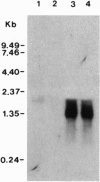
Abstract
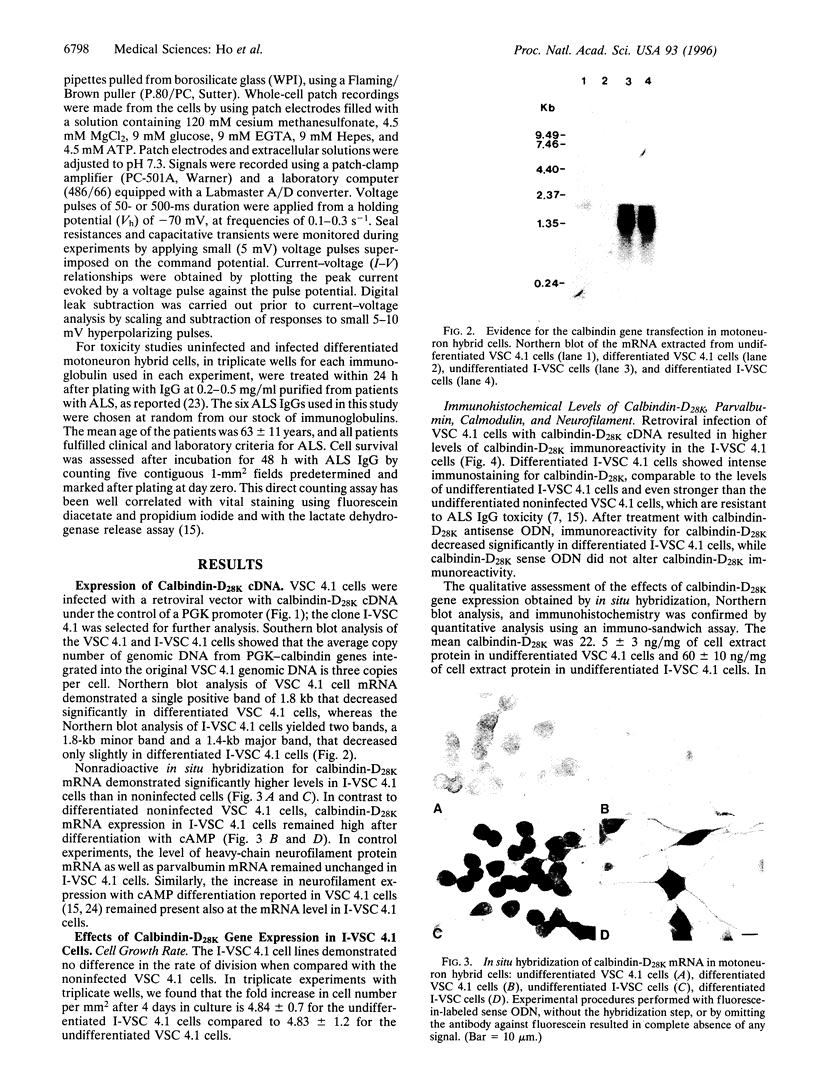
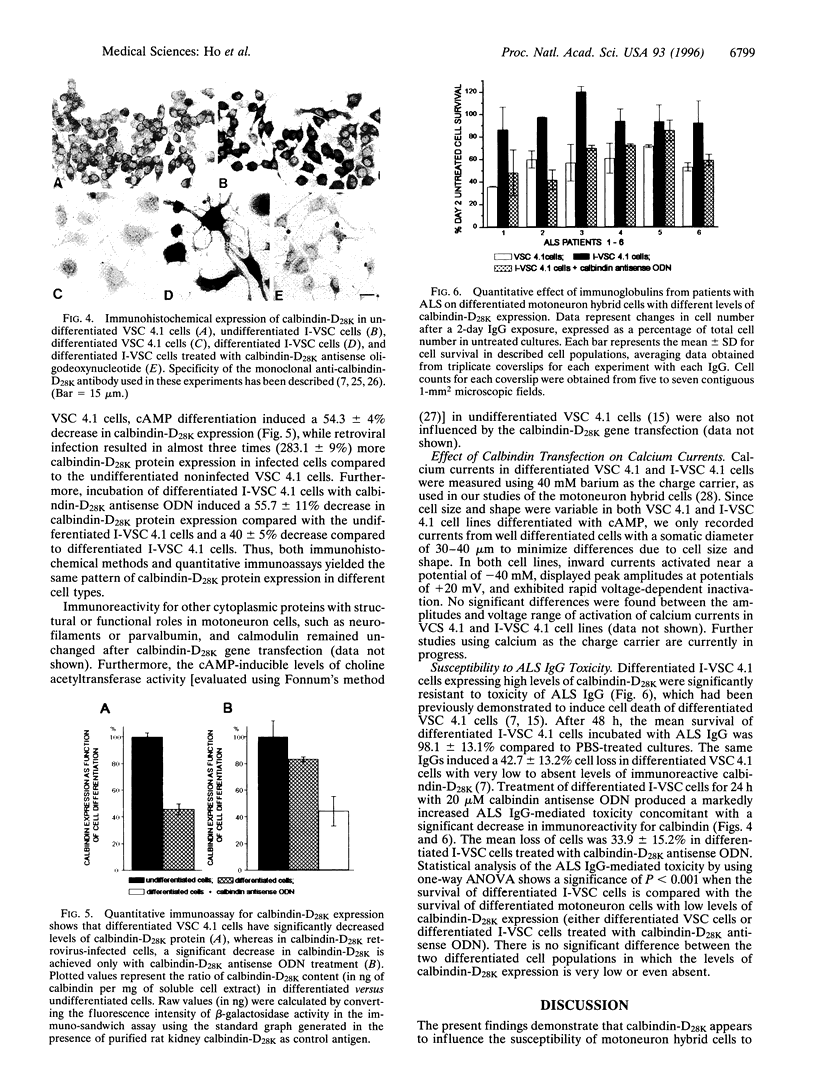
Calbindin-D28K and/or parvalbumin appear to influence the selective vulnerability of motoneurons in amyotrophic lateral sclerosis (ALS). Their immunoreactivity is undetectable in motoneurons readily damaged in human ALS, and in differentiated motoneuron hybrid cells [ventral spinal cord (VSC 4.1 cells)] that undergo calcium-dependent apoptotic cell death in the presence of ALS immunoglobulins. To provide additional evidence for the role of calcium-binding proteins in motoneuron vulnerability, VSC 4.1 cells were infected with a retrovirus carrying calbindin-D28K cDNA under the control of the promoter of the phosphoglycerate kinase gene. Differentiated calbindin-D28K cDNA-infected cells expressed high calbindin-D28K and demonstrated increased resistance to ALS IgG-mediated toxicity. Treatment with calbindin-D28K antisense oligodeoxynucleotides, which significantly decreased calbindin-D28K expression, rendered these cells vulnerable again to ALS IgG toxicity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexianu M. E., Ho B. K., Mohamed A. H., La Bella V., Smith R. G., Appel S. H. The role of calcium-binding proteins in selective motoneuron vulnerability in amyotrophic lateral sclerosis. Ann Neurol. 1994 Dec;36(6):846–858. doi: 10.1002/ana.410360608. [DOI] [PubMed] [Google Scholar]
- Alexianu M. E., Mohamed A. H., Smith R. G., Colom L. V., Appel S. H. Apoptotic cell death of a hybrid motoneuron cell line induced by immunoglobulins from patients with amyotrophic lateral sclerosis. J Neurochem. 1994 Dec;63(6):2365–2368. doi: 10.1046/j.1471-4159.1994.63062365.x. [DOI] [PubMed] [Google Scholar]
- Appel S. H., Smith R. G., Alexianu M., Engelhardt J., Mosier D., Colom L., Stefani E. Neurodegenerative disease: autoimmunity involving calcium channels. Ann N Y Acad Sci. 1994 Dec 15;747:183–194. doi: 10.1111/j.1749-6632.1994.tb44409.x. [DOI] [PubMed] [Google Scholar]
- Barr P. J., Tomei L. D. Apoptosis and its role in human disease. Biotechnology (N Y) 1994 May;12(5):487–493. doi: 10.1038/nbt0594-487. [DOI] [PubMed] [Google Scholar]
- Caceres A., Kosik K. S. Inhibition of neurite polarity by tau antisense oligonucleotides in primary cerebellar neurons. Nature. 1990 Feb 1;343(6257):461–463. doi: 10.1038/343461a0. [DOI] [PubMed] [Google Scholar]
- Caceres A., Mautino J., Kosik K. S. Suppression of MAP2 in cultured cerebellar macroneurons inhibits minor neurite formation. Neuron. 1992 Oct;9(4):607–618. doi: 10.1016/0896-6273(92)90025-9. [DOI] [PubMed] [Google Scholar]
- Celio M. R., Baier W., Schärer L., Gregersen H. J., de Viragh P. A., Norman A. W. Monoclonal antibodies directed against the calcium binding protein Calbindin D-28k. Cell Calcium. 1990 Oct;11(9):599–602. doi: 10.1016/0143-4160(90)90014-l. [DOI] [PubMed] [Google Scholar]
- Celio M. R., Baier W., Schärer L., de Viragh P. A., Gerday C. Monoclonal antibodies directed against the calcium binding protein parvalbumin. Cell Calcium. 1988 Apr;9(2):81–86. doi: 10.1016/0143-4160(88)90027-9. [DOI] [PubMed] [Google Scholar]
- Chard P. S., Bleakman D., Christakos S., Fullmer C. S., Miller R. J. Calcium buffering properties of calbindin D28k and parvalbumin in rat sensory neurones. J Physiol. 1993 Dec;472:341–357. doi: 10.1113/jphysiol.1993.sp019950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham D. E. Calcium signaling. Cell. 1995 Jan 27;80(2):259–268. doi: 10.1016/0092-8674(95)90408-5. [DOI] [PubMed] [Google Scholar]
- Collard J. F., Côté F., Julien J. P. Defective axonal transport in a transgenic mouse model of amyotrophic lateral sclerosis. Nature. 1995 May 4;375(6526):61–64. doi: 10.1038/375061a0. [DOI] [PubMed] [Google Scholar]
- Deng H. X., Hentati A., Tainer J. A., Iqbal Z., Cayabyab A., Hung W. Y., Getzoff E. D., Hu P., Herzfeldt B., Roos R. P. Amyotrophic lateral sclerosis and structural defects in Cu,Zn superoxide dismutase. Science. 1993 Aug 20;261(5124):1047–1051. doi: 10.1126/science.8351519. [DOI] [PubMed] [Google Scholar]
- Dowd D. R., MacDonald P. N., Komm B. S., Haussler M. R., Miesfeld R. L. Stable expression of the calbindin-D28K complementary DNA interferes with the apoptotic pathway in lymphocytes. Mol Endocrinol. 1992 Nov;6(11):1843–1848. doi: 10.1210/mend.6.11.1336124. [DOI] [PubMed] [Google Scholar]
- Engelhardt J. I., Siklós L., Kömüves L., Smith R. G., Appel S. H. Antibodies to calcium channels from ALS patients passively transferred to mice selectively increase intracellular calcium and induce ultrastructural changes in motoneurons. Synapse. 1995 Jul;20(3):185–199. doi: 10.1002/syn.890200302. [DOI] [PubMed] [Google Scholar]
- Epstein P., Means A. R., Berchtold M. W. Isolation of a rat parvalbumin gene and full length cDNA. J Biol Chem. 1986 May 5;261(13):5886–5891. [PubMed] [Google Scholar]
- Ferreira A., Kosik K. S., Greengard P., Han H. Q. Aberrant neurites and synaptic vesicle protein deficiency in synapsin II-depleted neurons. Science. 1994 May 13;264(5161):977–979. doi: 10.1126/science.8178158. [DOI] [PubMed] [Google Scholar]
- Fonnum F. A rapid radiochemical method for the determination of choline acetyltransferase. J Neurochem. 1975 Feb;24(2):407–409. doi: 10.1111/j.1471-4159.1975.tb11895.x. [DOI] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Guitteny A. F., Fouque B., Mougin C., Teoule R., Bloch B. Histological detection of messenger RNAs with biotinylated synthetic oligonucleotide probes. J Histochem Cytochem. 1988 Jun;36(6):563–571. doi: 10.1177/36.6.3259249. [DOI] [PubMed] [Google Scholar]
- Hillman D., Chen S., Aung T. T., Cherksey B., Sugimori M., Llinás R. R. Localization of P-type calcium channels in the central nervous system. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7076–7080. doi: 10.1073/pnas.88.16.7076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunziker W., Schrickel S. Rat brain calbindin D28: six domain structure and extensive amino acid homology with chicken calbindin D28. Mol Endocrinol. 1988 May;2(5):465–473. doi: 10.1210/mend-2-5-465. [DOI] [PubMed] [Google Scholar]
- Ince P., Stout N., Shaw P., Slade J., Hunziker W., Heizmann C. W., Baimbridge K. G. Parvalbumin and calbindin D-28k in the human motor system and in motor neuron disease. Neuropathol Appl Neurobiol. 1993 Aug;19(4):291–299. doi: 10.1111/j.1365-2990.1993.tb00443.x. [DOI] [PubMed] [Google Scholar]
- Julien J. P., Meyer D., Flavell D., Hurst J., Grosveld F. Cloning and developmental expression of the murine neurofilament gene family. Brain Res. 1986 Dec;387(3):243–250. doi: 10.1016/0169-328x(86)90030-6. [DOI] [PubMed] [Google Scholar]
- Kato K., Hamaguchi Y., Okawa S., Ishikawa E., Kobayashi K., Katunuma N. Use of rabbit antibody IgG-loaded silicone pieces for the sandwich enzymoimmunoassay of macromolecular antigens. J Biochem. 1977 May;81(5):1557–1566. [PubMed] [Google Scholar]
- Lledo P. M., Somasundaram B., Morton A. J., Emson P. C., Mason W. T. Stable transfection of calbindin-D28k into the GH3 cell line alters calcium currents and intracellular calcium homeostasis. Neuron. 1992 Nov;9(5):943–954. doi: 10.1016/0896-6273(92)90246-a. [DOI] [PubMed] [Google Scholar]
- Llinás R., Sugimori M., Cherksey B. D., Smith R. G., Delbono O., Stefani E., Appel S. IgG from amyotrophic lateral sclerosis patients increases current through P-type calcium channels in mammalian cerebellar Purkinje cells and in isolated channel protein in lipid bilayer. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11743–11747. doi: 10.1073/pnas.90.24.11743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masu Y., Wolf E., Holtmann B., Sendtner M., Brem G., Thoenen H. Disruption of the CNTF gene results in motor neuron degeneration. Nature. 1993 Sep 2;365(6441):27–32. doi: 10.1038/365027a0. [DOI] [PubMed] [Google Scholar]
- Mattson M. P., Rychlik B., Chu C., Christakos S. Evidence for calcium-reducing and excito-protective roles for the calcium-binding protein calbindin-D28k in cultured hippocampal neurons. Neuron. 1991 Jan;6(1):41–51. doi: 10.1016/0896-6273(91)90120-o. [DOI] [PubMed] [Google Scholar]
- Mosier D. R., Baldelli P., Delbono O., Smith R. G., Alexianu M. E., Appel S. H., Stefani E. Amyotrophic lateral sclerosis immunoglobulins increase Ca2+ currents in a motoneuron cell line. Ann Neurol. 1995 Jan;37(1):102–109. doi: 10.1002/ana.410370119. [DOI] [PubMed] [Google Scholar]
- Normand E., Bloch B. Simultaneous detection of two messenger RNAs in the central nervous system: a simple two-step in situ hybridization procedure using a combination of radioactive and non-radioactive probes. J Histochem Cytochem. 1991 Nov;39(11):1575–1578. doi: 10.1177/39.11.1918932. [DOI] [PubMed] [Google Scholar]
- Reisner P. D., Christakos S., Vanaman T. C. In vitro enzyme activation with calbindin-D28k, the vitamin D-dependent 28 kDa calcium binding protein. FEBS Lett. 1992 Feb 3;297(1-2):127–131. doi: 10.1016/0014-5793(92)80342-e. [DOI] [PubMed] [Google Scholar]
- Rosen D. R., Siddique T., Patterson D., Figlewicz D. A., Sapp P., Hentati A., Donaldson D., Goto J., O'Regan J. P., Deng H. X. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993 Mar 4;362(6415):59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- Rothstein J. D., Jin L., Dykes-Hoberg M., Kuncl R. W. Chronic inhibition of glutamate uptake produces a model of slow neurotoxicity. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6591–6595. doi: 10.1073/pnas.90.14.6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein J. D., Martin L. J., Kuncl R. W. Decreased glutamate transport by the brain and spinal cord in amyotrophic lateral sclerosis. N Engl J Med. 1992 May 28;326(22):1464–1468. doi: 10.1056/NEJM199205283262204. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. G., Alexianu M. E., Crawford G., Nyormoi O., Stefani E., Appel S. H. Cytotoxicity of immunoglobulins from amyotrophic lateral sclerosis patients on a hybrid motoneuron cell line. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):3393–3397. doi: 10.1073/pnas.91.8.3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. G., Hamilton S., Hofmann F., Schneider T., Nastainczyk W., Birnbaumer L., Stefani E., Appel S. H. Serum antibodies to L-type calcium channels in patients with amyotrophic lateral sclerosis. N Engl J Med. 1992 Dec 10;327(24):1721–1728. doi: 10.1056/NEJM199212103272405. [DOI] [PubMed] [Google Scholar]
- Wasserman R. H., Chandler J. S., Meyer S. A., Smith C. A., Brindak M. E., Fullmer C. S., Penniston J. T., Kumar R. Intestinal calcium transport and calcium extrusion processes at the basolateral membrane. J Nutr. 1992 Mar;122(3 Suppl):662–671. doi: 10.1093/jn/122.suppl_3.662. [DOI] [PubMed] [Google Scholar]
- Yoshitake S., Imagawa M., Ishikawa E., Niitsu Y., Urushizaki I., Nishiura M., Kanazawa R., Kurosaki H., Tachibana S., Nakazawa N. Mild and efficient conjugation of rabbit Fab' and horseradish peroxidase using a maleimide compound and its use for enzyme immunoassay. J Biochem. 1982 Nov;92(5):1413–1424. doi: 10.1093/oxfordjournals.jbchem.a134065. [DOI] [PubMed] [Google Scholar]
- Zhu Y. Y., Takashi M., Miyake K., Kato K. Sensitive enzyme immunoassay for human 28 kDa calbindin-D. Clin Chim Acta. 1991 Sep 30;201(3):183–192. doi: 10.1016/0009-8981(91)90369-n. [DOI] [PubMed] [Google Scholar]