Abstract
The adverse effects of azithromycin on the treatment of patients with chronic lung diseases (CLD) were evaluated in the present study. MEDLINE and other databases were searched for relevant articles published until August 2013. Randomized controlled trials that enrolled patients with chronic lung diseases who received long-term azithromycin treatment were selected, and data on microbiological studies and azithromycin-related adverse events were abstracted from articles and analyzed. Six studies were included in the meta-analysis. The risk of bacterial resistance in patients receiving long-term azithromycin treatment was increased 2.7-fold (risk ratio [RR], 2.69 [95% confidence interval {95% CI}, 1.249, 5.211]) compared with the risk in patients receiving placebo treatment. On the other hand, the risk of bacterial colonization decreased in patients receiving azithromycin treatment (RR, 0.551 [95% CI, 0.460, 0.658]). Patients receiving long-term azithromycin therapy were at risk of increased impairment of hearing (RR, 1.168 [95% CI, 1.030, 1.325]). This analysis provides evidence supporting the idea that bacterial resistance can develop with long-term azithromycin treatment. Besides the increasingly recognized anti-inflammatory role of azithromycin used in treating chronic lung diseases, we should be aware of the potential for adverse events with its long-term use.
INTRODUCTION
Recently, two reports analyzed leading causes of death and indicated that, in the United States and China in 2010, chronic obstructive pulmonary disease (COPD) ranked as the top 4th and 3rd, respectively, in terms of years of life lost and that lung cancer, which is closely related to chronic lung inflammatory diseases, ranked 2nd and 5th. Furthermore, compared with the declining numbers for death in patients with ischemic heart disease and stroke, the number of patients that died of COPD and lung cancer increased (1, 2). Hence, controlling the development of chronic inflammatory lung diseases, including COPD, asthma, interstitial lung diseases, bronchiectasis, and cystic fibrosis, has been increasingly recognized as a challenging but necessary task. However, we are still far from knowing how to control those diseases, and millions of lives will be lost to them in the future. Chronic lung diseases (CLD) produce a tendency for acute episodes. For instance, patients with COPD tend to have episodes of acute exacerbations which incur decreased lung function and increased morbidity and mortality. Each exacerbation needs upgraded medications which have tremendous health and economic costs. Despite endeavors to relieve patients by using various strategies, patients may still have as many as 1.4 acute exacerbations per year, on average (3). Therefore, any effort that results in improving the outcome of patients with CLD will be a great step forward.
Azithromycin, containing a macrocyclic 15-membered lactone ring with excellent tissue penetration and antimicrobial activity against a broad range of Gram-positive and Gram-negative bacteria (4), is widely used in the clinical setting. Besides its antibacterial activity, its anti-inflammatory roles have been increasingly recognized (3, 4). In recent years, macrolide antibiotics have been explored in several clinical trials for their effects on patients with CLD, including COPD, asthma, cystic fibrosis (CF), and non CF-bronchiectasis (3, 5–13). Recently, several meta-analyses collected the data and confirmed that the long-term use of macrolides improves the quality of life of CLD patients by improving lung function and decreasing the frequency of acute exacerbations (14, 15). However, no study has focused on their detrimental effects, especially on microbiological aspects. To evaluate the safety of long-term azithromycin use in CLD patients, we collected randomized controlled trials evaluating the microbiological changes and azithromycin-related adverse events among patients receiving long-term therapy.
MATERIALS AND METHODS
Search strategy.
We sought to identify all potentially relevant clinical trials using searches of Web-based databases (MEDLINE [1996 to 2013], ISI Web of Knowledge [1996 to 2013], The Cochrane Central Register of Controlled Trials [1996 to 2013], and EMBASE). The search was performed in August 2013. The search terms were “azithromycin,” “(chronic lung diseases OR asthma OR COPD OR cystic fibrosis OR bronchiectasis),” and “randomized controlled trials.” Potentially relevant studies were retrieved and reviewed by two reviewers.
Selection criteria.
Studies were included in our analysis if they met the following criteria: (i) their design was a prospective, randomized controlled trial (RCT); (ii) they included patients with chronic lung diseases, including COPD, asthma, cystic fibrosis, and bronchiectasis; (iii) they randomized patients to a strategy of azithromycin therapy for at least 3 months or a parallel control group; and (iv) they reported microbiological studies of either new colonization of bacteria in the upper and lower respiratory tract or isolation of resistant bacteria during the study.
Data extraction.
Two reviewers (H.L. and D.-H.L.) independently extracted the following data from each study: first author, year of publication, study design, number of individuals initially enrolled, number of participants evaluated, details of azithromycin use, and number of patients managed with versus without azithromycin therapy. Data on microbiological studies, including new colonization of bacteria and isolation of macrolide-resistant or overall-resistant bacteria during the study, mortality, hospitalization and antibiotic use during the study period, and azithromycin-related side effects, including gastrointestinal and hearing impairments, were also extracted from the articles. Regarding the assessment of the methodological quality for the RCTs included in the meta-analysis, we followed the recommendations in the Cochrane handbook for systematic reviews of interventions and summarized in a domain-based evaluation the following components: randomization, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and other sources of bias.
Outcomes.
Our primary outcomes were changes in microbiological patterns and the resistance of isolates from the respiratory tract between enrollment and the termination of the study. Our secondary outcomes were use of antibiotics and adverse events, including hearing impairment and gastrointestinal reactions.
Statistical analysis.
We assessed the heterogeneity between trials using the χ2 and I2 tests. The I2 statistic approximates the proportion of total variation in the effect size estimates that is due to heterogeneity rather than sampling error. I2 values above 25%, 50%, and 75% were taken as indicators of mild, modest, and high heterogeneity, respectively, and a P value lower than 0.10 to be statistically significant. We calculated pooled risk ratios (RR) and 95% confidence intervals (CI) for all primary and secondary outcomes using the Mantel-Haenszel fixed effects and random effects models. For all analyses, the results from the fixed effects model are presented only when there was no heterogeneity between trials; otherwise, the results from the random effects model are presented. Sensitivity analyses were undertaken when high heterogeneity appeared.
RESULTS
Selected studies.
The process of identifying eligible studies is presented in Fig. 1. The search criteria identified 91 potentially relevant articles. Of these 91 articles, 73 were excluded from this meta-analysis due to unrelated contents, non-English text, or other reasons (shown in Fig. 1). In addition, 2 studies that did not evaluate the relevant side effects, 1 RCT in which azithromycin was administered for less than 12 weeks, 2 RCTs which had no placebo control, 1 study that analyzed the long-term effect of azithromycin for chronic rhinosinusitis, and 2 RCTs for transplanted patients were excluded from this analysis. Overall, 10 articles reporting on RCTs were retained for further screening. However, 4 of these did not collect the microbiological data of interest (10–13). Thus, we ruled out these 4 studies for our analysis, since we were interested in the underlying adverse effects of long-term azithromycin use on microbiological changes in the treated patients. Therefore, 6 RCTs were retained for this analysis (3, 5–9).
FIG 1.
Flow chart depicting the number of studies included at each stage of the selection process. RCT, randomized controlled trial.
Study methodology and quality.
All 6 clinical trials were identified by our research strategy as prospective randomized controlled trials (Table 1). All of the studies randomized patients on a 1:1 basis to either an azithromycin treatment group or a placebo control group. All 6 studies used intention-to-treat analysis to analyze their endpoint during their statistical procedure. Of these, Altenburg's study used a modified intention-to-treat method, i.e., they removed the data for six patients, including two patients in the azithromycin group and four patients in the placebo group, for the final statistical analysis because these patients did not receive the related treatment at the beginning (9). Five studies used the double-blind method for blinding treated patients, and Albert's study was unblinded (5–9). Two of the 6 studies used azithromycin according to the weight of patients, because they enrolled children in their studies (5, 6). Another 3 of the 6 studies administered azithromycin at a dose of 250 mg for different time periods (3, 8, 9). Another study used 500 mg of azithromycin for their experimental groups (7). The patients in 2 studies took azithromycin for 1 year (3, 9), and the other 4 studies administered azithromycin for approximately 6 months (5–8). All trials treated their control group by using a placebo with the same appearance, according to the current published guidelines. Only two studies set up a run-up time period free of exacerbations to ensure the stability of the patients for at least 2 weeks before enrollment (8, 9). To exclude the influence of antibiotic therapy, 5 of the 6 studies set up exclusion criteria for patients treated with antibiotics, including macrolides and quinolones, for at least 14 days before enrollment (5–9). Albert et al. did not mentioned exclusion criteria of antibiotic use before enrollment, but patients in their study were at a stable stage of COPD which had not exhibited an acute exacerbation of COPD for at least 4 weeks before enrollment (3). Only 2 studies set up a washout time period to exclude or investigate the influence of prolonged effect of the treatment (3, 8).
TABLE 1.
Characterization of enrolled trials and extracted data
| Parametera | Groupb | Criterion or value from trial ofc: |
|||||
|---|---|---|---|---|---|---|---|
| Albert et al. (3) | Saiman et al. (5) | Saiman et al. (6) | Wong et al. (7) | Brusselle et al. (8) | Altenburg et al. (9) | ||
| CLD of patients | COPD | Cystic fibrosis | Cystic fibrosis | Bronchiectasis | Asthma | Bronchiectasis | |
| Azithromycin regimen | 250 mg/day for 1 yr | 250 mg (wt < 40 kg) or 500 mg (wt ≥ 40 kg) oral azithromycin 3 days/wk for 168 days | 250 mg (wt 18–35.9 kg) or 500 mg (wt ≥ 36 kg) oral azithromycin 3 days/wk for 168 days | 500 mg azithromycin 3 days/wk for 6 mo | 250 mg/day for 5 days followed by 3 days/wk for total of 26 wk | 250 mg/day for 1 yr | |
| Total no. of randomized patients | 1,142 | 185 | 263 | 141 | 109 | 89 | |
| No. of fully compliant patients | A | 495 | 83 | 125 | 67 | 53 | 42 |
| C | 502 | 92 | 124 | 60 | 49 | 39 | |
| Age [yr; mean ± SD or mean (IQR)] | A | 65 ± 9 | 20.2 ± 7.9 | 10.7 ± 3.25 | 60.9 ± 13.6 | 53 (46–64) | 59.9 ± 12.3 |
| C | 66 ± 8 | 20.6 ± 8.6 | 10.6 ± 3.10 | 59.0 ± 13.3 | 53 (36–60) | 64.6 ± 9.1 | |
| FEV1 (% of predicted; mean ± SD) | A | 39 ± 16 | 68.3 ± 21.3 | 97.7 ± 16.4 | 67.1 ± 21.2 | 80.1 ± 21.9 | 77.7 ± 24.4 |
| C | 40 ± 16 | 70.6 ± 23.7 | 99.65 ± 16.65 | 67.3 ± 23.2 | 84.8 ± 20.7 | 82.7 ± 27.2 | |
| FEV1/FVC (mean ± SD) | A | 42 ± 13 | 83.2 ± 18.4 | 65.4 ± 12.5 | 66.8 ± 12.3 | ||
| C | 43 ± 13 | 83.8 ± 20.0 | 64.7 ± 11.8 | 67.8 ± 12.1 | |||
| No. of subjects positive/no. tested for: | |||||||
| New bacterial colonization | A | 66/558 | 23/84 | 82/969 | 6/46 | ||
| C | 172/559 | 39/92 | 45/905 | 10/45 | |||
| Resistant bacteria | A | 38/47 | 4/84 | 52/331 | 2/46 | 20/23 | 53/60 |
| C | 44/108 | 4/92 | 13/293 | 0/45 | 8/23 | 29/112 | |
| No. of patients receiving: | |||||||
| Oral antibiotic | A | 60 | 65 | ||||
| C | 91 | 99 | |||||
| Intravenous antibiotic | A | 18 | 11 | ||||
| C | 30 | 14 | |||||
| No. of patients experiencing: | |||||||
| Hearing impairment | A | 142 | 1 | 5 | |||
| C | 110 | 1 | 4 | ||||
| Gastrointestinal symptoms | A | 15 | 76 | 17 | 19 | 6 | 23 |
| C | 21 | 54 | 23 | 9 | 18 | 8 | |
IQR, interquartile range; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity.
A, azithromycin group; C, control group.
All trials were multicenter, RCT, and double-blinded except that Albert et al. (3) was unblinded. All studies used intention-to-treat analysis.
Patient population.
A total of 1,929 patients were randomized to either azithromycin groups or placebo/control groups. Of these, 1,731 patients completed the full-course investigation. All studies declared that there were no significant differences between both study groups at baseline with respect to age, lung function, or smoking history. The details of population characteristics regarding the different studies are shown in Table 1. Two of the 6 trials enrolled patients, including children older than 6 years for their investigations because they investigated the long-term effect of azithromycin for patients with cystic fibrosis, which is a common, lethal, inherited disease among Caucasians that develops from early childhood (5, 6). Of these 6 trials, 1 trial studied patients with COPD (3) and 1 studied patients with asthma (8). In addition, 2 trials investigated patients with non-cystic fibrosis bronchiectasis (7, 9), and the last 2 focused on cystic fibrosis (5, 6). For COPD patients, the parameter of percentage of forced expiratory volume in 1 s (FEV1) of the predicted value indicated that patients with severity from the moderate to severe stage were enrolled. The other trials enrolled patients with mild to moderate impairment of lung function.
Effects of long-term azithromycin use on the changes in microbiological patterns.
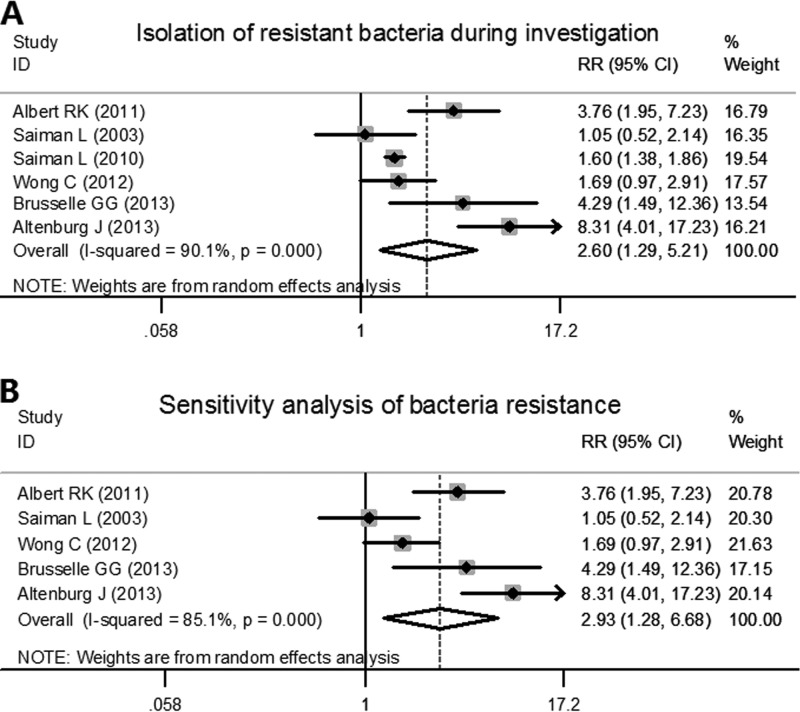
All of the studies performed bacterial resistance analyses. Of these, 4 studies determined the macrolide susceptibility of related bacteria isolated during the investigation, and the other 2 studies listed not only macrolide-resistant bacteria but also methicillin-resistant Staphylococcus aureus. We collected the data regardless of different species or resistance to different antibiotics. Significant heterogeneity was found among the trials analyzed (χ2 = 50.47, P < 0.001, I2 = 90.1%). By using the Mantel-Haenszel random effects model, patients in azithromycin groups were at a risk of a higher rate of bacterial resistance compared with patients in placebo groups during the study period (pooled RR, 2.597 [95% CI, 1.294, 5.211], z = 2.69, P = 0.007) (Fig. 2A). For the sensitivity analysis, we excluded the Saiman et al. study from 2010 (6) because they reported the results as bacterial colony counts rather than as the number of patients that carried resistant bacteria. With a decreased heterogeneity to some degree (χ2 = 26.93, P < 0.001, I2 = 85.1%), the results remained stable and exhibited the same trend regarding bacterial resistance during the study period for the azithromycin-treated patients (pooled RR, 2.927 [95% CI, 1.282, 6.683], z = 2.55, P = 0.011) (Fig. 2B).
FIG 2.
Forest plots for risk ratio (RR) of bacterial resistance during the investigations in azithromycin-treated patients and placebo patients. (A) Plot includes all six RCTs used in the meta-analysis. (B) For the sensitivity analysis of bacterial resistance, the 2010 study of Saimen et al. (6) was excluded. Albert, 3; Saiman 2003, 5; Saiman 2010, 6; Wong, 7; Brusselle, 8; Altenburg, 9. % weight represents the weight of each study among the included studies. RR represents the risk ratio. Horizontal lines represent the 95% CI. The pooled RR and its 95% CI are indicated by the unshaded diamond. The black dot indicates the RR in each study, with a gray square indicating the weight of this study.
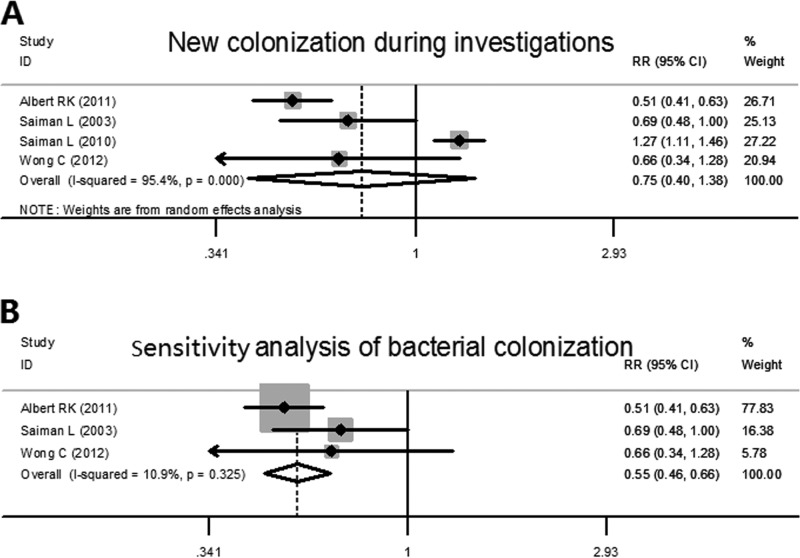
Four studies observed newly colonized bacteria from different sites or samples, including nasopharyngeal, sputum, and throat cultures. Significant heterogeneity was found among the trials analyzed (χ2 = 64.95, P < 0.001, I2 = 95.4%). Then, a random effect analyzer was used for further evaluation and indicated that there was no significant difference between azithromycin groups and placebo groups for newly detected colonization (pooled RR, 0.747 [95% CI, 0.404, 1.381], z = 0.93, P = 0.352) (Fig. 3A). As the study of Saiman et al. from 2010 used a different method to count new colonizations (newly detected bacteria rather than patients with new colonization), it might have led to the high heterogeneity and could have affected the result. We therefore excluded this study for the analysis and found that decreased heterogeneity existed among the studies (χ2 = 2.25, P = 0.325, I2 = 10.9%). The further analysis indicated that, compared with placebo groups, patients in azithromycin groups had a lower risk of new colonization by bacteria during the investigations (pooled RR, 0.551 [95% CI, 0.460, 0.658], z = 6.54, P < 0.001) (Fig. 3B).
FIG 3.
Forest plots for risk ratio of new detection of bacterial colonization in azithromycin-treated patients and placebo patients. (A) Plot includes four of the RCTs. (B) For the sensitivity analysis of new colonization, the 2010 study of Saimen et al. (6) was excluded.
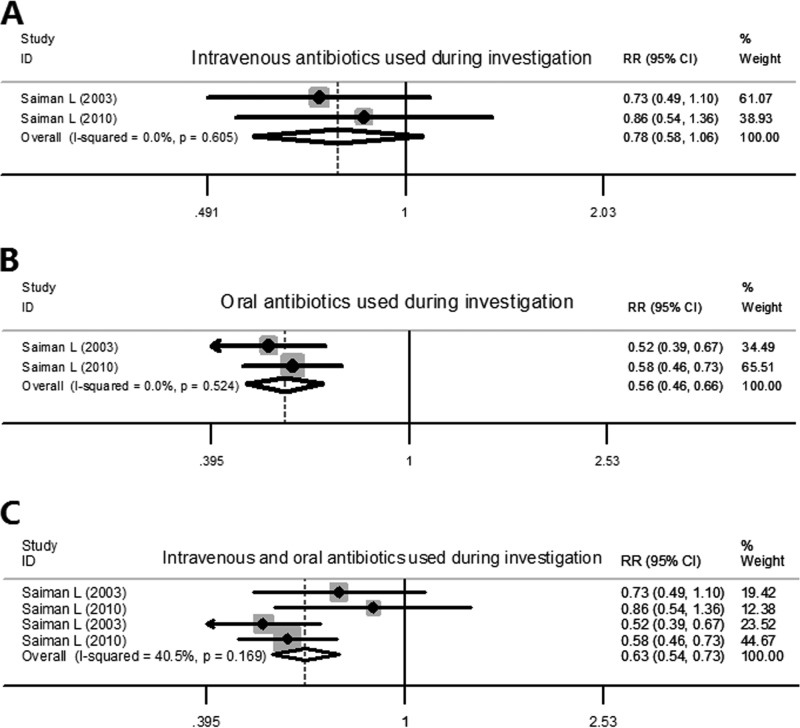
Since it seemed that patients in azithromycin groups had a trend toward carrying resistant bacteria, we speculated that the antibiotic prescription might have been increased for these patients. Only two trials recorded information regarding antibiotic treatment of patients. In patients that received intravenous antibiotics, we found that there were no significant differences between patients in the azithromycin and placebo groups (pooled RR, 0.784 [95% CI, 0.579, 1.060], z = 1.58, P = 0.114) (Fig. 4A). However, patients in azithromycin groups showed a reduction in oral antibiotic use, with a pooled RR value of 0.555 and a 95% CI of 0.465 to 0.664 (Fig. 4B). Then, we pooled the data for the numbers of patients that used intravenous antibiotics and oral antibiotics to see whether there was a difference in overall antibiotic use between patients in azithromycin groups and placebo groups. This analysis indicated that the overall antibiotic use for patients in azithromycin groups was still significantly reduced compared with that of patients in placebo groups (pooled RR, 0.628 [95% CI, 0.537, 0.735], z = 5.81, P < 0.001) (Fig. 4C).
FIG 4.
Forest plots for risk ratio of antibiotic use in azithromycin-treated patients and placebo patients. (A) Intravenous antibiotic use. (B) Oral antibiotic use. (C) Overall antibiotic use.
Comparison of azithromycin-related side effects.
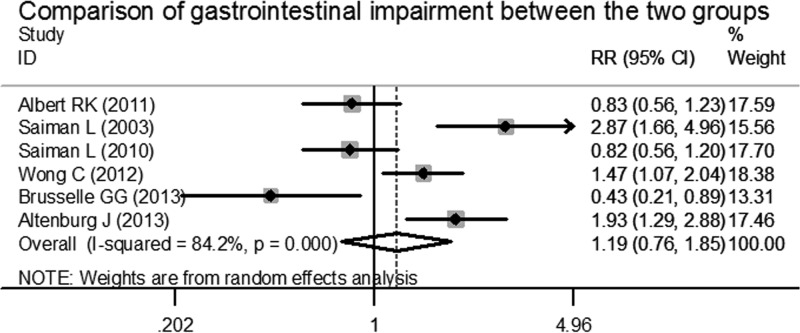
To analyze azithromycin-related side effects, we extracted the numbers of patients with gastrointestinal side effects, including vomiting, abdominal pain, diarrhea, and decreased appetite, from the original articles. Overall, this analysis exhibited a high degree of heterogeneity among these trials (χ2 = 31.68, P < 0.001, I2 = 84.2%). Therefore, we conducted an analysis using the random effects model. This indicated that there were no significant differences between the patients in azithromycin groups and the patients in placebo groups for gastrointestinal side effects during the study periods (pooled RR, 1.187 [95% CI, 0.761, 1.849], z = 0.76, P = 0.450) (Fig. 5).
FIG 5.
Forest plot for risk ratio of gastrointestinal impairment in azithromycin-treated patients and placebo patients.
Then, we compared the data for another reported side effect, hearing impairment. Three articles reported the relevant data, and the heterogeneity test showed that there was no difference among these data. The pooled RR was 1.168, with a 95% CI of 1.030 to 1.325, which indicated that, compared to placebo groups, patients in azithromycin groups showed a trend toward hearing impairment after receiving long-term azithromycin therapy (z = 2.42, P = 0.015) (Fig. 6).
FIG 6.
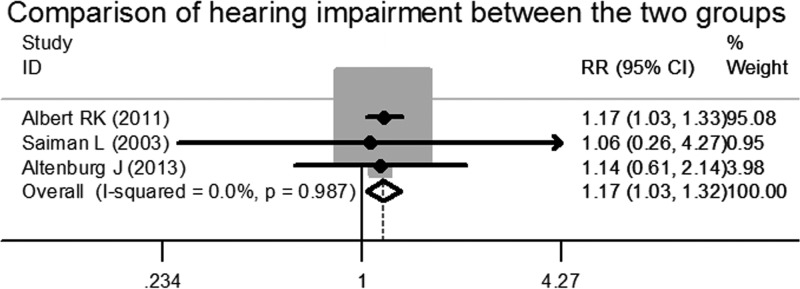
Forest plot for risk ratio of hearing impairment in azithromycin-treated patients and placebo patients.
DISCUSSION
Long-term use of macrolides has been found to be effective for patients with diffuse panbronchiolitis since the late 1980s (16). More recently, its advantages have been investigated among patients with chronic lung diseases, including cystic fibrosis, COPD, asthma, and non-cystic fibrosis bronchiectasis (3, 5–13). The mechanisms for the benefits of macrolides in the treatment of these diseases remain unexplained but may be due to their antibacterial and/or anti-inflammatory actions (4), which include reduction in proinflammatory cytokine production (9–11) and hastening of the phagocytosis ability of macrophages (17, 18). In addition, macrolides have potentially beneficial properties, including antiviral actions (19). However, recent research has been done on underlying adverse events of long-term macrolide therapy for patients with chronic lung diseases, and it has been found that patients treated with long-term macrolides could be at risk of increased infection with nontuberculous mycobacteria (20, 21). Furthermore, some macrolides may have underlying severe side effects, such as increased cardiovascular events (22).
In this analysis, we focused on the side effects of long-term use of azithromycin for patients with chronic lung diseases enrolled in randomized controlled trials. We found that long-term use of azithromycin (i) may lead to increased bacterial resistance in isolates from treated patients, (ii) might decrease colonization of bacteria and antibiotic use to some extent, and (iii) potentially results in hearing impairments. Hence, long-term use of azithromycin for patients with chronic lung diseases should be considered carefully in spite of its advantages of improving lung function and decreasing exacerbations of diseases.
We noticed that, in our analysis, the results for bacterial colonization exhibited some degree of variation and seemed unstable. After eliminating the 2010 study of Saiman et al. (6) from this analysis, the results showed decreased heterogeneity and indicated that bacterial colonization was reduced among patients treated with azithromycin. Presumably, the following explanations could account for the heterogeneity and variability. (i) Different methods were used for analyzing the bacterial colonization. In the 2010 study of Saiman et al. (6), the number of positive species was counted rather than the number of patients colonized with bacteria. As the lower respiratory tract in CF patients is rarely sterile, the difference in the numbers of patients colonized with bacteria in the two treatment groups could be insignificant. (ii) We also noticed that the increased rate of colonization in this study (6) was due to the increased numbers of resistant bacteria isolated from the azithromycin-treated patients, which is in accordance with the patterns of bacterial resistance analyzed for these patients. Indeed, species other than resistant bacteria did not show differences between the both groups. (iii) Pseudomonas aeruginosa itself is not killed by macrolides. However, macrolides are detrimental to P. aeruginosa's colonization by inhibiting the formation of biofilm which is produced by the strain (23). Hence, CF patients infected with P. aeruginosa might be benefiting from long-term azithromycin use because long-term azithromycin use could be reducing the suitability of the niche in the lung of CF patients infected with P. aeruginosa, whereas long-term azithromycin use in CF patients uninfected with P. aeruginosa might mediate bacterial resistance. This could be an interpretation of why the two studies of Saiman et al. (5, 6) had different microbiological results.
Our analysis indicates that long-term azithromycin use might lead to increased bacterial resistance. In accordance with the most recent literature (24), patients with bronchiectasis who use azithromycin are at risk of higher macrolide resistance than those with less exposure. Meanwhile, in that report (24), azithromycin use was correlated with significantly reduced carriage of Streptococcus pneumoniae (carried by about 60% of patients), Haemophilus influenzae, and Moraxella catarrhalis; however, it increased the carriage of S. aureus and macrolide-resistant strains of S. pneumoniae and S. aureus in a cumulative, dose-dependent manner. This report may partly explain the results of our analysis, showing decreased bacterial colonization and increased bacterial resistance. As colonization of resistant bacteria in the respiratory tract may not require escalation of the current therapy, the reduced antibiotic use in the patients treated with azithromycin might be due to the decreased frequency of exacerbation. However, resistance genes for azithromycin can be transferred between different pathogens (25). The increased colonization of resistant bacteria may facilitate the dissemination of bacterial resistance and, potentially, cause further therapy to fail.
As this is a meta-analysis, we must be concerned with the following limitations: (i) there was a small number of available trials, as only 6 randomized controlled trials were available for this analysis; (ii) there was potential publication bias in the studies included, since positive results are more likely to be published than negative results; and (iii) there may be an increase of type I error after many calculations.
In conclusion, long-term azithromycin use in chronic lung diseases should be carefully considered, and multicenter RCTs with a stricter and longer investigation weighing the adverse effects on bacteriological changes should be explored in the future.
ACKNOWLEDGMENT
The authors declare no conflicts of interest.
Footnotes
Published ahead of print 4 November 2013
REFERENCES
- 1.US Burden of Disease Collaborators 2013. The state of US health, 1990-2010: burden of diseases, injuries, and risk factors. JAMA 310:591–608. 10.1001/jama.2013.13805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang G, Wang Y, Zeng Y, Gao GF, Liang X, Zhou M, Wan X, Yu S, Jiang Y, Naghavi M, Vos T, Wang H, Lopez AD, Murray CJ. 2013. Rapid health transition in China, 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet 381:1987–2015. 10.1016/S0140-6736(13)61097-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albert RK, Connett J, Bailey WC, Casaburi R, Cooper JA, Jr., Criner GJ, Curtis JL, Dransfield MT, Han MK, Lazarus SC, Make B, Marchetti N, Martinez FJ, Madinger NE, McEvoy C, Niewoehner DE, Porsasz J, Price CS, Reilly J, Scanlon PD, Sciurba FC, Scharf SM, Washko GR, Woodruff PG, Anthonisen NR, COPD Clinical Research Network 2011. Azithromycin for prevention of exacerbations of COPD. N. Engl. J. Med. 365:689–698. 10.1056/NEJMoa1104623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanoh S, Rubin BK. 2010. Mechanisms of action and clinical application of macrolides as immunomodulatory medications. Clin. Microbiol. Rev. 23:590–615. 10.1128/CMR.00078-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saiman L, Marshall BC, Mayer-Hamblett N, Burns JL, Quittner AL, Cibene DA, Coquillette S, Fieberg AY, Accurso FJ, Campbell PW, III, Macrolide Study Group 2003. Azithromycin in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA 290:1749–1756. 10.1001/jama.290.13.1749 [DOI] [PubMed] [Google Scholar]
- 6.Saiman L, Anstead M, Mayer-Hamblett N, Lands LC, Kloster M, Hocevar-Trnka J, Goss CH, Rose LM, Burns JL, Marshall BC, Ratjen F, AZ0004 Azithromycin Study Group 2010. Effect of azithromycin on pulmonary function in patients with cystic fibrosis uninfected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA 303:1707–1715. 10.1001/jama.2010.563 [DOI] [PubMed] [Google Scholar]
- 7.Wong C, Jayaram L, Karalus N, Eaton T, Tong C, Hockey H, Milne D, Fergusson W, Tuffery C, Sexton P, Storey L, Ashton T. 2012. Azithromycin for prevention of exacerbations in non-cystic fibrosis bronchiectasis (EMBRACE): a randomized, double-blind, placebo-controlled trial. Lancet 380:660–667. 10.1016/S0140-6736(12)60953-2 [DOI] [PubMed] [Google Scholar]
- 8.Brusselle GG, Vanderstichele C, Jordens P, Deman R, Slabbynck H, Ringoet V, Verleden G, Demedts IK, Verhamme K, Delporte A, Demeyere B, Claeys G, Boelens J, Padalko E, Verschakelen J, Van Maele G, Deschepper E, Joos GF. 2013. Azithromycin for prevention of exacerbations in severe asthma (AZISAST): a multicenter randomized double-blind placebo-controlled trial. Thorax 68:322–329. 10.1136/thoraxjnl-2012-202698 [DOI] [PubMed] [Google Scholar]
- 9.Altenburg J, de Graaff CS, Stienstra Y, Sloos JH, van Haren EH, Koppers RJ, van der Werf TS, Boersma WG. 2013. Effect of azithromycin maintenance treatment on infectious exacerbations among patients with non-cystic fibrosis bronchiectasis: the BAT randomized controlled trial. JAMA 309:1251–1259. 10.1001/jama.2013.1937 [DOI] [PubMed] [Google Scholar]
- 10.Wolter J, Seeney S, Bell S, Bowler S, Masel P, McCormack J. 2002. Effect of long term treatment with azithromycin on disease parameters in cystic fibrosis: a randomized trial. Thorax 57:212–216. 10.1136/thorax.57.3.212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Equi A, Balfour-Lynn IM, Bush A, Rosenthal M. 2002. Long term azithromycin in children with cystic fibrosis: a randomized, placebo-controlled crossover trial. Lancet 360:978–984. 10.1016/S0140-6736(02)11081-6 [DOI] [PubMed] [Google Scholar]
- 12.Hahn DL, Grasmick M, Hetzel S, Yale S, AZMATICS (AZithroMycin-Asthma Trial In Community Settings) Study Group 2012. Azithromycin for bronchial asthma in adults: an effectiveness trial. J. Am. Board Fam. Med. 25:442–459. 10.3122/jabfm.2012.04.110309 [DOI] [PubMed] [Google Scholar]
- 13.Clement A, Tamalet A, Leroux E, Ravilly S, Fauroux B, Jais JP. 2006. Long term effects of azithromycin in patients with cystic fibrosis: a double blind, placebo controlled trial. Thorax 61:895–902. 10.1136/thx.2005.057950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donath E, Chaudhry A, Hernandez-Aya LF, Lit L. 2013. A meta-analysis on the prophylactic use of macrolide antibiotics for the prevention of disease exacerbations in patients with chronic obstructive pulmonary disease. Respir. Med. 107:1385–1392. 10.1016/j.rmed.2013.05.004 [DOI] [PubMed] [Google Scholar]
- 15.Reiter J, Demirel N, Mendy A, Gasana J, Vieira ER, Colin AA, Quizon A, Forno E. 2013. Macrolides for the long-term management of asthma: a meta-analysis of randomized clinical trials. Allergy 68:1040–1049. 10.1111/all.12199 [DOI] [PubMed] [Google Scholar]
- 16.Nagai H, Shishido H, Yoneda R, Yamaguchi E, Tamura A, Kurashima A. 1991. Long-term low-dose administration of erythromycin to patients with diffuse panbrochiolitis. Respiration 58:145–149. 10.1159/000195915 [DOI] [PubMed] [Google Scholar]
- 17.Feola DJ, Garvy BA, Cory TJ, Birket SE, Hoy H, Hayes D, Jr, Murphy BS. 2010. Azithromycin alters macrophage phenotype and pulmonary compartmentalization during lung infection with Pseudomonas. Antimicrob. Agent Chemother. 54:2437–2447. 10.1128/AAC.01424-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hodge S, Reynolds PN. 2012. Low-dose azithromycin improves phagocytosis of bacteria by both alveolar and monocyte-derived macrophages in chronic obstructive pulmonary disease subjects. Respirology 17:802–807. 10.1111/j.1440-1843.2012.02135.x [DOI] [PubMed] [Google Scholar]
- 19.Gielen V, Johnston SL, Edwards MR. 2010. Azithromycin induces anti-viral responses in bronchial epithelial cells. Eur. Respir. J. 36:646–654. 10.1183/09031936.00095809 [DOI] [PubMed] [Google Scholar]
- 20.Renna M, Schaffner C, Brown K, Shang S, Tamayo MH, Hegyi K, Grimsey NJ, Cusens D, Coulter S, Cooper J, Bowden AR, Newton SM, Kampmann B, Helm J, Jones A, Haworth CS, Basaraba RJ, DeGroote MA, Ordway DJ, Rubinsztein DC, Floto RA. 2011. Azithromycin blocks autophagy and may predispose cystic fibrosis patients to mycobacterial infection. J. Clin. Invest. 121:3554–3563. 10.1172/JCI46095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Binder AM, Adjemian J, Olivier KN, Prevots DR. 2013. Epidemiology of nontuberculous mycobacterial infections and associated chronic macrolide use among persons with cystic fibrosis. Am. J. Respir. Crit. Care Med. 188:807–812. 10.1164/rccm.201307-1200OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schembri S, Williamson PA, Short PM, Singanayagam A, Akram A, Taylor J, Singanayagam A, Hill AT, Chalmers JD. 2013. Cardiovascular events after clarithromycin use in lower respiratory tract infections: analysis of two prospective cohort studies. BMJ 346:f1235. 10.1136/bmj.f1235 [DOI] [PubMed] [Google Scholar]
- 23.Lutz L, Pereira DC, Paiva RM, Zavascki AP, Barth AL. 2012. Macrolides decrease the minimal inhibitory concentration of anti-pseudomonal agents against Pseudomonas aeruginosa from cystic fibrosis patients in biofilm. BMC Microbiol. 12:196. 10.1186/1471-2180-12-196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hare KM, Singleton RJ, Grimwood K, Valery PC, Cheng AC, Morris PS, Leach AJ, Smith-Vaughan HC, Chatfield M, Redding G, Reasonover AL, McCallum GB, Chikoyak L, McDonald MI, Brown N, Torzillo PJ, Chang AB. 2013. Longitudinal nasopharyngeal carriage and antibiotic resistance of respiratory bacteria in indigenous Australian and Alaska native children with bronchiectasis. PLoS One 8:e70478. 10.1371/journal.pone.0070478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roberts MC, Soge OO, No DB. 2011. Characterization of macrolide resistance genes in Haemophilus influenzae isolated from children with cystic fibrosis. J. Antimicrob. Chemother. 66:100–104. 10.1093/jac/dkq425 [DOI] [PubMed] [Google Scholar]