Abstract
Herpes simplex virus-2 (HSV-2) infection increases HIV susceptibility. We previously established a rhesus macaque model of vaginal HSV-2 preexposure followed by cochallenge with HSV-2 and simian/human immunodeficiency virus-reverse transcriptase (SHIV-RT). Using this model, we showed that a gel containing the nonnucleoside reverse transcriptase inhibitor (NNRTI) MIV-150 in carrageenan (CG) reduced SHIV-RT infection. To evaluate the efficacy of new generation microbicides against both viruses, we first established dual infection after single vaginal cochallenge with SHIV-RT and HSV-2 in HSV-2-naive macaques. All animals (6/6) became HSV-2 infected, with 4/6 coinfected with SHIV-RT. In a control group cochallenged with SHIV-RT and UV-inactivated HSV-2, 2/4 became SHIV-RT infected, and none had detectable HSV-2. Low-level HSV-2-specific antibody and T cell responses were detected in some HSV-2-infected animals. To test a CG gel containing MIV-150 and zinc acetate (MZC), which provided naive animals full protection from SHIV-RT for at least 8 h, MZC (vs. CG) was applied daily for 14 days followed by cochallenge 8 h later. MZC prevented SHIV-RT infection (0/9 infected, p=0.04 vs. 3/6 in CG controls), but only reduced HSV-2 infection by 20% (6/9 infected vs. 5/6 in CG, p=0.6). In HSV-2-infected animals, none of the gel-treated animals seroconverted, and only the CG controls had measurable HSV-2-specific T cell responses. This study shows the promise of MZC to prevent immunodeficiency virus infection (even in the presence of HSV-2) and reduce HSV-2 infection after exposure to a high-dose inoculum. Additionally, it demonstrates the potential of a macaque coinfection model to evaluate broad-spectrum microbicides.
Introduction
There is growing consensus that transmission, host response, and treatment of human immunodeficiency virus (HIV) infection is complicated by coinfection with other pathogens.1–5 Herpes simplex virus-2 (HSV-2) is a particular challenge, as it establishes lifelong infection associated with considerable morbidity and is very common, with an estimated global disease burden of greater than 500 million infected.6 Since HSV-2-infected individuals have enhanced susceptibility to HIV infection even after lesion healing,7,8 this is an enormous pool of people at elevated risk of dual infection.9,10 Among the coinfected, HIV may worsen HSV-2 disease, as evidenced by increased HSV-2 shedding in coinfected individuals,11–13 and HSV-2 has been a major potentiator of the HIV pandemic.14
Using a macaque vaginal challenge model, we showed that a first-generation microbicide gel composed of the nonnucleoside reverse transcriptase inhibitor (NNRTI) MIV-150 in carrageenan (CG) partially reduced infection by simian/human immunodeficiency virus-reverse transcriptase (SHIV-RT), an SIV expressing the reverse transcriptase (RT) gene of HIV, in both HSV-2-naive15,16 and HSV-2-infected animals.17 A promising next-generation microbicide gel with added zinc acetate (ZA) (MZC) has improved efficacy against SHIV-RT.16,18 Additionally, inclusion of ZA significantly reduces HSV-2 infection in mice after vaginal and rectal challenge.19,20 Following these promising observations obtained with a prototype gel, we have since modified the MZC formulation for vaginal use (adjusting buffers and cosolvents) in order to advance it for clinical testing. Modified MZC provided macaques complete protection against vaginal SHIV-RT infection for up to 8 h and protected mice against human papillomavirus (HPV) pseudovirus and HSV-2 infection.21 In the present study, we set out to test modified MZC for its ability to protect naive macaques against vaginal SHIV-RT and HSV-2 cochallenge.
We demonstrated coinfection, sporadic HSV-2 shedding, and adaptive immune responses against both pathogens after vaginal cochallenge of naive animals. Using this model, MZC was completely protective against SHIV-RT infection for up to 8 h but only slightly reduced HSV-2 infection. No HSV-2-specific antibody (Ab) responses were detected in animals infected after treatment with either gel, and T cell responses were observed only in the CG-treated animals. These studies help establish the rhesus macaque model of HSV-2/SHIV-RT cochallenge for the evaluation of microbicides and demonstrate the potential of MZC to limit infection with immunodeficiency virus and HSV-2.
Materials and Methods
Virus stocks
SHIV-RT stocks were grown in phytohemagglutinin (PHA)-activated rhesus macaque peripheral blood mononuclear cells (PBMCs) in RPMI (Invitrogen, Carlsbad, CA) with 10% fetal bovine serum (FBS) (Invitrogen, Carlsbad, CA), and infectious titer was determined in 174XCEM cells by the Reed and Muench formula.22 HSV-2 strain G was obtained from ATCC and was expanded and titered on Vero cells grown in DMEM (Invitrogen, Carlsbad, CA) with 10% FBS (Invitrogen, Carlsbad, CA).23 To prepare UV-inactivated HSV-2, virus was incubated on ice for 6 h, 6–10 cm from a 365-nm UV light (UVP, Upland, CA), and inactivation was verified by the absence of plaques in the plaque assay.24
Microbicide gels
MZC (lot number 120120A1005MR) was composed of 50 μM MIV-150, 14 mM zinc acetate dihydrate, and 3.1% CG (60:40 lambda:kappa). CG gel (lot number 120111A525MR) contained 3.4% CG (60:40 lambda:kappa). Each gel was made in 10 mM sodium acetate buffer with 2% propylene glycol and 0.2% methyl paraben as a preservative.21
Animals and treatments
Female adult Chinese and Indian rhesus macaques (Macaca mulatta) were housed at the Tulane National Primate Research Center (TNPRC) in Covington, LA. All protocols were reviewed and approved by the Institutional Animal Care and Use Committee of TNPRC (OLAW Assurance A4499-01). All animal care procedures were compliant with the Animal Welfare Act and the Guide for the Care and Use of Laboratory Animals.25,26
To reduce the potential of preexisting antiherpetic immunity, only herpes B-negative animals were selected for this study. Animals were injected with 30 mg intramuscular Depo-Provera 5 weeks before atraumatic vaginal challenge with mixed inocula of 103 TCID50 SHIV-RT and 2×108 pfu of live HSV-2 or an equivalent amount of UV-inactivated virus in a total volume of 1 ml serum-free RPMI. Blood, lymph nodes (LNs), vaginal swabs, and cervical and vaginal pinch biopsies were collected at various time points and were shipped to the Center for Biomedical Research, Population Council, NY by overnight courier.27 Animals were anesthetized with tiletimine/zolazepam (8 mg/kg body weight), with buprenorophine (0.01 mg/kg body weight) for analgesia for all biopsy procedures.
For microbicide efficacy studies, uninfected animals were Depo-Provera treated 5 weeks prior to virus challenge. Two weeks before challenge, they began receiving daily vaginal applications of 2 ml MZC or CG, for a total of 14 gel applications. Eight hours after the final gel application, they were challenged atraumatically with a vaginal inoculum of 103 TCID50 SHIV-RT and 2×108 pfu HSV-2 mixed in a total volume of 1 ml serum-free RPMI.
Sample collection
Blood from challenged animals was collected in 7.5-ml EDTA Vacutainer tubes, and plasma and PBMCs were isolated as previously described.17,28 Axillary and inguinal LN mononuclear cells (LNMCs) were collected by passing cell suspensions through 70-μm sieves and washing with cold PBS. Cervical and vaginal tissues were obtained as described,27 cut into 3 mm×3 mm×3 mm pieces, and frozen at −80°C in RNA later until DNA extraction (Qiagen, Valencia, CA).27 Vaginal swabs were collected and shipped as previously described.27 The cells/fluid mixtures were resuspended, aliquotted, and frozen at −80°C until DNA extraction.
Detection of HSV-2 shedding
DNA was extracted from vaginal swabs using the QIAamp DNA blood mini kit (Valencia, CA), or from cervical or vaginal biopsies from the Qiagen DNeasy blood and tissue kit (Valencia, CA) as per the manufacturer's instructions. Nested polymerase chain reaction (nPCR) was carried out as previously described using Qiagen HotStarTaqPlus (Valencia, CA)17 to amplify a 121-bp DNA product within the gD gene of HSV-2. Six replicates per sample were amplified, which was determined to be required to amplify samples at the minimum threshold for detection. Using purified HSV-2 DNA (Advanced Biotechnologies, Columbia, MD), we showed that the nPCR strategy can detect 1.2 DNA copies in six PCR replicates (not shown). PCR products were visualized on 2% agarose gels, and were photographed on a Kodak Carestream Molecular Imager (New Haven, CT). To verify that extracted DNA was of amplifiable quality, a control GAPDH PCR, amplifying a 226-bp product, was run in parallel from the same sample (not shown).17
SHIV-RT plasma viral load
Plasma obtained from EDTA-treated whole blood was used as a source for the determination of SIV gag RNA by quantitative reverse transcriptase polymerase chain reaction (RT-PCR) assay.29 The lower limit for quantification was 30 RNA copies/ml plasma.
Intracellular cytokine staining (ICS)
Five-color flow cytometry-based ICS was used to quantify antigen-specific CD4+ and CD8+ T lymphocytes in blood and LNs. The protocol for ICS was as previously described, using UV-inactivated HSV-2 as an antigen stimulus and microvesicles (MV) from HSV-2-infected Vero cells as a background control.17 Following antigen stimulation, cells were stained with anti-CD4-PerCP-Cy5.5 and anti-CD3-Pacific Blue (both BD Biosciences, San Jose, CA). CD3+CD8+ lymphocytes were gated from the singlet lymphocyte CD3+CD4− population. Subsequently, cells were permeabilized and stained with anti-TNF-α-FITC (Biolegend, San Diego, CA), anti-IL-2-PE (Miltenyi Biotec, Auburn, CA), and anti-IFN-γ-PE-Cy7 (BD Biosciences, San Jose, CA). Fluorescence was detected on an LSRII cytometer (BD Biosciences, San Jose, CA) and analyzed by FlowJo software (Tree Star, Ashland, OR). The percentage of positive cells expressing specific cytokines was calculated by subtracting the percentage of positive cells after incubation with MV control from HSV-2-stimulated cells. For HSV-2-infected animals, polyreactive cells were calculated by adding the percentage of cells positive for two (double-reactive) or three (triple-reactive) cytokines.
SIV and HSV-2 Ab ELISA
SIV and HSV-2 Abs were measured in plasma. Either HSV-2 (500 ng protein/well) or SIV (100 ng protein/well) particles was lysed in 10% Triton X-100 for 1 h at room temperature (RT), then diluted in 8 mM sodium carbonate/17 mM sodium bicarbonate (pH 9.6) buffer. Plates were coated with virus lysate overnight at 4°C, followed by blocking with 0.25% gelatin in phosphate-buffered saline (PBS). After washing with ELISA plate wash buffer (Perkin Elmer, Boston, MA), diluted plasma was incubated in wells for 2 h at 37°C. Plates were washed and 100 μl/well 1:2,500 antihuman IgG peroxidase conjugate (Sigma, St. Louis, MO) was added for 2 h at 37°C. SureBlue peroxidase substrate (KPL, Gaithersburg, MD) was added after washing plates. Samples were read at 450 nm and were defined as positive when elevated 2-fold above absorbance detected in the baseline time point sample. Positive controls were from an HSV-2 antiserum panel (Focus Diagnostics, Cypress, CA) or from the plasma of an SIV-infected animal with known high Ab titers. Samples from baseline, week 4, and week 8, and from baseline, week 4, week 8, week 12, and week 24 were analyzed for SIV and for HSV Ab, respectively.
Statistics
Due to the small sample size, Fisher's exact test (GraphPad Prism 5.02, San Diego, CA) was used to compare the number of animals infected with SHIV-RT or HSV-2 in the differently treated groups. A p value less than 0.05 was defined as statistically significant. Two-way ANOVA (GraphPad Prism 5.02, San Diego, CA) was used to compare viral loads from SHIV-infected animals.
Results
SHIV-RT/HSV-2 cochallenge results in productive infection with both viruses
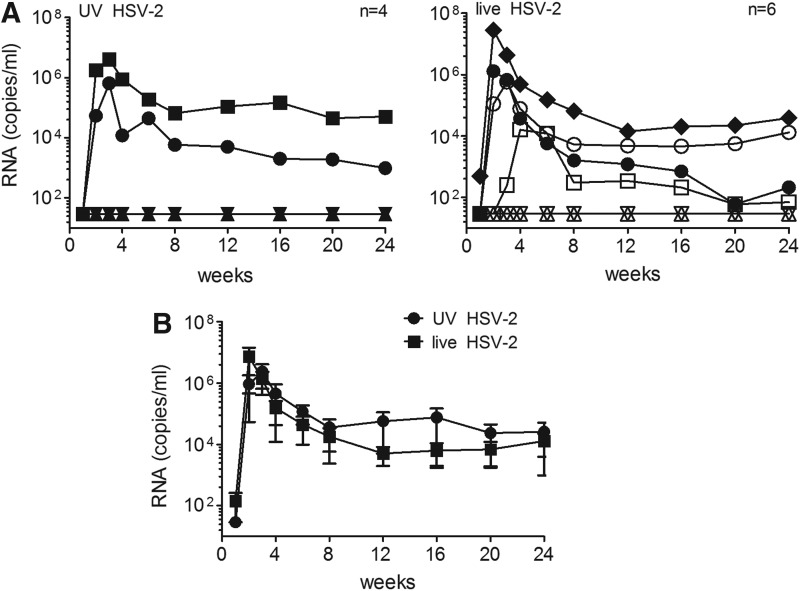
Previously, we showed that SHIV-RT/HSV-2 cochallenge of macaques after HSV-2 preexposure resulted in HSV-2 infection and an increased frequency of SHIV-RT infection.17 Here, we determined whether SHIV-RT and HSV-2 could infect animals when administered in one combined inoculum and if, under these conditions, the susceptibility to SHIV-RT infection was enhanced. A control arm of the study included an inoculum containing SHIV-RT with UV-inactivated HSV-2 to evaluate any ligand-induced effects of nonreplicating HSV-2. Figure 1A shows that 2/4 (50%) animals became infected with SHIV-RT when UV-HSV-2 was present in the inoculum, while with live HSV-2, 4/6 (67%) became SHIV-RT infected (p=1.0). Both rates of infection are within expectations for this SHIV-RT inoculum in HSV-2-naive animals.16,18,27,30 Thus, the presence of HSV-2 in the inoculum did not enhance SHIV-RT transmissibility in HSV-2-naive animals, which contrasts with the outcome after cochallenge of previously HSV-2-exposed animals.17 In each group, typical SHIV-RT viremia was observed, peaking during the first 4 weeks and resulting in postacute viral load levels consistent with prior experience with this virus (Fig. 1B). Peak viral loads between the groups did not significantly differ, as UV-HSV-2-challenged animals had a mean of 2.4×106±1.7×106 RNA copies/ml, while live HSV-2-challenged animals had 7.4×106±6.9×106 copies/ml in plasma (not significant). At 6 months postchallenge, the mean SHIV-RT viral load for live vs. UV-HSV-2 groups was 1.3×104±9.2×103 and 2.6×104±2.5×104 RNA copies/ml, respectively.
FIG. 1.
Simian human immunodeficiency virus-reverse transcriptase (SHIV-RT) productively infects animals cochallenged with SHIV-RT/herpes simplex virus-2 (HSV-2). (A) Viral load of SHIV-RT [copies of simian immunodeficiency virus (SIV) gag RNA per ml of plasma] in animals cochallenged with either UV-inactivated HSV-2 and SHIV-RT (UV-HSV-2) or live HSV-2 and SHIV-RT (live HSV-2). The number of animals in each group is indicated. (B) Mean (±SEM) SHIV-RT viral loads for SHIV-RT-infected animals after cochallenge with UV-HSV-2 or live HSV-2 and SHIV-RT.
HSV-2 shedding, in ∼20 swabs sampled between weeks 3 and 48 postchallenge, was detected in all six animals cochallenged with live HSV-2 and SHIV-RT. However, swabs in the four animals exposed to UV HSV-2 and SHIV-RT were consistently negative for HSV-2 DNA up to and including week 16, thus no further HSV-2 DNA sampling was carried out after this time point (Table 1). Samples were monitored from 3 weeks and thereafter to avoid false positives from inoculum HSV-2 DNA, which were detected in some animals over the first 2 weeks (5/6 exposed to live HSV-2 vs. 3/4 exposed to UV-HSV-2, not shown). HSV-2 DNA was detected over multiple time points in infected animals, the exception being IR54, who tested positive at only one time point (Table 1). Shedding was also sporadic, as animals whose swabs were positive on one day were frequently negative on successive days (not shown). To possibly stimulate HSV-2 shedding and enhance the probability of detecting all HSV-2-positive animals, we performed pinch biopsies of cervical and vaginal tissue at 8, 16, and 45 weeks postchallenge in animals challenged with SHIV-RT and live HSV-2 and collected swabs over the next 2 days. HSV-2 DNA was detected in the tissues of half of the animals, and in some instances, shedding was detected in swabs in the days following the biopsy (Table 1). Animals were examined at weeks 6, 12, and 21 for herpetic lesions, and none was observed. Among the six HSV-2+ animals, there was no difference in the mean percentage of HSV-2+ swabs between SHIV+ (12/82 swabs) or SHIV− (4/42 swabs) animals (p=0.57). Thus, SHIV infection did not enhance HSV-2 shedding.
Table 1.
Infection and Immune Status of Animals Cochallenged with Herpes Simplex Virus-2 and Simian Human Immunodeficiency Virus-Reverse Transcriptase
| HSV-2 | SHIV-RT | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Animal | Origin | HSV-2 | Number of weeks follow-up | HSV DNA in swabsa | HSV DNA in tissueb | Infection | Ab | Infection | Ab |
| IR18 | China | UV | 48 | 0/12 | ND | − | − | + | + |
| IR19 | China | UV | 48 | 0/9 | ND | − | − | + | + |
| IR20 | China | UV | 48 | 0/9 | ND | − | − | − | − |
| IR22 | China | UV | 48 | 0/12 | ND | − | − | − | − |
| IR23 | China | Live | 48 | 4/21 | 1/5 | + | + | + | + |
| IR24 | China | Live | 48 | 4/21 | 1/5 | + | + | + | + |
| IR54 | China | Live | 48 | 1/21 | 0/3 | + | + | − | − |
| IR55 | China | Live | 48 | 3/21 | 0/4 | + | − | − | − |
| IR56 | China | Live | 48 | 2/20 | 0/4 | + | − | + | + |
| IR57 | China | Live | 48 | 2/20 | 1/4 | + | − | + | + |
Number of positive samples/number of time points tested.
Number of positive tissue samples/total tissue samples.
HSV, herpes simplex virus; SHIV-RT, simian human immunodeficiency virus-reverse transcriptase; ND, not done.
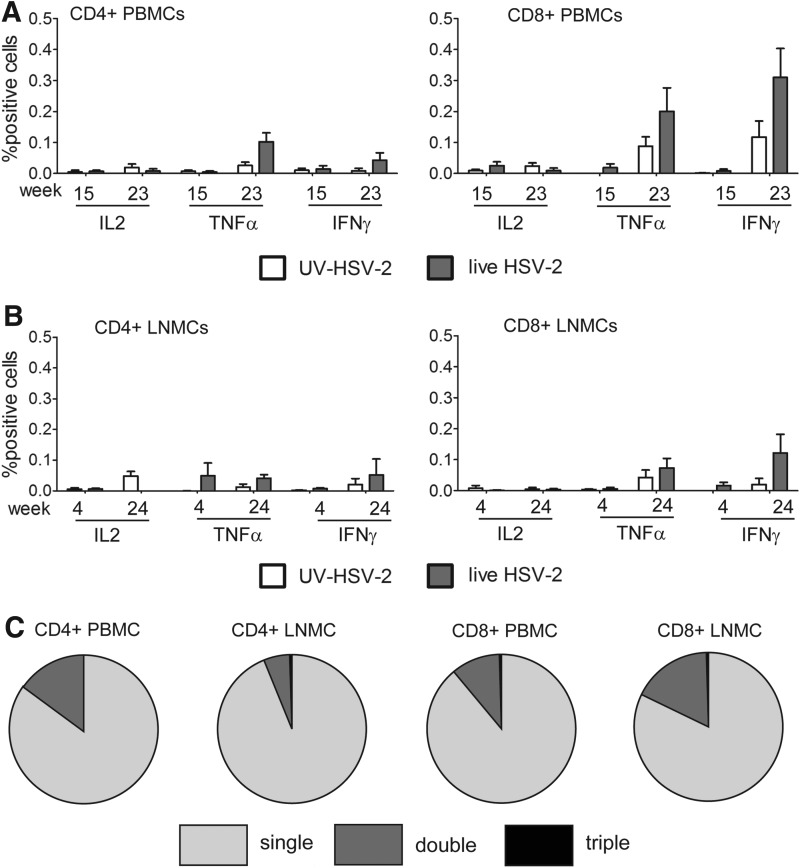
As observed previously,17 HSV-2-specific adaptive responses were detected in some HSV-2-infected animals. Low-level HSV-2-specific Ab responses were detected by ELISA in the plasma of the 3/6 HSV-2-infected animals, two of which were also coinfected with SHIV-RT (Table 1). Consistent with HSV-2 infection, HSV-2-specific tumor necrosis factor (TNF)-α-producing and interferon (IFN)-γ-producing CD8+ (and less so CD4+) T cells were detected in HSV-2-infected animals above the background levels detected in uninfected (UV HSV-2) animals (Fig. 2A and B). No interleukin (IL)-2 responses were detected. Responses were greater at later time points (week 23 and week 24, for PBMCs and LNMCs, respectively) than at earlier times. Most of the cytokine-producing cells in blood and LN produced only one factor, with a smaller fraction producing both TNF-α and IFN-γ (Fig. 2C). HSV-2-specific T cell responses were detected in all HSV-2-infected animals independent of their SHIV-RT infection status.
FIG. 2.
Detection of HSV-2-specific T cell responses in animals cochallenged with live HSV-2 and SHIV-RT. (A, B) Intracellular cytokine staining (ICS) of HSV-2-specific CD4+ or CD8+ T cells in peripheral blood mononuclear cells (PBMCs) (A) and lymph node mononuclear cells (LNMCs) (B) of animals cochallenged with UV HSV-2 (n=4) vs. live HSV-2 (n=6) and SHIV-RT. Shown are the means (±SEM) of the percent positive cells [calculated after subtracting background microvesicle (MV) control values from each] detected at the indicated weeks post cochallenge (weeks 15 and 23 for the PBMCs and weeks 4 and 24 for the LNMCs). (C) Polyreactive CD4+ and CD8+ lymphocytes from PBMCs or LNs relative to single cytokine-reactive cells.
MZC protection against SHIV-RT/HSV-2 cochallenge
We previously showed that prototype MZC is extremely effective at blocking vaginal SHIV-RT infection in rhesus macaques, providing significant protection for 8–24 h after repeated or single dosing.16,18 Advancing this gel for clinical testing, we modified the formulation (adjusting buffers and cosolvents) to obtain a safe, effective, and stable isoosmolar MZC formulation for human use and showed that it prevents vaginal and rectal SHIV-RT infection in macaques, as well as HPV pseudovirus and HSV-2 infection in mice.21 We set out to evaluate whether modified MZC remains effective against SHIV-RT in the cochallenge model described herein, and also whether it is active against HSV-2 in macaques.
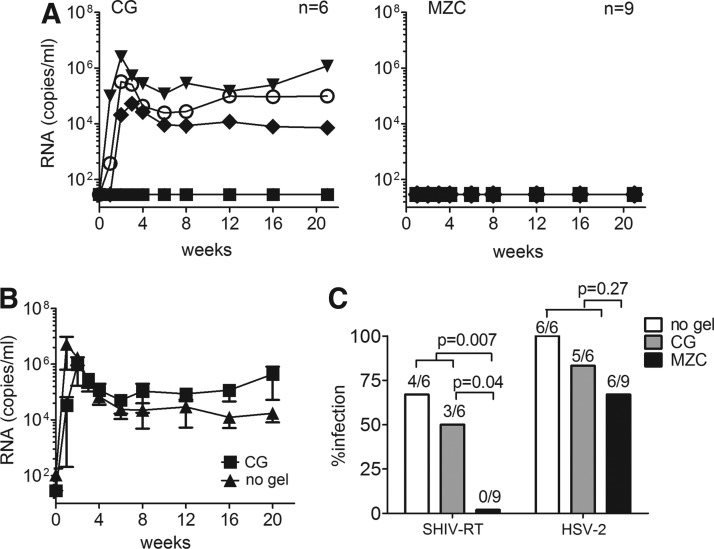
We used the regimen under which modified MZC was fully protective against SHIV-RT challenge.21 Macaques were Depo-Provera treated, then had MZC or CG placebo applied daily for 14 days. Eight hours after the final gel application, the animals were cochallenged with SHIV-RT and HSV-2. The virologic outcomes are summarized in Table 2 and Fig. 3. Much like SHIV-RT/HSV-2 cochallenge in the absence of gel and the single SHIV-RT challenge, half of the CG control animals (3/6) became infected with SHIV-RT (Fig. 3A). This is not significantly different from the 4/6 infected in the no gel controls (p=1.0) (Fig. 1A). SHIV-RT viral loads for CG-treated animals were comparable to those seen in the no gel SHIV-RT/HSV-2 cochallenged controls for all time points (p=0.5; Fig. 3B). In contrast, none of the nine animals treated with MZC became infected with SHIV-RT (p=0.04 vs. CG, and p=0.007 vs. the pooled no gel and CG controls, 7/12 infected) (Fig. 3A and C). Consistent with infection, all SHIV-RT-infected animals developed SIV-specific Ab responses, independent of their HSV-2 status (Table 2).
Table 2.
Infection and Immune Status of Animals Cochallenged After Microbicide Gel Application
| HSV-2 | SHIV-RT | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Animal | Origin | Gel | Number of weeks follow-up | HSV DNA in swabsa | HSV DNA in tissueb | Infection | Ab | Infection | Ab |
| BV57 | India | CG | 21 | 1/11 | 0/2 | + | − | − | − |
| DE32 | India | CG | 21 | 1/11 | 0/2 | + | − | − | − |
| DF20 | India | CG | 21 | 2/11 | 0/2 | + | − | − | − |
| FH48 | India | CG | 21 | 3/11 | 0/2 | + | − | + | + |
| GC25 | India | CG | 21 | 0/11 | 0/2 | − | − | + | + |
| IR45 | China | CG | 21 | 1/11 | 0/2 | + | − | + | + |
| CM08 | India | MZC | 21 | 3/11 | 0/2 | + | − | − | − |
| CM11 | India | MZC | 21 | 2/11 | 0/2 | + | − | − | − |
| CM72 | India | MZC | 21 | 0/11 | 0/2 | − | − | − | − |
| FH27 | India | MZC | 21 | 1/11 | 0/2 | + | − | − | − |
| GA18 | India | MZC | 21 | 1/11 | 0/2 | + | − | − | − |
| GC12 | India | MZC | 21 | 1/11 | 0/2 | + | − | − | − |
| GD92 | India | MZC | 21 | 0/11 | 0/2 | − | − | − | − |
| GM30 | India | MZC | 21 | 0/11 | 0/2 | − | − | − | − |
| IR50 | China | MZC | 21 | 4/11 | 0/2 | + | − | − | − |
Number of positive samples/number of time points tested.
Number of positive tissue samples/total tissue samples.
SHIV-RT infection was determined by SIV gag PCR on week 2 and 4 PBMC DNA, and by SIV RNA viral load detection.
FIG. 3.
Infection with SHIV-RT is blocked and HSV-2 reduced by MZC gel. (A) SHIV-RT viral loads of animals cochallenged with SHIV-RT/HSV-2 8 h after the last of 14 daily applications of either carrageenan (CG) control or MZC. The number of animals in each group is indicated. (B) Mean (±SEM) SHIV-RT viral loads of animals infected after application of CG (n=3) or no gel (n=4). (C) The percentage of animals infected by either SHIV-RT or HSV-2 after cochallenge without a gel or after application of CG or MZC. The numbers above each bar denote the number of infected animals over the total number cochallenged for each group. Fisher's exact test was performed to determine statistical significance.
We have shown that ZA/CG (ZC) gel is highly effective against high-dose vaginal HSV-2 challenge in mice.19,20 In the present experiments, 67% (6/9 infected) of the animals treated with MZC and 83% (5/6) of those in the CG control group shed HSV-2 DNA (Fig. 3C and Table 2) (p=0.6). While this was reduced relative to the 100% of animals that became infected in the no gel group (Table 1) it was not significantly decreased for either gel (p=1.0 for CG, p=0.23 for MZC). Additionally, the comparison of MZC vs. pooled no gel and CG groups did not achieve significance (6/9 infected vs. 11/12 infected, p=0.27). HSV-2 DNA was detected in ∼12% of all swabs analyzed in both the CG (8/66 total swabs) and MZC (12/99 total swabs) groups, indicating similar rates of virus shedding (Table 2). Cervical and vaginal biopsies, obtained at week 8, were all negative for HSV-2 DNA (Table 2). In one animal (IR50), HSV-2 DNA was detected in the vaginal swab sample at this time point but not in the tissues, suggesting that anatomic location of sampling may be important in detection of shedding.
Given the complete protection by MZC against SHIV-RT, the number of dually infected animals was significantly different for MZC (0/9 coinfected) compared to no gel controls (4/6 coinfected; p=0.011). Although reduced, the number of dually infected animals in the CG group (2/6) was not significantly different from the 4/6 coinfected in the no gel controls (p=0.57).
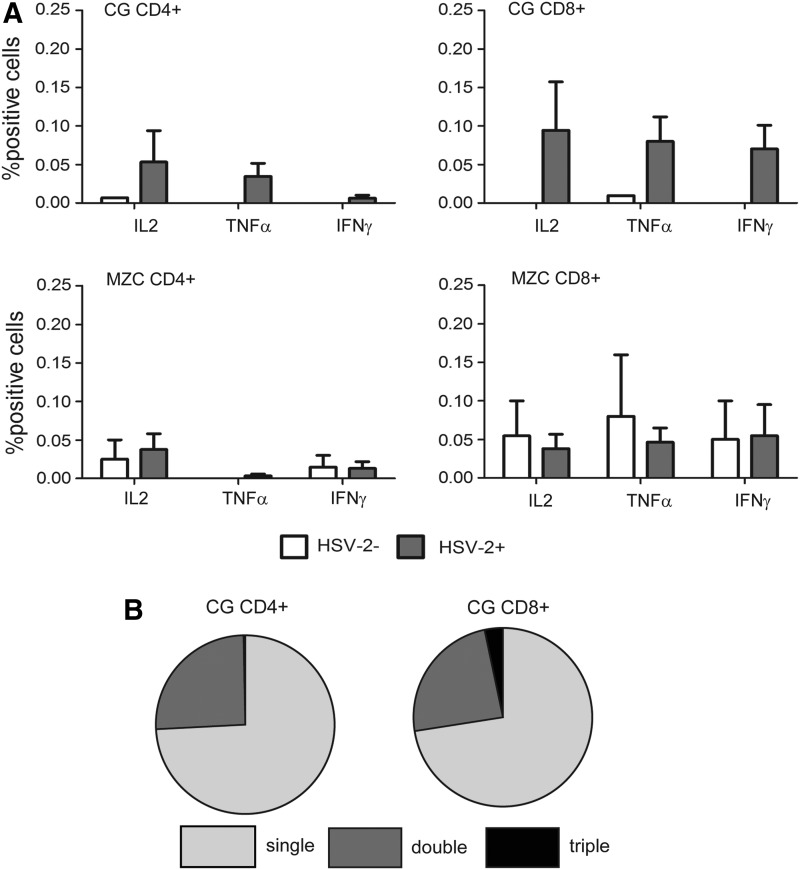
As expected, the absence of SHIV-RT infection coincided with the lack of SIV-specific Ab responses (Table 2). However, although we observed HSV-2 shedding in both MZC and CG-treated animals, HSV-2 Ab responses were not detected in any of the 11 HSV-2-infected, gel-treated animals at the time points measured. In HSV-2-infected animals, compared to the no gel control animals, lower level HSV-2-specific CD4+ and CD8+ T cell responses were detected after the application of CG, and were near background levels after MZC application (compare HSV-2-infected vs. -uninfected animals, Fig. 4). In CG-treated animals, the reactive cells were predominantly single cytokine-producing T cells. Taken together, these results show that animals treated with MZC were fully protected from SHIV-RT infection even in the face of only somewhat reduced HSV-2 infection.
FIG. 4.
Lower HSV-2-specific cellular response in animals infected after application of MZC compared to CG. (A) ICS staining of HSV-2-responsive CD4+ or CD8+ T cells in blood taken 23 weeks post-SHIV-RT/HSV-2 cochallenge from animals treated with MZC (n=6 HSV-2+ and n=2 HSV-2−) or CG (n=5 HSV-2+ and n=1 HSV-2−). Three animals were HSV-2− in the MZC group, but a blood sample from one was unavailable at this time point. (B) Polyreactive CD4+ and CD8+ lymphocytes relative to single cytokine-reactive cells in blood from CG-treated HSV-2+ animals.
Discussion
An animal model for both HIV and HSV-2 would help to define the parameters of cotransmission that result in enhanced susceptibility and to serve as a rational platform on which to evaluate broad spectrum microbicides.31 This study set out to show first that HSV-2-naive macaques could become infected by both SHIV-RT and HSV-2 after a single vaginal cochallenge before evaluating MZC for protection against both pathogens.
We found that a similar proportion of HSV-2-naive animals became infected with SHIV-RT after cochallenge with either HSV-2 (4/6 animals) or UV-inactivated HSV-2 (2/4 animals), and there was no significant difference in peak SHIV-RT viremia or chronic viral load. This suggests that while preexisting HSV-2 infection is associated with enhanced SHIV transmission,17 supporting clinical studies of enhanced HIV infection in HSV-2+ individuals,2,9,32 coexposure might have less impact on HIV acquisition.
Similar to what we have observed in previous studies with this virus,16–18,20,21,27,30 50–67% of control group animals became infected with SHIV-RT. Although this is only a moderate infection frequency, the inoculum is still 100–1,000 times greater than that present in human semen33–36 and thus represents a stringent test of microbicide efficacy. That the protection offered by MZC gel compared to CG (with only 3/6 infections) was statistically significant is a testament to the strength of the microbicide protection. We have now developed a repeated lower dose SHIV-RT/HSV-2 cochallenge model using non-Depo-Provera-treated animals (J. Kenney and M. Robbiani, unpublished), which more closely mimics real world HIV (and HSV-2) exposure and which will aid our evaluation of microbicide efficacy in macaques.
A potential caveat is the use of Rhesus macaques of Chinese origin (used in Crostarosa et al.17 and in the first study herein) vs. Indian origin (used in the second study herein). We have previously demonstrated that animals of both origins are similarly susceptible to SHIV-RT and experience identical viremia.16,18 In the context of HSV-2 exposure in the study reported here, we observed no significant differences in the SHIV infection frequency in Chinese (no gel, 4/6 infected) vs. Indian (CG-treated, 3/6 infected) macaques, and as stated above, both of these infection frequencies are within the expected range for this virus. These data suggest that the macaque origin did not alter the effect of HSV-2 exposure on SHIV infection. With regard to HSV-2 infection, 6/6 Chinese no gel macaques and 5/6 CG-treated Indian macaques became HSV-2 infected in this study, suggesting that there are not likely to be any substantial differences in susceptibility of the two macaque types to HSV-2 infection. Nevertheless, we need to study more animals before we draw conclusions about HSV-2 susceptibility or the impact of HSV-2 infection on SHIV susceptibility.
HSV-2 infected all six animals challenged with live HSV-2 and SHIV-RT. Similar to what we observed in our earlier study,17 HSV-2 shedding was episodic, and we did not observe herpetic lesions at any of the times examined. Intermittent virus shedding is consistent with human HSV-2 infection, particularly in the absence of symptoms.37–39 In contrast to human HSV-2/HIV coinfections,38 the presence of SHIV-RT infection did not enhance HSV-2 shedding in our infected animals. However, as the median duration of human HSV-2 shedding is estimated to be between 6 and 48 h,38,40,41 logistical constraints on obtaining more frequent vaginal swabs could have limited the detection of HSV-2 shedding and lesions.
Low-level B and T cell responses against HSV-2 were observed, in agreement with our previous study.17 Plasma HSV-2 Abs during acute infection were found in some but not all animals that shed HSV-2 DNA, which is consistent with human HSV-2 infection wherein seroconversion is not universal.42 In humans, cellular responses are likely to be important for virus control,40 potentially with polyfunctional T cells directed against multiple HSV-2 targets.43 Less is known in macaques, in which we previously showed predominantly production of TNF-α and IL-2 by CD4+ T cells after cochallenge of HSV-2-exposed animals.17 In the present study, we found mostly low HSV-2-specific responses by CD8+ lymphocytes (with few polyreactive cells) in blood and LNs of HSV-2-infected (but not UV-HSV-2-treated) macaques. It is possible that staining with HSV-2 peptide pools rather than inactivated HSV-2 particles would have been a more sensitive method for the detection of HSV-2-specific CD8+ T cell responses, although in nonhuman primates, reagents are limiting. Additionally, testing mucosal biopsies to determine whether HSV-2 infection led to recruitment of HSV-2-specific T cells would have been interesting, but was beyond the scope of this study. Notably, the SIV-specific Ab response appeared to be unaffected by HSV-2 infection.
Using this vaginal cochallenge model, we tested the efficacy of modified MZC. Just as with the prototype16,18 and modified21 MZC against a single SHIV-RT challenge, modified MZC fully protected macaques against SHIV-RT for up to 8 h, even in the presence of HSV-2 cochallenge. Although modified MZC significantly protects mice against HSV-2,21 it only partially reduced HSV-2 infection in the macaques after SHIV-RT/HSV-2 cochallenge. Testing of ZA-containing gels in the mouse revealed that protection was dependent on the HSV-2 inoculum dose and time at which the gel is applied relative to virus challenge.19–21 Specifically, against a high-dose inoculum of 106 pfu, the gel protected if applied 10 min prior to challenge, while for low-dose (5×103 pfu) vaginal HSV-2 challenge, the gel's greatest efficacy was when applied between 8 h before and 4 h after virus challenge.21 In the present study, the 2×108 pfu inoculum was potentially too high, especially since the last gel was applied 8 h before challenge. This challenge dose contains ∼2×108 copies of HSV-2 DNA (not shown), which is orders of magnitude greater than the observed 103.3–104.9 DNA copies/ml in anogenital swabs of HSV-2-infected humans37,38 and 1.58×102 copies of HSV DNA/ml of semen of HIV/HSV-2-coinfected men.44 Despite this, there was a trend to reduced HSV-2 infection. The repeated lower dose cochallenge model may more closely resemble human exposure to both viruses and provide more clinically relevant data on protection from HSV-2.
Although animals were infected by HSV-2 after treatment with CG or MZC, the HSV-2-specific adaptive responses overall appeared somewhat lower than for control animals infected in the absence of gel. This was most apparent in the MZC-treated animals, where the HSV-2-specific responses were comparable in the HSV-2-infected vs. uninfected animals. Since HSV-2 shedding was sporadic, it is possible that at least some of the animals that were scored as uninfected (within the timeframe tested) were actually infected and therefore had HSV-2-specific T cell responses. Shedding intermittency combined with the lack of a quantitative measure of HSV-2 infection levels did not allow us to determine whether a lower-level infection and/or frequency of shedding in the MZC-treated (and possibly less so in CG-treated) animals limited the magnitude of the adaptive responses induced compared to that in the no gel controls. Measuring immune responses at additional time points after infection might further illuminate the postinfection host response after gel treatment.
We have shown that single coexposure to SHIV-RT and HSV-2 results in dual infection, and that modified MZC still exerts significant protection against immunodeficiency virus infection in the face of HSV-2 exposure, while partially reducing coincident HSV-2 infection. These results highlight the promise for further development of MZC as a microbicide. Clearly, further studies are needed to determine the optimal timing of MZC application as well as HSV-2 dose to be efficacious against both pathogens.
Acknowledgments
The assistance of Catherine Rapelje and the Population Council's Cell Biology and Flow Cytometry Facility and Kevin Roberts for statistical support is gratefully acknowledged. The authors thank the Veterinary and Comparative Medicine staff of TNPRC for their continued support. This work was funded with the support of the United States Agency for International Development (USAID) Cooperative Agreement GPO-A-00-04-00019-00, the NIH base grant RR00164, and with federal funds from the National Cancer Institute, NIH, under contract HHSN261200800001E. This research is made possible by the generous support of the American people through the USAID. The contents of this article are the sole responsibility of the Population Council and do not necessarily reflect the views or policies of USAID or of the U.S. government. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. None of the material in this article has been published or is under consideration elsewhere, including the Internet. Melissa Robbiani is a 2002 Elizabeth Glaser Scientist.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Operskalski EA. and Kovacs A: HIV/HCV co-infection: Pathogenesis, clinical complications, treatment, and new therapeutic technologies. Curr HIV/AIDS Rep 2011;8(1):12–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnabas RV, Webb EL, Weiss HA, and Wasserheit JN: The role of co-infections in HIV epidemic trajectory and positive prevention: A systematic review and meta-analysis. AIDS 2011;25(13):1559–1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalichman SC, Pellowski J, and Turner C: Prevalence of sexually transmitted co-infections in people living with HIV/AIDS: Systematic review with implications for using HIV treatments for prevention. Sex Transm Infect 2011;87(3):183–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Modjarrad K. and Vermund SH: Effect of treating co-infections on HIV-1 viral load: A systematic review. Lancet Infect Dis 2010;10(7):455–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Houlihan CF, Larke NL, Watson-Jones D, et al.: Human papillomavirus infection and increased risk of HIV acquisition. A systematic review and meta-analysis. AIDS 2012;26(17):2211–2222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Looker KJ, Garnett GP, and Schmid GP: An estimate of the global prevalence and incidence of herpes simplex virus type 2 infection. Bull World Health Organ 2008;86(10):805–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu J, Hladik F, Woodward A, et al.: Persistence of HIV-1 receptor-positive cells after HSV-2 reactivation is a potential mechanism for increased HIV-1 acquisition. Nat Med 2009;15(8):886–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corey L, Wald A, Celum CL, and Quinn TC: The effects of herpes simplex virus-2 on HIV-1 acquisition and transmission: A review of two overlapping epidemics. J Acquir Immune Defic Syndr 2004;35(5):435–445 [DOI] [PubMed] [Google Scholar]
- 9.Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, and Hayes RJ: Herpes simplex virus 2 infection increases HIV acquisition in men and women: Systematic review and meta-analysis of longitudinal studies. AIDS 2006;20(1):73–83 [DOI] [PubMed] [Google Scholar]
- 10.Glynn JR, Biraro S, and Weiss HA: Herpes simplex virus type 2: A key role in HIV incidence. AIDS 2009;23(12):1595–1598 [DOI] [PubMed] [Google Scholar]
- 11.Nagot N, Ouedraogo A, Konate I, et al.: Roles of clinical and subclinical reactivated herpes simplex virus type 2 infection and human immunodeficiency virus type 1 (HIV-1)-induced immunosuppression on genital and plasma HIV-1 levels. J Infect Dis 2008;198(2):241–249 [DOI] [PubMed] [Google Scholar]
- 12.Van de Perre P, Segondy M, Foulongne V, et al.: Herpes simplex virus and HIV-1: Deciphering viral synergy. Lancet Infect Dis 2008;8(8):490–497 [DOI] [PubMed] [Google Scholar]
- 13.Tanton C, Weiss HA, LeGoff J, et al.: Patterns of herpes simplex virus shedding over 1 month and the impact of acyclovir and HIV in HSV-2-seropositive women in Tanzania. Sex Transm Infect 2011;87(5):406–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abu-Raddad LJ, Magaret AS, Celum C, et al.: Genital herpes has played a more important role than any other sexually transmitted infection in driving HIV prevalence in Africa. PLoS One 2008;3(5):e2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turville SG, Aravantinou M, Miller T, et al.: Efficacy of Carraguard-based microbicides in vivo despite variable in vitro activity. PLoS One 2008;3(9):e3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kenney J, Aravantinou M, Singer R, et al.: An antiretroviral/zinc combination gel provides 24 hours of complete protection against vaginal SHIV infection in macaques. PLos One 2011;6(1):e15835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crostarosa F, Aravantinou M, Akpogheneta OJ, et al.: A macaque model to study vaginal HSV-2/immunodeficiency virus co-infection and the impact of HSV-2 on microbicide efficacy. PLoS One 2009;4(11):e8060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kenney J, Singer R, Derby N, et al.: A single dose of a MIV-150/zinc acetate gel provides 24 h of protection against vaginal simian human immunodeficiency virus reverse transcriptase infection, with more limited protection rectally 8–24 h after gel use. AIDS Res Hum Retroviruses 2012;28(11):1476–1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fernandez-Romero JA, Abraham CJ, Rodriguez A, et al.: Zinc acetate/carrageenan gels exhibit potent activity in vivo against high-dose herpes simplex virus 2 vaginal and rectal challenge. Antimicrob Agents Chemother 2012;56(1):358–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kenney J, Rodriguez A, Kizima L, et al.: A promising non-ARV microbicide—an optimized zinc acetate gel is safe and effective against SHIV-RT and HSV-2 infection in vivo. Antimicrobial Agents and Chemotherapy. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kizma L, Rodniguez A, Kenney J, et al.: A potent combination microbicide gel that is effective against SHIV-RT, HSV-2, and HPV infection in vivo. Submitted.
- 22.Singer R, Derby N, Rodriguez A, et al.: The nonnucleoside reverse transcriptase inhibitor MIV-150 in carrageenan gel prevents rectal transmission of simian/human immunodeficiency virus infection in macaques. J Virol 2011;85(11):5504–5512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aurelian L: Herpes simplex virus. In: Clinical Virology Manual, 3rd ed. (Specter SHR, Young SA, eds.). ASM Press, Washington, DC, 2000, pp. 384–409 [Google Scholar]
- 24.Ashley RL: Herpes simplex viruses. In: Diagnostic Procedures for Viral, Rickettsial, and Chlamydial Infections, 7 ed. (Schmidt NJ, Emmons RW, eds.). American Public Health Association, Washington, DC, 1995, pp. 375–395 [Google Scholar]
- 25.Animal Welfare Act and Regulation of 2001: In: Code of Federal Regulations, Chapter 1, Subchapter A: Animals and Animal Products. U.S. Department of Agriculture, Beltsville, MD, 2001 [Google Scholar]
- 26.Committee on Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources Guide for the Care and Use of Laboratory Animals: Vol Publication no 85-23. U.S. Department of Health and Human Services, National Institutes of Health, Bethesda, MD, 1985, pp. 1–83 [Google Scholar]
- 27.Singer R, Mawson P, Derby N, et al.: An intravaginal ring that releases the NNRTI MIV-150 reduces SHIV transmission in macaques. Sci Transl Med 2012;4(150):150ra123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frank I, Piatak M, Jr, Stoessel H, et al.: Infectious and whole inactivated simian immunodeficiency viruses interact similarly with primate dendritic cells (DCs): Differential intracellular fate of virions in mature and immature DCs. J. Virol 2002;76(6):2936–2951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cline AN, Bess JW, Piatak M, Jr, and Lifson JD: Highly sensitive SIV plasma viral load assay: Practical considerations, realistic performance expectations, and application to reverse engineering of vaccines for AIDS. J Med Primatol 2005;34(5–6):303–312 [DOI] [PubMed] [Google Scholar]
- 30.Aravantinou M, Singer R, Derby N, et al.: The nonnucleoside reverse transcription inhibitor MIV-160 delivered from an intravaginal ring, but not from a carrageenan gel, protects against simian/human immunodeficiency virus-RT Infection. AIDS Res Hum Retroviruses 2012;28(11):1467–1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Veazey RS: Microbicide safety/efficacy studies in animals—macaques and small animal models. Curr Opin HIV AIDS 2008;3(5):567–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hughes JP, Baeten JM, Lingappa JR, et al.: Determinants of per-coital-act HIV-1 infectivity among African HIV-1-serodiscordant couples. J Infect Dis 2012;205(3):358–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gupta P, Mellors J, Kingsley L, et al.: High viral load in semen of human immunodeficiency virus type 1-infected men at all stages of disease and its reduction by therapy with protease and nonnucleoside reverse transcriptase inhibitors. J Virol 1997;71(8):6271–6275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Halfon P, Giorgetti C, Khiri H, et al.: Semen may harbor HIV despite effective HAART: Another piece in the puzzle. PLoS One 2010;5(5):e10569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pasquier C, Saune K, Raymond S, et al.: Determining seminal plasma human immunodeficiency virus type 1 load in the context of efficient highly active antiretroviral therapy. J Clin Microbiol 2009;47(9):2883–2887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Butler DM, Delport W, Kosakovsky Pond SL, et al.: The origins of sexually transmitted HIV among men who have sex with men. Sci Transl Med 2010;2(18):18re11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tronstein E, Johnston C, Huang ML, et al.: Genital shedding of herpes simplex virus among symptomatic and asymptomatic persons with HSV-2 infection. JAMA 2011;305(14):1441–1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mark KE, Wald A, Magaret AS, et al.: Rapidly cleared episodes of oral and anogenital herpes simplex virus shedding in HIV-infected adults. J Acquir Immune Defic Syndr 2010;54(5):482–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sacks SL, Griffiths PD, Corey L, et al.: Introduction: Is viral shedding a surrogate marker for transmission of genital herpes? Antiviral Res 2004;63(Suppl 1):S3–9 [DOI] [PubMed] [Google Scholar]
- 40.Zhu J, Koelle DM, Cao J, et al.: Virus-specific CD8+T cells accumulate near sensory nerve endings in genital skin during subclinical HSV-2 reactivation. J Exp Med 2007;204(3):595–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sacks SL, Griffiths PD, Corey L, et al.: HSV shedding. Antiviral Res 2004;63(Suppl 1):S19–26 [DOI] [PubMed] [Google Scholar]
- 42.Ashley-Morrow R, Krantz E, and Wald A: Time course of seroconversion by HerpeSelect ELISA after acquisition of genital herpes simplex virus type 1 (HSV-1) or HSV-2. Sex Transm Dis 2003;30(4):310–314 [DOI] [PubMed] [Google Scholar]
- 43.Ouwendijk WJ, Laing KJ, Verjans GM, and Koelle DM: T-cell immunity to human alphaherpesviruses. Curr Opin Virol 2013;3(4):452–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gianella S, Strain MC, Rought SE, et al.: Associations between virologic and immunologic dynamics in blood and in the male genital tract. J Virol 2012;86(3):1307–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]