Abstract
Southeast Asia (the Thailand-Cambodia border) has been considered the primal epicenter for most antimalarial drug resistance; however, numerous molecular epidemiological studies have successively reported multiple independent origins of sulfadoxine-pyrimethamine (SP) resistance-associated Plasmodium falciparum dhfr (pfdhfr) and pfdhps alleles in other areas. To better understand the origin and evolutionary pathway of the SP resistance in Southeast Asia, a total of 374 P. falciparum field isolates from the Yunnan-Burma border and Hainan Island in southern China have been collected for comprehensive investigations on the mutation patterns of the pfdhfr/pfdhps genes as well as their microsatellite haplotypes. By comparative analysis of single-nucleotide polymorphism (SNP) genotyping and flanking microsatellite haplotypes, we reveal a unique origin of pyrimethamine-resistant mutations in Pfdhfr gene in Hainan Island and an oriented spread route of the pyrimethamine resistance from the Thailand-Cambodia border into the Hainan area, which reflects the geographical traits and SP administration histories in the two geographically independent areas. Moreover, genetic linkages between the high-level SP resistance-conferring pfdhfr/pfdhps alleles have been established in the isolates from the Yunnan-Burma border, raising the concern of a genetic basis in adopting combination chemotherapies against falciparum malaria.
INTRODUCTION
Since the prevalence of chloroquine resistance in malaria chemotherapy worldwide, the wide use of combination therapies such as sulfadoxine-pyrimethamine (SP), an affordable alternative to chloroquine, had led to increasing multidrug resistance in malarial parasites, which has hampered therapeutic efficacy in many areas (1, 2). Recently, resistance to artimisinin (ART) in Plasmodium falciparum has been reported in Southeast Asia (SEA), causing the current situation of first-line treatment in malaria control to deteriorate (3–5). As one of the partner drugs of artemisinin-based combination therapy (ACT), SP combination is still the only drug treatment recommended by WHO for intermittent preventive treatment (IPT) in those vulnerable populations because of its safety in pregnant women and infants and its long action. Therefore, the fundamental understanding of how SP resistance emerged and spread globally will definitely contribute to the development of novel intervention strategies against widespread multidrug resistance and prevention of potential drug resistances in malarial parasites.
Resistance to SP in P. falciparum parasites was established mainly by site mutations in the genes encoding dihydrofolate reductase (dhfr) at codons 50, 51, 59, 108, and 164 and dihydropteroate synthase (dhps) at codons 436, 437, 540, 581, and 613 (6–10). These key mutations are suggested to appear in a stepwise manner and to be able to act synergistically to enhance the level of SP resistance both in vivo and in vitro (10, 11). Molecular epidemiological studies such as site-mutation genotyping and haplotype analysis of microsatellites surrounding the target gene locus have provided crucial information in tracing the origin, evolutionary history, and spread route of drug resistance in various areas where malaria is endemic. In this way, a common ancestry (CNRNL) of the triple mutant P. falciparum dhfr (pfdhfr) allele was discovered at the Thailand-Cambodia border, the epicenter of pyrimethamine resistance. This mutant allele evolved and was introduced into other regions in Southeast Asia and Africa through a similar spread pathway of chloroquine resistance (12–15). Nevertheless, multiple indigenous origins of some single, double, or triple pfdhfr alleles were also reported in Southeast Asia, Africa, South America, and Papua New Guinea (16–19). To date, the evolution and spread of resistant lineages are traced by the pfdhfr rather than the pfdhps gene, but there are still several surveys that showed that unlike the pfdhfr gene, various sulfadoxine-resistant pfdhps alleles originated independently in each of those areas, including Thailand, Cambodia, Kenya, Cameroon, and Venezuela (17, 18, 20–22).
In Asia, resistance to SP was first found on the Thailand-Cambodia border in 1960s and quickly spread to neighboring countries in Southeast Asia (SEA) (23, 24). In the 1970s and 1980s, there existed a wide range of SP resistance in this area, where Fansidar was widely used. Consequently, the efficacy of SP decreased sharply and the cure rates were very low in SEA regions, especially on the Thailand-Burma (42%) and Thailand-Cambodia (32%) borders (2). As one of the regions in Southeast Asia where malaria is endemic, the southern areas of China, including the Yunnan-Burma border and Hainan Province (a geographically isolated island located in the South China Sea), have also experienced the use of SP combination therapy and thereby have encountered the occurrence and spread of SP resistance. However, the SP therapy programs in the two regions were not identical, as one was introduced as an antimalarial prophylactic remedy in Yunnan from the middle of 1960s until the early 1990s, whereas in Hainan Island, pyrimethamine was first introduced in 1959 and then combined with sulfadoxine in several villages from 1967 to 1972. Due to the distinct histories of drug administration, geographical environments, and population migration in these two areas, the field isolates of P. falciparum collected from Yunnan and Hainan are of great interest for characterization of the mutation patterns of the pfdhfr/pfdhps genes and related flanking microsatellite loci and thereby tracking the origin and evolution of SP resistance-associated alleles. To date, no such comparative investigation of SP drug resistance in the two areas has been performed, though a few small-scale genotyping studies on the pfdhfr or pfdhps genes have been described (25).
In the present study, we have addressed this issue by profiling the SP resistance-associated pfdhfr/pfdhps alleles, as well as the microsatellite haplotypes flanking the pfdhfr gene, in P. falciparum isolates from Yunnan and Hainan Provinces and further evaluating the relationship between the two populations. Our results present evidence of the different selection on pfdhfr and pfdhps alleles in the two geographically independent areas and reveal an independent origin of the pfdhfr ANCNI and ANRNI alleles in Hainan Island. Moreover, we show significant genetic linkages between the high-level SP-resistant pfdhfr/pfdhps alleles in the population of the Yunnan-Burma border, suggesting that multiple mutations in both pfdhfr and pfdhps play an critical role in establishing the genetic linkage across chromosomal boundaries which reciprocally stabilize the existing multiple mutations in the population even after use of the drugs has been ceased for longer than 20 years.
MATERIALS AND METHODS
Study sites and sample collection.
The malaria patients involved in this study were all local residents in the Yunnan-Burma border area and Hainan Island with a primary diagnosis of falciparum malaria. After obtaining written informed consent and ethical approval, we collected 374 P. falciparum clinical isolates from symptomatic malaria patients seeking care at the local Center for Disease Control and Prevention (CDC) in Yunnan and Hainan from 2003 to 2008. Six sampling sites were included in this study, three located on the China-Burma border (Yunnan Province) and the others on the west coast of the island (Hainan Province). The Yunnan isolates (n = 230) were mainly from venous blood, while the Hainan isolates (n = 119) were mostly from finger prick blood (adsorbed onto Whatman filter paper). The study was approved by the Ethical Review Board of Second Military Medical University, China.
DNA isolation and genotyping methods.
The parasite DNA was extracted from 200 μl venous blood or finger prick blood spot by using the QIAamp DNA Blood Minikit according to the manufacturer's recommendations (Qiagen, Germany). All these samples were genotyped for pfdhfr mutations at codons 16, 51, 59, 108, and 164 and pfdhps mutations at codons 436, 437, 540, 581, 613, 640, and 645 by pyrosequencing. The sequences of primers used for pfdhfr and pfdhps genotyping were described by Zhou et al. (26). Each DNA sample was tested by nested PCRs. The primary amplification of pfdhfr was done with the following parameters: 95°C for 3 min; 35 cycles of 95°C for 30 s, 55°C for 30 s, and 65°C for 60 s; and 65°C for 5 min. The second amplification of pfdhps was done with the following parameters: 95°C for 3 min; 35 cycles of 95°C for 30 s, 52°C for 30 s, and 65°C for 60 s; and 65°C for 5 min. The 650-bp product of pfdhfr and 750-bp product of pfdhps were subjected to sequencing on the ABI 3130xl Genetic Analyzer (Applied Biosystems, Foster City, CA, USA). Samples with multiple peaks at any genotyped single-nucleotide polymorphism (SNP) codon (mixed genotype) were excluded from SNP analysis and microsatellite genotyping.
Microsatellite analysis.
To assess the selective sweeps of pfdhfr resistance determinants, we investigated polymorphic microsatellite repeats (TA) within a 60-kb flanking region of the pfdhfr gene. Seven loci located on chromosome 4 linked to pfdhfr were chosen, i.e., 50 kb, 30 kb, 3.87 kb, and 0.1 kb upstream and 1.48 kb, 5.87 kb, and 54 kb downstream. Nested PCR was performed using fluorescence end-labeled primers; the sequences of primers and the cycling parameters were previously described by Nair et al. (27). As a reference of the baseline heterozygosity of all these isolates, 13 loci on the other chromosomes which were regarded as putatively neutral microsatellite markers were integrated into this study (28). The amplified products were then detected by electrophoresis on an ABI 377 sequencer and analyzed with GeneScan software v3.7 (Applied Biosystems, Foster City, CA, USA). Samples with two or more peaks at the same locus were treated as exceptions.
Statistical analysis.
The Pearson correlation between pfdhfr and pfdhps resistance alleles which represented the hierarchy among different genes was calculated with Cluster version 3.0 and Treeview (http://rana.lbl.gov/EisenSoftware.htm). The results were shown in terms of a heat map indicating the hierarchical information, where pfdhfr was shown in columns and pfdhps in rows. Each box represented the frequency of the isolate carrying a certain pfdhfr/pfdhps allele (a χ2 test had been done in advance; P < 0.01). Linkage disequilibrium (LD) analysis between pairs of pfdhfr/pfdhps point mutations was performed with TASSL software. Samples with multiple infections detected at any SNP site were excluded from the LD analysis. Significant LD between the loci should meet the requirements of D′ > 0.8, r2 > 0.2, and P < 0.01 simultaneously.
To measure the genetic diversity, we evaluated the expected heterozygosity (He) at all pfdhfr and neutral microsatellite loci by using Genalex software, version 6. He was calculated by using the formula He = [n/(n − 1)](1 − ∑pi2) (29), where n is the number of infections sampled and pi is the frequency of the ith allele. The sampling variance of He was calculated according to the formula with a slight modification of the standard diploid variance, 2(n −1)/n3{2(n − 2)[∑pi3 − (∑pi2)2]} (29). Different mean He values were compared by using the Mann-Whitney U test in SPSS (version 19.0). A P value of <0.05 was considered statistically significant. To track genetic lineages of pfdhfr alleles, we constructed a median-joining tree based on 7-locus microsatellite haplotypes through NETWORK version 4.5.1.0. In addition, for determining the number of origins precisely, the haplotypes of 4 loci at positions 3.87 kb and 0.1 kb upstream and 1.48 kb and 5.87 kb downstream within 10 kb were selected for this analysis.
RESULTS
Identification of infection complexity in field isolates.
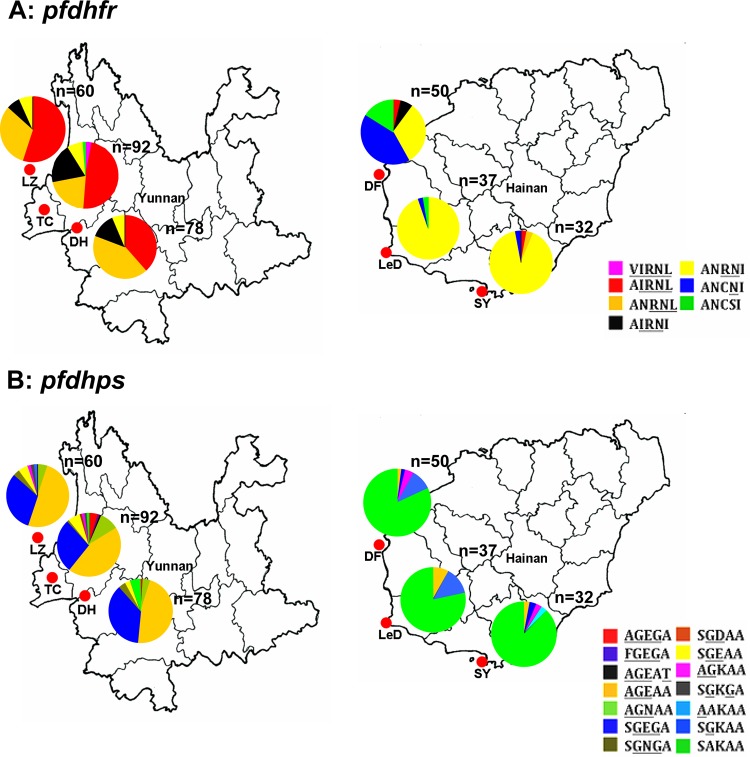
The isolates of Plasmodium falciparum were collected from the Yunnan-Burma border and Hainan Island, two geographically independent regions in southern China (Fig. 1A). Each region included three sampling sites: Tengchong City (n = 92), Dehong City (n = 78), and Lazan County (n = 60) on the Yunnan-Burma border and Dongfang County (n = 50), Ledong County (n = 37), and Sanya City (n = 32) on Hainan Island (see Table S1 in the supplemental material). Among them, 25 isolates (6.68%) were excluded from this study because of multiple infections detected by SNP assay (data not shown). The rest of the 349 samples were subsequently subjected to pyrosequencing. Of them, 100 were found to contain multiple alleles at one or more neutral loci through microsatellite analysis (13 loci). Therefore, a total of 249 isolates were finally recruited in the microsatellite haplotype analysis around the pfdhfr gene locus on chromosome 4.
FIG 1.
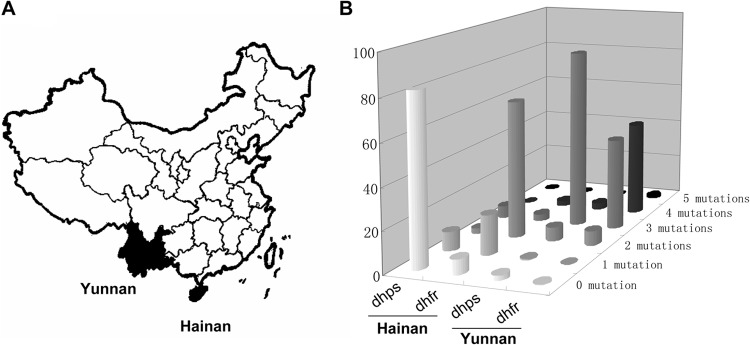
Distribution patterns of drug resistance-associated pfdhfr and pfdhps mutations in Plasmodium falciparum isolates from the Hainan and Yunnan areas. (A) The black parts of the map represent the two high-risk regions of endemicity of falciparum malaria in southern China (Yunnan and Hainan Provinces). (B) The number of site mutations represents various SNP haplotypes as listed in Table S1 in the supplemental material.
Regional distribution of pfdhfr and pfdhps resistance alleles in the two populations.
By specific amplification and sequencing of the pfdhfr/pfdhps alleles, we found a total of 7 pfdhfr and 14 pfdhps haplotypes in this study, most of which have been described in previous reports. There were two novel pfdhps mutants (SGDAA and FGEGA) with extremely low frequencies in Tengchong City in Yunnan. In addition, two newly reported triple mutants, AGNAA and SGNGA, were also found in this site (20, 30), indicating a relatively high rate of transmission rate P. falciparum parasites in this area.
Approximately, 92.61% of the Yunnan isolates harbored pfdhfr triple (AIRNI and ANRNL), quadruple (AIRNL), and quintuple (VIRNL) mutants. There was only one isolate with the wild-type pfdhfr allele (ANCSI) (0.43%), and no single mutants were found in this region. In contrast, the Hainan isolates exhibited predominantly single (ANCNI, 19.33%) and double (ANRNI, 67.23%) mutants, whereas both high-level drug resistance-related mutants (AIRNI/ANRNL/VIRNL) and the wild-type allele (ANCSI) were rare (5.88% and 7.56%, respectively). Similar to the case for the pfdhfr alleles, high-level resistant pfdhps mutants, including two predominant triple mutants, AGEAA and SGEGA, and other mutants such as AGEGA, FGEGA, AGEAT, AGNAA, and SGNGA, were observed in 90.43% of the Yunnan-derived isolates. The frequencies of pfdhps double mutants (SGEAA/SGDAA/AGKAA/SGKGA), single mutants (SGKAA), and the wild type (SAKAA) were 6.97%, 0.43%, and 2.17%, respectively. For the P. falciparum isolates collected from Hainan Island, however, the pfdhps alleles showed a distinct distribution pattern where the wild type (SAKAA) was highly predominant (82.35%) compared to the high-level drug resistance alleles (5.89% for triple mutants and 2.52% for double mutants) (Fig. 1B; see Table S1 in the supplemental material).
With respect to individual sampling sites, further analysis showed similar distribution patterns of pfdhfr alleles in Lazan, Tengchong, and Dehong in Yunnan, where the quadruple mutant AIRNL and the triple mutant ANRNL were the major alleles in all the three sites. In Hainan Island, however, it is notable that Dongfang City exhibited a varied status of the pfdhfr mutants, which were composed mainly of ANCNI, ANRNI, and wild-type ANCSI alleles, while only the double mutant ANRNI allele was prevalent in the other sites (Ledong and Sanya) (Fig. 2A). For the pfdhps gene, no significant difference in the mutation profiles was observed among the three sites in either Yunnan or Hainan (Fig. 2B).
FIG 2.
Local distributions of pfdhfr and pfdhps resistance alleles in Yunnan and Hainan. (A) Pie charts of the six sampling sites on the China-Burma border and western coast of Hainan Island illustrate the proportions of all 7 pfdhfr alleles. (B) Proportions of all 14 pfdhps alleles in the 6 sampling sites. The various pfdhfr or pfdhps alleles are shown in different colors. LZ, Lazan (n = 60); TC, Tengchong (n = 92); DH, Dehong (n = 78); DF, Dongfang (n = 50); LeD, Ledong (n = 37); SY, Sanya (n = 32).
Genetic linkage between sites in pfdhfr and pfdhps.
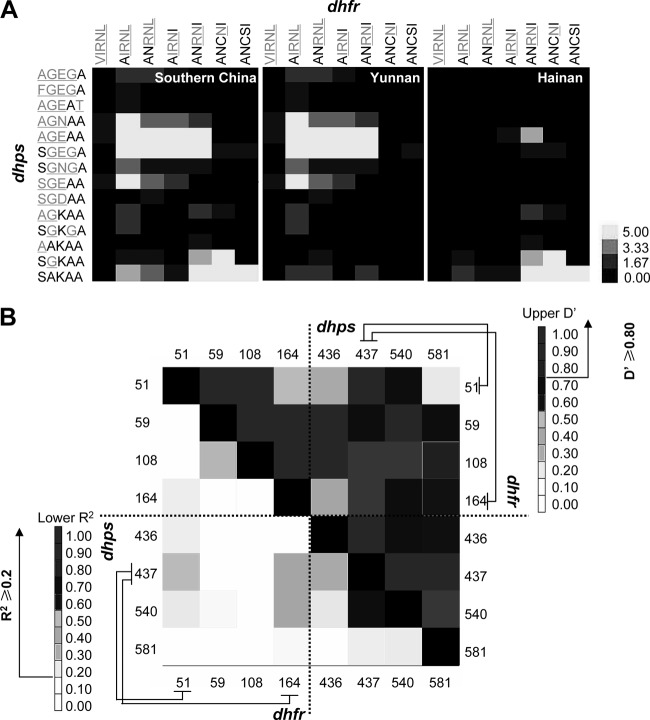
The similar SP resistance status observed in the sites in Yunnan indicated a potential genetic linkage between the pfdhfr and pfdhps gene loci, which appeared and was fixed during the multidrug selections, as was discovered in P. vivax recently (31). In order to address this key point for P. falciparum isolates, we rearranged all these alleles determined in this study into a cross table to determine the linkages between pfdhfr and pfdhps alleles (Fig. 3A). Strong linkage was established in two major groups, AGEAA-AIRNL/ANRNL/AIRNI and SGEGA-AIRNL/ANRNL/AIRNI (dhps-dhfr correlation, >5), which are linked to high-level SP-resistant mutants, and SAKAA-ANRNI/ANCNI/ANCSI and SGKAA-ANCNI (dhps-dhfr correlation, >5), which represent low-level SP resistance or wild type (χ2 test, P < 0.01) (Fig. 3A, Southern China). Considering the geographical differences among these isolates, we further analyzed the pfdhfr/pfdhps alleles from Yunnan (n = 230) and Hainan (n = 119) via the same strategy. The results showed that most of the Yunnan isolates belonged to the aforementioned high-level SP-resistant groups and that the Hainan isolates fell in the SP-sensitive groups (Fig. 3A, Yunnan and Hainan). Several other pfdhfr-pfdhps pairs showed weaker linkages without statistical significance.
FIG 3.
Genetic linkage between pfdhfr and pfdhps alleles. (A) Linkage clustering. The level of linkage was measured by the Pearson correlation and is displayed in different colors (a χ2 test had been performed in advance, P < 0.01). The black, gray-yellow, and yellow grids represent zero, weakly positive, and strongly positive correlations, respectively. The numbers in the bar chart refer to the values of Pearson correlation grades. (B) LD analysis. The numbers on the x and y axes indicate SNP sites in pfdhfr and pfdhps. The upper right shows D′ values for all the SNP pairs, while the lower left represents r2. The grids in different colors indicate the LD level between each pairs. The arrows show the baseline in determining the significant LDs.
The significant linkages between pfdhfr and pfdhps mutants strongly supported the role of linkage disequilibrium (LD) between sites of the two genes on different chromosomes in stabilizing and fixing the existed multidrug resistance within these parasite populations even after the removal of the SP drugs in these areas for a long time. Thus, we further analyzed the LD of SNP mutations among them. Using TASSEL software, we showed a significant LD between pfdhps codon 437 and pfdhfr codon 51 (D′ = 0.91, r2 = 0.25, P < 0.001) or pfdhfr codon 164 (D′ = 0.85, r2 = 0.36, P < 0.001) in this population (Fig. 3B). It is notable that, unlike the previous finding by McCollum et al. (22), no significant association was observed between pfdhps codon 437 and the key pfdhfr mutation site 59 or 108 (P > 0.01). In addition, for the alleles within the pfdhfr or pfdhps gene, significant LDs were observed between pfdhfr codons 59 and 108 (D′ = 1, r2 = 0.28, P < 0.001) and pfdhps codons 437 and 436 (D′>0.8, r2 > 0.2, P < 0.001) or 540 (D′ = 1, r2 = 0.64, P < 0.001), suggesting that the synchronous mutations on adjacent SNP sites might increase the level of SP resistance synergistically.
Selective sweeps of pfdhfr drug resistance alleles.
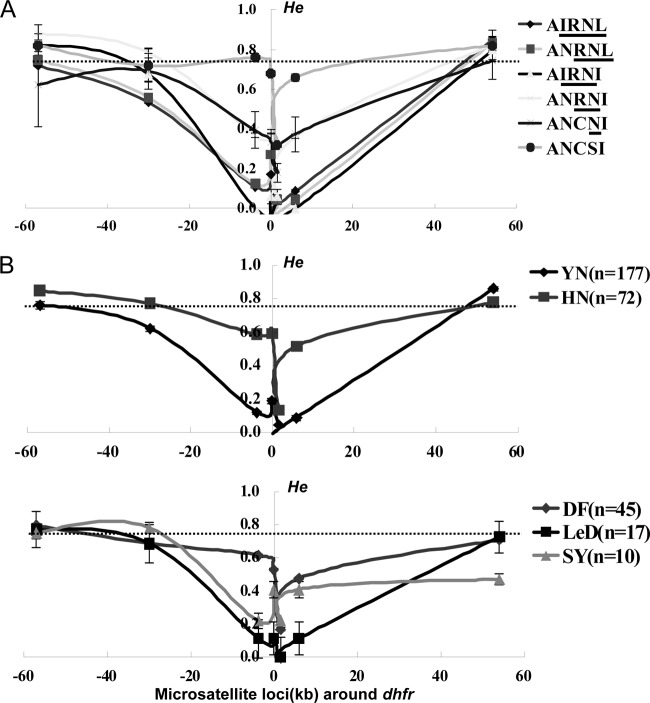
The distinct regional distribution pattern of pfdhfr alleles in Hainan suggests a more complex origin and evolutionary pathway of pyrimethamine resistance on this island. To clarify this issue, we examined the genetic diversity and selective sweeps around pfdhfr alleles in 349 field isolates by microsatellite analysis of 7 loci around each of five major pfdhfr mutations and the wide-type allele. Due to PCR amplification failure or multiple infections on some microsatellite loci, 249 isolates were finally subjected for this analysis. We first determined the heterozygosity (He) of all these alleles on each of the 4 loci, i.e., 5.87 kb and 0.1 kb upstream and 1.48 kb and 3.84 kb downstream from the pfdhfr gene within a range of 10 kb. The mean He of the wild-type allele (ANCSI) was greater (He = 0.605 ± 0.013) than those of alleles with single (ANCNI, He = 0.321 ± 0.068; P = 0.010), double (ANRNI, He = 0.290 ± 0.014; P = 0.013), triple (AIRNI, He = 0.030 ± 0.017 [P < 0.001] and ANRNL, He = 0.121 ± 0.019 [P < 0.001]), or quadruple (He = 0.103 ± 0.006; P < 0.001) mutations. This observation is compatible with the previously established model of positive directional selection; i.e., a progressive decline in He correlates with an increase in the number of favorable mutations of the pfdhfr gene (32). For three other loci, 57 kb and 30 kb upstream and 54 kb downstream from the pfdhfr gene, however, no significant differences in He were observed between the wild-type and other mutant alleles, probably because the diversity of these loci was not influenced by drug selection since they located far from the pfdhfr locus (Fig. 4A). In addition, we also measured 10 neutral loci on chromosomes 1, 3, 5, 6, 7, 9, and 10 as references. The mean He of these loci (He = 0.751 ± 0.062) was significantly greater than that of the 4 loci flanking the mutant pfdhfr gene locus (P < 0.001) but similar to that of the wild type (P = 0.103).
FIG 4.
Selective sweep around dhfr alleles in different regions. (A) He comparison of the wild type and five mutant groups (single, double, triple, quadruple, and quintuple mutations) on loci 57 kb, 30 kb, 5.87 kb, and 0.1 kb upstream and 1.48 kb, 3.84 kb, and 54 kb downstream around the pfdhfr locus. The dotted line crossing the y axis indicates the mean He at 10 neutral microsatellite markers on other chromosomes. (B) Selection patterns of pfdhfr alleles in Yunnan (black line) and Hainan (red line) are showed in the upper panel. A detailed description of the selection patterns in three sampling sites of Hainan is shown in the lower panel. The error bars indicate standard deviations (SD).
Next, we further compared the overall diversity of the pfdhfr locus between the two regions in southern China. Figure 4B shows a significant lower mean He in Yunnan (0.383 ± 0.003) than in Hainan (0.603 ± 0.014) (P = 0.016), suggesting a stronger effect of drug selection in the Yunnan-Burma area. Moreover, a site-based analysis revealed a distinct profile of the genetic diversity of the pfdhfr locus in Dongfang isolates (He = 0.568 ± 0.008), whereas the other two sites in Hainan showed a pattern similar to that in Yunnan (HeLedong = 0.359 ± 0.034; HeSanya = 0.464 ± 0.107) (P = 0.021). This is interesting since it indicates an independent origin or evolutionary pathway of pfdhfr mutant alleles in the Dongfang region. A further analysis of the evolutionary pathway of the resistance-conferring mutations in the pfdhfr and pfdhps genes did not show an apparent difference between Hainan (Dongfang) and Yunnan (see Fig. S1 in the supplemental material), which points to a unique origin of the pfdhfr drug resistance mutations in the Dongfang region.
Microsatellite haplotype and genetic relationship of dhfr alleles.
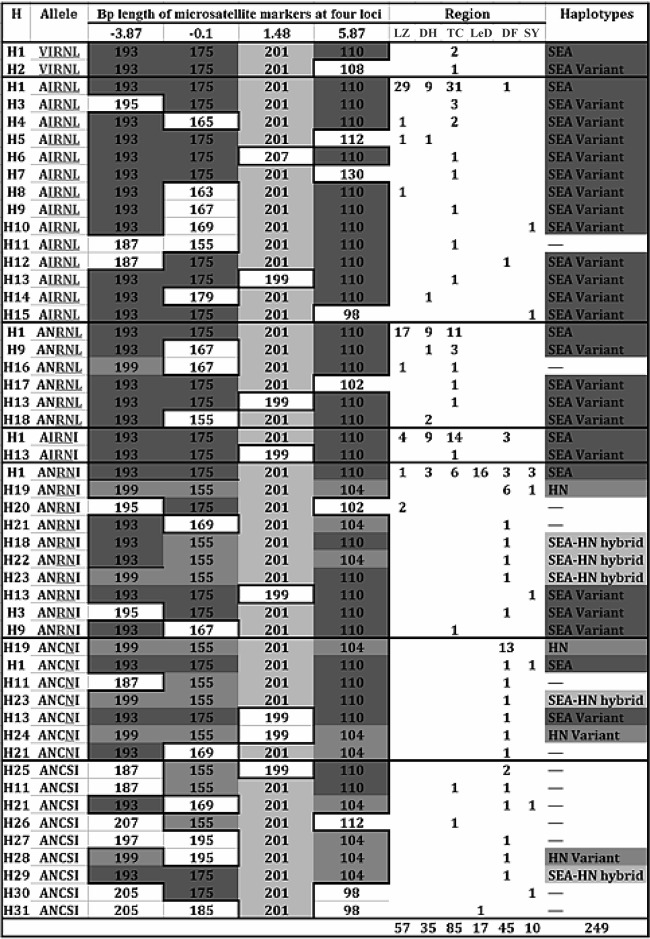
To classify the origin of pfdhfr mutant alleles in Yunnan and Hainan isolates, we performed analysis of microsatellite haplotypes with the 4 loci within 10 kb flanking the pfdhfr locus. In total, there were 31 various microsatellite haplotypes, H1 to H31, in the recruited 249 isolates, and according to the relationship among them, they were able to be classified into two major groups corresponding to H1 and H19 (Fig. 5). The haplotype H1, displaying a microsatellite combination of 193-175-201-110 bp on 4 loci, was described previously as the Southeast Asia (SEA) haplotype (19, 28). The H19 haplotype, 199-155-201-104, however, had not been reported yet. Accordingly, this novel microsatellite haplotype of the pfdhfr locus was designated the HN haplotype here. The derivatives of the two haplotypes were called SEA variant and HN variant haplotypes, respectively, and that containing mixed microsatellite types was designated the SEA-HN hybrid haplotype. Figure 6 lists the categories of all microsatellite haplotypes in the Yunnan and Hainan isolates analyzed in this study. It shows that SEA haplotype derivatives were mainly detected in 96% of the Yunnan isolates and 47.2% of Hainan isolates, corresponding to the triple or quadruple mutant pfdhfr alleles. Meanwhile, approximately 30.6% of the Hainan isolates presented the HN haplotype and its variants, most of which were from Dongfang samples with single or double mutations of the pfdhfr gene.
FIG 5.
Microsatellite haplotype profiles (H1 to H31) on four loci flanking dhfr in isolates from southern China. Each box represents the microsatellite type at codons 16, 51, 59, 108, and 164 of each pfdhfr allele in the six sampling sites. Identical colors (green and blue) represent proposed common lineages. Blue grids represent the SEA lineage, whereas the HN lineage is shown in green. The yellow grids under the column “Haplotypes” indicate a hybrid of the SEA and HN haplotypes based on microsatellite characteristics at loci −3.87, −0.1, and 5.87. The numbers under the column “Region” indicate the frequency of each haplotype in various regions. LZ, Lazan; TC, Tengchong; DH, Dehong; DF, Dongfang; LeD, Ledong; SY, Sanya.
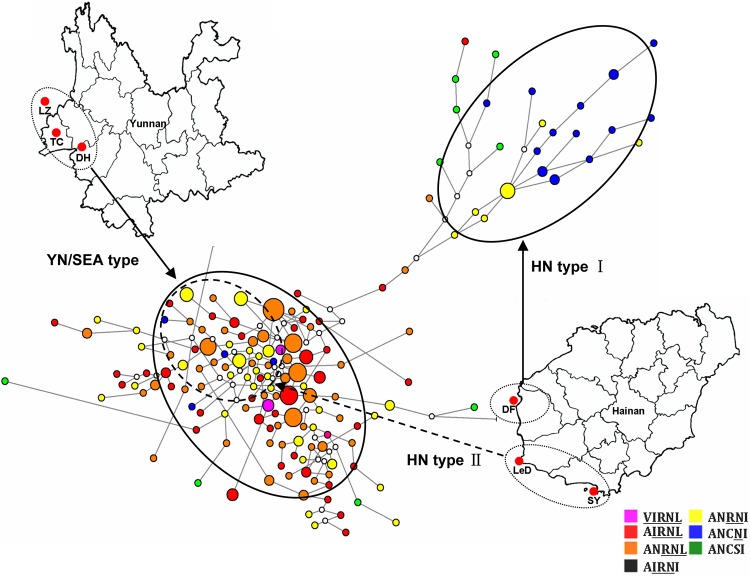
FIG 6.
Genetic relationships among pfdhfr alleles in southern China. Based on 7-loci microsatellite polymorphism around pfdhfr, all these isolates (n = 249) were classified into 174 haplotypes. The sizes of the circles are proportional to the number of isolates showing particular 7-loci microsatellite haplotypes. Six types of pfdhfr alleles are showed in different colors. White dots connecting haplotypes within the network are hypothetical median vectors generated by the software. Two major independent networks, designated YN/SEA types and HN type I, are formed simultaneously in the population (circles with solid line). The HN type II circle (dashed line) represents partial Hainan isolates with double mutants belong to the YN/SEA type.
To further address this point, we also drew a median-joining network diagram tree of these microsatellite haplotypes based on the polymorphism on those loci flanking the pfdhfr gene. Two major independent groups were constructed (Fig. 6) and represent the high-level (YN/SEA type) and low-level (HN type I) drug resistance alleles. It should be noted that a few pfdhfr double mutants with SEA haplotypes from Hainan isolates, mainly from the Ledong site, also migrated into the Yunnan group (HN type II), suggesting a common ancestor of the pfdhfr mutant alleles in Ledong and Yunnan. Taken together, this clearly showed that in Yunnan, the pyrimethamine resistance of P. falciparum parasites could be traced to an ancestor originating in the well-characterized Southeast Asia isolates. However, at least two origins of pfdhfr mutations existed in the geographically isolated Hainan Island under multidrug selections. The Dongfang isolates represented a novel source of the pyrimethamine resistance in P. falciparum field isolates.
DISCUSSION
Hainan and Yunnan Provinces are the two representative high-risk regions of endemicity of falciparum malaria in southern China. In the past 2 decades, resistance to pyrimethamine-sulfadoxine has appeared in a stepwise fashion since the widespread use of SP combination chemotherapy in these areas. In this study, samples of field isolates were collected from 2003 to 2008, when SP were replaced by artemisinin-based combination therapy for a long period. The genotyping analysis of drug resistance-conferring pfdhfr and pfdhps alleles revealed a more severe situation of SP resistance in the isolates from Yunnan area. Compared to the pyrimethamine resistance, it is notable that there is a delay in the emergence and development of sulfadoxine resistance alleles in both areas. This observation supports the presumption that the pfdhps resistance takes place only after a substantial fraction of the population has been selected for pyrimethamine resistance (22, 33, 34), and it likely reaffirms the previous clinical finding that mutations in the pfdhfr gene plays a major role in the failure of SP treatment against falciparum malaria (35, 36). Though the SP drug selection was assumed to act independently and differently on dhfr and dhps loci (37, 38), the asymmetry in the selection pattern suggests a potential genetic linkage between the two loci across chromosomal boundaries during the coselection of the drug combination (22, 31). In this study, LD analysis of the pfdhfr and pfdhps alleles discovered strong linkages of some SNPs between the major high-level resistance alleles in the population. In another, parallel study on P. vivax isolates from Yunnan, we have shown that the high-resistance mutations in positions 57, 61, and 117 of pvdhfr (chromosome 5) and position 383 of pvdhps (chromosome 14) are genetically linked (31). Little is known about when and how these genetic linkages of certain SNPs in the dhfr and dhps genes took place across chromosomal boundaries, but it has been proved that such genetic relationships tend to induce the emergence and development of multiple drug resistance mutations and stabilize them within a parasite population after use of the drugs has been ceased for as long as 20 years. Therefore, this should be taken into account before the adoption of a combined chemotherapy against malaria.
In China, Yunnan and Hainan Provinces are two geographically independent areas that both suffer from a prevalence of drug-resistant P. falciparum as well as P. vivax, though the drug selection pressure forced by SP combination chemotherapy has been ceased for approximately 20 years. The nonidentical distribution patterns of pfdhfr and pfdhps alleles in the parasite populations from the two areas partially reflect their history of drug administration. Unlike the case for the pfdhps alleles, it is intriguing to observe heterogeneity of prevalent patterns of the pfdhfr alleles within the sites in Hainan Island, which points to a unique evolution pathway of pyrimethamine resistance gene in the Dongfang site. Consistent with this finding, the systematic microsatellite-based analysis clearly distinguishes the origin and evolution pathway of Dongfang isolates from those of the other isolates. Particularly, we found a novel “HN” microsatellite haplotype in Dongfang City, which included nearly one-third of the Hainan isolates, whereas the other sites in Hainan and all the sites in the Yunnan area shared a common ancestor, the SEA haplotype, with Southeast Asia isolates. The observation that the HN haplotype was absent in all of the Yunnan isolates suggests that it is still limited to this geographically isolated island, though the representative SEA type from SEA epicenter, the Thailand-Cambodia border, has invaded the Dongfang site in Hainan, resulting in full or hybrid types. This phenomenon is further supported by the map of genetic relationships among pfdhfr alleles in southern China, where nearly all the Dongfang isolates belong to the HN type I cluster and other Hainan isolates fall into the YN/SEA subgroup. These data suggest a spread route of the SEA-type resistant pfdhfr alleles from Southeast Asian countries to China-Burma border and then to Hainan Island. Currently, how the unique HN-type pyrimethamine resistance in the Dongfang region originated and evolved and how the SEA type spread from the SEA epicenter to this isolated island are still not clear, but factors including the genetic characteristics of parasites and host, transmission of the local mosquito vector, and population migration among various areas where malaria is endemic might be involved in this process.
In conclusion, the results presented here show that (i) the geographically different distributions of drug-resistant pfdhfr and pfdhps alleles are fixed in southern China, which reflects their distinct histories of antimalaria chemotherapy, (ii) genetic linkage exists between certain pfdhfr and pfdhps alleles, and (iii) unlike in the Yunnan area where the common origin (SEA type) is prevalent, multiple origins of pyrimethamine resistance, including the novel HN type I, are present in Hainan (Dongfang), though the evolutionary pathway is similar to that in Yunnan. Taking the findings together, we have provided here a better understanding of SP resistance in the area in Asia where primary drug-resistant malaria is endemic.
Supplementary Material
ACKNOWLEDGMENTS
We thank all the staff at the Yunnan CDC and the Hainan CDC who participated in sampling. We are also grateful to Run Ye and Bing Rui for monitoring the parasitemia. Special thanks go to Shuai Ding, Lili Zhang, and Dianne Wellems for providing critical discussions and suggestions for data analysis and article writing.
This study was supported by the National Natural Science Foundation of China (81220108019) and the National Basic Research Program (973 Program) in China (2007CB513100).
Y.Z., Q.Z., and W.P. conceived and designed the experiments. Y.Z. and H.Y. performed the experiments. Y.Z., H.Y., Q.Z., and W.P. analyzed the data. G.W. and Y.H. contributed reagents, materials, and analysis tools. Y.Z., Q.Z., and W.P. wrote the paper.
Footnotes
Published ahead of print 21 October 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.00972-13.
REFERENCES
- 1.Talisuna AO, Bloland P, D'Alessandro U. 2004. History, dynamics, and public health importance of malaria parasite resistance. Clin. Microbiol. Rev. 17:235–254. 10.1128/CMR.17.1.235-254.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pinichpongse S, Doberstyn EB, Cullen JR, Yisunsri L, Thongsombun Y, Thimasarn K. 1982. An evaluation of five regimens for the outpatient therapy of falciparum malaria in Thailand 1980-81. Bull. World Health Organ. 60:907–912 [PMC free article] [PubMed] [Google Scholar]
- 3.Denis MB, Tsuyuoka R, Poravuth Y, Narann TS, Seila S, Lim C, Incardona S, Lim P, Sem R, Socheat D, Christophel EM, Ringwald P. 2006. Surveillance of the efficacy of artesunate and mefloquine combination for the treatment of uncomplicated falciparum malaria in Cambodia. Trop. Med. Int. Health 11:1360–1366. 10.1111/j.1365-3156.2006.01690.x [DOI] [PubMed] [Google Scholar]
- 4.Denis MB, Tsuyuoka R, Lim P, Lindegardh N, Yi P, Top SN, Socheat D, Fandeur T, Annerberg A, Christophel EM, Ringwald P. 2006. Efficacy of artemether-lumefantrine for the treatment of uncomplicated falciparum malaria in northwest Cambodia. Trop. Med. Int. Health 11:1800–1807. 10.1111/j.1365-3156.2006.01739.x [DOI] [PubMed] [Google Scholar]
- 5.Noedl H, Se Y, Schaecher K, Smith BL, Socheat D, Fukuda MM. 2008. Evidence of artemisinin-resistant malaria in western Cambodia. N. Engl. J. Med. 359:2619–2620. 10.1056/NEJMc0805011 [DOI] [PubMed] [Google Scholar]
- 6.Cowman AF, Morry MJ, Biggs BA, Cross GA, Foote SJ. 1988. Amino acid changes linked to pyrimethamine resistance in the dihydrofolate reductase-thymidylate synthase gene of Plasmodium falciparum. Proc. Natl. Acad. Sci. U. S. A. 85:9109–9113. 10.1073/pnas.85.23.9109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cortese JF, Plowe CV. 1998. Antifolate resistance due to new and known Plasmodium falciparum dihydrofolate reductase mutations expressed in yeast. Mol. Biochem. Parasitol. 94:205–214. 10.1016/S0166-6851(98)00075-9 [DOI] [PubMed] [Google Scholar]
- 8.Triglia T, Menting JG, Wilson C, Cowman AF. 1997. Mutations in dihydropteroate synthase are responsible for sulfone and sulfonamide resistance in Plasmodium falciparum. Proc. Natl. Acad. Sci. U. S. A. 94:13944–13949. 10.1073/pnas.94.25.13944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang P, Read M, Sims PF, Hyde JE. 1997. Sulfadoxine resistance in the human malaria parasite Plasmodium falciparum is determined by mutations in dihydropteroate synthetase and an additional factor associated with folate utilization. Mol. Microbiol. 23:979–986. 10.1046/j.1365-2958.1997.2821646.x [DOI] [PubMed] [Google Scholar]
- 10.Plowe CV, Kublin JG, Doumbo OK. 1998. P. falciparum dihydrofolate reductase and dihydropteroate synthase mutations: epidemiology and role in clinical resistance to antifolates. Drug Resist. Updates 1:389–396. 10.1016/S1368-7646(98)80014-9 [DOI] [PubMed] [Google Scholar]
- 11.Gregson A, Plowe CV. 2005. Mechanisms of resistance of malaria parasites to antifolates. Pharmacol. Rev. 57:117–145. 10.1124/pr.57.1.4 [DOI] [PubMed] [Google Scholar]
- 12.Bjorkman A, Phillips-Howard PA. 1990. The epidemiology of drug-resistant malaria. Trans. R. Soc. Trop. Med. Hyg. 84:177–180. 10.1016/0035-9203(90)90246-B [DOI] [PubMed] [Google Scholar]
- 13.Roper C, Pearce R, Bredenkamp B, Gumede J, Drakeley C, Mosha F, Chandramohan D, Sharp B. 2003. Antifolate antimalarial resistance in southeast Africa: a population-based analysis. Lancet 361:1174–1181. 10.1016/S0140-6736(03)12951-0 [DOI] [PubMed] [Google Scholar]
- 14.Maïga O, Djimdé AA, Hubert V, Renard E, Aubouy A, Kironde F, Nsimba B, Koram K, Doumbo OK, Le Bras J, Clain J. 2007. A shared Asian origin of the triple-mutant dhfr allele in Plasmodium falciparum from sites across Africa. J. Infect. Dis. 196:165–172. 10.1086/518512 [DOI] [PubMed] [Google Scholar]
- 15.Mita T, Tanabe K, Takahashi N, Culleton R, Ndounga M, Dzodzomenyo M, Akhwale WS, Kaneko A, Kobayakawa T. 2009. Indigenous evolution of Plasmodium falciparum pyrimethamine resistance multiple times in Africa. J. Antimicrob. Chemother. 63:252–255. 10.1093/jac/dkn482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bacon DJ, McCollum AM, Griffing SM, Salas C, Soberon V, Santolalla M, Haley R, Tsukayama P, Lucas C, Escalante AA, Udhayakumar V. 2009. Dynamics of malaria drug resistance patterns in the Amazon basin region following changes in Peruvian national treatment policy for uncomplicated malaria. Antimicrob. Agents Chemother. 53:2042–2051. 10.1128/AAC.01677-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cortese JF, Caraballo A, Contreras CE, Plowe CV. 2002. Origin and dissemination of Plasmodium falciparum drug-resistance mutations in South America. J. Infect. Dis. 186:999–1006. 10.1086/342946 [DOI] [PubMed] [Google Scholar]
- 18.McCollum AM, Mueller K, Villegas L, Udhayakumar V, Escalante AA. 2007. Common origin and fixation of Plasmodium falciparum dhfr and dhps mutations associated with sulfadoxine-pyrimethamine resistance in a lowtransmission area in South America. Antimicrob. Agents Chemother. 51:2085–2091. 10.1128/AAC.01228-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mita T, Tanabe K, Takahashi N, Tsukahara T, Eto H, Dysoley L, Ohmae H, Kita K, Krudsood S, Looareesuwan S, Kaneko A, Björkman A, Kobayakawa T. 2007. Independent evolution of pyrimethamine resistance in Plasmodium falciparum isolates in Melanesia. Antimicrob. Agents Chemother. 51:1071–1077. 10.1128/AAC.01186-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vinayak S, Alam MT, Mixson-Hayden T, McCollum AM, Sem R, Shah NK, Lim P, Muth S, Rogers WO, Fandeur T, Barnwell JW, Escalante AA, Wongsrichanalai C, Ariey F, Meshnick SR, Udhayakumar V. 2010. Origin and evolution of sulfadoxine resistant Plasmodium falciparum. PLoS Pathog. 6:e1000830. 10.1371/journal.ppat.1000830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pearce RJ, Pota H, Evehe MS, Bâ el-H, Mombo-Ngoma G, Malisa AL, Ord R, Inojosa W, Matondo A, Diallo DA, Mbacham W, van den Broek IV, Swarthout TD, Getachew A, Dejene S, Grobusch MP, Njie F, Dunyo S, Kweku M, Owusu-Agyei S, Chandramohan D, Bonnet M, Guthmann JP, Clarke S, Barnes KI, Streat E, Katokele ST, Uusiku P, Agboghoroma CO, Elegba OY, Cissé B, A-Elbasit IE, Giha HA, Kachur SP, Lynch C, Rwakimari JB, Chanda P, Hawela M, Sharp B, Naidoo I, Roper C. 2009. Multiple origins and regional dispersal of resistant dhps in African Plasmodium falciparum malaria. PLoS Med. 6:e1000055. 10.1371/journal.pmed.1000055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCollum AM, Basco LK, Tahar R, Udhayakumar V, Escalante AA. 2008. Hitchhiking and selective sweeps of Plasmodium falciparum sulfadoxine and pyrimethamine resistance alleles in a population from central Africa. Antimicrob. Agents Chemother. 52:4089–4097. 10.1128/AAC.00623-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hurwitz ES, Johnson D, Campbell CC. 1981. Resistance of Plasmodium falciparum malaria to sulfadoxine-pyrimethamine (‘Fansidar’) in a refugee camp in Thailand. Lancet i:1068–1070 [DOI] [PubMed] [Google Scholar]
- 24.Verdrager J. 1986. Epidemiology of the emergence and spread of drug-resistant, falciparum malaria in South-East Asia and Australasia. J. Trop. Med. Hyg. 89:277–289 [PubMed] [Google Scholar]
- 25.Huang F, Tang L, Yang H, Zhou S, Liu H, Li J, Guo S. 2012. Molecular epidemiology of drug resistance markers of Plasmodium falciparum in Yunnan Province, China. Malar. J. 11:243. 10.1186/1475-2875-11-243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou Z, Poe AC, Limor J, Grady KK, Goldman I, McCollum AM, Escalante AA, Barnwell JW, Udhayakumar V. 2006. Pyrosequencing, a high-throughput method for detecting single nucleotide polymorphisms in the dihydrofolate reductase and dihydropteroate synthetase genes of Plasmodium falciparum. J. Clin. Microbiol. 44:3900–3910. 10.1128/JCM.01209-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nair S, Williams JT, Brockman A, Paiphun L, Mayxay M, Newton PN, Guthmann JP, Smithuis FM, Hien TT, White NJ, Nosten F, Anderson TJ. 2003. A selective sweep driven by pyrimethamine treatment in Southeast Asian malaria parasites. Mol. Biol. Evol. 20:1526–1536. 10.1093/molbev/msg162 [DOI] [PubMed] [Google Scholar]
- 28.Anderson TJ, Haubold B, Williams JT, Estrada-Franco JG, Richardson L, Mollinedo R, Bockarie M, Mokili J, Mharakurwa S, French N, Whitworth J, Velez ID, Brockman AH, Nosten F, Ferreira MU, Day KP. 2000. Microsatellite markers reveal a spectrum of population structures in the malaria parasite Plasmodium falciparum. Mol. Biol. Evol. 17:1467–1482. 10.1093/oxfordjournals.molbev.a026247 [DOI] [PubMed] [Google Scholar]
- 29.Nei M. 1987. Molecular evolutionary genetics. Columbia University Press, New York, NY [Google Scholar]
- 30.Alam MT, Vinayak S, Congpuong K, Wongsrichanalai C, Satimai W, Slutsker L, Escalante AA, Barnwell JW, Udhayakumar V. 2011. Tracking origins and spread of sulfadoxine-resistant Plasmodium falciparum dhps alleles in Thailand. Antimicrob. Agents Chemother. 55:155–164. 10.1128/AAC.00691-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ding S, Ye R, Zhang D, Sun X, Zhou H, McCutchan TF, Pan W. 2013. Anti-folate combination therapies and their effect on the development of drug resistance in Plasmodium vivax. Sci. Rep. 3:1008. 10.1038/srep01008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pearce R, Malisa A, Kachur SP, Barnes K, Sharp B, Roper C. 2005. Reduced variation around drug-resistant dhfr alleles in African Plasmodium falciparum. Mol. Biol. Evol. 22:1834–1844. 10.1093/molbev/msi177 [DOI] [PubMed] [Google Scholar]
- 33.Bonizzoni M, Afrane Y, Baliraine FN, Amenya DA, Githeko AK, Yan G. 2009. Genetic structure of Plasmodium falciparum populations between lowland and highland sites and antimalarial drug resistance in western Kenya. Infect. Genet. Evol. 9:806–812. 10.1016/j.meegid.2009.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nzila AM, Nduati E, Mberu EK, Hopkins Sibley C, Monks SA, Winstanley PA, Watkins WM. 2000. Molecular evidence of greater selective pressure for drug resistance exerted by the long-acting antifolate pyrimethamine/sulfadoxine compared with the shorter-acting chlorproguanil/dapsone on Kenyan Plasmodium falciparum. J. Infect. Dis. 181:2023–2028. 10.1086/315520 [DOI] [PubMed] [Google Scholar]
- 35.Gatton ML, Martin LB, Cheng Q. 2004. Evolution of resistance to sulfadoxine-pyrimethamine in Plasmodium falciparum. Antimicrob. Agents Chemother. 48:2116–2123. 10.1128/AAC.48.6.2116-2123.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mockenhaupt FP, Teun Bousema J, Eggelte TA, Schreiber J, Ehrhardt S, Wassilew N, Otchwemah RN, Sauerwein RW, Bienzle U. 2005. Plasmodium falciparum dhfr but not dhps mutations associated with sulphadoxine-pyrimethamine treatment failure and gametocyte carriage in northern Ghana. Trop. Med. Int. Health 10:901–908. 10.1111/j.1365-3156.2005.01471.x [DOI] [PubMed] [Google Scholar]
- 37.Nzila AM, Mberu EK, Sulo J, Dayo H, Winstanley PA, Sibley CH, Watkins WM. 2000. Towards an understanding of the mechanism of pyrimethamine-sulfadoxine resistance in Plasmodium falciparum: genotyping of dihydrofolate reductase and dihydropteroate synthase of Kenyan parasites. Antimicrob. Agents Chemother. 44:991–996. 10.1128/AAC.44.4.991-996.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sibley CH, Hyde JE, Sims PF, Plowe CV, Kublin JG, Mberu EK, Cowman AF, Winstanley PA, Watkins WM, Nzila AM. 2001. Pyrimethamine-sulfadoxine resistance in Plasmodium falciparum: what next? Trends Parasitol. 17:582–588. 10.1016/S1471-4922(01)02085-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.