPV1 channels, and Chinese hamster ovary cells expressing TRPA1 channels respond to H2S (61).
Gliotransmitters are released by exocytosis (84). Glutamate is synthesized via the tricarboxylic acid cycle, and ATP is produced by glycolysis and by oxidative phosphorylation in astrocytes. Both glutamate and ATP are loaded into vesicles by vesicular transporters. Vacuolar-type proton ATPase (V-ATPase) drives protons into vesicles to create the proton concentration gradient required for the transport of glutamate and ATP into the vesicles (84). In contrast to gliotransmitters, H2S, which readily passes through the plasma membrane of astrocytes, is released into the extracellular space between neurons and astrocytes (47) (Fig. 4).
Microglia also transmit Ca2+- mediated signaling to astrocytes, and the Ca2+ waves induced in astrocytes, in turn, propagate to the neighboring microglia. In microglia, the Ca2+ imaging in the presence of specific inhibitors of PKA and PKC showed that H2S activates Ca2+ channels in the plasma membrane as well as Ca2+ activated intracellular Ca2+ channels by activating cyclic adenosine monophosphate (cAMP)/PKA pathway (44).
Suppression of Ca2+ influx in retinal photoreceptor cells and cardiomyocytes
In contrast to its effect of increasing the Ca2+ influx in neurons and astrocytes, H2S suppresses Ca2+ influx in the retinal photoreceptor cells to which 3MST and CAT are co-localized (50). The intracellular concentration of Ca2+ in cells is ∼100 nM when cells are under quiescent conditions, and increases to approximately 3 μM when Ca2+ channels are opened. The intracellular concentrations of Ca2+ are shifted to lower concentrations specifically in the retinal neurons. When retinal photoreceptor cells are exposed to light, cyclic guanosine monophosphate-gated channels are closed and the intracellular concentration of Ca2+ is decreased to 10 nM. This is increased to approximately 600 nM in darkness (43). The production of H2S by the 3MST/CAT pathway is dramatically altered in this range of Ca2+ concentrations. At low concentrations of intracellular Ca2+, the 3MST/CAT pathway is activated to produce H2S in both horizontal cells and photoreceptor cells, which make synaptic contact with the horizontal cells. H2S activates V-ATPase on horizontal cells. V-ATPase has a cysteine disulfide at its active site. When this disulfide is reduced, V-ATPase is in the active state. The activated V-ATPase releases protons, which suppress voltage-gated Ca2+ channels on the photoreceptor cell membrane, thereby maintaining intracellular Ca2+ concentrations at much lower levels than seen in other cell types (50) (Fig. 3).
In cardiomyocytes, H2S directly suppresses L-type Ca2+ channels (71, 82). The depolarization induced by the electrical stimulation returns to the resting level faster in the presence of H2S than in its absence in cardiomyocytes, suggesting that H2S suppresses L-type Ca2+ channels. In contrast, Ca2+ release from the intracellular Ca2+ stores such as sarcoplasmic reticulum is not affected by H2S, judging from the observation that H2S did not have any effect on caffeine-induced increase in the intracellular Ca2+ concentrations (71). Sulfhydration may be involved in the suppression of L-type Ca2+ channels by H2S (82).
Cytoprotective Effect
Reducing cascade by producing glutathione
There are two forms of glutamate toxicity: oxidative toxicity and ionotropic toxicity. Oxidative glutamate toxicity is caused by high concentrations of glutamate, which suppress the glutamate/cystine antiporter and thereby decrease the import of cystine into cells (52). Cystine is reduced in the cells to cysteine and incorporated into glutathione. Therefore, lack of cysteine causes a decrease in the production of glutathione, the major intracellular antioxidant. H2S enhances the activity of the cystine/glutamate antiporter, thereby increasing the transport of cystine into the cells (40). In blood or extracellular fluid, cystine is the predominant form, but ∼20 μM cysteine is also present. H2S enhances the activity of the cysteine transporter as well as the cystine transporter to contribute to the production of glutathione (39, 40).
Glutathione is a tripeptide that consists of glutamate, cysteine, and glycine. Of these three amino acids, cysteine has the lowest level in the cytosol; therefore, glutamylcysteine synthase, which produces glutamylcysteine, is a limiting enzyme for glutathione synthesis. H2S, which enhances the activity of glutamylcysteine synthase, is required to exist in extracellular spaces to increase the production of glutamylcysteine (39, 40). The exposure of the enzyme to H2S, causing the direct interaction of the enzyme with H2S, does not increase the production of glutathione, suggesting that H2S activates some receptor localized to the cell surface to induce a signal which leads to the production of glutathione. Oxidative glutamate toxicity has been studied without the effect of excitotoxicity in cultured embryonic neurons, in which ionotropic glutamate receptors are not expressed. The recovery of decreased glutathione levels caused by ischemia-reperfusion injury in embryonic brains was also observed in vivo by the application of sodium hydrosulfide (NaHS) (39).
Excitotoxicity is caused by the entry of an excess amount of Ca2+ into cells through NMDA receptors or voltage-activated Ca2+ channels, which are opened by depolarization induced by the activation of alpha-amino-3-hydroxy-5-methy-4 isoxazole proprionic acid (AMPA) receptors (13). Under physiological conditions, NMDA receptors are not activated by their cognate ligand, glutamate, due to suppression by Mg2+. Glutamate initially activates AMPA receptors, causing depolarization of the membrane, which, in turn, releases the Mg2+ block and activates NMDA receptors. In brain slices of adult animals, H2S alone has no effect on neuronal activity at concentrations lower than 130 μM NaHS, but the activation of NMDA receptors by their ligand is enhanced by H2S (1). Even at toxic levels (up to 640 μM), H2S completely but reversibly suppresses synaptic transmission, leaving Na channels unaffected (1).
Recently, it was reported that H2S and NMDA, which cause excitotoxicity, induce the expression of 1649 genes in common, accounting for more than 80% of the genes induced by NMDA (11). The gene families involved include those related to cell death, endoplasmic stress, calcium homeostasis, cell cycle, heat shock proteins, and chaperones. Based on these observations, Chen et al. concluded that H2S causes neuronal cell death by activating NMDA receptors.
Scavenging free radical species
Another mechanism by which H2S protects neurons is by scavenging free radical species induced by glutamate, hydrogen peroxide, NO, peroxynitrite, and hypochlorous acid (39, 78, 79). Significant amounts of ROS are produced in mitochondria, to which 3MST and CAT are localized. Cells expressing 3MST and CAT are resistant to oxidative stress caused by glutamate and hydrogen peroxide, suggesting that H2S produced by both enzymes may scavenge ROS in mitochondria (39). Since mitochondria contain ∼1 mM cysteine, which is greater than the level seen in the cytoplasm, appropriate amounts of H2S can be produced in mitochondria (29, 72, 74). However, compared with glutathione, which exists at several mM in cells and has greater reducing potential than H2S, the levels of H2S are much lower. Therefore, the glutathione cascade induced by H2S may contribute to the suppression of oxidative stress more efficiently than the scavenging of free radical species by H2S itself.
Regulation of transcription and translation
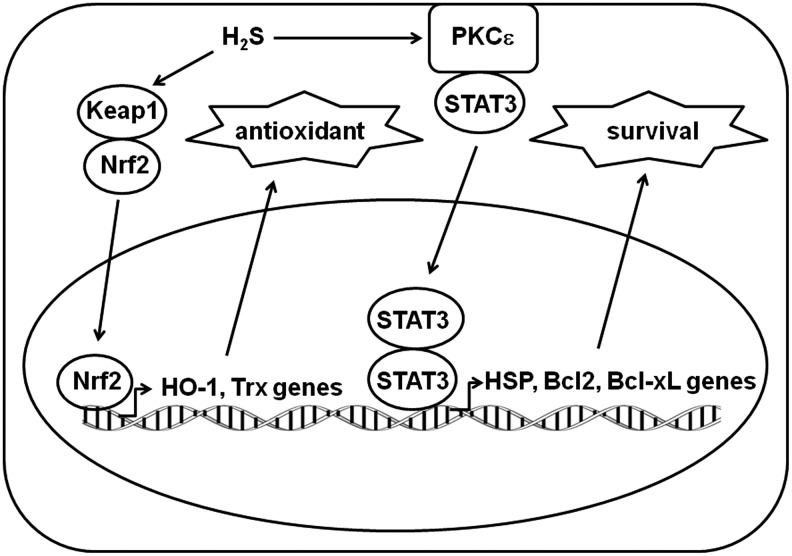
H2S protects cardiac muscle from ischemia-reperfusion injury by preserving mitochondrial function (21). H2S reduces cardiomyocyte apoptosis, as demonstrated by a decreased number of terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)-positive nuclei, by decreasing the activity of caspase3 in the presence of H2S. The transcriptional regulation of this cardioprotective effect was recently elucidated (9). H2S increases the nuclear localization of nuclear factor erythroid 2-related factor 2 (Nrf2), which is a nuclear basic leucine zipper transcription factor that regulates a number of antioxidants and related enzymes, including thioredoxin and heme oxygenase-1. H2S also increases the phosphorylation of proteins in the protein kinase Cɛ/signal transducers and activators of transcription 3 (STAT-3) signaling cascade, leading to the up-regulation of the expression of the survival factors Bcl-2 and Bcl-xL and the down-regulation of the apoptotic factor Bad (Fig. 5) (9).
FIG. 5.
H2S up-regulates antioxidant- and survival-related genes. H2S translocates Nrf2 into the nucleus to activate antioxidant-related genes such as HO-1 and Trx. It also activates PKCɛ and STAT3 to translocate dimerized STAT3 to the nucleus, which then activates survival-related genes such as HSP, Bcl2, and Bcl-xL. RPS3, ribosomal protein S3.
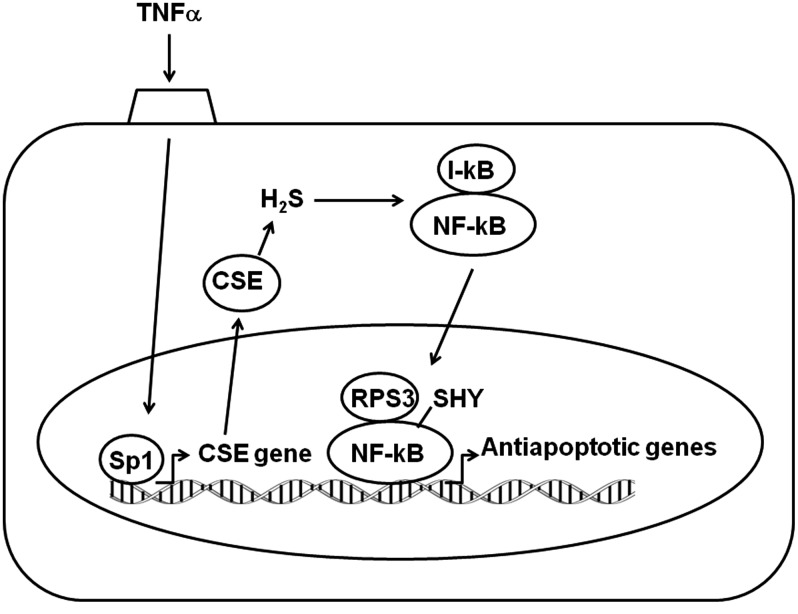
Another instance of transcriptional regulation in the antiapoptotic action of H2S was recently revealed in the effect of the multifunctional proinflammatory cytokine tumor necrosis factor-α (TNF-α) (65). TNF-α activates the IκB kinase complex that phosphorylates IκB, leading to IκB degradation and the subsequent translocation of NF-κB to the nucleus. TNF-α also stimulates the binding of a transcription factor, SP1, to the CSE promoter to increase the production of H2S (33, 65). H2S, in turn, sulfhydrates the p65 subunit of NF-κB, which promotes its binding to the coactivator ribosomal protein S3 (RPS3). The NF-κB/RPS3 complex binds to the promoters of several antiapoptotic genes, leading to the suppression of apoptosis (Fig. 6).
FIG. 6.
Antiapoptotic action of H2S. TNFα activates the transcription of CSE gene to increase the levels of CSE. H2S produced by CSE sulfhydrates NF-kB, which leads to the translocation of NF-kB into the nucleus to activate the transcription of antiapoptotic genes. CSE, cystathionine γ-lyase; Nrf2, nuclear factor erythroid 2-related factor 2; STAT3, signal transducers and activators of transcription 3;TNF-α, tumor necrosis factor-α.
The endoplasmic reticulum (ER) is the primary site of protein synthesis, and it controls the folding and modification of newly synthesized proteins. After being properly folded and modified, proteins are trafficked to the Golgi apparatus. However, if proteins are misfolded or improperly modified, a condition known as ER stress, the unfolded protein response (UPR) is activated (46). UPR is triggered by transmembrane sensor proteins that detect unfolded proteins in the ER and transduce signals to fine tune protein synthesis and folding. If this tuning is not successful, it triggers proapoptotic cascades. ER stress increases the production of H2S by CSE, which sulfhydrates and thereby inactivates protein tyrosine phosphatase, resulting in the protection of protein kinase-like ER kinase (PERK) from dephosphorylation. Activated PERK inhibits global translation by phosphorylating eukaryotic translational initiation factor 2 alpha (elF2α), resulting in promoting the restoration of ER homeostasis (42).
Ca2+ homeostasis
Light-induced photoreceptor cell apoptosis represents an animal model for the study of human retinal degenerations. Apoptosis is the mode of photoreceptor cell death in both human cases and animal models. An increase in intracellular calcium levels occurs during apoptotic cell death, and this may activate calcium-dependent proteases, such as calpain. Voltage-gated Ca2+ channel blockers such as diltiazem suppress photoreceptor cell apoptosis, and the lack of the V-ATPase a3 subunit causes retinal degeneration, suggesting that voltage-gated Ca2+ channels and V-ATPase are involved in photoreceptor degeneration (19, 38). H2S activates V-ATPase in horizontal cells to release protons, which suppress voltage-gated Ca2+ channels in photoreceptor cells, thereby maintaining intracellular Ca2+concentrations at low levels in photoreceptor cells (Fig. 3). H2S greatly suppresses the intensive light-induced photoreceptor degeneration (50). The number of TUNEL-positive photoreceptor cells and those containing 8-hydroxy-2′-deoxyguanosine, a product of ROS-induced DNA damage, is decreased in animals administered H2S. The retina is susceptible to oxidative stress because of its high consumption of oxygen and daily exposure to light. Under normal conditions, H2S maintains the intracellular Ca2+ concentrations at low levels. However, this regulation by H2S may fail when photoreceptor cells are exposed to excessive levels of light. Even under such conditions, H2S supplementation protects cells from degeneration.
Universal defense mechanism
The cytoprotective effect of H2S produced by CBS, CSE, and 3MST is a phenomenon that is observed not only in mammalian cells but also in prokaryote bacteria, as demonstrated by Nudler and colleagues (66). For centuries, it has been well known that bacteria produce H2S, but it was recognized as a byproduct of metabolic pathways, and not considered as having a particular function. In four clinically relevant and evolutionarily distant pathogenic species of bacteria, all three enzymes were demonstrated to produce H2S. The overexpression of 3MST increases spectinomycin resistance; whereas the chemical inhibition of CBS, CSE, or 3MST renders bacteria more sensitive to antibiotics. NaHS suppresses the antibiotic sensitivity of CBS-, CSE-, and 3MST-deficient bacteria. These observations suggest that endogenously produced H2S enhances the resistance of bacteria to antibiotics. H2S protects bacteria from oxidative stress and antibiotics via two mechanisms; suppressing DNA breaks and enhancing the activity of catalase and superoxide dismutase. H2O2 and ampicillin caused linearization of the chromosome in 3MST-deficient bacteria; while overexpression of 3MST suppressed this linearization, suggesting that H2S produced by 3MST protects chromosomal DNA.
The cytoprotective effect of H2S is similar to that of NO in bacteria (66). Bacteria deficient in both bacterial NO synthase and CBS or CSE are unable to establish colonies, suggesting that bacteria cannot survive in the absence of both NO and H2S. The levels of H2S are increased in NO-deficient bacteria, and vice versa, suggesting that one substance compensates for the lack of the other. There is also a synergistic effect in the protection afforded by H2S and NO against antibiotics. Nudler also noted that NO synthase is present only in a small number of gram-positive bacteria, while H2S-producing enzymes are essentially universal. H2S can be a diffusible defense agent in the entire population of bacteria. Since bacterial CBS, CSE, and 3MST have substantially diverged from their mammalian counterparts, designing specific inhibitors of these bacterial enzymes may enhance the effect of antibiotics against a broad range of pathogens (66).
Concluding Remarks
CBS and CSE have been intensively studied as H2S-producing enzymes. Both enzymes are mainly localized to the cytosol in cells. In contrast, 3MST is localized to mitochondria as well as the cytosol, and CAT is present in two forms: mitochondrial and cytosolic. Therefore, the 3MST/CAT pathway can produce H2S both in mitochondria and the cytosol. CBS and CSE require only substrates, while 3MST requires a reducing cofactor, as well as a substrate, to produce H2S. Since the endogenous reducing cofactor for 3MST has not been known so far, the 3MST/CAT pathway was not recognized to be involved in H2S production. Now that the cofactors thioredoxin and DHLA have been identified, the role of the 3MST/CAT pathway in the nervous and vascular systems as well as in other systems will be clarified (49, 50).
H2S sometimes exerts contrasting effects in different tissues. For example, Ca2+ influx is increased by H2S in neurons and astrocytes by enhancing the activity of NMDA receptors, and by activating TRP channels, respectively (1, 57). In contrast, Ca2+ influx is decreased in retinal neurons via the activation of V-ATPase, which releases H+ to suppress voltage-gated Ca2+ channels (50). H2S reduces the cysteine disulfide bonds of NMDA receptors and V-ATPase to modulate their function, whereas it may produce persulfide formation at the cysteine residues in the regulatory site of TRP channels.
In addition to the direct activation or modulation of channels, receptors, and enzymes, H2S modulates enzyme activity through intracellular signaling. For example, the enhancement of γ-glutamylcysteine synthase by H2S is observed only when H2S is applied to the outside of the cells, but not when the enzyme is directly exposed to H2S, suggesting that H2S activates a cell surface receptor to induce a second messenger, which then activates the enzyme (39, 40).
The cytoprotective effect of H2S was initially found in the mammalian brain and heart, and subsequently in many other organs (21, 40, 78). This effect is not restricted in mammals. It is well known that bacteria produce H2S, but it has been considered only a byproduct of metabolic pathways. Recently, H2S was found to protect bacteria from antibiotics (66). The enzymes that produce H2S in bacteria are the bacterial counterparts of CBS, CSE, and 3MST. The disruption of these enzymes renders bacteria sensitive to antibiotics. Since H2S is diffusible, it is effective in protecting surrounding bacteria as well. These findings indicate that the cytoprotective effect of H2S is a universal defense mechanism in organisms from bacteria to mammals. Since mammalian enzymes have diverged from their bacterial counterparts, it is possible to make specific inhibitors of bacterial enzymes that leave mammalian counterparts unaffected, and conjugate them to currently used antibiotics to produce novel antibiotics which do not elicit resistance from bacteria.
The equilibrium between H2S and HS− changes depending on the pH. Since the pH varies in each organelle, various ratios of H2S/HS− are seen in cells. The pH of extracellular fluids such as blood is slightly alkaline compared with that of the cytosol; therefore, HS− is more dominant in blood. Since H2S but not HS− readily passes though the lipid bilayer membrane, this ratio may limit its efficiency to pass through the membrane (47). The levels of H2S are also affected by the rate of H2S absorption in the form of bound sulfane (32). For example, H2S is immediately absorbed in liver homogenates, but it takes minutes in brain homogenates, suggesting that H2S may remain longer in some tissues than in others.
It is possible that some of these observed effects which are considered induced by H2S might be induced by other forms of sulfur compounds (58, 73). It is necessary to address these problems in order to establish H2S as a physiological mediator and to identify its appropriate therapeutic applications to diseases in which H2S is involved.
During the revision of this article, we discovered the fourth pathway for H2S production (67). 3MST produces H2S from 3MP, which is produced from D-cysteine by d-amino acid oxidase. This pathway operates predominantly in the cerebellum and the kidney. Administration of D-cysteine protects primary cultures of cerebellar neurons from oxidative stress induced by H2O2 and attenuates ischemia-reperfusion injury in the kidney more than l-cysteine. The novel pathway of H2S production provides a new therapeutic approach to deliver H2S to specific tissues.
We also discovered that polysulfides are possible H2S-derived signaling molecules (40a).
Abbreviations Used
- 3MP
3-mercaptopyruvate
- 3MST
3-mercaptopyruvate sulfurtransferase
- AMPA
alpha-amino-3-hydroxy-5-methy-4 isoxazole proprionic acid
- ATP
adenosine triphosphate
- CAT
cysteine aminotransferase
- CBS
cystathionine β-synthase
- CSE
cystathionine γ-lyase
- DHLA
dihydrolipoic acid
- DTT
dithiothreitol
- elF2α
eukaryotic translational initiation factor 2 alpha
- ER
endoplasmic reticulum
- GABA
γ-aminobutyric acid
- H2S
hydrogen sulfide
- NADH (NAD)
nicotinamide adenine dinucleotide
- NADPH
nicotinamide adenine dinucleotide phosphate
- NaHS
sodium hydrosulfide
- NMDA
N-methyl-d-aspartate
- NO
nitric oxide
- Nrf2
nuclear factor erythroid 2-related factor 2
- PERK
protein kinase-like ER kinase
- PLP
pyridoxal 5′-phosphate
- ROS
reactive oxygen species
- RPS3
ribosomal protein S3
- STAT3
signal transducers and activators of transcription 3
- TNF-α
tumor necrosis factor-α
- TRP
transient receptor potential
- UPR
unfolded protein response
- V-ATPase
vacuolar-type proton ATPase
Acknowledgments
This work was supported by a grant from the National Institute of Neuroscience and KAKENHI (23659089) from Grant-in-Aid for Challenging Exploratory Research to H.K.
References
- 1.Abe K. and Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci 16: 1066–1071, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aizenman E, Lipton DA, and Loring RH. Selective modulation of NMDA responses by reduction and oxidation. Neuron 2: 1257–1263, 1989 [DOI] [PubMed] [Google Scholar]
- 3.Akagi R. Purification and characterization of cysteine aminotransferase from rat liver cytosol. Acta Med Okayama 36: 187–197, 1982 [DOI] [PubMed] [Google Scholar]
- 4.Alphey MS, Williams RAM, Mottram JC, Coombs GH, and Hunter WN. The crystal structure of Leishmania major 3-mercaptopyruvate sulfurtransferase. J Biol Chem 278: 48219–48227, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Arner ES, Sarioglu H, Lottspeich F, Holmgren A, and Bock A. High-level expression in Escherichia coli of selenocysteine-containing rat thioredoxin reductase utilizing gene fusions with engineered bacterial-type SECIS elements and co-expression with the selA, selB and selC genes. J Mol Biol 292: 1003–1016, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Bezzi P. and Volterra A. A neuron-glia signaling network in the active brain. Curr Opin Neurobiol 11: 387–394, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Bredt DA. and Snyder SH. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci U S A 87: 682–685, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cai WJ, Wang MJ, Moore PK, Jin HM, Yao T, and Zhu YC. The novel proangiogenic effect of hydrogen sulfide is dependent on Akt phosphorylation. Cardiovasc Res 76: 29–40, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Calvert JW.Jha S, Gundewar S, Elrod JW.Ramachandran A, Pattillo CB, Kevil CG, and Lefer DJ. Hydrogen sulfide mediates cardioprotection through Nrf2 signaling. Circ Res 105: 365–374, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casey JR, Grinstein S, and Orlowski J. Sensors and regulators of intracellular pH. Nat Rev Mol Cell Biol 11: 50–61, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Chen MJ, Peng ZF, Manikandan J, Melendez AJ, Tan GS, Chung CM, Li QT, Tan TM, Deng LW, Whiteman M, Beart PM, Moore PK, and Cheung NS. Gene profiling reveals hydrogen sulphide recruits death signaling via the N-methy-D-aspartate receptor identifying commonalities with excitotoxicity. J Cell Physiol 226: 1308–1322, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Chen X, Jhee KH, and Kruger WD. Production of the neuromodulator H2S by cystathionine beta-synthase via the condensation of cysteine and homocystein. J Biol Chem 279: 52082–52086, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Choi DW. Ionic dependence of glutamate neurotoxicity in cortical cell culture. J Neurosci 7: 369–379, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clapham DE, Runnels LW, and Strubing C. The TRP ion channel family. Nat Rev Neurosci 2: 387–396, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Cook SP, Galve-Roperh I, Martinez del Pozo A, and Rodriguez-Crespo I. Direct calcium binding results inactivation of brain serine racemase. J Biol Chem 277: 27782–27792, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Cooper AJL. Biochemistry of sulfur-containing amino acids. Annu Rev Biochem 52: 187–222, 1983 [DOI] [PubMed] [Google Scholar]
- 17.Czyzewski BK. and Wang D-N. Identification and characterization of a bacterial hydrosulphide ion channel. Nature 483: 494–497, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Denton RM. Regulation of mitochondrial dehydrogenases by calcium ions. Biochim Biophys Acta 1787: 1309–1316, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Donovan M. and Cotter TG. Caspase-independent photoreceptor apoptosis in vivo and differential expression of apoptotic protease activating factor-1 and caspase-3 during retinal development. Cell Death Differ 9: 1220–1231, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Doyle JM, Schinina ME, Bossa F, and Doonan S. The amino acid sequence of cytosolic aspartate aminotransferase from human liver. Biochem J 270: 651–657, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elrod JW, Calvert JW, Morrison J, Doeller JE, Kraus DW, Tao L, Jiao X, Scalia R, Kiss L, Szabo C, et al. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc Natl Acad Sci U S A 104: 15560–15565, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujiwara N, Fujii T, Fujii J, and Taniguchi N. Functional expression of rat thioredoxin reductase: selenocysteine insertion sequence element is essential for the active enzyme. Biochem J 340: 439–444, 1999 [PMC free article] [PubMed] [Google Scholar]
- 23.Furchgott RF. and Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 288: 373–376, 1980 [DOI] [PubMed] [Google Scholar]
- 24.Furne J, Saeed A, and Levitt MD. Whole tissue hydrogen sulfide concentrations are orders of magnitude lower than presently accepted values. Am J Physiol Regul Integr Comp Physiol 295: R1479–R1498, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Gadalla MM. and Snyder SH. Hydrogen sulfide as a gasotransmitter. J Neurochem 113: 14–26, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garcia-Bereguiain MA, Samhan-Arias AK, Martin-Romero FJ, and Gutierrez-Merino C. Hydrogen sulfide raises cytosolic calcium in neurons through activation of L-type Ca2+ channels. Antioxid Redox Signal 10: 31–41, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Garthwaite J, Charles SL, and Chess-Williams R. Endothelium-derived relaxing factor release on activation of NMDA receptors suggests role as intercellular messenger in the brain. Nature 336: 385–388, 1988 [DOI] [PubMed] [Google Scholar]
- 28.Goodwin LR, Francom D, Dieken FP, Taylor JD, Warenycia MW, Reiffenstein RJ, and Dowling G. Determination of sulfide in brain tissue by gas dialysis/ion chromatography: postmortem studies and two case reports. J Anal Toxicol 13: 105–109, 1989 [DOI] [PubMed] [Google Scholar]
- 29.Griffith OW. Biologic and pharmacologic regulation of mammalian glutathione synthesis. Free Radic Biol Med 27: 922–935, 1999 [DOI] [PubMed] [Google Scholar]
- 30.Holmgren A. The thioredoxin system. In: Redox Biochemistry, edited by Banerjee R. Hoboken, NJ: John Wiley & Sons, 2008, pp. 68–74 [Google Scholar]
- 31.Hosoki R, Matsuki N, and Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem Biophys Res Commun 237: 527–531, 1997 [DOI] [PubMed] [Google Scholar]
- 32.Ishigami M, Hiraki K, Umemura K, Ogasawara Y, Ishii K, and Kimura H. A source of hydrogen sulfide and a mechanism of its release in the brain. Antioxid Redox Signal 11: 205–214, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Ishii I, Akahoshi N, Yu X-N, Kobayashi Y, Namekata K, Komaki G, and Kimura H. Murine cystathionine γ–lyase: complete cDNA and genomic sequences, promoter activity, tissue distribution and developmental expression. Biochem J 381: 113–123, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kabil O, Vitvitsky V, Xie P, and Banerjee R. The quantitative significance of the transsulfuration enzymes for H2S production in murine tissues. Antioxid Redox Signal 15: 363–372, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaneko Y, Kimura T, Taniguchi S, Souma M, Kojima Y, Kimura Y, Kimura H, and Niki I. Glucose3-induced production of hydrogen sulfide may protect the pancreatic beta-cells from apoptotic cell death by high glucose. FEBS Lett 583: 377–382, 2009 [DOI] [PubMed] [Google Scholar]
- 36.Kaneko Y, Kimura Y, Kimura H, and Niki I. L-cysteine inhibits insulin release from the pancreatic beta-cell: Possible involvement of metabolic production of hydrogen sulfide, a novel gasotransmitter. Diabetes 55: 1391–1397, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Kataoka H, Hirabayashi N, and Makita M. Analysis of lipoic acid in biological samples by gas chromatography with flame photometric detection. J Chromatogr 615: 197–202, 1993 [DOI] [PubMed] [Google Scholar]
- 38.Kawamura N, Tabata H, Sun-Wada GH, and Wada Y. Optic nerve compression and retinal degeneration in Tcirg1 mutant mice lacking the vacuolar-type H+-ATPase a3 subunit. PLoS One 5: e12086, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kimura Y, Goto Y-I, and Kimura H. Hydrogen sulfide increases glutathione production and suppresses oxidative stress in mitochondria. Antioxid Redox Signal 12: 1–13, 2010 [DOI] [PubMed] [Google Scholar]
- 40.Kimura Y. and Kimura H. Hydrogen sulfide protects neurons from oxidative stress. FASEB J 18: 1165–1167, 2004 [DOI] [PubMed] [Google Scholar]
- 40a.Kimura Y, Mikami Y, Osumi K, Tsugane M, Oka J-I, Kimura H. Polysulfides are possible H2S-derived signaling molecules in rat brain. FASEB J. 2013 [Epub ahead of print]; DOI: 10.1096/fj.12-226415 [DOI] [PubMed] [Google Scholar]
- 41.Koj A, Frendo J, and Janik Z. [35S]thiosulphate oxidation by rat liver mitochondria in the presence of glutathione. Biochem J 103: 791–795, 1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krishnan N, Fu C, Pappin DJ, and Tonks NK. H2S-induced sulfhydration of the phosphatase PTP1B and its role in the endoplasmic reticulum stress response. Sci Signal 4: ra86, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krizaj D. and Copenhagen DR. Calcium regulation in photoreceptors. Front Biosci 7: d2023–d2044, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee SW, Hu Y-S, Hu L-F, Lu Q, Dawe GS, Moore PK, Wong PT-H, and Bian J-S. Hydrogen sulphide regulates calcium homeostasis in microglial cells. Glia 54: 116–124, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Li L, Bhatia M, Zhu YZ, Zhu YC, Ramnath RD, Wang ZJ, Anuar FB, Whiteman M, Salto-Tellez M, and Moore PK. Hydrogen sulfide is a novel mediator of lipopolysaccharide-induced inflammation in the mouse. FASEB J 19: 1196–1198, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Malhotra JD. and Kaufman RJ. Endoplasmic reticulum stress and oxidative stress:A vicious cycle or a double-edged sword? Antioxid Redox Signal 9: 2277–2293, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Mathai JC, Missner A, Kugler P, Saparov SM, Zeidel ML, Lee JK, and Pohl P. No facilitator required for membrane transport of hydrogen sulfide. Proc Natl Acad Sci U S A 106: 16633–16638, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meister A, Fraser PE, and Tice SV. Enzymatic desulfuration of β-mercaptopyruvate to pyruvate. J Biol Chem 206: 561–575, 1954 [PubMed] [Google Scholar]
- 49.Mikami Y, Shibuya N, Kimura Y, Nagahara N, Ogasawara Y, and Kimura H. Thioredoxin and dihydrolipoic acid are required for 3-mercaptopyruvate sulfurtransferase to produce hydrogen sulfide. Biochem J 439: 479–485, 2011 [DOI] [PubMed] [Google Scholar]
- 50.Mikami Y, Shibuya N, Kimura Y, Nagahara N, Yamada M, and Kimura H. Hydrogen sulfide protects the retina from light-induced degeneration by the modulation of Ca2+ influx. J Biol Chem 286: 39379–39386, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miller DM, Delgado R, Chirgwin JM, Hardies SC, and Horowitz PM. Expression of cloned bovine adrenal rhodanese. J Biol Chem 266: 4686–4691, 1991 [PubMed] [Google Scholar]
- 52.Murphy TH, Miyamoto M, Sastre A, Schnaar RL, and Coyle JT. Glutamate toxicity in a neuronal cell line involves inhibition of cystine transport leading to oxidative stress. Neuron 2: 1547–1558, 1989 [DOI] [PubMed] [Google Scholar]
- 53.Mustafa AK, Sikka G, Gazi SK, Steppan J, Jung SM, Bhunia AK, Barodka VM, Gazi FK, Barrow RK, Wang R, Amzel LM, Berkowitz DE, and Snyder SH. Hydrogen sulfide as endothelium-derived hyperpolarizing factor sulfhydrates potassium channels. Circ Res 109: 1259–1268, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nagahara N, Ito T, Kitamura H, and Nishino T. Tissue and subcellular distribution of mercaptopyruvate sulfurtransferase in the rat: confocal laser fluorescence and immunoelectron microscopic studies combined with biochemical analysis. Histochem Cello Biol 110: 243–250, 1998 [DOI] [PubMed] [Google Scholar]
- 55.Nagahara N. and Nishino T. Role of amino acid residues in the active site of rat liver mercaptopyruvate sulfurtransferase. cDNA cloning, overexpression, and site-directed mutagenesis. J Biol Chem 271: 27395–27401, 1996 [DOI] [PubMed] [Google Scholar]
- 56.Nagahara N, Okazaki T, and Nishino T. Cytosolic mercaptypyruvate sulfurtransferase is evolutionarily related to mitochondrial rhodanese. Striking similarity in active site amino acid sequence and the increase in the mercaptopyruvate sulfurtransferase activity of rhodanese by site-directed mutagenesis. J Biol Chem 270: 16230–16235, 1995 [DOI] [PubMed] [Google Scholar]
- 57.Nagai Y, Tsugane M, Oka J, and Kimura H. Hydrogen sulfide induces calcium waves in astrocytes. FASEB J 18: 557–559, 2004 [DOI] [PubMed] [Google Scholar]
- 58.Nagy P. and Winterbourn CC. Rapid reaction of hydrogen sulfide with the neutrophil oxidant hypochlorous acid to generate polysulfides. Chem Res Toxicol 23: 1541–1543, 2010 [DOI] [PubMed] [Google Scholar]
- 59.Olson KR, Dombkowski RA, Russell MJ, Doellman MM, Head SK, Whitfield NL, and Madden JA. Hydrogen sulfide as an oxygen sensor/transducer in vertebrate hypoxic vasoconstriction and hypoxic vasodilation. J Exp Biol 209: 4011–4023, 2006 [DOI] [PubMed] [Google Scholar]
- 60.Papapetropoulos A, Pyriochou A, Altaany Z, Yang G, Marazioti A, Zhou Z, Jeschke MG, Branski LK, Herndon DN.Wang R, and Szabo C. Hydrogen sulfide is an endogenous stimulator of angiogenesis. Proc Natl Acad Sci U S A 106: 21972–21977, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Patacchini R, Santicioli P, Giuliani S, and Maggi CA. Pharmacological investigation of hydrogen sulfide (H2S) contractile activity in rat detrusor muscle. Eur J Pharmacol 509: 171–177, 2005 [DOI] [PubMed] [Google Scholar]
- 62.Peng YJ, Nanduri J, Raghuraman G, Souvannakitti D, Gadalla MM, Kumar GK, Snyder SH, and Prabhakar NR. H2S mediates O2 sensing in the carotid body. Proc Natl Acad Sci U S A 107: 10710–10724, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Petersen Shay K, Moreau R, Smith E, and Hagen T. Is α-lipoic acid a scavenger of reactive oxygen species in vivo? Evidence for its initiation of stress signaling pathways that promote endogenous antioxidant capacity. IUBMB Life 60: 362–367, 2008 [DOI] [PubMed] [Google Scholar]
- 64.Savage JC. and Gould DH. Determination of sulfide in brain tissue and rumen fluid by ion-interaction reversed-phase high-performance liquid chromatography. J Chromatogr 526: 540–545, 1990 [DOI] [PubMed] [Google Scholar]
- 65.Sen N, Paul BD, Gadalla MM, Mustafa AK, Sen T, Xu R, Kim S, and Snyder SH. Hydrogen sulfide-linked sulfhydration of NF-κB mediates its antiapoptotic actions. Mol Cell 45: 13–24, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shatalin K, Shatalina E, Mironov A, and Nudler E. H2S: a universal defense against antibiotics in bacteria. Science 334: 986–990, 2011 [DOI] [PubMed] [Google Scholar]
- 67.Shibuya N, Koike S, Tanaka M, Ishigami-Yuasa M, Kimura Y, Ogasawara Y, Fukui K, Nagahara N, and Kimura H. A novel pathway for the production of hydrogen sulfide from D-cysteine in mammalian cells. Nat Commun 4: 1366, 2013 [DOI] [PubMed] [Google Scholar]
- 68.Shibuya N, Mikami Y, Kimura Y, Nagahara N, and Kimura H. Vascular endothelium exresses 3-mercaptopyruvate sulfurtransferase and produces hydrogen sulfide. J Biochem 146: 623–626, 2009 [DOI] [PubMed] [Google Scholar]
- 69.Shibuya N, Tanaka M, Yoshida M, Ogasawara Y, Togawa T, Ishii K, and Kimura H. 3-Mercaptopyruvate sulfurtransferease produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxid Redox Signal 11: 703–714, 2009 [DOI] [PubMed] [Google Scholar]
- 70.Spyrou G, Enmark E, Miranda-Vizuete A, and Gustafsson JA. Cloning and expression of a novel mammalian thioredoxin. J Biol Chem 272: 2936–2941, 1997 [DOI] [PubMed] [Google Scholar]
- 71.Sun Y-G, Cao Y-X, Wang W-W, Ma S-F, Yao T, and Zhu Y-C. Hydrogen sulphide is an inhibitor of L-type calcium channels and mechanical contraction in rat cardiomyocytes. Cardiovasc Res 79: 632–641, 2008 [DOI] [PubMed] [Google Scholar]
- 72.Tateishi N, Higashi T, Naruse A Nakashima K, and Shiozaki H. Rat liver glutathione: possible role as a reservoir of cysteine. J Nutr 107: 51–60, 1977 [DOI] [PubMed] [Google Scholar]
- 73.Toohey JI. Sulfur signaling: is the agent sulfide or sulfane? Anal Biochem 413: 1–7, 2011 [DOI] [PubMed] [Google Scholar]
- 74.Ubuka T, Ohta J, Yao W, Abe T, Teraoka T, and Kurozumi Y. L-Cysteine metabolism via 3-mercaptopyruvate pathway and sulfate formation in rat liver mitochondria. Amino Acids 2: 143–155, 1992 [DOI] [PubMed] [Google Scholar]
- 75.Ubuka T, Umemura S, Yuasa S, Kinuta M, and Watanabe K. Purification and characterization of mitochondrial cysteine aminotransferase from rat liver. Physiol Chem Phys 10: 483–500, 1978 [PubMed] [Google Scholar]
- 76.Vitvitsky V, Kabil O, and Banerjee R. High turnover rates for hydrogen sulfide allow for rapid regulation of its tissue concentrations. Antioxid Redox Signal 17: 22–31, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Warenycia MW, Goodwin LR, Benishin CG, Reiffenstein RJ, Grancom DM, Taylor JD, and Dieken FP. Acute hydrogen sulfide poisoning. Demonstration of selective uptake of sulfide by the brainstem by measurement of brain sulfide levels. Biochem Pharmacol 38: 973–981, 1989 [DOI] [PubMed] [Google Scholar]
- 78.Whiteman M, Armstrong JS, Chu SH, Jia-Ling S, Wong BS, Hheung NS, Halliwell B, and Moore PK. The novel neuromodulator hydrogen sulfide: an endogenous peroxynitrite ‘scavenger’? J Neurochem 90: 765–768, 2004 [DOI] [PubMed] [Google Scholar]
- 79.Whiteman M, Cheung NS, Zhu YZ, Chu SH, Siau JL, Wong BS, Armstrong JS, and Moore PK. Hydrogen sulphide: a novel inhibitor of hypochlorous acid-mediated oxidative damage in the brain? Biochem Biophys Res Commun 326: 794–798, 2005 [DOI] [PubMed] [Google Scholar]
- 80.Yang W, Yang G, Jia X, Wu L, and Wang R. Activation of KATP channels by H2S in rat insulin-secreting cells and the underlying mechanisms. J Physiol 569: 519–531, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zanardo RC, Brancaleone V, Distrutti E, Fiorucci S, Cirino G, and Wallace JL. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. FASEB J 20: 2118–2120, 2006 [DOI] [PubMed] [Google Scholar]
- 82.Zhang R, Sun Y, Tsai H, Tang C, Jin H, and Du J. Hydrogen sulfide inhibits L-type calcium currents depending upon the protein sulfhydryl state in rat cardiomyocytes. PLoS One 7: e37073, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhao W, Zhang J, Lu Y, and Wang R. The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opener. EMBO J 20: 6008–6016, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zorec R, Araque A, Carmignoto G, Haydon PG, Verkhratsky A, and Parpura V. Astroglial excitability and gliotransmission: an appraisal of Ca2+ as a signaling route. ASN Neuro 4: 103–119 [DOI] [PMC free article] [PubMed] [Google Scholar]