Abstract
Colistin, administered intravenously as its inactive prodrug colistin methanesulfonate (CMS), is increasingly used as last-line therapy to combat multidrug-resistant Gram-negative bacteria. CMS dosing needs to be adjusted for renal function. The impact of continuous ambulatory peritoneal dialysis (CAPD) on the pharmacokinetics of both CMS and colistin has not been studied. No CMS dosing recommendations are available for patients receiving CAPD. Eight CAPD patients received a single intravenous CMS dose (150 mg colistin base activity [CBA]) over 30 min. Serial blood and dialysate samples, and cumulative urine where applicable, were collected over 25 h. CMS and colistin concentrations were determined by high-performance liquid chromatography. Population pharmacokinetic modeling and Monte Carlo simulations were conducted. The total body clearance of CMS (excluding CAPD clearance) was 1.77 liters/h (44%) [population mean (between-subject variability)], while CAPD clearance was 0.088 liter/h (64%). The population mean terminal half-life of CMS was 8.4 h. For colistin, the total clearance/fraction of CMS metabolized to colistin (fm) (excluding CAPD clearance) was 2.74 liters/h (50%), the CAPD clearance was 0.101 liter/h (34%), and the mean terminal half-life was 13.2 h. Monte Carlo simulations suggested a loading dose of 300 mg CBA on day 1 and a maintenance dose of either 150 mg or 200 mg CBA daily to achieve a target average steady-state plasma colistin concentration of 2.5 mg/liter. Clearance by CAPD was low for both CMS and formed colistin. Therefore, CMS doses should not be increased during CAPD. Modeling and simulation enabled us to propose the first evidence-based CMS dosage regimen for CAPD patients.
INTRODUCTION
Colistin (polymyxin E) is a cationic lipopeptide antimicrobial agent with activity against multidrug-resistant (MDR) Gram-negative pathogens (1). Colistin is administered parenterally as colistin methanesulfonate (CMS), an inactive prodrug (2) that was approved by the U.S. FDA in 1959. Clinical use of CMS/colistin declined in the 1970s due to concern about its propensity to cause nephrotoxicity and neurotoxicity (3, 4). The emergence of strains of Pseudomonas aeruginosa, Acinetobacter baumannii, and Enterobacteriaceae that are resistant to β-lactams (including carbapenems), fluoroquinolones, and aminoglycosides is increasing worldwide at an alarming rate (5). This situation has led to a resurgence in the use of colistin in many health care centers around the world (1, 6). Within the last decade, a number of clinical studies have shown favorable outcomes of CMS/colistin treatment (7–10). Our recent study showed that patients infected with MDR A. baumannii and P. aeruginosa who received parenteral CMS had more favorable clinical response (80.8% versus 26.7%) and lower overall mortality (46.2% versus 80.0%) than those who were not treated with CMS/colistin (11).
Infections due to MDR Gram-negative bacteria are very common in critically ill patients with compromised renal function. Although colistin is predominantly cleared by nonrenal pathways, its prodrug (CMS) is mainly renally eliminated, (1, 4), and therefore, CMS dosing regimens need to be adjusted in patients with compromised renal function (12). The pharmacokinetics (PK) of CMS and formed colistin in critically ill patients and suggested dosing regimens of CMS in these patients, including those receiving temporary (acute) renal replacement therapy with intermittent hemodialysis or continuous extracorporeal support, have only recently become available (12). Patients with end-stage renal disease (ESRD) receiving chronic renal replacement therapy are also at increased risk of developing hospital-acquired infections caused by MDR Gram-negative bacteria, which may require parenteral CMS. Continuous ambulatory peritoneal dialysis (CAPD) is a common renal replacement therapy in ESRD patients. However, there has not been a PK study using specific assay methods for the administered prodrug (CMS) and the colistin formed from it in patients receiving peritoneal dialysis; also, there are no PK data or dosage recommendations for these patients in the product information. Three studies (13–15), published in 1967 and 1968, reported information on plasma and dialysate concentrations of “colistin” and corresponding dialysis clearance in a relatively small number of patients who were receiving acute (temporary) peritoneal dialysis according to procedures in use at that time. It is very important to note that a microbiological assay was used to quantify “colistin” in all of these studies (13–15), but such bioassay methods cannot differentiate colistin (the active antibacterial) that was present in a sample at the time of its collection from a patient and colistin that formed during the microbiological incubation (1).
The impact of CAPD on the disposition of CMS and formed colistin, and the possible need for CMS dosage adjustment, in patients receiving this renal support modality have not been reported. Thus, the aims of the present study were to determine the PK, including peritoneal dialysis clearance, of CMS and formed colistin after intravenous administration of CMS and to explore via Monte Carlo simulations optimal CMS dosage regimens in ESRD patients receiving CAPD.
MATERIALS AND METHODS
Patients and study design.
The study was approved by the Siriraj Institutional Review Board and conducted at the renal dialysis unit and Siriraj Clinical Research Center, Siriraj Hospital, Bangkok, Thailand. Written informed consent was obtained from each subject. The eligible subjects were adults with ESRD without acute concurrent illness who were receiving long-term CAPD. Subjects were excluded if they had a hematocrit of less than 20% or known allergy to colistin or polymyxin B or had received polymyxin therapy within 2 months before enrollment. Enrollment of eligible subjects occurred from 18 January to 12 February 2010.
CMS administration, pharmacokinetic sampling, and quantification of CMS and colistin.
Each subject received a single intravenous infusion of 150 mg of colistin base activity (CBA) (Colistate; Atlantic Pharmaceutical Co., Ltd.) over 30 min. This dose of CBA (150 mg) is equivalent to ∼5 million IU and to ∼400 mg of CMS. Serial blood samples (predose and at 0.5, 1, 2, 3, 5, 7, 13, and 25 h post-start of CMS infusion) were collected through an indwelling venous cannula in the arm contralateral to that used for the CMS infusion. CAPD was performed by each subject (2-liter dwell volume, replaced every 6 h). The peritoneal dialysate (PD) was recovered at the end of each 6-h CAPD dwell time over the study period, the volume was measured, and a sample was retained. Six of the subjects were able to produce urine; for these subjects, the total urine voided over 24 h was collected, the volume was recorded, and a sample was retained. The plasma, dialysate, and urine samples were stored at −70°C in order to prevent in vitro conversion of CMS to colistin (16). The concentrations of CMS and colistin were quantified by high-performance liquid chromatography (HPLC) within 4 months of collection (16–18). Adverse events experienced by the subjects were recorded throughout the study.
Population pharmacokinetic analysis.
Population PK modeling of CMS and formed colistin in both plasma and peritoneal dialysate was performed in S-ADAPT (version 1.57) with the Monte Carlo parametric expectation maximization algorithm (MC-PEM) (importance sampling, pmethod = 4) (19). SADAPT-TRAN was utilized for pre- and postprocessing (20, 21). Less than 2% of samples were below the quantification limit, and they were taken into account by the Beal M3 method as implemented in S-ADAPT (22). Models with one, two, or three disposition compartments for CMS and one or two disposition compartments for formed colistin were explored. The distributions of CMS and formed colistin between plasma and peritoneal dialysate, as well as the formation of colistin from CMS in plasma and peritoneal dialysate, were described by first-order processes. The individual measured volumes of dialysate recovered from each subject and dwell period were recorded (median, 2.2 liters) and included in the model. The concentrations of CMS and formed colistin in plasma and peritoneal dialysate from all subjects were fitted simultaneously. The interindividual variability was assumed to be log-normally distributed. Models with full variance-covariance matrices and diagonal variance-covariance matrices were evaluated. The residual unidentified variability was described by a combined additive- and proportional-error model. The possible effect of total body weight on the disposition of CMS and formed colistin was explored. For model evaluation, plots of observed versus individual-fitted and observed versus population-fitted concentrations, visual predictive checks, the normalized prediction distribution error, and the objective function in S-ADAPT were utilized. The recovery of CMS and colistin in dialysate and urine was expressed as the amount of unchanged drug recovered over 24 h divided by the dose.
Monte Carlo simulations.
Based upon the population PK model, Monte Carlo simulations with between-subject variability (BSV) were performed for various potential dosage regimens. The simulated regimens were (i) 100 mg CBA as a 30-min infusion every 24 h, (ii) 200 mg CBA as a 30-min infusion loading dose followed 24 h later by 100 mg CBA as a 30-min infusion every 24 h, (iii) 150 mg CBA as a 30-min infusion every 24 h, (iv) 300 mg CBA as a 30-min infusion loading dose followed 24 h later by 150 mg CBA as a 30-min infusion every 24 h, (v) 200 mg CBA as a 30-min infusion every 24 h, and (vi) 300 mg CBA as a 30-min infusion loading dose followed 24 h later by 200 mg CBA as a 30-min infusion every 24 h. For each dosage regimen, 500 virtual subjects were simulated using Berkeley Madonna (version 8.3.18) (23).
RESULTS
Patient characteristics.
A total of 8 subjects (7 male) were included in the study. The median (range) age and body weight were 69 (54 to 77) years and 61 (45.7 to 81.3) kg, respectively. The median (range) Kt/V value (where K is the dialyzer clearance of urea, t is the dialysis time, and V is the volume of distribution of urea) for the CAPD was 2.5 (1.8 to 3.2) per week (24). Some subjects developed transient adverse events (3 with mild dizziness and 1 with minor skin rash) that resolved within 24 h. One subject had unstable gait at 19 h post-CMS infusion. Physical examination of the subject revealed that he had cerebellar ataxia, and brain imaging showed multiple old lacunar cerebral infarctions at the left caudate, left pons, and right cerebellum. His ataxia disappeared within 45 h after CMS infusion.
Concentrations of CMS and colistin and amounts recovered.
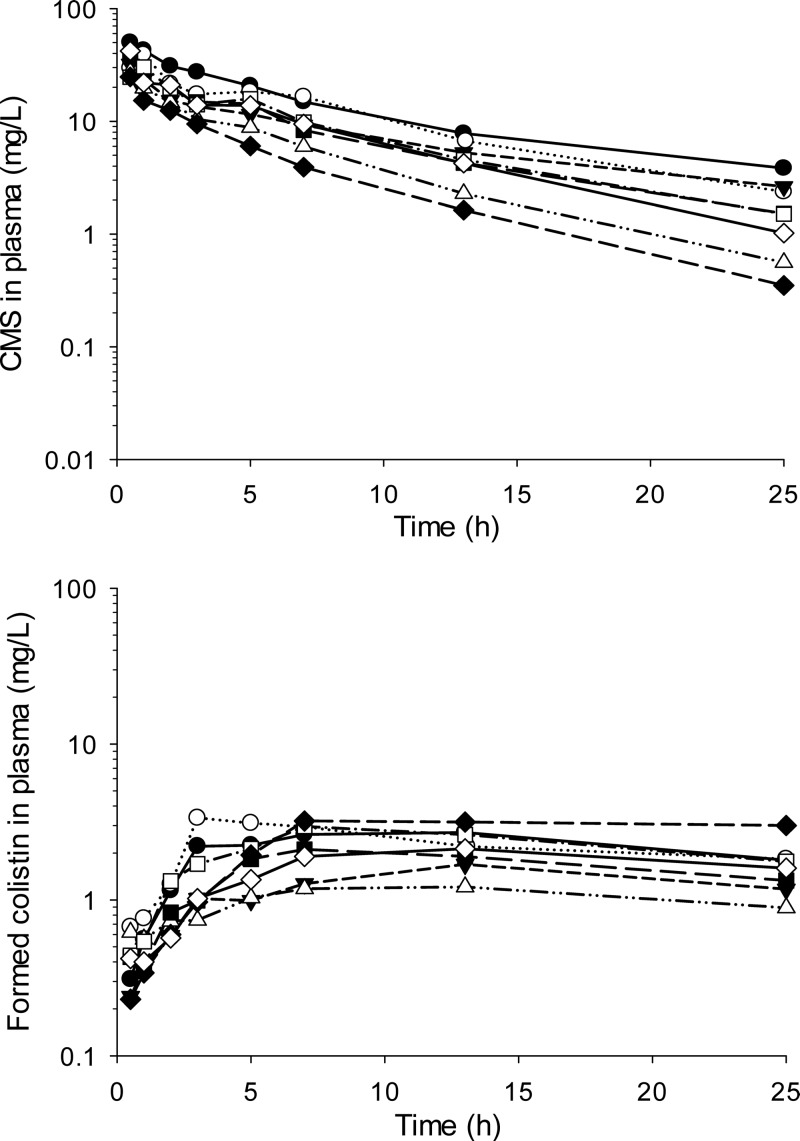
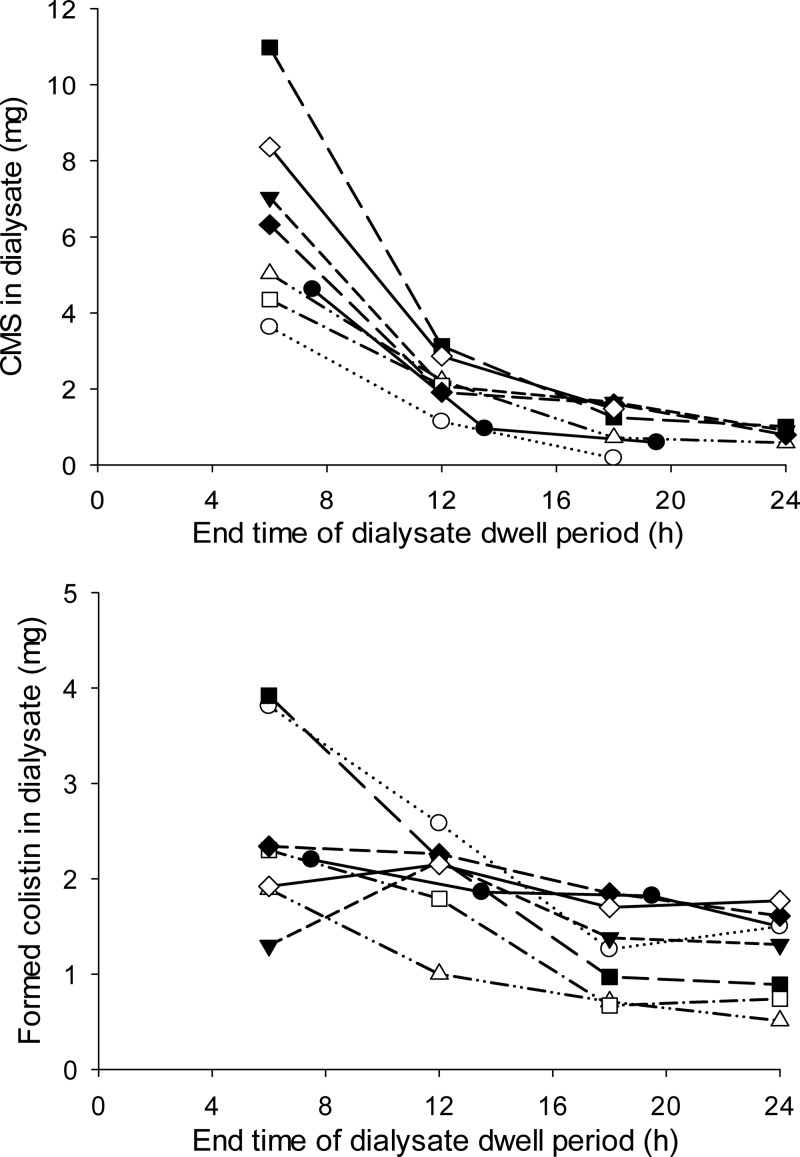
The plasma concentration-time profiles of CMS and formed colistin for the individual subjects are presented in Fig. 1. Visual examination of the profiles for the observed data indicated that the plasma concentrations of CMS in each subject declined in a multiexponential manner. Meanwhile, the concentration of colistin in plasma increased over time to achieve a mean (range) maximum observed concentration (Cmax) of 2.4 (1.2 to 3.4) mg/liter at 9.5 (3 to 13) h (Fig. 1). The observed amounts of CMS and colistin recovered in PD fluid over each 6-h dwell period are presented in Fig. 2. The mean cumulative recovery of the dose across the four dwell periods was 2.58% (1.32 to 4.37%) as CMS and 2.61% (1.54 to 3.43%) as colistin, with a combined recovery of 5.20% (3.78 to 7.36%). On average, in the six subjects able to produce urine, 6.1% of the dose was recovered in urine, 3.3% as CMS, and 2.8% as colistin.
FIG 1.
Observed plasma CMS (top) and formed colistin (bottom) concentrations in individual subjects.
FIG 2.
Observed amounts of CMS (top) and formed colistin (bottom) recovered in peritoneal dialysate fluid plotted against the end time of each 6-h dialysate dwell period.
Population pharmacokinetics.
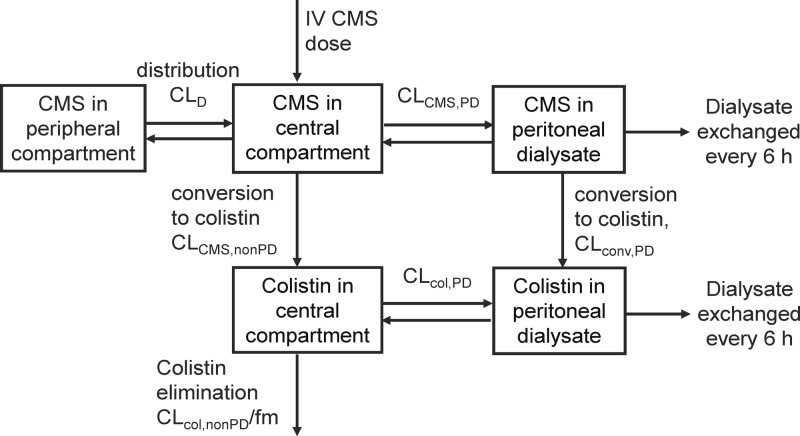
The time course of plasma concentrations of CMS and formed colistin and of the amounts of these two species recovered in PD fluid at the end of each dwell period were well described by the disposition model developed (Fig. 3). The final model comprised two equilibrating disposition compartments for CMS and one compartment for colistin and one compartment each for CMS and colistin representative of PD fluid, for which exchange occurred every 6 h, corresponding to the dwell time. As the fraction of the nondialysis clearance of CMS (CLCMS,nonPD) resulting in formation of colistin (fm) is unknown, the colistin clearance (CLcol,nonPD/fm) and volume of distribution (Vcol/fm) are conditioned on fm and are therefore apparent values. The conversion of CMS to colistin was adequately described by a first-order process in both plasma and dialysate. The inclusion of this conversion process in dialysate was necessary to describe the time courses of CMS and colistin in dialysate and also enabled estimation of the actual PD clearances of both CMS and colistin, rather than their apparent clearances.
FIG 3.
Pharmacokinetic model developed to describe the disposition of CMS and formed colistin. PK parameter names are defined in the footnotes to Table 1. IV, intravenous.
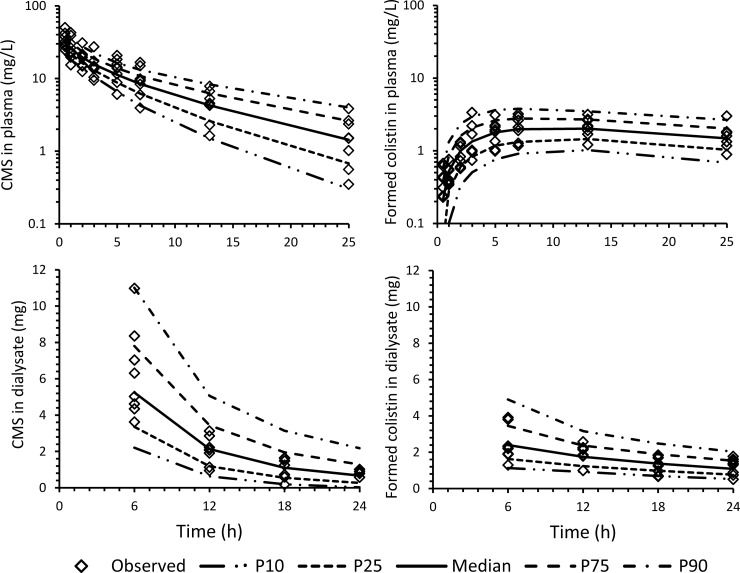
The population PK model provided excellent fits to the observed concentration-time profiles and amounts recovered in dialysate for individual subjects. The proportional and additive residual-error components for CMS in plasma (dialysate) were 15% (15%) and 0.010 mg/liter (0.22 mg/liter) and were 8.6% (23%) and 0.19 mg/liter (0.038 mg/liter) for colistin in plasma (dialysate). The visual predictive checks demonstrated a very good predictive performance of the model for both CMS and colistin (Fig. 4). The population PK parameter estimates and corresponding BSV arising from application of the model are presented in Table 1. The final model included a diagonal variance-covariance matrix. Scaling of CMS and colistin clearances and volumes of distribution by total body weight had no substantial effect on decreasing the BSV for the various parameters overall.
FIG 4.
Visual predictive checks for CMS and formed colistin: plasma concentrations (top) and amounts recovered in peritoneal dialysate (bottom). The lines represent the model predicted 10th percentile (P10), 25th percentile (P25), median, 75th percentile (P75), and 90th percentile (P90).
TABLE 1.
Population pharmacokinetic parameter estimates and BSV for CMS and formed colistin in plasma and dialysate
| Parameter | Estimate (% BSV) | SE (%)l |
|---|---|---|
| CMS | ||
| CLCMS,nonPDa (liters/h) | 1.77 (44) | 17 |
| CLCMS,PDb (liter/h) | 0.0883 (64) | 26 |
| V1CMSc (liters) | 11.0 (26) | 11 |
| V2CMSd (liters) | 7.11 (14) | 17 |
| CLDe (liters/h) | 1.53 (99) | 45 |
| T1/2βf (h) | 8.40 | |
| Formed colistin | ||
| CLcol,nonPD/fmg (liters/h) | 2.74 (50) | 23 |
| CLcol,PDh (liter/h) | 0.100 (34) | 27 |
| Vcol/fmi (liters) | 54.2 (54) | 18 |
| T1/2k (h) | 13.2 | |
| CLconv,PDj (liter/h) | 0.220 (37) | 25 |
CLCMS,nonPD, total body clearance of CMS excluding peritoneal dialysis (PD) clearance.
CLCMS,PD, clearance of CMS by PD.
V1CMS, central volume of distribution of CMS.
V2CMS, peripheral volume of distribution of CMS.
CLD, distribution clearance of CMS between the central and peripheral compartments.
T1/2β, terminal half-life of CMS.
CLcol,nonPD/fm, total body clearance of colistin excluding clearance by PD, conditioned on the unknown fraction (fm) of CLCMS,nonPD resulting in the formation of colistin.
CLcol,PD, clearance of colistin by PD.
Vcol/fm, volume of distribution of colistin, conditioned on fm.
T1/2, half-life of colistin.
CLconv,PD, clearance describing conversion of CMS to colistin in peritoneal dialysate fluid.
SE, standard error of the estimate, expressed as percent coefficient of variation.
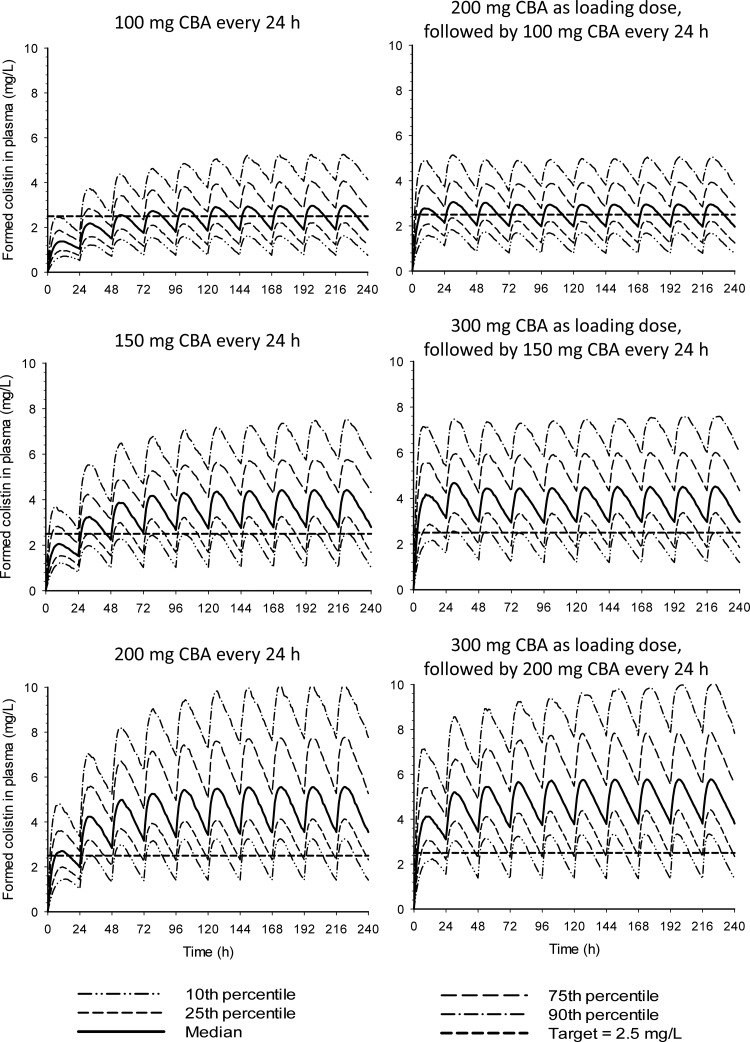
The plasma concentration-time profiles of formed colistin for the six CMS dosage regimens evaluated by Monte Carlo simulations are presented in Fig. 5.
FIG 5.
Monte Carlo simulations of plasma concentrations of formed colistin for six clinically relevant dosage regimens of CBA designed to achieve a colistin Css,avg of at least 2.5 mg/liter in a large proportion of patients.
DISCUSSION
The previously reported studies on colistin in patients undergoing peritoneal dialysis were performed in the 1960s (13–15). At that time, it was not well recognized that CMS is an inactive prodrug that is converted to colistin both in vivo and in vitro (2, 16, 25, 26). The microbiological assay used in the aforementioned studies (13–15) to quantify “colistin” was incapable of separately quantifying CMS and formed colistin. In the present study, concentrations of CMS and colistin in the samples were quantified separately by HPLC assays. To our knowledge, the present study is the first to define the PK of CMS and formed colistin in ESRD patients receiving CAPD and will assist in optimization of dosage regimens in this group of patients.
The intravenous dose of 150 mg CBA of CMS was generally well tolerated; the transient mild dizziness in 3 subjects was in keeping with previous observations (6). One subject had a preexisting cerebrovascular disease and developed transient cerebellar ataxia. The observed plasma colistin Cmax and the area under the plasma colistin concentration time curve over 25 h (AUC0–25) (calculated by the linear up/log down trapezoidal rule) in this subject were 2.13 mg/liter and 41.5 mg · h/liter, respectively. This exposure was well within the range of Cmax (1.21 to 3.36 mg/liter) and AUC0–25 (25.5 to 65.6 mg · h/liter) observed in the subjects who did not experience neurological signs or symptoms after administration of CMS. This observation implied that preexisting cerebrovascular disease might be a risk factor for developing colistin-related neurotoxicity.
In subjects with normal kidney function, approximately 70% of an intravenous dose of CMS is recovered in urine over 24 h; a substantial proportion is recovered as colistin that is formed in urine after excretion of CMS in the kidney (27). Indeed, CMS is predominantly renally cleared, and its clearance is therefore dependent upon renal function (1, 12). In the present study, in six subjects able to produce urine, we observed low urinary recovery of CMS and colistin over 24 h, approximately 3% (each) of the administered dose was recovered as CMS and colistin. Across all 8 subjects, the cumulative recovery of CMS and colistin in peritoneal dialysate was similarly low (average, ∼5% of the CMS dose), again with CMS and colistin contributing approximately equally to this recovery. Conversion of CMS to colistin in dialysate during the 6-h dwell time was taken into account in the population PK model. The population estimates of the peritoneal dialysate clearance for CMS (0.088 liter/h) and colistin (0.101 liter/h) were similar, low, and only a small percentage of the respective non-peritoneal-dialysate clearance values (CLCMS,nonPD, 1.77 liters/h, and CLcol,nonPD/fm, 2.74 liters/h). Collectively, these urinary and dialysate recovery and clearance data indicate that in subjects with ESRD receiving CAPD, as yet unknown pathways other than renal and dialysis clearance account for the elimination of CMS and formed colistin. Both CMS and formed colistin are cleared efficiently by intermittent hemodialysis or continuous venovenous hemodialysis or hemodiafiltration (12, 28). In contrast, the low peritoneal dialysis clearance values for CMS and colistin observed in the present study (∼0.1 liter/h or ∼1.5 ml/min) are in keeping with the general inefficiency of peritoneal dialysis in regard to drug elimination (29, 30).
In the present study, CAPD subjects with ESRD received a single intravenous dose of CMS (150 mg CBA) and achieved an average (±standard deviation [SD]) concentration of colistin, the active antibacterial, of 0.82 ± 0.28 mg/liter in peritoneal dialysate at the end of the 6-h dwell time. The subjects in this study did not have peritonitis, and caution is required in considering these concentrations in relation to MICs of an organism that may be causing peritonitis. In a single case report involving a patient with severe peritonitis administered 180 mg CBA per day, Mimoz et al. observed steady-state colistin concentrations in peritoneal fluid in the range of 2.5 to 3 mg/liter (31). The notable differences between the present and the previous reports (31) are the single-dose versus multiple-dose nature of the studies, the presence of peritonitis in the single case report and the possible effect of associated inflammation of the peritoneal membrane on drug permeation, and the ongoing presence in the peritoneal cavity of 2 liters of dialysis fluid in the subjects in the present report.
The major objective of the present study was to elucidate the systemic PK of CMS and formed colistin in ESRD patients receiving CAPD and to provide guidance on dosage selection for treatment of infections other than peritonitis. After taking into account the lack of, or minimal residual, renal function in the CAPD patients in the current study, the disposition of CMS and colistin in these patients was similar to that reported by Garonzik et al. in a large study in 105 critically ill non-CAPD patients with very diverse renal function (12). The CLCMS,nonPD in CAPD patients was 1.77 liters/h (Table 1) compared to 1.9 liters/h for the nonrenal CMS clearance reported by Garonzik et al. (12). The CMS terminal half-life of 8.4 h in CAPD patients is within the range of half-lives previously reported for non-CAPD critically ill patients (12). At 2.74 liters/h, the CLcol,nonPD/fm value (Table 1) was slightly higher than the apparent nonrenal colistin clearance of 2.19 liters/h in critically ill patients not receiving CAPD (12), while the colistin terminal half-life of 13.2 h (Table 1) was very similar to the median value (13 h) reported by Garonzik et al. in patients with creatinine clearance of less than 10 ml/min. These comparisons highlight the consistency between the studies, as renal clearance was very small or nonexistent in the CAPD patients in the present study. Due to the time required for conversion of CMS to colistin and the slow decline of colistin concentrations during the observation period, there might be some uncertainty attached to the estimate of the terminal half-life of formed colistin. Importantly, as noted above, the peritoneal-dialysis clearances of both CMS and colistin were very small relative to the respective non-PD clearance values. Thus, CAPD has a negligible effect on the overall clearance of CMS and colistin in subjects with ESRD.
The population PK model developed in the current study provides the first information on which to base proposed colistin dosage regimens in CAPD patients. The population PK model was employed in Monte Carlo simulations to predict the distribution of plasma concentration-time profiles of formed colistin (the active antibacterial) for six potential dosage regimens, which are presented in Fig. 5. The range of plasma colistin concentrations predicted for all dosage regimens is similar to those observed in critically ill patients (12). Due to the small sample size of 8 patients in the current study, predictions for the lower and upper ends of the distribution (i.e., 10th and 90th percentiles) should be interpreted with caution. We also recognize that the simulations are based upon single-dose data; however, there is no indication that CMS or colistin exhibits nonlinear PK (12, 32, 33). The average steady-state concentration (Css,avg) of formed colistin achieved by each of these regimens was compared to a “target” plasma colistin Css,avg of 2.5 mg/liter (12). A CMS maintenance dosage regimen of 150 mg or 200 mg CBA administered every 24 h was predicted to achieve a colistin Css,avg of 2.5 mg/liter in the majority of a CAPD patient population with characteristics similar to those of the subjects in our study. However, even with the 200-mg/day regimen, less than half of the patients would reach a colistin Css,avg of 2.5 mg/liter on day 1 and 75% on day 2, due to the slow accumulation of colistin after its formation from CMS, necessitating consideration of initiating therapy with a loading dose of CMS (12, 32, 33). With a CMS loading dose of 300 mg CBA (which is at the upper limit of the current product-recommended daily dose range) on day 1, the majority of patients receiving a maintenance regimen of either 150 mg or 200 mg CBA per day are predicted to reach a colistin Css,avg of 2.5 mg/liter on day 1. Therefore, population PK modeling and simulations based upon the observed data from CAPD patients suggest a loading dose of 300 mg CBA on day 1 and a maintenance dose of either 150 mg or 200 mg CBA per day.
In conclusion, our study demonstrated that clearances by CAPD were low for both CMS and formed colistin. Therefore, CMS doses should not be increased during CAPD. Modeling and simulation analyses enabled us to propose the first evidence-based CMS dosage regimen for CAPD patients. Further clinical evaluations are warranted to confirm the effectiveness and safety of the suggested CMS dosage regimen in ESRD patients on CAPD therapy.
ACKNOWLEDGMENTS
This work was partially supported by Atlantic Pharmaceutical Co., Ltd. J.L. is an Australian National Health and Medical Research Council (NHMRC) Senior Research Fellow.
We thank Ratana Chawanasuntorapoj and the responsible personnel at the renal dialysis unit, Siriraj Hospital, Siriluck Suddhichupaiboon, for their assistance, and also Jovan Jacob at Monash University for his contribution to analysis of colistin in biological fluids.
Footnotes
Published ahead of print 4 November 2013
REFERENCES
- 1.Li J, Nation RL, Turnidge JD, Milne RW, Coulthard K, Rayner CR, Paterson DL. 2006. Colistin: the re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect. Dis. 6:589–601. 10.1016/S1473-3099(06)70580-1 [DOI] [PubMed] [Google Scholar]
- 2.Bergen PJ, Li J, Rayner CR, Nation RL. 2006. Colistin methanesulfonate is an inactive prodrug of colistin against Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 50:1953–1958. 10.1128/AAC.00035-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hermsen ED, Sullivan CJ, Rotschafer JC. 2003. Polymyxins: pharmacology, pharmacokinetics, pharmacodynamics, and clinical applications. Infect. Dis. Clin. North Am. 17:545–562. 10.1016/S0891-5520(03)00058-8 [DOI] [PubMed] [Google Scholar]
- 4.Nation RL, Li J. 2009. Colistin in the 21st century. Curr. Opin. Infect. Dis. 22:535–543. 10.1097/QCO.0b013e328332e672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spellberg B, Blaser M, Guidos RJ, Boucher HW, Bradley JS, Eisenstein BI, Gerding D, Lynfield R, Reller LB, Rex J, Schwartz D, Septimus E, Tenover FC, Gilbert DN. 2011. Combating antimicrobial resistance: policy recommendations to save lives. Clin. Infect. Dis. 52(Suppl 5):S397–S428. 10.1093/cid/cir153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Landman D, Georgescu C, Martin DA, Quale J. 2008. Polymyxins revisited. Clin. Microbiol. Rev. 21:449–465. 10.1128/CMR.00006-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paksu MS, Paksu S, Karadag A, Sensoy G, Asilioglu N, Yildizdas D, Akyildiz BN, Kendirli T, Demirkol D, Akgun M, Alp E, Ciftci E, Guney AK, Murat N. 2012. Old agent, new experience: colistin use in the paediatric intensive care unit—a multicentre study. Int. J. Antimicrob. Agents 40:140–144. 10.1016/j.ijantimicag.2012.04.010 [DOI] [PubMed] [Google Scholar]
- 8.Markou N, Apostolakos H, Koumoudiou C, Athanasiou M, Koutsoukou A, Alamanos I, Gregorakos L. 2003. Intravenous colistin in the treatment of sepsis from multiresistant Gram-negative bacilli in critically ill patients. Crit. Care 7:R78–R83. 10.1186/cc2358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bassetti M, Repetto E, Righi E, Boni S, Diverio M, Molinari MP, Mussap M, Artioli S, Ansaldi F, Durando P, Orengo G, Bobbio Pallavicini F, Viscoli C. 2008. Colistin and rifampicin in the treatment of multidrug-resistant Acinetobacter baumannii infections. J. Antimicrob. Chemother. 61:417–420. 10.1093/jac/dkm509 [DOI] [PubMed] [Google Scholar]
- 10.Reina R, Estenssoro E, Saenz G, Canales HS, Gonzalvo R, Vidal G, Martins G, Das Neves A, Santander O, Ramos C. 2005. Safety and efficacy of colistin in Acinetobacter and Pseudomonas infections: a prospective cohort study. Intensive Care Med. 31:1058–1065. 10.1007/s00134-005-2691-4 [DOI] [PubMed] [Google Scholar]
- 11.Koomanachai P, Tiengrim S, Kiratisin P, Thamlikitkul V. 2007. Efficacy and safety of colistin (colistimethate sodium) for therapy of infections caused by multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii in Siriraj Hospital, Bangkok, Thailand. Int. J. Infect. Dis. 11:402–406. 10.1016/j.ijid.2006.09.011 [DOI] [PubMed] [Google Scholar]
- 12.Garonzik SM, Li J, Thamlikitkul V, Paterson DL, Shoham S, Jacob J, Silveira FP, Forrest A, Nation RL. 2011. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob. Agents Chemother. 55:3284–3294. 10.1128/AAC.01733-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Curitis JR, Eastwood JB. 1968. Colistin sulphomethate sodium administration in the presence of severe renal failure and during haemodialysis and peritoneal dialysis. Br. Med. J. 1:484–485. 10.1136/bmj.1.5590.484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greenberg PA, Sanford JP. 1967. Removal and absorption of antibiotics in patients with renal failure undergoing peritoneal dialysis. Tetracycline, chloramphenicol, kanamycin, and colistimethate. Ann. Intern. Med. 66:465–470 [DOI] [PubMed] [Google Scholar]
- 15.Goodwin NJ, Friedman EA. 1968. The effects of renal impairment, peritoneal dialysis, and hemodialysis on serum sodium colistimethate levels. Ann. Intern. Med. 68:984–994. 10.7326/0003-4819-68-5-984 [DOI] [PubMed] [Google Scholar]
- 16.Dudhani RV, Nation RL, Li J. 2010. Evaluating the stability of colistin and colistin methanesulphonate in human plasma under different conditions of storage. J. Antimicrob. Chemother. 65:1412–1415. 10.1093/jac/dkq134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li J, Milne RW, Nation RL, Turnidge JD, Coulthard K, Valentine J. 2002. Simple method for assaying colistin methanesulfonate in plasma and urine using high-performance liquid chromatography. Antimicrob. Agents Chemother. 46:3304–3307. 10.1128/AAC.46.10.3304-3307.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li J, Milne RW, Nation RL, Turnidge JD, Coulthard K, Johnson DW. 2001. A simple method for the assay of colistin in human plasma, using pre-column derivatization with 9-fluorenylmethyl chloroformate in solid-phase extraction cartridges and reversed-phase high-performance liquid chromatography. J. Chromatogr. B Biomed. Sci. Appl. 761:167–175. 10.1016/S0378-4347(01)00326-7 [DOI] [PubMed] [Google Scholar]
- 19.Bauer RJ. 2010. S-ADAPT/MCPEM user's guide (version 1.57). Software for Pharmacokinetic, Pharmacodynamic and Population Data Analysis, Berkeley, CA [Google Scholar]
- 20.Bulitta JB, Bingolbali A, Shin BS, Landersdorfer CB. 2011. Development of a new pre- and post-processing tool (SADAPT-TRAN) for nonlinear mixed-effects modeling in S-ADAPT. AAPS J. 13:201–211. 10.1208/s12248-011-9257-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bulitta JB, Landersdorfer CB. 2011. Performance and robustness of the Monte Carlo importance sampling algorithm using parallelized S-ADAPT for basic and complex mechanistic models. AAPS J. 13:212–226. 10.1208/s12248-011-9258-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beal SL. 2001. Ways to fit a PK model with some data below the quantification limit. J. Pharmacokinet. Pharmacodyn. 28:481–504. 10.1023/A:1012299115260 [DOI] [PubMed] [Google Scholar]
- 23.Macey RI, Oster GF. 2010. Berkeley Madonna version 8.3.18 (1996–2010). University of California, Berkeley, CA [Google Scholar]
- 24.Peritoneal Dialysis Adequacy Work Group 2006. Clinical practice guidelines for peritoneal dialysis adequacy. Am. J. Kidney Dis. 48(Suppl 1):S98–S129. 10.1053/j.ajkd.2006.04.006 [DOI] [PubMed] [Google Scholar]
- 25.Li J, Coulthard K, Milne R, Nation RL, Conway S, Peckham D, Etherington C, Turnidge J. 2003. Steady-state pharmacokinetics of intravenous colistin methanesulphonate in patients with cystic fibrosis. J. Antimicrob. Chemother. 52:987–992. 10.1093/jac/dkg468 [DOI] [PubMed] [Google Scholar]
- 26.Li J, Milne RW, Nation RL, Turnidge JD, Smeaton TC, Coulthard K. 2004. Pharmacokinetics of colistin methanesulphonate and colistin in rats following an intravenous dose of colistin methanesulphonate. J. Antimicrob. Chemother. 53:837–840. 10.1093/jac/dkh167 [DOI] [PubMed] [Google Scholar]
- 27.Couet W, Gregoire N, Gobin P, Saulnier PJ, Frasca D, Marchand S, Mimoz O. 2011. Pharmacokinetics of colistin and colistimethate sodium after a single 80-mg intravenous dose of CMS in young healthy volunteers. Clin. Pharmacol. Ther. 89:875–879. 10.1038/clpt.2011.48 [DOI] [PubMed] [Google Scholar]
- 28.Markou N, Fousteri M, Markantonis SL, Zidianakis B, Hroni D, Boutzouka E, Baltopoulos G. 2012. Colistin pharmacokinetics in intensive care unit patients on continuous venovenous haemodiafiltration: an observational study. J. Antimicrob. Chemother. 67:2459–2462. 10.1093/jac/dks257 [DOI] [PubMed] [Google Scholar]
- 29.Ronco C, Clark W. 2001. Factors affecting hemodialysis and peritoneal dialysis efficiency. Semin. Dial. 14:257–262. 10.1046/j.1525-139X.2001.00065.x [DOI] [PubMed] [Google Scholar]
- 30.Rowland M, Tozer TN. 2011. Individualization, p 434–436 In Rowland M, Tozer TN. (ed), Clinical pharmacokinetics and pharmacodynamics: concepts and applications, 4th ed. Lippincott Williams & Wilkins, Philadephia, PA [Google Scholar]
- 31.Mimoz O, Petitpas F, Gregoire N, Gobin P, Marchand S, Couet W. 2012. Colistin distribution in the peritoneal fluid of a patient with severe peritonitis. Antimicrob. Agents Chemother. 56:4035–4036. 10.1128/AAC.00478-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mohamed AF, Karaiskos I, Plachouras D, Karvanen M, Pontikis K, Jansson B, Papadomichelakis E, Antoniadou A, Giamarellou H, Armaganidis A, Cars O, Friberg LE. 2012. Application of a loading dose of colistin methanesulfonate in critically ill patients: population pharmacokinetics, protein binding, and prediction of bacterial kill. Antimicrob. Agents Chemother. 56:4241–4249. 10.1128/AAC.06426-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Plachouras D, Karvanen M, Friberg LE, Papadomichelakis E, Antoniadou A, Tsangaris I, Karaiskos I, Poulakou G, Kontopidou F, Armaganidis A, Cars O, Giamarellou H. 2009. Population pharmacokinetic analysis of colistin methanesulfonate and colistin after intravenous administration in critically ill patients with infections caused by gram-negative bacteria. Antimicrob. Agents Chemother. 53:3430–3436. 10.1128/AAC.01361-08 [DOI] [PMC free article] [PubMed] [Google Scholar]