Abstract
Ganciclovir-resistant cytomegalovirus (CMV) infections are reported infrequently among lung transplant recipients receiving extended valganciclovir prophylaxis. We performed a single-center, retrospective review of ganciclovir-resistant CMV infections in a program that employed valganciclovir prophylaxis for ≥6 months after lung transplant. CMV infections were diagnosed in 28% (170/607) of patients. UL97 mutations were detected in 9.4% (16/170) of CMV-infected patients at a median of 8.5 months posttransplant (range, 5 to 21) and despite prophylaxis for a median of 7 months (range, 4 to 21). UL97 mutations were canonical; 25% (4/16) of strains carried concurrent UL54 mutations. Ganciclovir-resistant CMV was more likely with breakthrough infections (75% [12/16] versus 19% [30/154]; P = 0.00001) and donor positive/recipient negative (D+/R−) serostatus (75% versus 45% [69/154]; P = 0.03). The median whole-blood CMV load was 4.13 log10 copies/cm3 (range, 2.54 to 5.53), and 93% (14/15) of patients had low-moderate immune responses (Cylex Immunoknow). Antiviral therapy was successful, failed, or eradicated viremia followed by relapse in 12% (2/16), 31% (5/16), and 56% (9/16) of patients, respectively. Eighty-seven percent (14/16) of patients were treated with foscarnet-containing regimens; toxicity developed in 78% (11/14) of these. Median viral load half-life and time to viremia eradication among foscarnet-treated patients were 2.6 and 23 days, respectively, and did not correlate with protection from relapse. Sixty-nine percent (11/16) of patients developed CMV pneumonitis, and 25% (4/16) died of it. Serum viral load was independently associated with death among foscarnet-treated patients (P = 0.04). In conclusion, ganciclovir-resistant CMV infections remained a major cause of morbidity and mortality following lung transplantation. Foscarnet-based regimens often eradicated viremia rapidly but were ineffective in the long term and limited by toxicity.
INTRODUCTION
Cytomegalovirus (CMV) infections develop in 80% of lung transplant recipients in the absence of preventive antiviral therapy (1). The optimal preventive regimen against CMV infection following lung transplantation remains undefined, but most programs employ universal antiviral prophylaxis (2). Most prophylaxis studies in lung transplant recipients have employed ganciclovir, alone or in combination with CMV hyperimmunoglobulin G (3–10). Valganciclovir, an oral prodrug that achieves ganciclovir concentrations comparable to those of intravenous (i.v.) ganciclovir (11), is at least as effective and safe in reducing CMV infections after lung transplantation (8, 12–18). Controversy persists, however, over the recommended duration of valganciclovir prophylaxis. Several reports, including one from our program, demonstrated that courses ≥6 months are superior to shorter courses (8, 14, 19). More recently, a multicenter study of 136 lung transplant recipients showed that valganciclovir prophylaxis for 12 months significantly reduced active infections compared to a 3-month course (17). While these data have been used to support 12 months of routine valganciclovir prophylaxis (2), it is uncertain how much additional benefit is garnered after 6 months.
Overall, ganciclovir-resistant CMV has been reported in 0 to 15% of lung transplant recipients who develop active infections (17, 19–24). Genotypic resistance stems from mutations at hot spots in the UL97 gene, which encodes the viral DNA phosphotransferase that monophosphorylates ganciclovir, and/or the UL54 gene, which encodes viral DNA polymerase (25). UL54 mutations are less common and may confer cross-resistance to foscarnet or cidofovir. Foscarnet, which inhibits viral DNA polymerase, is the preferred agent for patients failing ganciclovir therapy due to resistance, but it carries significant renal toxicity (20, 26). The published experience with foscarnet in the treatment of ganciclovir-resistant CMV infections among solid-organ transplant (SOT) recipients, in particular lung transplant recipients, is limited, and clinical results have been mixed (23–25, 27–31).
Our objectives were to describe the clinical manifestations and outcomes of ganciclovir-resistant CMV infections among lung transplant recipients in the era of extended valganciclovir prophylaxis. In particular, we sought to describe our experience in treating patients with foscarnet-containing regimens.
MATERIALS AND METHODS
Patients.
Lung or heart/lung transplant recipients at the University of Pittsburgh Medical Center from 2006 through 2010 who underwent CMV genotypic resistance testing were identified. Electronic medical records were reviewed retrospectively through 31 December 2011. The study was approved by the University of Pittsburgh Institutional Review Board. The standard induction and maintenance immunosuppression were alemtuzumab and tacrolimus (induction) and mofetil mycophenolate (MMF) and prednisone (maintenance). Valganciclovir prophylaxis (900 mg/day or the renally adjusted equivalent) was recommended for ≥6 months for donor positive/recipient negative (D+/R−) and R+ transplants. In December 2008, valganciclovir prophylaxis was recommended for ≥12 months for D+/R− transplants. Valganciclovir prophylaxis was reinstituted for ≥3 months upon receipt of augmented immunosuppression for treatment of acute rejection. CMV whole-blood viral load monitoring by PCR was recommended bimonthly during valganciclovir prophylaxis (weekly if off prophylaxis in the first 6 months) and monthly thereafter. Resistance testing was ordered at the providers' discretion and consisted of detection of UL97 and UL54 mutations (codons 363 to 698 and 184 to 1017, respectively). Viral load measurements were performed using an in-house real-time PCR assay (32). Resistance testing was performed as a send-out test at Quest Diagnostics (San Juan Capistrano, CA). Decisions on antiviral therapy were made by providers.
Definitions.
CMV viremia was defined by the detection of viral DNA using our in-house assay (low-copy-number cutoff of 50 copies), in the absence of attributable symptoms. CMV disease was defined as the occurrence of clinical symptoms in the presence of histopathologic findings consistent with tissue invasion by CMV. Ganciclovir resistance was defined by the detection of mutations within UL97 and/or UL54, as described above. Virologic suppression was defined as the achievement of a nondetectable viral load in response to treatment, as measured by our in-house assay. Acute cellular rejection was documented as recorded in biopsy reports and defined as International Society of Heart and Lung Transplantation grade ≥2, treated with augmented immunosuppression. Drug toxicity was identified by the treating physician and corroborated by review of pertinent labs. The Immunoknow Immune Cell Function assay (Cylex, Inc., Columbia, MD) was ordered by providers, and results were interpreted as low, moderate, or strong using standard criteria (33).
Viral load kinetics.
Serial CMV viral loads were plotted over time, where time zero represented the initiation of antiviral therapy against ganciclovir-resistant CMV (Fig. 1. Viral loads followed a logarithmic decay curve, and the best-fit line was derived based on the equation y = y0e−ax, where y0 is the viral load at the start of foscarnet, x is the time from the start of foscarnet, and a is the decay constant. Viral load half-life was calculated according to the formula: t1/2 = (ln2)/a (34).
FIG 1.
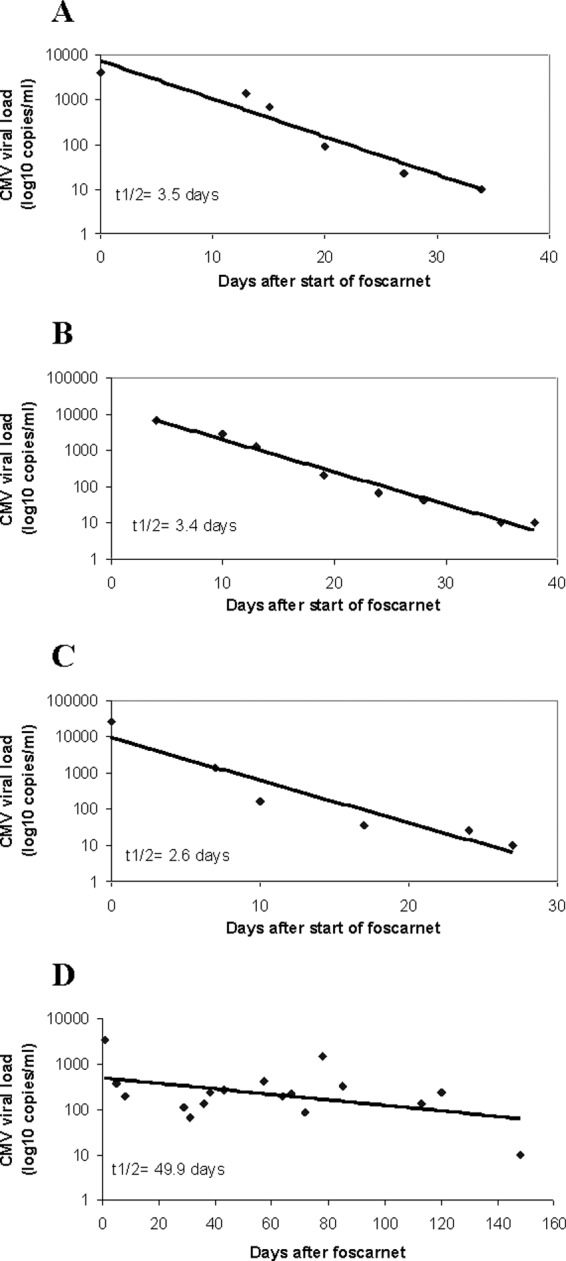
Viral load kinetics in response to foscarnet-containing treatment regimens. Viral load kinetics did not correlate with protection from relapsing infections. Data are presented for 4 representative patients. (A) Patient 1 (Tables 2 and 3) achieved serum viral suppression in 34 days and remained suppressed on foscarnet until death due to other causes on day 120. (B) Patient 9 achieved serum viral suppression in 35 days but died of CMV pneumonia on day 40. (C) Patient 5 achieved serum viral suppression in 27 days but suffered multiple relapses. (D) Patient 15 required 146 days to achieve serum viral suppression and suffered multiple relapses.
Statistics.
Instat Software (Graphpad Software Inc., San Diego, CA) was used. Comparisons of dichotomous variables were made with Fisher's exact and chi-square tests. Continuous variables were reported as medians with ranges, and differences between groups were calculated using the Mann-Whitney U test. P values of <0.05 were considered significant. Logistic regression analysis was performed to identify independent risk factors for death using variables with P values of <0.20 by univariate analysis.
RESULTS
Patients infected with ganciclovir-resistant CMV.
Twenty-eight percent (170/607) of lung transplant recipients developed CMV infections. Ganciclovir resistance mutations were detected in 9.4% (16/170) of CMV-infected patients, and 7.9% (12/152) and 2.6% (16/607) of D+/R− and all lung transplant recipients, respectively (Table 1). Compared to the rest of the CMV-infected cohort in our program, patients with ganciclovir-resistant CMV were more likely to have had initial breakthrough infections (75% [12/16] versus 19% [30/154]; P = 0.00001) and D+/R− status (75% [12/16] versus 45% [69/154]; P = 0.03). Patients infected with resistant CMV received valganciclovir for a median of 7 months (range, 2 to 21).
TABLE 1.
Demographics and clinical data for lung transplant recipients infected with ganciclovir-resistant CMVa
| Characteristic | Value |
|---|---|
| Male gender, % (n) | 50 (8) |
| Age at transplant, median (range), yr | 56 (22–68) |
| Age at transplant >60, % (n) | 44 (7) |
| Indication for transplant, % (n) | |
| Chronic obstructive pulmonary disease | 38 (6) |
| Idiopathic pulmonary fibrosis | 38 (6) |
| Cystic fibrosis | 19 (3) |
| Connective tissue disorder | 6 (1) |
| Retransplant | 6 (1) |
| Other | 0 |
| Type of transplant, % (n) | |
| Double lung transplant | 75 (12) |
| Single lung transplant | 31 (5) |
| Heart/lung transplant | 0 |
| Induction immunosuppression, % (n) | |
| Alemtuzumab | 87 (14) |
| Basiliximab | 12 (2) |
| Other | 0 |
| Maintenance immunosuppression, % (n) | |
| FK506/MMF/Prednisone | 81 (13) |
| Acute cellular rejection (treated), % (n) | 38 (6) |
| Duration of VGCV prophylaxis, median (range), months | 7 (4–21) |
| Patients with incorrect (low) VGCV dose, % (n) | 18 (3) |
| CMV D+/R−, % (n) | 81 (13) |
| Breakthrough infection, % (n) | 75 (12) |
| CMV load at time of genotypic test, median (range), log10 copies/cm3 | 4.13 (2.19–5.53) |
| Time from transplant to CMV infection, median (range), mo | 8 (5–13) |
Abbreviations: FK506, tacrolimus; MMF, mofetil mycophenolate; VGCV, valganciclovir; D+/R−, donor positive/receptor negative.
Clinical data and treatment regimens prior to documentation of ganciclovir resistance (Table 2).
TABLE 2.
CMV infections prior to detection of ganciclovir resistancea
| Patient no. | D/R serostatus | Breakthrough infection (yes/no) | Type of initial CMV infection | Time after transplant (mo) | Initial treatment regimen | Response to initial regimen | Subsequent course | Time from initial infection to detection of resistance (days) |
|---|---|---|---|---|---|---|---|---|
| 1 | D+/R− | Yes | Viremia | 6 | VGC | Not suppressed | Ongoing viremia | 49 |
| 2 | D+/R+ | Yes | Viremia | 4 | VGC, then GCV | Not suppressed | Diagnosed with pneumonitis | 21 |
| 3 | D+/R− | Yes | Viremia | 7 | VGC, then GCV | Not suppressed | Ongoing viremia | 32 |
| 5 | D+/R− | Yes | Viremia | 3 | VGC | Suppressed | Relapsed with viremia on maintenance VGC, then diagnosed with pneumonitis and bronchial polyp on GCV | 106 |
| 7 | D+/R− | No | Viremia | 3 | VGC, then GCV | Suppressed | Relapsed with pneumonitis on GCV | 90 |
| 8 | D+/R− | Yes | Viremia | 2 | VGC, then GCV | Suppressed | Relapsed with viremia on maintenance VGC | 120 |
| 9 | D+/R− | Yes | Viremia | 6 | VGC | Suppressed | Relapsed with pneumonitis on maintenance VGC | 90 |
| 10 | D+/R− | Yes | Pneumonitis | 17 | NA (presented with GCV-R) | NA | NA | NA |
| 11 | D+/R− | Yes | Viremia | 4 | VGC | Suppressed | Relapsed with pneumonitis and GI disease on maintenance VGC | 87 |
| 12 | D+/R− | No | Pneumonitis | 13 | GCV | Not suppressed | Ongoing pneumonitis, then diagnosed with GI disease | 82 |
| 13 | D+/R− | No | GI | 8 | GCV | Not suppressed | Ongoing GI disease | 131 |
| 14 | D+/R− | Yes | Viremia | 5 | VGC | Suppressed | Relapsed with viremia on maintenance VGC | 105 |
| 15 | D-/R− | Yes | Primary viremia | 4 | VGC, then GCV | Not suppressed | Ongoing viremia | 44 |
| 16 | D+/R− | Yes | Viremia | 20 | VGC | Not suppressed | Ongoing viremia | 15 |
| 6 | D+/R+ | No | Viremia | 7 | VGC | Suppressed | Relapsed with viremia on maintenance VGC | 92 |
| 4 | D+/R+ | Yes | Pneumonitis and GI | 12 | NA (presented with GCV-R) | NA | NA | NA |
D/R, donor/recipient; VGC, valganciclovir; GCV, ganciclovir; R, resistant; NA, not applicable; GI, gastrointestinal.
Median follow-up for the 16 patients infected with ganciclovir-resistant CMV was 19 months (range, 7 to 55) posttransplant. Patients first presented with CMV infection at a median of 6 months posttransplant (range, 2 to 20). Seventy-five percent (12/16) and 25% (4/16) of patients presented initially with CMV viremia and disease, respectively. In 75% (12/16) of patients, the initial CMV infection occurred as a breakthrough at a median of 5.5 months (range, 2 to 20) of valganciclovir prophylaxis. In the remaining 25% (4/16) of patients, the initial CMV infection occurred after discontinuation of valganciclovir prophylaxis. Among these patients, prophylactic valganciclovir was administered for a median of 7.5 months (range, 3 to 13) and then discontinued for a median of 1.2 months (range, 0.8 to 2) prior to CMV infection.
Twelve percent (2/16) of patients were demonstrated to be infected with ganciclovir-resistant virus upon their initial presentation for CMV infection (patients 4 and 10, who had breakthrough pneumonitis/gastrointestinal [GI] disease and breakthrough pneumonitis, respectively). The other 87% (14/16) of patients were diagnosed with ganciclovir-resistant infections a median of 88 days (range, 15 to 131) after their initial presentations. For the latter group, treatment of CMV viremia and disease prior to the documentation of resistance consisted of induction dose valganciclovir and intravenous ganciclovir, respectively. Fifty percent (7/14) of these patients failed to achieve complete viral suppression prior to the documentation of resistance mutations; 50% (7/14) had suppression but subsequently relapsed with resistant CMV.
Mutations and clinical data for ganciclovir-resistant CMV infections (Table 3).
TABLE 3.
Ganciclovir-resistant CMV infections, treatment regimens and outcomesa
| Patient no. | Mutation(s) (drug resistance) | Type of infection (serum log10 viral load) | Cylex (ng/ml ATP) | Treatment regimen | Viral load response | Decay half-life (days) | Subsequent course | Follow-up (mo) | Outcome classification |
|---|---|---|---|---|---|---|---|---|---|
| Treatment with foscarnet-containing regimens | |||||||||
| 1 | UL97 C603W (GCV) | Viremia (3.60 log) | 634 | FOS, GCV, CMV Ig | Suppressed (34 days) | 3.5 | Maintained on FOS until death (120 days; not CMV related) | 4 | Suppressed on FOS |
| 2 | UL97 H520Q (GCV) | Pneumonitis (3.01 log) | 138 | FOS, CMV Ig | Suppressed (14 days) | 1.8 | Relapsed on CMV Ig + LEF maintenance. Retreated with FOS. Retransplanted due to allograft failure. | 30 | Relapse |
| 3 | UL97 M460V (GCV) | Viremia (2.54 log) | 115 | FOS, CMV Ig | Suppressed (15 days) | 2.1 | Relapsed with pneumonitis, then ongoing viremia despite FOS + CMV Ig,+LEF + RAP | 17 | Relapse |
| 5 | UL97 M460V (GCV) | Pneumonitis and bronchial polyp (4.41 log) | 397 | FOS, CMV Ig | Suppressed (27 days) | 2.6 | Relapsed on CMV Ig + RAP maintenance | 8 | Relapse |
| 7 | UL97 M460V (GCV) | Pneumonitis (4.88 log) | 76 | FOS | Not suppressed | NA | Died of pneumonitis (28 days) | NA | Failure |
| 8 | UL97 L595S (GCV) | Viremia (4.88 log) | 87 | FOS, GCV, LEF | Not suppressed | NA | Died of pneumonitis (153 days) | NA | Failure |
| 9 | UL97 603W/C (GCV) | Pneumonitis (4.21 log) | 341 | FOS, CMV Ig | Suppressed (35 days) | 3.4 | Died of pneumonitis (40 days) | NA | Failure |
| 10 | UL97 A594P (GCV) | Pneumonitis (4.04 log) | 340 | FOS | Suppressed (16 days) | 1.8 | Relapsed off FOS. Retreated with FOS + CMV Ig + RAP | 9 | Relapse |
| 11 | UL97 M460I (GCV) UL54 L545S (CID) | Pneumonitis and GI (5.53 log) | 3 | FOS, CMV Ig | Not suppressed | NA | Died of pneumonitis (28 days) | NA | Failure |
| 12 | UL97 M460V (GCV) | Pneumonitis (4.00 log) | 366 | FOS, CMV Ig, RAP | Not suppressed | NA | Pneumonitis responded to FOS + GCV + CMV Ig + RAP + CMX-001, but ongoing viremia. | 7 | Failure |
| 13 | UL97 L595S, C603W/C (GCV) | Pneumonitis and GI (3.62 log) | 25 | FOS, CMV Ig | Suppressed (16 days) | 2.6 | Multiple relapses, maintained on FOS | 11 | Relapse |
| 14 | UL97 M460I (GCV) UL54 F412L (CID) | Viremia (3.22 log) | 260 | FOS, CMV Ig, RAP | Suppressed (20 days) | 2.1 | Relapsing viremia on RAP maintenance | 4 | Relapse |
| 15 | UL97 C603W (GCV) UL54 K513N (CID) | Viremia (3.52 log) | 145 | FOS, GCV, CMV Ig, RAP | Suppressed (146 days) | 49.9 | Relapsed on VGC + CMV Ig + RAP. Persistent viremia on VGC + RAP | 21 | Relapse |
| 16 | UL97 C603W (GCV) UL54 L501F (CID) | Viremia (4.30 log) | NA | FOS, LEF | Suppressed (80 days) | 10 | Relapsed on LEF. Retreated with FOS | 7 | Relapse |
| Treated with IV ganciclovir | |||||||||
| 6 | UL97 C603W (GCV) | Viremia (4.23 log) | 48 | GCV, then VGC | Suppressed (20 days) | 2.2 | Multiple relapses off antivirals, treated with VGC | 47 | Relapse |
| 4 | UL97 (GCV)b | Pneumonitis and GI infection (4.23 log) | 162 | GCV, then VGC | Suppressed (20 days) | 2.4 | Sustained suppression off antivirals | 43 | Success |
Abbreviations: GCV, ganciclovir; FOS, foscarnet; LEF, leflunomide; CID, cidofovir; RAP, rapamycin; CMV Ig, CMV immune globulin; NA, not applicable.
Codon information not provided.
Ganciclovir resistance was diagnosed at a median of 8.5 months (range, 5 to 21) posttransplant. Specific UL97 mutations were reported in 94% (15/16) of patients; in the remaining patient (patient 4), a UL97 mutation was detected but specific codon data were not provided. In 25% (4/16) of patients, concurrent ganciclovir and cidofovir resistance-conferring UL54 mutations were detected. At the time ganciclovir resistance was documented, 56% (9/16) and 44% (7/16) of patients had CMV pneumonitis and viremia, respectively. Median CMV whole-blood viral load at the time of resistance was 4.13 log10 (range, 2.19 to 5.53). Cylex Immunoknow assay data were available for 94% (15/16) of patients. The median level was 145 ATP ng/ml (range, 3 to 634). Sixty percent (9/15), 33% (5/15), and 6% (1/15) of patients had low, moderate, and strong responses (levels of ≤225, 226 to 524, and ≥525 ng/ml, respectively).
Treatment and outcomes for ganciclovir-resistant CMV infections (Table 3).
Antiviral therapy directed against ganciclovir-resistant CMV was instituted a median of 1 day (range, −1 to 86) after genotypic testing was ordered. Eighty-seven percent (14/16) of patients were treated with foscarnet, either alone (12%, 2/16) or combined with various agents (75%, 12/16). The median duration of treatment with foscarnet-containing regimens was 38 days (range, 17 to 210). Twenty-nine percent (4/14) of patients treated with a foscarnet-containing regimen failed to achieve serum virologic suppression (median duration, 28 days; range, 24 to 153). This group included three patients who died from CMV pneumonitis and one patient who recovered from pneumonitis but had persistent viremia over 7 months' follow-up. The remaining 71% (10/14) of patients treated with a foscarnet-containing regimen achieved virologic suppression after a median of 23 days (range, 14 to 146). The median duration of treatment was 39 days (range, 17 to 153). The median viral load half-life in these patients was 2.6 days (range, 1.8 to 49.9). Twenty percent (2/10) of patients who had virologic suppression subsequently died; one patient died of persistent CMV pneumonitis 5 days after clearing viremia, and one patient died of allograft failure without evidence of active CMV infection. The other 80% (8/10) of patients who had virologic suppression suffered relapsing infections. There was no association between specific UL97 mutations or the combination of UL97 and UL54 mutations and outcomes among patients treated with foscarnet. Viral load half-life or time to complete virologic suppression did not correlate with protection from relapsing infections (Fig. 1).
Twelve percent (2/16) of patients were treated with ganciclovir for resistant infections, including viremia and pneumonitis/GI disease (one patient each). These patients both achieved serum virologic suppression at 20 days, with viral load half-lives of 2.2 and 2.4 days, respectively. The patient with viremia had multiple episodes of relapsing viremia after stopping ganciclovir (C630W). The patient with pneumonitis and GI disease had sustained suppression off antivirals after completing 2 months of ganciclovir (UL97 codon not reported). Both patients were alive at the end of the study.
Overall, 69% (11/16) of patients developed pneumonitis at some stage of their infection. Treatment of ganciclovir-resistant CMV was considered unsuccessful in 87% (14/16), including treatment failures (31%, 5/16) and relapsing infections (56%, 9/16). Twenty-five percent (4/16) of patients died from CMV pneumonitis. One of the patients treated successfully for viremia died 3 months later from other causes. Only one patient had sustained suppression of CMV infection off antiviral maintenance therapy.
By univariate analysis, serum viral load at the time of diagnosis of ganciclovir resistance and a failure to achieve serum viral suppression were significant risk factors for death among patients treated with foscarnet (Table 4). By multivariate analysis, serum viral load was the only independent risk factor for death.
TABLE 4.
Risk factors for death due to ganciclovir-resistant CMV disease among patients treated with foscarnet
| Risk factor | Value for patients who: |
P value |
|||
|---|---|---|---|---|---|
| Died | Lived | Univariate analysis | Logistic regression analysis (model 1)a | Logistic regression analysis (model 2)b | |
| Mean time (mo) from transplant to infection with resistant CMV | 17.6 ± 2.9 | 32.0 ± 16.7 | 0.12 | Not included | 0.10 |
| CMV viral load (log10 copies/ml) at time resistance was diagnosed | 4.54 ± 0.54 | 3.61 ± 0.72 | 0.004 | 0.04 | 0.03 |
| Failure to achieve serum viral suppression | 75% (3/4) | 10% (1/10) | 0.04 | 0.12 | 0.19 |
| CMV disease at time resistance was diagnosed | 75% (3/4) | 50% (5/10) | 0.58 | Not included | Not included |
| D+/R− serostatus | 0% (0/2) | 80% (8/10) | 1.00 | Not included | Not included |
| Mean Cylex (ng/ml ATP) | 127 ± 148 | 269 ± 187 | 0.62 | Not included | Not included |
Model 1 included factors with P values of <0.10 in univariate analysis.
Model 2 included factors with P values of <0.20 in univariate analysis.
Drug toxicity.
Seventy-eight percent (11/14) of patients treated with foscarnet experienced toxicity, including renal injury (71%, 10/14), electrolyte abnormalities (71%, 10/14), and/or GI disturbances (28%, 3/14) (9 patients had multiple toxicities). One patient required hemodialysis. Radiographic changes or hypoxia developing as a consequence of fluid management and electrolyte abnormalities were not reported. Foscarnet was discontinued due to toxicity in 36% (5/14) of patients. One patient treated with ganciclovir developed drug-related neutropenia that required granulocyte colony-stimulating factor (G-CSF).
DISCUSSION
To the best of our knowledge, this is the largest series of lung transplant recipients with ganciclovir-resistant CMV infections in the era of extended valganciclovir prophylaxis. Several findings from our study are particularly notable. First, ganciclovir-resistant CMV infections were associated with poor outcomes, including the development of pneumonitis in 69% (11/16) of patients and attributable mortality of 25% (4/16). Second, foscarnet-containing treatment regimens performed poorly, limited by therapeutic failures, relapses, and toxicity. Serum viral load at the time of treatment was the major predictor of death due to ganciclovir-resistant CMV disease among patients receiving foscarnet-containing regimens. Third, the rates of CMV infection and ganciclovir resistance among CMV-infected patients were 28% and 9.4%, respectively, despite receipt of valganciclovir prophylaxis for a median of 7 months (range, 4 to 21 months). The rate of CMV infection was higher than in a recent multicenter study of extended valganciclovir prophylaxis (17, 18). Taken together, the data demonstrate that ganciclovir-resistant CMV infections remained a major cause of morbidity and mortality.
Ganciclovir resistance was diagnosed at a median of 8.5 months and as late as 21 months following lung transplantation. While late-onset ganciclovir-resistant CMV infections, high rates of organ disease, and poor outcomes are well recognized among SOT recipients (22, 25, 31, 35), our rates of pneumonitis and mortality were particularly dire. The performance of foscarnet-based treatment regimens in prior studies has varied. In several studies of kidney and lung transplant recipients, foscarnet and foscarnet-containing regimens achieved striking reductions and complete eradication of ganciclovir-resistant infections in the majority of patients without causing significant toxicity (23, 27, 28). In other studies, however, SOT patients treated with foscarnet had high rates of death, therapeutic failure, renal and electrolyte toxicity, allograft loss, and/or prolonged hospitalizations (24, 25, 29–31). Moreover, relapsing infections are well recognized in the literature (23, 28) and were encountered in almost all of our patients who initially achieved virologic suppression.
Of note, most patients who suffered relapsing infections had brisk initial serum viral load responses and rapid eradication of viremia. Viral load kinetics followed an exponential decay curve, with median serum half-life and time to eradication of 2.6 and 23 days, respectively. In this regard, our experience differed from previous studies. Viral load kinetics were predictive of outcomes in one study of nonresistant CMV infections treated with ganciclovir (34), as mean serum half-lives of 3.2 and 8.8 days were associated with cures and recurrent disease among SOT recipients, respectively. In a study of kidney transplant recipients treated with foscarnet for ganciclovir-resistant CMV infections, mean viral load half-life and time to eradication were similar to ours (3.7 and 30.5 days, respectively) but none of the patients had relapses (27). Our data corroborate that foscarnet-based regimens, in general, are potent at suppressing CMV viral loads. However, the frequent relapses speak to the importance of host immune function in determining outcomes, particularly among highly susceptible D+/R− lung transplant recipients. The better outcomes with foscarnet in the previous study may reflect the fact that kidney transplant recipients are typically less immunosuppressed and at lower risk for CMV infections than lung transplant recipients.
Unfortunately, alternative therapeutic options to foscarnet are lacking. Cidofovir is also limited by severe renal toxicity and neutropenia (26), and as illustrated by our experience, almost all UL54 mutations that confer ganciclovir resistance are associated with cidofovir cross-resistance (36). Other agents are in development, but none has completed phase III studies successfully (37, 38). Two of our patients achieved viral load suppression after treatment with ganciclovir and reduction of immunosuppression. Ganciclovir was continued in these patients after clinicians received the reports of UL97 mutations because of good therapeutic responses. Indeed, some lung transplant recipients infected with ganciclovir-resistant CMV in a previous study were managed with induction ganciclovir without untoward consequences (23). In the previously cited study of ganciclovir-resistant CMV infections following kidney transplantation, the majority of patients did not receive foscarnet and were successfully treated with valganciclovir and reduction of MMF (27). Despite these successes, we do not recommend treating known or suspected ganciclovir-resistant CMV by switching to i.v. ganciclovir and/or increasing valganciclovir/ganciclovir doses. The best course of action for these patients may be treatment with a combination of foscarnet and ganciclovir, as was advocated in a recent study of lung transplant recipients (28). In this context, it is incumbent upon clinicians to recognize the risk factors for the emergence of ganciclovir resistance and promptly institute aggressive therapy. The importance of starting foscarnet and foscarnet-containing regimens early is highlighted by our finding that viral load at the time of treatment was the only independent predictor of mortality.
It is also important for clinicians to properly interpret genotypic data in making treatment decisions. The UL97 mutations reported for our patients were among the seven canonical mutations that account for >80% of resistant CMV strains (25, 39–41). Most importantly, each of the UL97 and UL54 mutations is known to confer phenotypic drug resistance. The UL97 mutations are associated with 2.9- to 10-fold reductions in ganciclovir susceptibility and the UL54 mutations with 3.5- to 6-fold and 9.1- to 12.5-fold reductions in ganciclovir and cidofovir susceptibility, respectively (25, 40). As more clinical strains are studied, an increasing number of uncharacterized UL97 and UL54 sequence variants that have unclear relevance to drug resistance have been described (40). Moreover, it is clear that sequence polymorphisms can be detected in both genes in the absence of prior drug exposure (40). Therefore, providers should ensure that mutations identified in clinical strains have been shown to correlate with antiviral resistance. Along these lines, one of our patients (patient 4) was infected with a strain for which specific codon data were not available. Despite developing breakthrough pneumonitis and GI infection, the patient responded fully to ganciclovir therapy and had no evidence of active infection at 43 months follow-up. As such, it is plausible that the patient's CMV strain was not phenotypically resistant to ganciclovir.
The risk factors for the emergence of resistance were consistent with previous reports, including prolonged antiviral exposure, intense immunosuppression, D+/R− serostatus, and breakthrough CMV infections. In contrast to our experience, breakthrough infections were rare in the multicenter study cited above (17). The reasons that our results differed are not immediately apparent but may reflect differences in patient populations. Of particular note, almost all of our patients received induction immunosuppression, compared to only 33% of patients in the extended-prophylaxis arm of the clinical trial. Moreover, the standard induction agent in our program was alemtuzumab, a particularly potent anti-CD52 monoclonal antibody that predisposes patients to active CMV infections due to profound and sustained T cell depletion and dysfunction (42). In fact, over 90% of our patients infected with ganciclovir-resistant CMV had low or moderate immune responses at the time of diagnosis, as assessed by the Cylex Immunoknow assay. Interestingly, UL97 mutations were detected in 2.9% of lung transplant recipients randomized to the extended valganciclovir arm of the multicenter study (17), which was virtually identical to the 2.6% rate among our lung transplant recipients. Ganciclovir resistance was not reported in a follow-up study of a subset of patients from the earlier trial, who were from a single center (18). Nevertheless, our data showcase that concerns over the potential for ganciclovir resistance with extended valganciclovir prophylaxis are legitimate (2, 17, 20). Programs employing these strategies should collect data on their outcomes, in order to understand the costs and benefits among their own patients. In response to our high rates of CMV infection and ganciclovir resistance, we have switched from alemtuzumab to basiliximab as the induction agent among D+/R− patients.
This report is limited by its single-center, retrospective study design, and results may not be applicable to other programs or types of SOT. Moreover, our study and others may be biased by the fact that genotypic testing was performed at providers' discretion, generally in response to persistent viral loads and symptoms. In several clinical trials in which CMV loads and genotypes were monitored without regard to symptoms, resistance mutations were also detected in relatively asymptomatic patients who responded to therapy (43, 44). Therefore, the clinical spectrum and outcomes of ganciclovir-resistant CMV infections may be broader than suggested by the data. Clearly, more-effective, better-tolerated, and less resource-intensive preventive strategies than extended valganciclovir are needed. Investigational methods such as CMV-specific T cell profiling may enable clinicians to target prophylaxis to the highest-risk patients, while minimizing unnecessary antiviral exposure. Such tools would eliminate the need for universal extended valganciclovir prophylaxis or other one-size-fits-all approaches and decrease pressure for antiviral resistance.
ACKNOWLEDGMENTS
No funding was received for this study. The authors do not have any relevant conflicts of interest.
We thank our study coordinator, Diana Pakstis, and database manager, Lloyd Clarke, for their assistance.
Footnotes
Published ahead of print 21 October 2013
REFERENCES
- 1.Ettinger NA, Bailey TC, Trulock EP, Storch GA, Anderson D, Raab S, Spitznagel EL, Dresler C, Cooper JD. 1993. Cytomegalovirus infection and pneumonitis. Impact after isolated lung transplantation. Washington University Lung Transplant Group. Am. Rev. Respir. Dis. 147:1017–1023 [DOI] [PubMed] [Google Scholar]
- 2.Patel N, Snyder LD, Finlen-Copeland A, Palmer SM. 2012. Is prevention the best treatment? CMV after lung transplantation. Am. J. Transplant. 12:539–544. 10.1111/j.1600-6143.2011.03837.x [DOI] [PubMed] [Google Scholar]
- 3.Duncan SR, Grgurich WF, Iacono AT, Burckart GJ, Yousem SA, Paradis IL, Williams PA, Johnson BA, Griffith BP. 1994. A comparison of ganciclovir and acyclovir to prevent cytomegalovirus after lung transplantation. Am. J. Respir. Crit. Care Med. 150:146–152. 10.1164/ajrccm.150.1.8025741 [DOI] [PubMed] [Google Scholar]
- 4.Gerbase MW, Dubois D, Rothmeier C, Spiliopoulos A, Wunderli W, Nicod LP. 1999. Costs and outcomes of prolonged cytomegalovirus prophylaxis to cover the enhanced immunosuppression phase following lung transplantation. Chest 116:1265–1272. 10.1378/chest.116.5.1265 [DOI] [PubMed] [Google Scholar]
- 5.Gutierrez J, Piedrola G, Maroto MC. 1998. Value of cytomegalovirus (CMV) IgG avidity index for the diagnosis of primary CMV infection. J. Infect. Dis. 178:599–600. 10.1086/517464 [DOI] [PubMed] [Google Scholar]
- 6.Speich R, Thurnheer R, Gaspert A, Weder W, Boehler A. 1999. Efficacy and cost effectiveness of oral ganciclovir in the prevention of cytomegalovirus disease after lung transplantation. Transplantation 67:315–320. 10.1097/00007890-199901270-00023 [DOI] [PubMed] [Google Scholar]
- 7.Weill D, Lock BJ, Wewers DL, Young KR, Zorn GL, Early L, Kirklin JK, McGiffin DC. 2003. Combination prophylaxis with ganciclovir and cytomegalovirus (CMV) immune globulin after lung transplantation: effective CMV prevention following daclizumab induction. Am. J. Transplant. 3:492–496. 10.1034/j.1600-6143.2003.00074.x [DOI] [PubMed] [Google Scholar]
- 8.Zamora MR, Nicolls MR, Hodges TN, Marquesen J, Astor T, Grazia T, Weill D. 2004. Following universal prophylaxis with intravenous ganciclovir and cytomegalovirus immune globulin, valganciclovir is safe and effective for prevention of CMV infection following lung transplantation. Am. J. Transplant. 4:1635–1642. 10.1111/j.1600-6143.2004.00571.x [DOI] [PubMed] [Google Scholar]
- 9.Wreghitt TG, Abel SJ, McNeil K, Parameshwar J, Stewart S, Cary N, Sharples L, Large S, Wallwork J. 1999. Intravenous ganciclovir prophylaxis for cytomegalovirus in heart, heart-lung, and lung transplant recipients. Transpl. Int. 12:254–260. 10.1111/j.1432-2277.1999.tb01210.x [DOI] [PubMed] [Google Scholar]
- 10.Valentine VG, Weill D, Gupta MR, Raper B, Laplace SG, Lombard GA, Bonvillain RW, Taylor DE, Dhillon GS. 2008. Ganciclovir for cytomegalovirus: a call for indefinite prophylaxis in lung transplantation. J. Heart Lung Transplant. 27:875–881. 10.1016/j.healun.2008.05.009 [DOI] [PubMed] [Google Scholar]
- 11.Hodson EM, Barclay PG, Craig JC, Jones C, Kable K, Strippoli GF, Vimalachandra D, Webster AC. 2005. Antiviral medications for preventing cytomegalovirus disease in solid organ transplant recipients. Cochrane Database Syst. Rev. 2:CD003774. 10.1002/14651858.CD003774.pub3 [DOI] [PubMed] [Google Scholar]
- 12.Couchoud C. 2000. Cytomegalovirus prophylaxis with antiviral agents for solid organ transplantation. Cochrane Database Syst. Rev. 2:CD001320. 10.1002/14651858.CD001320.pub2 [DOI] [PubMed] [Google Scholar]
- 13.Humar A, Kumar D, Preiksaitis J, Boivin G, Siegal D, Fenton J, Jackson K, Nia S, Lien D. 2005. A trial of valganciclovir prophylaxis for cytomegalovirus prevention in lung transplant recipients. Am. J. Transplant. 5:1462–1468. 10.1111/j.1600-6143.2005.00866.x [DOI] [PubMed] [Google Scholar]
- 14.Jaksch P, Zweytick B, Kerschner H, Hoda AM, Keplinger M, Lang G, Aigner C, Klepetko W. 2009. Cytomegalovirus prevention in high-risk lung transplant recipients: comparison of 3- vs 12-month valganciclovir therapy. J. Heart Lung Transplant. 8:670–675. 10.1016/j.healun.2009.03.012 [DOI] [PubMed] [Google Scholar]
- 15.Monforte V, Lopez C, Santos F, Zurbano F, de la Torre M, Sole A, Gavalda J, Ussetti P, Lama R, Cifrian J, Borro JM, Pastor A, Len O, Bravo C, Roman A. 2009. A multicenter study of valganciclovir prophylaxis up to day 120 in CMV-seropositive lung transplant recipients. Am. J. Transplant. 9:1134–1141. 10.1111/j.1600-6143.2009.02574.x [DOI] [PubMed] [Google Scholar]
- 16.Chmiel C, Speich R, Hofer M, Michel D, Mertens T, Weder W, Boehler A. 2008. Ganciclovir/valganciclovir prophylaxis decreases cytomegalovirus-related events and bronchiolitis obliterans syndrome after lung transplantation. Clin. Infect. Dis. 46:831–839. 10.1086/528689 [DOI] [PubMed] [Google Scholar]
- 17.Palmer SM, Limaye AP, Banks M, Gallup D, Chapman J, Lawrence EC, Dunitz J, Milstone A, Reynolds J, Yung GL, Chan KM, Aris R, Garrity E, Valentine V, McCall J, Chow SC, Davis RD, Avery R. 2010. Extended valganciclovir prophylaxis to prevent cytomegalovirus after lung transplantation: a randomized, controlled trial. Ann. Intern. Med. 152:761–769. 10.7326/0003-4819-152-12-201006150-00003 [DOI] [PubMed] [Google Scholar]
- 18.Finlen Copeland CA, Davis WA, Snyder LD, Banks M, Avery R, Davis RD, Palmer SM. 2011. Long-term efficacy and safety of 12 months of valganciclovir prophylaxis compared with 3 months after lung transplantation: a single-center, long-term follow-up analysis from a randomized, controlled cytomegalovirus prevention trial. J. Heart Lung Transplant. 30:990–996. 10.1016/j.healun.2011.02.017 [DOI] [PubMed] [Google Scholar]
- 19.Mitsani D, Nguyen MH, Kwak EJ, Silveira FP, Vadnerkar A, Pilewski J, Crespo M, Toyoda Y, Bermudez C, Clancy CJ. 2010. Cytomegalovirus disease among donor-positive/recipient-negative lung transplant recipients in the era of valganciclovir prophylaxis. J. Heart Lung Transplant. 29:1014–1020. 10.1016/j.healun.2010.04.022 [DOI] [PubMed] [Google Scholar]
- 20.Limaye AP. 2002. Ganciclovir-resistant cytomegalovirus in organ transplant recipients. Clin. Infect. Dis. 35:866–872. 10.1086/342385 [DOI] [PubMed] [Google Scholar]
- 21.Limaye AP, Raghu G, Koelle DM, Ferrenberg J, Huang ML, Boeckh M. 2002. High incidence of ganciclovir-resistant cytomegalovirus infection among lung transplant recipients receiving preemptive therapy. J. Infect. Dis. 185:20–27. 10.1086/338143 [DOI] [PubMed] [Google Scholar]
- 22.Limaye AP, Corey L, Koelle DM, Davis CL, Boeckh M. 2000. Emergence of ganciclovir-resistant cytomegalovirus disease among recipients of solid-organ transplants. Lancet 356:645–649. 10.1016/S0140-6736(00)02607-6 [DOI] [PubMed] [Google Scholar]
- 23.Kruger RM, Shannon WD, Arens MQ, Lynch JP, Storch GA, Trulock EP. 1999. The impact of ganciclovir-resistant cytomegalovirus infection after lung transplantation. Transplantation 68:1272–1279. 10.1097/00007890-199911150-00010 [DOI] [PubMed] [Google Scholar]
- 24.Isada CM, Yen-Lieberman B, Lurain NS, Schilz R, Kohn D, Longworth DL, Taege AJ, Mossad SB, Maurer J, Flechner SM, Mawhorter SD, Braun W, Gordon SM, Schmitt SK, Goldman M, Long J, Haug M, Avery RK. 2002. Clinical characteristics of 13 solid organ transplant recipients with ganciclovir-resistant cytomegalovirus infection. Transplant Infect. Dis. 4:189–194. 10.1034/j.1399-3062.2002.t01-1-02008.x [DOI] [PubMed] [Google Scholar]
- 25.Lurain NS, Chou S. 2010. Antiviral drug resistance of human cytomegalovirus. Clin. Microbiol. Rev. 23:689–712. 10.1128/CMR.00009-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Biron KK. 2006. Antiviral drugs for cytomegalovirus diseases. Antiviral Res. 71:154–163. 10.1016/j.antiviral.2006.05.002 [DOI] [PubMed] [Google Scholar]
- 27.Myhre HA, Haug Dorenberg D, Kristiansen KI, Rollag H, Leivestad T, Asberg A, Hartmann A. 2011. Incidence and outcomes of ganciclovir-resistant cytomegalovirus infections in 1244 kidney transplant recipients. Transplantation 92:217–223. 10.1097/TP.0b013e31821fad25 [DOI] [PubMed] [Google Scholar]
- 28.Reddy AJ, Zaas AK, Hanson KE, Palmer SM. 2007. A single-center experience with ganciclovir-resistant cytomegalovirus in lung transplant recipients: treatment and outcome. J. Heart Lung Transplant. 26:1286–1292. 10.1016/j.healun.2007.09.012 [DOI] [PubMed] [Google Scholar]
- 29.Bhorade SM, Lurain NS, Jordan A, Leischner J, Villanueva J, Durazo R, Creech S, Vigneswaran WT, Garrity ER. 2002. Emergence of ganciclovir-resistant cytomegalovirus in lung transplant recipients. J. Heart Lung Transplant. 21:1274–1282. 10.1016/S1053-2498(02)00463-1 [DOI] [PubMed] [Google Scholar]
- 30.Eid AJ, Arthurs SK, Deziel PJ, Wilhelm MP, Razonable RR. 2008. Emergence of drug-resistant cytomegalovirus in the era of valganciclovir prophylaxis: therapeutic implications and outcomes. Clin. Transplant. 22:162–170. 10.1111/j.1399-0012.2007.00761.x [DOI] [PubMed] [Google Scholar]
- 31.Li F, Kenyon KW, Kirby KA, Fishbein DP, Boeckh M, Limaye AP. 2007. Incidence and clinical features of ganciclovir-resistant cytomegalovirus disease in heart transplant recipients. Clin. Infect. Dis. 45:439–447. 10.1086/519941 [DOI] [PubMed] [Google Scholar]
- 32.Sanghavi SK, Abu-Elmagd K, Keightley MC, St George K, Lewandowski K, Boes SS, Bullotta A, Dare R, Lassak M, Husain S, Kwak EJ, Paterson DL, Rinaldo CR. 2008. Relationship of cytomegalovirus load assessed by real-time PCR to pp65 antigenemia in organ transplant recipients. J. Clin.Virol. 42:335–342. 10.1016/j.jcv.2008.03.031 [DOI] [PubMed] [Google Scholar]
- 33.Husain S, Raza K, Pilewski JM, Zaldonis D, Crespo M, Toyoda Y, Shutt K, Spichty K, Bentlejewski C, Pakstis DL, Carey ME, McCurry KR, Zeevi A. 2009. Experience with immune monitoring in lung transplant recipients: correlation of low immune function with infection. Transplantation 87:1852–1857. 10.1097/TP.0b013e3181a75ad2 [DOI] [PubMed] [Google Scholar]
- 34.Humar A, Kumar D, Boivin G, Caliendo AM. 2002. Cytomegalovirus (CMV) virus load kinetics to predict recurrent disease in solid-organ transplant patients with CMV disease. J. Infect. Dis. 186:829–833. 10.1086/342601 [DOI] [PubMed] [Google Scholar]
- 35.Acosta B, Ferrer D, Jordan M, Gonzalez D, Gobernado M. 1991. [Detection of cytomegalovirus in urine by the shell-vial technique: usefulness of diluting the sample]. Enferm. Infecc. Microbiol. Clin. 9:561–563 (In Spanish.) [PubMed] [Google Scholar]
- 36.Lurain NS, Fox AM, Lichy HM, Bhorade SM, Ware CF, Huang DD, Kwan SP, Garrity ER, Chou S. 2006. Analysis of the human cytomegalovirus genomic region from UL146 through UL147A reveals sequence hypervariability, genotypic stability, and overlapping transcripts. Virol. J. 3:4. 10.1186/1743-422X-3-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Snydman DR, Limaye AP, Potena L, Zamora MR. 2011. Update and review: state-of-the-art management of cytomegalovirus infection and disease following thoracic organ transplantation. Transplant. Proc. 43:S1–S17. 10.1016/j.transproceed.2011.02.069 [DOI] [PubMed] [Google Scholar]
- 38.Sharland M, Luck S, Griffiths P, Cotton M. 2011. Antiviral therapy of CMV disease in children. Adv. Exp. Med. Biol. 697:243–260. 10.1007/978-1-4419-7185-2_17 [DOI] [PubMed] [Google Scholar]
- 39.Chou S, Waldemer RH, Senters AE, Michels KS, Kemble GW, Miner RC, Drew WL. 2002. Cytomegalovirus UL97 phosphotransferase mutations that affect susceptibility to ganciclovir. J. Infect. Dis. 185:162–169. 10.1086/338362 [DOI] [PubMed] [Google Scholar]
- 40.Hakki M, Chou S. 2011. The biology of cytomegalovirus drug resistance. Curr. Opin. Infect. Dis. 24:605–611. 10.1097/QCO.0b013e32834cfb58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schreiber A, Harter G, Schubert A, Bunjes D, Mertens T, Michel D. 2009. Antiviral treatment of cytomegalovirus infection and resistant strains. Expert Opin. Pharmacother. 10:191–209. 10.1517/14656560802678138 [DOI] [PubMed] [Google Scholar]
- 42.Chakrabarti S, Mackinnon S, Chopra R, Kottaridis PD, Peggs K, O'Gorman P, Chakraverty R, Marshall T, Osman H, Mahendra P, Craddock C, Waldmann H, Hale G, Fegan CD, Yong K, Goldstone AH, Linch DC, Milligan DW. 2002. High incidence of cytomegalovirus infection after nonmyeloablative stem cell transplantation: potential role of Campath-1H in delaying immune reconstitution. Blood 99:4357–4363. 10.1182/blood.V99.12.4357 [DOI] [PubMed] [Google Scholar]
- 43.Martin M, Goyette N, Ives J, Boivin G. 2010. Incidence and characterization of cytomegalovirus resistance mutations among pediatric solid organ transplant patients who received valganciclovir prophylaxis. J. Clin. Virol. 47:321–324. 10.1016/j.jcv.2010.01.009 [DOI] [PubMed] [Google Scholar]
- 44.Boivin G, Goyette N, Gilbert C, Covington E. 2005. Analysis of cytomegalovirus DNA polymerase (UL54) mutations in solid organ transplant patients receiving valganciclovir or ganciclovir prophylaxis. J. Med. Virol. 77:425–429. 10.1002/jmv.20471 [DOI] [PubMed] [Google Scholar]


