Abstract
For Mycobacterium tuberculosis, phenotypic methods for drug susceptibility testing of second-line drugs are poorly standardized and technically challenging. The Sensititre MYCOTB MIC plate (MYCOTB) is a microtiter plate containing lyophilized antibiotics and configured for determination of MICs to first- and second-line antituberculosis drugs. To evaluate the performance of MYCOTB for M. tuberculosis drug susceptibility testing using the Middlebrook 7H10 agar proportion method (APM) as the comparator, we conducted a two-site study using archived M. tuberculosis isolates from Uganda and the Republic of Korea. Thawed isolates were subcultured, and dilutions were inoculated into MYCOTB wells and onto 7H10 agar. MYCOTB results were read at days 7, 10, 14, and 21; APM results were read at 21 days. A total of 222 isolates provided results on both platforms. By APM, 106/222 (47.7%) of isolates were resistant to at least isoniazid and rifampin. Agreement between MYCOTB and APM with respect to susceptibility or resistance was ≥92% for 7 of 12 drugs when a strict definition was used and ≥96% for 10 of 12 drugs when agreement was defined by allowing a ± one-well range of dilutions around the APM critical concentration. For ethambutol, agreement was 80% to 81%. For moxifloxacin, agreement was 83% to 85%; incorporating existing DNA sequencing information for discrepant analysis raised agreement to 91% to 96%. For MYCOTB, the median time to plate interpretation was 10 days and interreader agreement was ≥95% for all drugs. MYCOTB provided reliable results for M. tuberculosis susceptibility testing of first- and second-line drugs except ethambutol, and results were available sooner than those determined by APM.
INTRODUCTION
The emergence and spread of drug-resistant strains of Mycobacterium tuberculosis comprise a serious threat to tuberculosis (TB) control (1, 2). Knowledge of M. tuberculosis drug susceptibility is important in optimizing individual patient management and TB control in populations. Genotypic methods have the potential for a very short time to results, but to date, the knowledge of the full spectrum of genetic loci and mutations associated with resistance to many antituberculosis drugs is incomplete (3, 4, 5, 6, 7). Phenotypic methods therefore remain important. The reference phenotypic method—the indirect agar proportion method (APM) using Middlebrook solid media—is qualitative and based on drug critical concentrations. Limitations of the APM and related methods include lack of standardization and in some cases the need for in-laboratory preparation of drug stocks and agar plates, which can be a source of variability over time and between laboratories. Critical concentrations are based on historical epidemiological data and for some drugs are not well-aligned with achievable drug serum concentrations or accurate in predicting clinical failure (8, 9, 10). Studies on molecular drug resistance mechanisms in M. tuberculosis have shown that, at least for some antibiotics, different mutations are associated with different MICs, further emphasizing the importance of bacterial factors in pharmacodynamic relationships and treatment optimization (11, 12, 13).
The Trek Sensititre MYCOTB MIC plate (MYCOTB; Trek Diagnostic Systems, Cleveland, OH) is a dry microdilution plate containing lyophilized antibiotics, with concentrations prepared and quality controlled by the manufacturer. The MYCOTB plate is configured for determination of MICs of first- and second-line anti-TB drugs (Fig. 1). Previous studies comparing use of the MYCOTB plate to solid-medium drug susceptibility testing (DST) methods have shown good agreement between methods (14, 15). However, there is little published information about test performance with a large and diverse isolate population that includes highly resistant M. tuberculosis isolates.
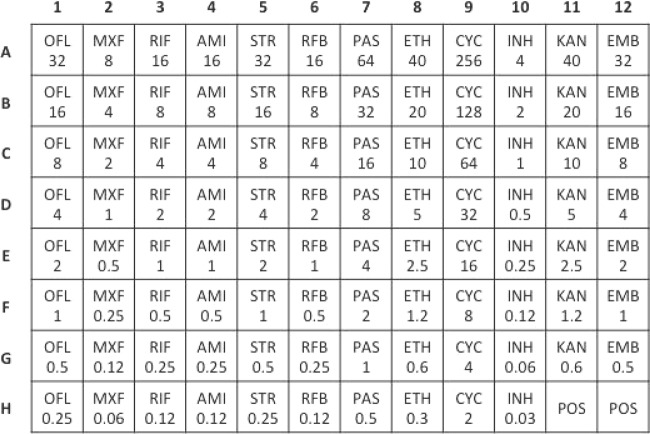
FIG 1.
Schematic of the Sensititre MYCOTB MIC plate showing antibiotics and concentrations in micrograms/milliliter. Abbreviations: OFL, ofloxacin; MXF, moxifloxacin; RIF, rifampin; AMI, amikacin; STR, streptomycin; RFB, rifabutin; PAS, para-aminosalicylic acid; ETH, ethionamide; CYC, cycloserine; INH, isoniazid; KAN, kanamycin; EMB, ethambutol; POS, antibiotic-free positive-control wells.
Therefore, we conducted a study to assess the performance and feasibility of the use of the MYCOTB plate for drug susceptibility testing of M. tuberculosis strains using a large set of M. tuberculosis isolates from Uganda and the Republic of Korea, with conventional APM as the reference comparator.
MATERIALS AND METHODS
Design, settings, and M. tuberculosis isolates.
This was a laboratory study using existing archived M. tuberculosis isolates with previously characterized DST patterns. Existing DST information was used solely to select isolates for study testing, which was subsequently conducted using the MYCOTB plates and APM performed simultaneously. M. tuberculosis isolates had been obtained from clinical specimens submitted previously to the study laboratories; an additional 25 challenge strains from the TB Supranational Reference Laboratory Network were included. The cohort of test isolates was selected to contain at least 50 isolates resistant to and at least 50 isolates susceptible to each test drug, although there was limited existing DST information about ethionamide. M. tuberculosis isolates used in this study have been deposited at BEI Resources (www.beiresources.org) and are undergoing whole-genome sequencing at the Broad Institute Genomic Sequencing Center for Infectious Diseases; isolates and sequencing data will be shared with the scientific community. For the present study, all testing was conducted in reference mycobacteriology laboratories in Masan, Republic of Korea, or in Kampala, Uganda, by personnel who underwent study-specific training for the APM and MYCOTB methods. Personnel performing the MYCOTB tests were blind to APM results, and vice versa. Approval to conduct the study was provided by ethics committees for each study site.
DST methods. (i) MYCOTB.
Tests were performed according to the manufacturer's instructions. Briefly, strains previously isolated from sputum were thawed and subcultured twice onto Middlebrook 7H10 agar (Becton, Dickinson and Co., Sparks, MD). Colonies with bacteria in log-phase growth were added to a saline-Tween solution (Trek Diagnostic Systems) with glass beads, adjusted to turbidity at a McFarland standard of 0.5, and allowed to settle for 15 min. One hundred microliters was transferred to 11 ml of Middlebrook 7H9 broth containing oleic acid-albumin-dextrose-catalase (Trek Diagnostic Systems) and vortex mixed for 20 s; 100 μl of this material was inoculated into each well of the MYCOTB plate. Plates were covered with permanent plastic seals provided with the test kit and incubated at 37°C in 5% CO2. Plates were checked after 24 and 48 h for contamination. Thereafter, growth was monitored at days 7, 10, 14, and 21 by examining unopened plates on the benchtop using a mirrored viewer. For each antibiotic, the lowest concentration with no visible growth was considered to be the MIC. Unless otherwise specified, MYCOTB test results are those for the first time point at which there was adequate growth in the drug-free control wells. Each MYCOTB plate was read and interpreted by two independent readers. The MIC result recorded by the first reader was considered to be the test result, and the result recorded by the second reader was used to assess agreement between readers. For each isolate inoculated, the numbers of CFU were determined to verify that the inoculum was within a targeted amount (approximately 105 CFU/ml). To assess reproducibility of the MYCOTB method, isolates for which results for one or more drugs showed an absence of conditional (or categorical) agreement between MYCOTB and APM underwent repeat testing by the MYCOTB method using a freshly prepared inoculum.
(ii) APM.
Middlebrook 7H10 plates were prepared according to the Clinical and Laboratory Standards Institute (CLSI) methods for indirect APM (16). BD Drug Sensi-Disks (Becton, Dickinson and Co.) were used for ethambutol, ethionamide, isoniazid, moxifloxacin at 2.0 μg/ml, ofloxacin, rifampin, and streptomycin. For other drugs, stock solutions were prepared and then utilized for preparation of the critical concentrations. Critical concentrations are listed in Table 1. For preparation of bacterial inocula, pure strains were thawed and subcultured twice onto Middlebrook 7H10 agar (Becton, Dickinson and Co.). Bacteria were inoculated into 7H9 broth and adjusted to a turbidity of a 1.0 McFarland standard, and serial dilutions were made in sterile distilled water. Agar plates were checked weekly for contamination and incubated for 21 days at 37°C in 5% CO2 prior to result interpretation. For each isolate inoculated, drug-free control quadrants containing 10−4 CFU/ml and 10−2 CFU/ml were prepared to allow countable colony growth for interpretation. All test batches of APM media were used within 3 weeks of preparation. A portion of the inoculum was streaked onto sheep blood agar plates and incubated at 37°C for 48 to 72 h to assess the purity of the initial stock cultures. Media were tested for sterility by incubating a subset of freshly made agar plates at 37°C for 48 h. Quality was controlled by using H37Rv as a reference strain for each set of agar proportion media tested.
TABLE 1.
Results for testing of 222 M. tuberculosis isolates using the Sensititre MYCOTB MIC and agar proportions methodsa
| Drug | APM critical concn (μg/ml) | MYCOTB result | No. of isolates resistant on APM | No. of isolates susceptible on APM | % sensitivity (95% CI) | % specificity (95% CI) | % agreement—categorical | % agreement—conditional |
|---|---|---|---|---|---|---|---|---|
| Isoniazid | 0.2 | Resistant | 141 | 3 | 100.0 (100, 100) | 96.3 (92.2, 100.4) | 98.7 | 99.6 |
| Susceptible | 0 | 78 | ||||||
| Isoniazid | 1 | Resistant | 108 | 2 | 88.5 (82.9, 94.2) | 98.0 (95.3, 100.7) | 92.8 | 99.1 |
| Susceptible | 14 | 98 | ||||||
| Rifampin | 1 | Resistant | 121 | 0 | 98.4 (96.1, 100.6) | 100.0 (100, 100) | 99.1 | 100.0 |
| Susceptible | 2 | 99 | ||||||
| Rifabutin | 0.5 | Resistant | 90 | 15 | 98.9 (96.8, 101.0) | 88.6 (83.1, 94) | 92.8 | 96.0 |
| Susceptible | 1 | 116 | ||||||
| Ethambutol | 5 | Resistant | 72 | 5 | 64.3 (55.4, 73.2) | 95.5 (91.6, 99.4) | 79.7 | 92.8 |
| Susceptible | 40 | 105 | ||||||
| Ethambutol | 10 | Resistant | 18 | 3 | 31.0 (19.1, 42.9) | 98.2 (96.1, 100.2) | 80.6 | 92.8 |
| Susceptible | 40 | 161 | ||||||
| Ofloxacin | 2 | Resistant | 63 | 13 | 100.0 (100, 100) | 91.8 (87.6, 96.1) | 94.1 | 97.8 |
| Susceptible | 0 | 146 | ||||||
| Moxifloxacin | 0.5 | Resistant | 56 | 34 | 100.0 (100, 100) | 79.5 (73.4, 85.7) | 84.7 | 91.9 |
| Susceptible | 0 | 132 | ||||||
| Moxifloxacin | 2 | Resistant | 23 | 37 | 95.8 (87.8, 103.8) | 81.3 (75.9, 86.7) | 82.9 | 89.6 |
| Susceptible | 1 | 161 | ||||||
| Streptomycin | 2 | Resistant | 45 | 28 | 97.8 (93.6, 102.0) | 84.1 (78.7, 89.5) | 86.9 | 98.2 |
| Susceptible | 1 | 148 | ||||||
| Streptomycin | 10 | Resistant | 34 | 5 | 91.9 (83.1, 100.7) | 97.3 (95.0, 99.6) | 96.4 | 98.2 |
| Susceptible | 3 | 180 | ||||||
| Amikacin | 4 | Resistant | 52 | 0 | 96.3 (91.3, 101.3) | 100.0 (100, 100) | 99.1 | 99.6 |
| Susceptible | 2 | 168 | ||||||
| Kanamycin | 5 | Resistant | 60 | 2 | 96.8 (92.4, 101.2) | 98.8 (97.0, 100.5) | 98.2 | 99.6 |
| Susceptible | 2 | 158 | ||||||
| Cycloserine | 25 | Resistant | 4 | 22 | 18.2 (2.1, 34.3) | 89.0 (84.7, 93.3) | 82.0 | 96.0 |
| Susceptible | 18 | 178 | ||||||
| Ethionamide | 5 | Resistant | 68 | 6 | 80.0 (71.5, 88.5) | 95.6 (92.2, 99.1) | 89.6 | 98.7 |
| Susceptible | 17 | 131 | ||||||
| PAS | 2 | Resistant | 58 | 7 | 89.2 (81.7, 96.8) | 95.5 (92.3, 98.8) | 93.7 | 98.2 |
| Susceptible | 7 | 150 |
Abbreviations: APM, agar proportion method; CI, confidence interval; PAS, para-aminosalicylic acid.
Detection of mutations in DNA gyrase genes.
For a subset of M. tuberculosis isolates from the Republic of Korea, genomic DNA sequencing of a 320-bp gyrA gene fragment of the quinolone resistance-determining region and a 375-bp gyrB gene fragment had been previously performed according to published methods (17).
Definitions. (i) Susceptibility by MYCOTB.
For determinations of susceptibility by MYCOTB, an isolate was considered susceptible if the MYCOTB MIC was lower than or equivalent to the APM critical concentration.
(ii) Resistance by MYCOTB.
For determinations of resistance by MYCOTB, an isolate was considered resistant if the MYCOTB MIC was higher than the APM critical concentration.
(iii) Susceptibility by APM.
For determinations of susceptibility by APM, an isolate was considered susceptible if growth in the drug-containing quadrant was less than growth in the control (10−4 dilution) well.
(iv) Resistance by APM.
For determinations of resistance by APM, an isolate was considered resistant if growth in the drug-containing quadrant was greater than or equal to growth in the control (10−4 dilution) well.
(v) Categorical agreement between MYCOTB and APM.
For determinations of categorical agreement between MYCOTB and APM for an isolate, there was considered to be categorical agreement if both MYCOTB and APM characterized the isolate as susceptible or if both MYCOTB and APM characterized the isolate as resistant.
(vi) Conditional agreement between MYCOTB and APM.
For determinations of “conditional agreement” between MYCOTB and APM for an isolate, there was considered to be conditional agreement if APM characterized the isolate as susceptible and the MYCOTB MIC was lower than or equivalent to the APM critical concentration plus 1 doubling dilution or if APM characterized the isolate as resistant and the MYCOTB MIC was equivalent to or higher than the APM critical concentration.
(vii) Sensitivity of MYCOTB.
For determinations of the sensitivity of MYCOTB for the detection of resistance to a drug, the data were expressed as follows: sensitivity = (number of APM-resistant isolates identified as resistant by MYCOTB)/[(number of APM-resistant isolates identified as resistant by MYCOTB) + (number of APM-resistant isolates identified as susceptible by MYCOTB)].
(viii) Specificity of MYCOTB.
For determinations of the specificity of MYCOTB for detection of resistance to a drug, the data were expressed as follows: specificity = (number of APM-susceptible isolates identified as susceptible by MYCOTB)/[(number of APM-susceptible isolates identified as susceptible by MYCOTB) + (number of APM-susceptible isolates identified as resistant by MYCOTB)].
Statistical methods.
Test results were based on comparison of MICs derived from the MYCOTB plate to critical concentrations found using the APM. Sensitivity and specificity were calculated, with 95% confidence intervals (CI). Results for two MYCOTB readers were compared using both a categorical and conditional definition of agreement. For each drug, receiver operating characteristic (ROC) analysis was performed to evaluate MYCOTB sensitivity and specificity based on different MYCOTB concentrations as the “critical concentration,” using APM results as the reference comparator. Data capture and validation were done using TeleForm 10.6 (Lake Forest, CA). SAS 9.3 (Cary, NC) was used for data manipulation as well as for computation of tables.
RESULTS
M. tuberculosis isolates (n = 234) were inoculated onto APM and MYCOTB plates. A total of 11 (4.7%) isolates had insufficient growth on APM plates, and 1 (0.4%) isolate had insufficient growth in the MYCOTB plate; these 12 isolates were excluded from the analysis. The analysis group therefore was comprised of 222 isolates having results on both DST platforms.
APM results.
Among these 222 isolates, the numbers (%) of isolates resistant by drug were 141 (63.5%) for isoniazid at 0.2 μg/ml, 122 (55.0%) for isoniazid at 1.0 μg/ml, 123 (55.4%) for rifampin, 91 (41.0%) for rifabutin, 112 (50.5%) for ethambutol at 5.0 μg/ml, 58 (26.1%) for ethambutol at 10.0 μg/ml, 63 (28.4%) for ofloxacin, 56 (25.2%) for moxifloxacin at 0.5 μg/ml, 24 (10.8%) for moxifloxacin at 2.0 μg/ml, 46 (20.7%) for streptomycin at 2.0 μg/ml, 37 (16.7%) for streptomycin at 10.0 μg/ml, 54 (24.3%) for amikacin, 62 (27.9%) for kanamycin, 22 (9.9%) for cycloserine, 85 (38.3%) for ethionamide, and 65 (29.3%) for para-aminosalicylic acid (PAS). By study APM testing, 43/222 (19.4%) isolates were classified as susceptible to all drugs tested, 64/222 (28.8%) were classified as multidrug-resistant TB (MDR-TB) isolates but not extensively drug-resistant TB (XDR-TB) isolates, 42/222 (18.9%) were classified as XDR-TB isolates, and 73/222 (32.9%) had other resistance patterns.
MYCOTB DST results.
Table 1 shows results for MYCOTB testing for all 222 M. tuberculosis isolates categorized by drug together with results for the reference comparator APM. Categorical agreement between methods was highest for rifampin (99.1%), amikacin (99.1%), isoniazid at 0.2 μg/ml (98.7%), kanamycin (98.2%), and streptomycin at 10.0 μg/ml (96.4%) and lowest for ethambutol (79.7% and 80.6% for ethambutol at 5.0 μg/ml and 10.0 μg/ml, respectively), moxifloxacin (84.7% and 82.9% for moxifloxacin at 0.5 μg/ml and 2.0 μg/ml, respectively), and cycloserine (81.9%). Results stratified by M. tuberculosis isolate source (Republic of Korea, Uganda, or challenge isolate) are provided in the supplemental material. Conditional agreement between methods was 96% or greater for all drugs except ethambutol (92.8% for both 5.0 μg/ml and 10.0 μg/ml) and moxifloxacin (91.9% and 89.6% for 0.5 μg/ml and 2.0 μg/ml, respectively). The range of agreement between MYCOTB results and APM results was also reflected in the ROC curve results for each drug, as shown in Fig. S1 and Table S4 in the supplemental material (16). Drugs with the highest agreement, such as rifampin, had a large area under the curve (AUC), while at the other end of the spectrum cycloserine had an AUC of 0.56.
MYCOTB plate sensitivity and specificity are also shown in Table 1. Of note are sensitivity for detection of resistance to cycloserine (18.2% [4/22]), to ethambutol at 5 μg/ml (64.3% [72/112]), to ethambutol at 10 μg/ml (31.0% [18/58]), and to ethionamide (80.0% [68/85]). Also of note are the low MYCOTB specificity results for moxifloxacin at 0.5 μg/ml (79.5% [132/166]) and for moxifloxacin at 2.0 μg/ml (81.3% [161/198]).
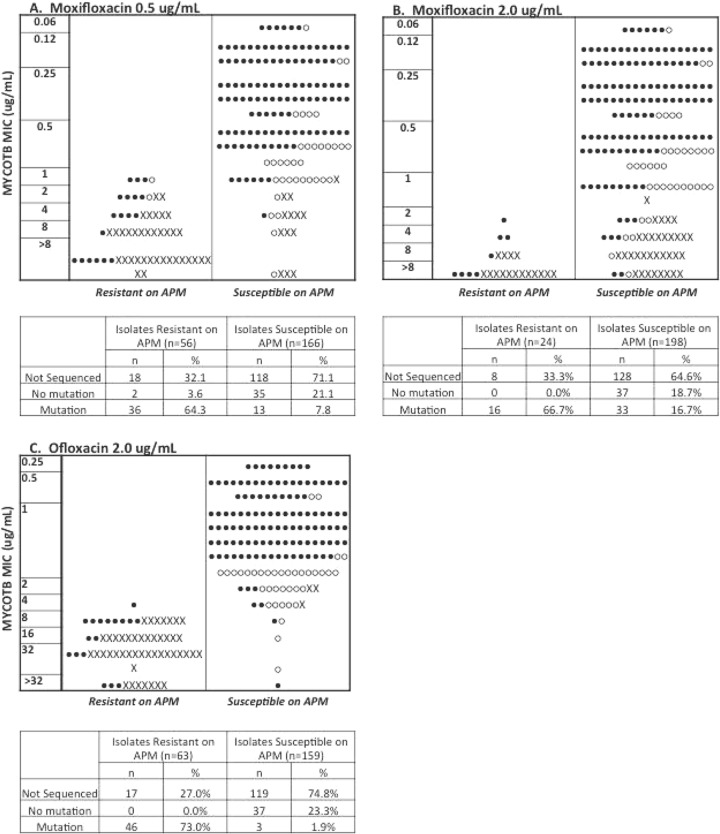
To better understand the nature of the observed discordances between the dichotomous APM results and MYCOTB MIC results, we plotted MYCOTB MIC values against APM results. For moxifloxacin at 0.5 μg/ml, 34/34 (100%) discordant isolates were MYCOTB resistant/APM susceptible (Fig. 2A), and for moxifloxacin at 2.0 μg/ml, 37/38 (97.3%) discordant isolates were MYCOTB resistant/APM susceptible (Fig. 2B). For moxifloxacin, the MYCOTB MICs were not clustered around the APM critical concentrations; for moxifloxacin at 0.5 μg/ml, 24/34 (70.6%) discordant isolates were MDR-TB with or without additional drug resistance, and for moxifloxacin at 2.0 μg/ml, 35/37 (94.6%) were MDR-TB with or without additional drug resistance. Results of previously performed DNA sequencing of the gyrA and gyrB quinolone resistance-determining region were available for 86 (38.7%) of the M. tuberculosis isolates (Fig. 2). When DNA sequencing results were considered a means of discrepant analysis, agreement between MYCOTB MIC and the reference comparator rose to 201/222 (90.5%) for moxifloxacin at 0.5 μg/ml and 213/222 (95.9%) for moxifloxacin at 2.0 μg/ml. There was no relationship between discordance and the interval between APM plate preparation and inoculation (data not shown).
FIG 2.
MICs as determined using the Sensititre MYCOTB MIC plate versus agar proportion method results for all tested isolates for moxifloxacin at 0.5 μg/ml (A), moxifloxacin at 2.0 μg/ml (B), and ofloxacin at 2.0 (C) μg/ml. Closed circles, DNA sequencing of gyrA and gyrB was not performed; open circles, DNA sequencing was performed but no mutation was identified; X, DNA sequencing was performed and a resistance-associated mutation was identified.
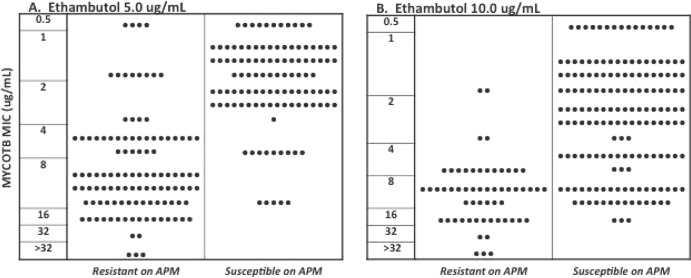
For ethambutol at 5.0 μg/ml, 40/45 (88.9%) discordant isolates were MYCOTB susceptible/APM resistant (Fig. 3A), and for ethambutol at 10.0 μg/ml, 40/43 (93.0%) discordant isolates were MYCOTB susceptible/APM resistant (Fig. 3B). As shown in Table 1, defining agreement to allow for ±1 doubling dilution (conditional agreement) substantially improved agreement between MYCOTB and APM by resolving discordance for 29/45 (64.2%) isolates not in categorical agreement for ethambutol at 5.0 μg/ml and for 27/43 (62.8%) isolates not in categorical agreement for ethambutol at 10.0 μg/ml, since many of the discordant isolates had MYCOTB MICs that were around the APM critical concentration (Fig. 3). There was no relationship between discordance and the interval between APM plate preparation and inoculation (data not shown).
FIG 3.
MICs as determined using the Sensititre MYCOTB MIC plate versus agar proportion method results for all tested isolates for (A) ethambutol at 5.0 μg/ml and (B) ethambutol at 10.0 μg/ml.
MYCOTB feasibility.
The median time to interpretation of MYCOTB plates was 10 days versus 21 days for APM (P < 0.0001). A total of 30 of 222 (13.5%) MYCOTB plates could be interpreted as early as day 7 of incubation, and 35/222 (15.8%) required 21 days of incubation before being deemed interpretable based on control well growth. Between two independent readers, overall agreement for 222 isolates with 12 drugs/isolate (2,664 readings) was 2,617/2,664 (98.2%) with respect to susceptibility or resistance, 2,307/2,664 (86.6%) for the exact MIC, and 2,624/2,664 (98.5%) for MIC ± 1 doubling dilution. Interreader agreement for each drug is shown in Table 2.
TABLE 2.
Interreader agreement for Sensititre MYCOTB MIC plate interpretation, by drug
| Drug (n = 222) | % agreement as susceptible or resistant | % agreement for exact MIC | % agreement for MIC ± 1 doubling dilution |
|---|---|---|---|
| Isoniazid | 98.7 | 89.6 | 98.7 |
| Rifampin | 100.0 | 96.0 | 100.0 |
| Rifabutin | 98.7 | 90.5 | 99.1 |
| Ethambutol | 97.3 | 86.5 | 99.6 |
| Ofloxacin | 98.2 | 84.7 | 99.1 |
| Moxifloxacin | 96.4 | 84.7 | 98.2 |
| Streptomycin | 99.1 | 88.3 | 99.1 |
| Amikacin | 99.5 | 89.2 | 98.7 |
| Kanamycin | 99.5 | 89.2 | 99.6 |
| Cycloserine | 96.4 | 81.5 | 99.6 |
| Ethionamide | 99.5 | 82.0 | 98.2 |
| PASa | 95.5 | 77.5 | 92.3 |
Abbreviation: PAS, para-aminosalicylic acid.
Reproducibility of MYCOTB results was assessed by repeat MYCOTB testing of the 69 isolates for which one or more drugs had an absence of conditional (or categorical) agreement between MYCOTB and APM on initial testing. Fresh inocula were prepared each time. Between the initial and repeat MYCOTB tests, the overall agreement for 69 isolates with 12 drugs/isolate (828 readings) was 766/828 (92.6%) with respect to susceptibility or resistance, 475/828 (57.4%) for the exact MIC, and 734/828 (88.7%) for the MIC ± 1 doubling dilution. Reproducibility of results for each drug is shown in Table 3.
TABLE 3.
Reproducibility of results for the initial and repeat testing by Sensititre MYCOTB MIC plate, by drug
| Drug (n = 69) | % agreement as susceptible or resistant | % agreement for exact MIC | % agreement for MIC ± 1 doubling dilution |
|---|---|---|---|
| Isoniazid | 100.0 | 68.1 | 95.7 |
| Rifampin | 98.6 | 82.6 | 92.8 |
| Rifabutin | 92.8 | 50.7 | 76.8 |
| Ethambutol | 95.7 | 65.1 | 98.6 |
| Ofloxacin | 92.8 | 47.8 | 89.9 |
| Moxifloxacin | 94.2 | 50.7 | 92.8 |
| Streptomycin | 81.2 | 39.1 | 87.0 |
| Amikacin | 100.0 | 63.8 | 95.7 |
| Kanamycin | 97.1 | 69.6 | 95.7 |
| Cycloserine | 84.1 | 56.5 | 92.8 |
| Ethionamide | 85.5 | 47.8 | 82.6 |
| PASa | 89.9 | 46.4 | 65.2 |
Abbreviation: PAS, para-aminosalicylic acid.
DISCUSSION
We assessed the feasibility and accuracy of a novel commercially available microtiter plate for testing of the susceptibility of M. tuberculosis to first- and second-line drugs. Among the cohort of tested isolates, almost half were resistant to at least isoniazid and rifampin by the reference method. Main findings from our study were that agreement between the investigational MYCOTB MIC method and the comparator APM was ≥92% for 7/12 drugs when a strict definition of agreement was used and was ≥96% (except for ethambutol and moxifloxacin) when agreement was defined by allowing a ±1 doubling dilution around the APM critical concentration. The median time to MYCOTB plate interpretation was 10 days, which was shorter than the 21 days used for APM. Agreement, with respect to susceptibility or resistance of each isolate, between two MYCOTB readers was ≥95% for all drugs.
Performance of the MYCOTB plate was least good for moxifloxacin, ethambutol, cycloserine, and ethionamide. For moxifloxacin, discordance was driven by the relatively large numbers of isolates that were categorized as resistant by MYCOTB and susceptible by APM. DNA sequencing resolved the discrepancy in favor of the MYCOTB plate for most of these isolates, and there was good correlation between ofloxacin results by MYCOTB and APM and DNA sequencing, thereby indicating a potential problem with the moxifloxacin APM testing. Most of the isolates with discordant results were resistant to multiple other drugs and were noted to grow slowly, and yet growth on drug-free APM media was considered adequate for APM interpretation.
Ethambutol phenotypic DST is well-known to be challenging, with low interlaboratory agreement (18, 19). There are several issues that warrant consideration. The APM critical concentrations of 5 μg/ml and 10 μg/ml each split the upper end of the wild-type MIC distribution, and thus, even among wild-type strains, small variations in laboratory methodology can contribute to oscillation of results between susceptible and resistant assessments (9, 20). This may have played a role in our study, since we observed that a large proportion of the discordant isolates had MYCOTB MICs within 1 doubling dilution of the critical concentration. In addition, in a proficiency program conducted by the U.S. Centers for Disease Control and Prevention in which cultures of known ethambutol susceptibility or resistance status were sent to participating laboratories over a 15-year period, there were substantial differences in performance between the 7H10 APM and the liquid culture MGIT method (critical concentration of 5 μg/ml for each method). Specifically, MGIT detected as resistant only 48.3% of strains with expected ethambutol resistance, leading the authors to conclude that re-evaluation of ethambutol critical concentrations is warranted (19). Notably, in our study, using the liquid MYCOTB method, the direction of discordance (driven mostly by isolates categorized as susceptible by MYCOTB and resistant by APM) was the same as that observed in the CDC program. In light of the findings of others and the fact that our study used a cohort of M. tuberculosis isolates enriched for drug resistance, our finding of reduced agreement between MYCOTB and APM is therefore not surprising.
A strength of our study was the large number of highly resistant M. tuberculosis isolates, thereby extending the existing studies published by Hall et al. and Abuali et al. (14, 15). A limitation of our study was that we assessed MYCOTB reproducibility only for the 69/222 (31.1%) isolates for which one or more drugs had an absence of conditional (or categorical) agreement between MYCOTB and APM. This approach selected for a subset of isolates that were “challenging” from a phenotypic susceptibility testing perspective, and our reproducibility results likely represent a worst-case scenario.
A challenge that we encountered was that of interpreting MYCOTB results for drugs for which the APM critical concentration was not contained on the MYCOTB plate (i.e., isoniazid at 2.0 μg/ml, ethambutol, streptomycin at 10.0 μg/ml, and cycloserine). For uniform handling across drugs, we used strict parameters to define “susceptibility” and “resistance” by MYCOTB, recognizing that doing so might, in some instances, result in an absence of agreement where agreement might truly exist. To account for this, and to provide information about proximity of observed MYCOTB MICs to the prescribed APM critical concentrations, we also used an alternative definition of agreement—“conditional agreement”—that allowed for a ±1 MYCOTB doubling dilution around the APM critical concentration. The ROC curve analyses provide additional insight into the relationship between MYCOTB and APM results and can help MYCOTB users select drug critical concentrations that are most appropriate for specific clinical situations, especially for those drugs for which no one MYCOTB drug concentration provided strong agreement with the APM reference comparator.
For MYCOTB, the decrement in the median time to result compared with APM was modest (10 days versus 21 days). However, in practice, M. tuberculosis DST, when performed, is typically conducted in a stepwise fashion, with testing of second-line drugs done only after testing of first-line drugs. Delays in recognition and appropriate treatment for polydrug-resistant TB can result in morbidity and further acquisition of resistance for the patient, as well as ongoing transmission (21, 22). While emerging rapid molecular tests for rifampin resistance represent a leap forward for TB diagnostics, they nevertheless underscore the need for accurate and prompt second-line DST for optimization of therapy to facilitate treatment success and avoid acquisition of further resistance. The MYCOTB plate includes first-line as well as second-line drugs and therefore has the potential to provide, all at once, a comprehensive picture of the therapeutic options for a patient.
The MYCOTB plate is an attractive platform for M. tuberculosis drug susceptibility testing for several additional reasons. First, it incorporates second-line drugs in a testing kit that is manufactured using quality controls, thereby eliminating the need for local preparation and maintenance of drug stocks and solutions—a source of error and variability over time and across laboratories and of technical complexity and labor requirements that have relegated second-line DST to a small number of specialized laboratories. Second, determination of MICs can facilitate regimen optimization, a critical issue for management of polydrug-resistant TB. Knowing precisely the susceptibility of an isolate is particularly important for those drugs, such as isoniazid, for which heterogeneity in phenotypic resistance occurs and for which the drug dose can be escalated safely in order to overcome the phenotype of decreased drug susceptibility (11, 13). This principle was recognized 50 years ago by Canetti, who wrote the following. “We consider that the best type of sensitivity test is a fully quantitative determination in which the organisms' capability of growth on medium containing a wide range of drug concentrations is known. This type of test would provide full information on the degree of resistance. However, since such a test requires large amounts of medium and is time-consuming, it cannot be recommended as a routine procedure” (8). The MYCOTB plate represents meaningful progress in overcoming these problems and is an important step toward enabling therapeutic drug monitoring for TB (23). Adaptation of the MYCOTB plate to incorporate a readout that can be detected more quickly after inoculation than with the present readout of visible growth would be useful, as would incorporation of pyrazinamide into the plate. In accordance with World Health Organization biosafety guidelines, because of the required manipulations of culture isolates, the MYCOTB method would be suitable for biosafety level 2-plus laboratories (24). Use of the MYCOTB plate in conjunction with rapid molecular tests for isoniazid and/or rifampin resistance is particularly attractive, as specimens (or patients) identified as having resistance by rapid molecular testing could be targeted immediately for phenotypic testing against the MYCOTB array of commonly used first- and second-line drugs.
Supplementary Material
ACKNOWLEDGMENTS
We gratefully acknowledge the following individuals who contributed to this study: in Uganda, Eric Bugumirwa and Maria Nassolo, and in South Korea, Hyunkyung Kwak and Eunjin Cho. We also gratefully acknowledge David Hom for logistical support. We thank Armand Van Deun at the International Union Against Tuberculosis and Lung Disease for permission to use 25 M. tuberculosis challenge isolates.
Sensititre MYCOTB MIC plates were generously donated by Trek Diagnostic Systems. Nadine Sullivan at Trek Diagnostic Systems and Emma Scopes at ThermoFisher Scientific provided assistance with training related to MYCOTB plates.
Neither Trek Diagnostic Systems personnel nor ThermoFisher Scientific personnel had a role in the study design, implementation, analysis, manuscript writing, or decision to submit the study findings for publication.
This work was funded by contract HHSN2722000900050C from the National Institute of Allergy and Infectious Diseases (NIAID) and in part by the U.S. Intramural Research Program of NIAID, National Institutes of Health, Department of Health and Human Services.
Footnotes
Published ahead of print 7 October 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.01209-13.
REFERENCES
- 1.World Health Organization 2012. Global tuberculosis report. World Health Organization, Geneva, Switzerland [Google Scholar]
- 2.Centers for Disease Control and Prevention 2006. Emergence of Mycobacterium tuberculosis with extensive resistance to second-line drugs-worldwide, 2000–2004. Mortal MMWR Morb. Mortal. Wkly. Rep. 55:301–305 [PubMed] [Google Scholar]
- 3.Boehme CC, Nabeta P, Hillemann D, Nicol MP, Shanai S, Krapp F, Allen J, Tahirli R, Blakemore R, Rustomjee R, Milovic A, Jones M, O'Brien SM, Persing DH, Ruesch-Gerdes S, Gotuzzo E, Rodrigues C, Alland D, Perkins MD. 2010. Rapid molecular detection of tuberculosis and rifampin resistance. N. Engl. J. Med. 363:1005–1015. 10.1056/NEJMoa0907847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boehme CC, Nicol MP, Nabeta P, Michael JS, Gotuzzo E, Tahirli R, Gler MT, Blakemore R, Worodria W, Gray C, Huang L, Caceres T, Mehdiyev R, Raymond L, Whitelaw A, Sagadevan K, Alexander H, Albert H, Cobelens F, Cox H, Alland D, Perkins MD. 2011. Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicentre implementation study. Lancet 377:1495–1505. 10.1016/S0140-6736(11)60438-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanrahan CF, Dorman SE, Erasmus L, Koornhof H, Coetzee G, Golub JE. 2012. The impact of expanded testing for multidrug resistant tuberculosis using Genotype MTBDRplus in South Africa: an observational cohort study. PLoS One. 7:e49898. 10.1371/journal.pone.0049898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barnard M, Warren R, Van Pittius NG, van Helden P, Bosman M, Streicher E, Coetzee G, O'Brien R. 2012. GenoType MTBDRsl line probe assay shortens time to diagnosis of extensively drug-resistant tuberculosis in a high-throughput diagnostic laboratory. Am. J. Respir. Crit. Care Med. 186:1298–1305. 10.1164/rccm.201205-0960OC [DOI] [PubMed] [Google Scholar]
- 7.Ramaswamy S, Musser JM. 1998. Molecular genetic basis of antimicrobial agent resistance in Mycobacterium tuberculosis: 1998 update. Tuber. Lung Dis. 79:3–29. 10.1054/tuld.1998.0002 [DOI] [PubMed] [Google Scholar]
- 8.Canetti G, Froman S, Grosset J, Hauduroy P, Langerova M, Mahler HT, Meissner G, Mitchison DA, Sula L. 1963. Mycobacteria: laboratory methods for testing drug sensitivity and resistance. Bull. World Health Organ. 29:565–578 [PMC free article] [PubMed] [Google Scholar]
- 9.Schön T, Juréen P, Giske CG, Chryssanthou E, Sturegård E, Werngren J, Kahlmeter G, Hoffner SE, Angeby KA. 2009. Evaluation of wild-type MIC distributions as a tool for determination of clinical breakpoints for Mycobacterium tuberculosis. J. Antimicrob. Chemother. 64:786–793. 10.1093/jac/dkp262 [DOI] [PubMed] [Google Scholar]
- 10.Böttger EC. 2011. The ins and outs of Mycobacterium tuberculosis drug susceptibility testing. Clin. Microbiol. Infect. 17:1128–1134. 10.1111/j.1469-0691.2011.03551.x [DOI] [PubMed] [Google Scholar]
- 11.Nuermberger E, Grosset J. 2004. Pharmacokinetic and pharmacodynamic issues in the treatment of mycobacterial infections. Eur. J. Clin. Microbiol. Infect. Dis. 23:243–255. 10.1007/s10096-004-1109-5 [DOI] [PubMed] [Google Scholar]
- 12.Meier A, Sander P, Schaper KJ, Scholz M, Böttger EC. 1996. Correlation of molecular resistance mechanisms and phenotypic resistance levels in streptomycin-resistant Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 40:2452–2454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Soolingen D, de Haas PE, van Doorn HR, Kuijper E, Rinder H, Borgdorff MW. 2000. Mutations at amino acid position 315 of the katG gene are associated with high-level resistance to isoniazid, other drug resistance, and successful transmission of Mycobacterium tuberculosis in the Netherlands. J. Infect. Dis. 182:1788–1790. 10.1086/317598 [DOI] [PubMed] [Google Scholar]
- 14.Hall L, Jude KP, Clark SL, Dionne K, Merson R, Boyer A, Parrish NM, Wengenack NL. 2012. Evaluation of the Sensititre MycoTB plate for susceptibility testing of the Mycobacterium tuberculosis complex against first- and second-line agents. J. Clin. Microbiol. 50:3732–3734. 10.1128/JCM.02048-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abuali MM, Katariwala R, LaBombardi VJ. 2012. A comparison of the Sensititre MYCOTB panel and the agar proportion method for the susceptibility testing of Mycobacterium tuberculosis. Eur. J. Clin. Microbiol. Infect. Dis. 31:835–839. 10.1007/s10096-011-1382-z [DOI] [PubMed] [Google Scholar]
- 16.Clinical Laboratory Standards Institute 2003. Susceptibility testing of mycobacteria, nocardia, and other aerobic actinomycetes: approved standard. CLSI document M24-A (ISBN 1-56238-550-3) Clinical Laboratory Standards Institute, Wayne, PA: [PubMed] [Google Scholar]
- 17.Takiff HE, Salazar L, Guerrero C, Philipp W, Huang WM, Kreiswirth B, Cole ST, Jacobs WR, Jr, Telenti A. 1994. Cloning and nucleotide sequence of Mycobacterium tuberculosis gyrA and gyrB genes and detection of quinolone resistance mutations. Antimicrob. Agents Chemother. 38:773–780. 10.1128/AAC.38.4.773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Madison B, Robinson-Dunn B, George I, Gross W, Lipman H, Metchock B, Sloutsky A, Washabaugh G, Mazurek G, Ridderhof J. 2002. Multicenter evaluation of ethambutol susceptibility testing of Mycobacterium tuberculosis by agar proportion and radiometric methods. J. Clin. Microbiol. 40:3976–3979. 10.1128/JCM.40.11.3976-3979.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Angra PK, Taylor TH, Iademarco MF, Metchock B, Astles JR, Ridderhof JC. 2012. Performance of tuberculosis drug susceptibility testing in U.S. laboratories from 1994 to 2008. J. Clin. Microbiol. 50:1233–1239. 10.1128/JCM.06479-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ängeby K, Juréen P, Kahlmeter G, Hoffner SE, Schön T. 2012. Challenging a dogma: antimicrobial susceptibility testing breakpoints for Mycobacterium tuberculosis. Bull. World Health Organ 90:693–698. 10.2471/BLT.11.096644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calver AD, Falmer AA, Murray M, Strauss OJ, Streicher EM, Hanekom M, Liversage T, Masibi M, van Helden PD, Warren RM, Victor TC. 2010. Emergence of increased resistance and extensively drug-resistant tuberculosis despite treatment adherence, South Africa. Emerg. Infect. Dis. 16:267–271. 10.3201/eid1602.090968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cox HS, Sibilia K, Feuerriegel S, Kalon S, Polonsky J, Khamraev AK, Rusch-Gerdes S, Mills C, Niemann S. 2008. Emergence of extensive drug resistance during treatment for multidrug-resistant tuberculosis. N. Engl. J. Med. 359:2398–2400. 10.1056/NEJMc0805644 [DOI] [PubMed] [Google Scholar]
- 23.Babalik A, Babalik A, Mannix S, Francis D, Menzies D. 2011. Therapeutic drug monitoring in the treatment of active tuberculosis. Can. Respir. J. 18:225–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization 2012. Tuberculosis laboratory biosafety manual. WHO/HTM/TB/2012.11 World Health Organization, Geneva, Switzerland: [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.