Abstract
Interest in antifungal therapeutic-drug monitoring has increased due to studies demonstrating associations between concentrations and outcomes. We reviewed the antifungal drug concentration database at our institution to gain a better understanding of achievable triazole drug levels. Antifungal concentrations were measured by high-performance liquid chromatography (HPLC), ultraperformance liquid chromatography and single-quadrupole mass spectrometry (UPLC/MS), or a bioassay. For this study, only confirmed human bloodstream (serum or plasma) and cerebral spinal fluid (CSF) concentrations of voriconazole, posaconazole, and itraconazole were analyzed. The largest numbers of bloodstream and CSF samples were found for voriconazole (14,370 and 173, respectively). Voriconazole bloodstream concentrations within the range of 1 to 5.5 μg/ml represented 50.6% of samples. Levels below the lower limit of quantification (0.2 μg/ml) were observed in 14.6% of samples, and 10.4% of samples had levels of ≥5.5 μg/ml. CSF voriconazole levels ranged from undetectable to 15.3 μg/ml and were <0.2 μg/ml in 11% of samples. Posaconazole bloodstream concentrations were ≥0.7 and ≥1.25 μg/ml in 41.6% and 18.9% of samples, respectively. Posaconazole was detected in only 4 of 22 CSF samples (undetectable to 0.56 μg/ml). Itraconazole levels, as measured by UPLC/MS, were ≥0.5 μg/ml in 43.3% and were undetectable in 33.9% of bloodstream samples. In contrast, when measured by a bioassay, itraconazole/hydroxyitraconazole bloodstream concentrations were ≥1.0 μg/ml in 72.9% of samples and were undetectable in 18% of samples. These results indicate that there is marked variability in bloodstream concentrations achieved with these three azoles. In addition, many levels within the bloodstream for each azole and for voriconazole and posaconazole in the CSF were undetectable or below thresholds associated with efficacy.
INTRODUCTION
Over the last 5 years, there has been increased interest in therapeutic-drug monitoring of systemic antifungals. Much of this interest stems from clinical evidence that suggests that this practice may improve outcomes for patients treated with voriconazole and posaconazole. For both agents, studies have reported a relationship between clinical response and certain threshold concentrations (1–6). In addition, marked interpatient variability has been observed with both of these antifungals (1, 7–11). For voriconazole, a clear relationship also exists between elevated concentrations and certain toxicities (1, 12, 13). Monitoring of voriconazole concentrations has also been suggested as part of patient management in the fungal meningitis outbreak associated with contaminated steroids due to the high doses of this agent that are recommended (14, 15). Although this practice has garnered recent attention, therapeutic-drug monitoring is not new, as previous studies have suggested relationships between itraconazole and flucytosine concentrations and clinical outcomes (16–19).
One limitation of the currently available literature for antifungal therapeutic-drug monitoring is that many of the data come from small single-center studies (20). The Fungus Testing Laboratory at the University of Texas Health Science Center at San Antonio is a large mycology reference laboratory that receives samples for the measurement of antifungal concentrations in different biological tissues from institutions across the United States. Here we review our experience with clinical samples submitted to this laboratory for voriconazole, posaconazole, and itraconazole concentrations within the bloodstream (plasma or serum) and cerebral spinal fluid (CSF).
MATERIALS AND METHODS
Samples.
The antifungal drug concentration database in the Fungus Testing Laboratory at the University of Texas Health Science Center at San Antonio was queried. Data from clinical samples sent to this laboratory for measurement from 1 January 2001 to 1 April 2013 were reviewed. Veterinary samples were excluded, as were results from samples measured for proficiency testing. Samples were stored frozen until assayed, and all samples were handled and processed according to established standard operating procedures.
Voriconazole and posaconazole concentrations.
Samples were assayed for voriconazole by using a previously described high-performance liquid chromatography (HPLC) analytical method with solid-phase extraction (SPE) (1-ml/100-mg C18 columns) (21). Posaconazole samples were analyzed by using SPE (Bond Elut Plexa) columns and HPLC. Standard curves were prepared by spiking blank human plasma with voriconazole or posaconazole. An internal standard (UK-115,794 and m-nitrophenol, respectively) was added to each sample. Voriconazole samples were buffered with 0.2 M borate buffer (pH 9.0) and loaded onto the SPE columns, followed by independent washings with borate buffer and methanol-water (50:50, vol/vol) to remove retained interferences. The samples were eluted with 1 ml of an acidic methanolic mixture. Posaconazole samples were loaded onto the SPE columns, washed independently with 20% and 50% methanol, and eluted with 100% methanol. Both voriconazole and posaconazole eluates were dried under a stream of nitrogen. The dried residues were reconstituted with each assay mixture's mobile phase (55:45 N,N,N′,N′-tetramethylethylenediamine [TEMED] buffer–acetonitrile for voriconazole and 35:65 water-acetonitrile for posaconazole). The reconstituted samples were analyzed isocratically by HPLC with UV detection (254 nm for voriconazole and 265 nm for posaconazole). The lowest limit of quantitation (LLQ) was 0.2 μg/ml and 0.125 μg/ml, respectively.
Itraconazole and hydroxyitraconazole concentrations.
Itraconazole concentrations were initially measured by a bioassay (22). Candida kefyr (ATCC 46764) was added to a solution of yeast nitrogen base (YNB) broth and incubated at 37°C for 6 h. The Candida kefyr solution was adjusted to a 2 McFarland standard, and 0.5 ml was added to 35 ml of melted YNB agar deeps. YNB was poured into 150- by 15-mm petri plates. Each 7-mm well bored into the agar was filled with 50 μl of standards (0.5, 2, 5, and 20 μg/ml) and controls (1.0 and 10.0 μg/ml), and samples were pipetted into individual wells. The plates were incubated overnight at 37°C. Diameters of the zones of inhibition were measured in millimeters, and itraconazole concentrations were determined by using a standard curve. The bioassay method is unable to distinguish between itraconazole and the active metabolite hydroxyitraconazole.
An analytical method that uses ultraperformance liquid chromatography and single-quadrupole mass spectrometry (UPLC/MS) for the determination of itraconazole and hydroxyitraconazole was developed and is currently used by our laboratory. Standard curves were prepared by spiking blank human plasma with itraconazole and hydroxyitraconazole. An internal standard (valethamate) was added to each sample. All samples were buffered and loaded onto conditioned SPE columns, which were then washed with independent washings of 5.0% NH4OH and 15.0% methanolic water. The samples were eluted with 1.0 ml of methanol and 1.0 ml of an acidic methanolic mixture (2% formic acid in methanol [MeOH]), and combined eluates were dried under a stream of nitrogen. The dried residues were reconstituted with a 60:40 dilution of acetonitrile-water, injected, and analyzed under specified conditions using mass-to-charge ratios (m/z) for both itraconazole and hydroxyitraconazole of 705.2 and 721.3, respectively. The lowest LOQ is 0.25 μg/ml for both itraconazole and hydroxyitraconazole.
RESULTS
Voriconazole and posaconazole.
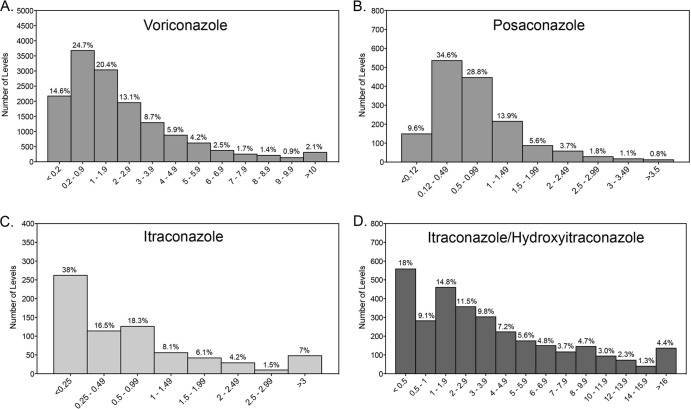
The majority of samples (73%) were received for the measurement of voriconazole concentrations within the bloodstream. As of the beginning of April 2013, 14,923 plasma or serum samples had been submitted to the Fungus Testing Laboratory for this purpose. The median quantifiable level (i.e., concentrations above the lower limit of quantification) was 1.86 μg/ml, and overall voriconazole concentrations within the bloodstream ranged between undetectable and 44.9 μg/ml (Table 1). As shown in Fig. 1A, the majority of samples had concentrations toward the lower end of this range. Concentrations below the limit of quantification (0.2 μg/ml) occurred in 14.6% of samples, and 39.2% had levels below 1 μg/ml. Approximately one-half of concentrations (50.3%) were below 1.5 μg/ml. In contrast, voriconazole concentrations above 5.5 μg/ml, a level reported to be associated with central nervous system toxicity in one study, were observed in only 10.4% of samples.
TABLE 1.
Total voriconazole concentrations in serum and plasma samples and concentrations in samples received from pediatric hospitalsa
| Population | No. of levels measured | Concn range (μg/ml) | Median quantifiable concn (μg/ml) | % of measurements below LLQ (<0.2 μg/ml) | % of measurements below 1 μg/ml | % of measurements below 1.5 μg/ml | % of measurements above 5.5 μg/ml |
|---|---|---|---|---|---|---|---|
| Total | 14,923 | Undetectable–44.9 | 1.86 | 14.60 | 39.20 | 50.30 | 10.40 |
| Pediatric | 1,700 | Undetectable–44.9 | 1.46 | 21.50 | 50.80 | 61.50 | 9.90 |
Concentrations were measured by HPLC. LLQ, lower limit of quantification.
FIG 1.
Bloodstream concentration distributions for voriconazole (A), posaconazole (B), itraconazole as measured by UPLC/MS (C), and itraconazole/hydroxyitraconazole as measured by bioassay (D). Antifungal concentrations were measured in clinical plasma or serum samples sent to our reference laboratory by validated assays.
Voriconazole concentrations were also measured in 173 CSF samples. Within this biological fluid, the median quantifiable level was 2.47 μg/ml, and concentrations ranged between undetectable and 15.3 μg/ml. As with the bloodstream concentrations, CSF concentrations were more heavily distributed toward the lower end of this range (Fig. 2A). However, concentrations of >5 μg/ml were observed in approximately 21% of samples. Matched bloodstream levels were available for 82 CSF samples (Fig. 2B). In these, the median CSF/bloodstream ratio was 0.52 (range, 0 to 1.22).
FIG 2.
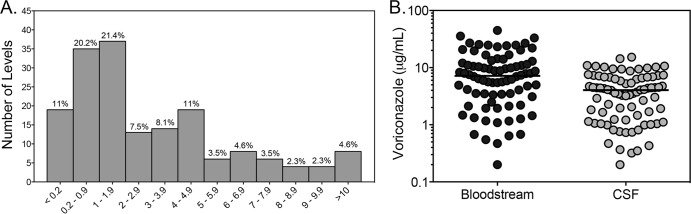
Distribution of voriconazole concentrations within the cerebral spinal fluid (CSF) (A) and voriconazole bloodstream and CSF concentrations in matched clinical samples (B).
Posaconazole concentrations were measured in 1,548 plasma or serum samples. The median quantifiable concentration was 0.64 μg/ml, and levels ranged between undetectable and 6.57 μg/ml (Table 2). Similar to voriconazole, posaconazole concentrations within the bloodstream were positively skewed (i.e., more heavily distributed toward the lower end of the range) (Fig. 1B). Levels below the lower limit of quantification (0.125 μg/ml) were observed in 9.6% of samples. In approximately one-third of samples, concentrations were below 0.5 μg/ml, and 58% had levels of <0.7 μg/ml. In contrast, concentrations above 1.25 μg/ml were observed in <20% of samples. The posaconazole concentration was also measured in 22 CSF samples, 18 of which had concentrations below the lower limit of quantification. In the four samples in which posaconazole was quantifiable, the concentrations ranged from 0.14 to 0.56 μg/ml.
TABLE 2.
Total posaconazole concentrations in serum and plasma samples and concentrations in samples received from pediatric hospitalsa
| Population | No. of levels measured | Concn range (μg/ml) | Median quantifiable concn (μg/ml) | % of measurements below LLQ (<0.125 μg/ml) | % of measurements below 0.5 μg/ml | % of measurements below 0.7 μg/ml | % of measurements below 1.25 μg/ml |
|---|---|---|---|---|---|---|---|
| Total | 1,548 | Undetectable–6.57 | 0.64 | 9.60 | 34.60 | 58.40 | 81.10 |
| Pediatric | 152 | Undetectable–3.99 | 0.52 | 12.50 | 54.60 | 63.80 | 80.30 |
Concentrations were measured by HPLC. LLQ, lower limit of quantification.
In order to determine if the overall median detectable values and the percentages of levels below or above certain thresholds were influenced by changes in practice based on the availability of newly published studies, we evaluated bloodstream concentrations of voriconazole and posaconazole by year (Fig. 3). Overall, the median detectable levels and percentages of samples with concentrations below the lower limit of quantification and various thresholds for efficacy and toxicity for both agents remained relatively similar over time, without dramatic changes from year to year. We also reviewed concentrations of voriconazole and posaconazole in samples that we received from 6 stand-alone pediatric institutions located in different geographic areas of the United States, including the East and West Coasts and southern, midwestern, and western states. As shown in Tables 1 and 2, concentrations of voriconazole and posaconazole in these samples were somewhat lower than the total values. For both agents, higher percentages of concentrations for the pediatric samples fell below various threshold levels. In addition, the median detectable bloodstream level of voriconazole in these pediatric samples (1.46 μg/ml) was significantly lower than the total median value (1.86 μg/ml; P < 0.0001 by the Mann-Whitney test), and there was a similar trend with posaconazole (0.52 μg/ml versus 0.64 μg/ml; P = 0.077).
FIG 3.
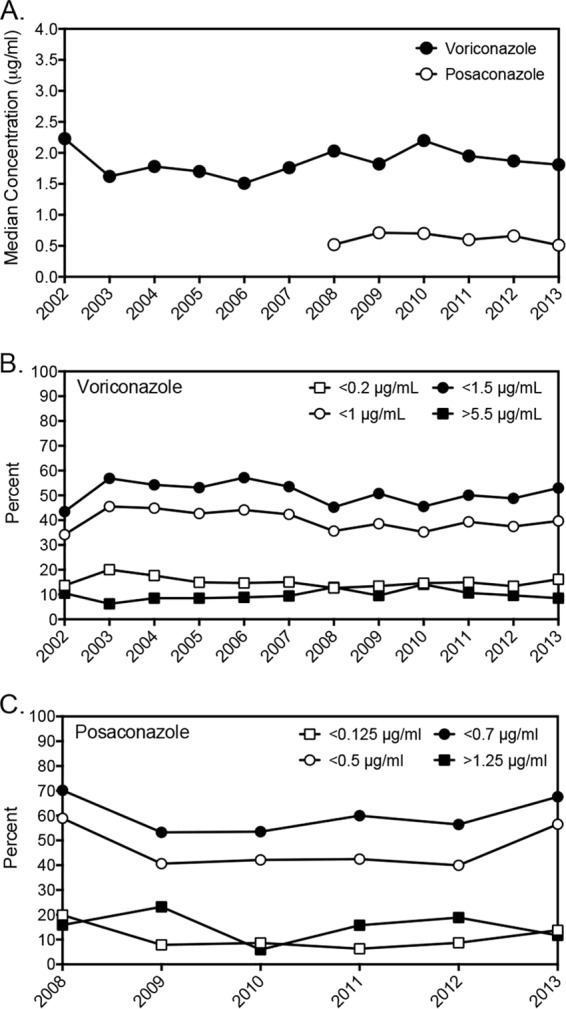
Voriconazole and posaconazole median bloodstream concentrations and percentages of samples above or below a certain threshold by year.
Itraconazole.
As in other laboratories, itraconazole concentrations were initially measured by a bioassay. Recently, a UPLC/MS assay has been validated and is now used to measure both itraconazole and hydroxyitraconazole. As the bioassay is unable to distinguish between itraconazole and the active metabolite hydroxyitraconazole, differences in various pharmacokinetic parameters were observed (Table 3). These included a wider range of concentrations and a higher median detectable level by the bioassay than by the UPLC/MS method. In addition, fewer levels fell below the lower limit of quantification for the bioassay (<0.5 μg/ml; 18%) than for UPLC/MS (<0.25 μg/ml; 38%). We also evaluated the concentration data for hydroxyitraconazole alone as well as the sum of itraconazole plus hydroxyitraconazole, as measured by UPLC/MS. Alone, the median quantifiable concentration of the active metabolite was 1.13 μg/ml, and the median level of the combined concentrations was 1.71 μg/ml. Approximately one-quarter of the hydroxyitraconazole levels fell below the lower limit of quantification, 0.25 μg/ml, and the median hydroxyitraconazole/itraconazole ratio for matched samples was 1.59. As was observed for voriconazole and posaconazole, itraconazole concentrations, as measured by both bioassay and UPLC/MS, were more heavily distributed toward the lower end of the concentration range. In contrast, only 3% of concentrations that were measured by bioassay were above 17.1 μg/ml, a threshold previously reported to be associated with significant toxicities with this agent (23).
TABLE 3.
Itraconazole and itraconazole/hydroxyitraconazole concentrations in serum and plasma samples
| Assay and drug | No. of levels measured | Concn range (μg/ml) | Median quantifiable concn (μg/ml) | % of measurements below LLQa | % of measurements below 0.5 μg/ml | % of measurements above 17.1 μg/ml | Median matched hydroxyitraconazole/itraconazole ratio (range) |
|---|---|---|---|---|---|---|---|
| UPLC/MS | |||||||
| Itraconazole | 689 | Undetectable–7.37 | 0.84 | 38 | 54.60 | 1.59 (0.2–3.63) | |
| Hydroxyitraconazole | 676 | Undetectable–7.31 | 1.13 | 25.30 | |||
| Itraconazole + hydroxyitraconazole | 651 | Undetectable–14.01 | 1.71 | 26.30 | 42.40 | ||
| Bioassay | |||||||
| Itraconazole/hydroxyitraconazole | 3,105 | Undetectable–>20 | 4.75 | 18 | 3.00 |
The lower limit of quantification (LLQ) was <0.25 μg/ml for UPLC/MS and <0.5 μg/ml for bioassay.
DISCUSSION
Therapeutic-drug monitoring of any drug involves the measurement of concentrations within biological fluids or tissues and the interpretation of the concentrations based on well-described pharmacokinetic parameters (24). In order for therapeutic-drug monitoring to be considered, certain criteria must be met. These include the availability of a valid and timely assay for the drug in question and a clear relationship between the concentration that is achieved and a clinical outcome (24–27). This practice may also be of value when significant intra- and/or interpatient variability exists between the dose that is administered and the drug concentration that is achieved.
Antifungal therapeutic-drug monitoring has recently gained much attention, and this practice is routinely used at some institutions. Although assays to measure flucytosine concentrations are available, the majority of antifungal therapeutic-drug monitoring is with the azoles voriconazole, posaconazole, and itraconazole. In this study, we report our experience with measuring concentrations of these three azoles in clinical samples sent to our reference mycology laboratory.
Several groups have reported associations between voriconazole concentrations and both clinical efficacy and toxicity. A potential relationship between bloodstream concentrations and outcomes was initially suggested in an early open-label, noncomparative, multicenter study of voriconazole for the treatment of invasive aspergillosis (13). Several single-center studies have also described associations between voriconazole concentrations and clinical outcome. In a retrospective analysis of patients who underwent voriconazole therapeutic-drug monitoring, Smith et al. reported that 44% of patients with voriconazole levels of ≤2.05 μg/ml failed therapy, in comparison to none with levels higher than this value (2). Similarly, Pascual et al. reported that 6 of 13 patients failing therapy had voriconazole trough concentrations of <1 μg/ml, compared to only 5 of 39 with higher trough concentrations (1). Following dose escalation, the median trough concentration in the 6 patients who were failing therapy increased to 2.1 μg/ml, and all patients had a complete or partial response. In addition, a significant association between neurotoxicity (e.g., encephalopathy) and voriconazole trough concentrations of >5.5 μg/ml was also observed, which is consistent with other reports (12, 28). Using multivariate regression analysis, that group recently identified an independent association between voriconazole troughs and the probability of response or neurotoxicity (11). In that study, a therapeutic range of between 1.5 μg/ml (>85% probability of response) and 4.5 μg/ml (<15% probability of neurotoxicity) was suggested. This range is similar to the voriconazole trough/MIC ratio of 2 to 5 estimated by Troke et al. to be associated with a near-maximal probability of response (29). In addition to the exposure-neurotoxicity relationship, there is some evidence that high voriconazole levels may be associated with hepatotoxicity (1, 13, 30). However, this is controversial and has not been consistently demonstrated (11, 12).
In the current study, a wide range of voriconazole bloodstream concentrations was observed. However, in many of the samples, the voriconazole levels were either below the lower limit of quantification of our assay or less than the thresholds associated with clinical response, as described above. Previous studies have reported both significant inter- and intrapatient variability with voriconazole levels (1, 7, 8, 31). As several factors influence voriconazole pharmacokinetics, it is difficult to predict what levels will be achieved based on the dose that patients receive. These factors include drug-drug interactions, nonlinear pharmacokinetics in adults, and CYP 2C19 polymorphisms. A weak correlation between the dose administered on a weight basis and the voriconazole concentration that is achieved in the bloodstream has been reported (8). In addition, it was recently reported that the oral bioavailability of this agent in patients may be less than previously thought (11). Previous studies also suggested that autoinduction of voriconazole metabolism may lead to subtherapeutic concentrations. Although this is known to occur in rodents, this was thought not to occur in humans (32). However, others have suggested that autoinduction, sometimes referred to as accelerated metabolism, of voriconazole may occur in some individuals (7, 31, 33).
We also report our experience with voriconazole concentrations in the CSF. Antifungal concentrations within the central nervous system (CNS) have recently been in the spotlight due to the outbreak of Exserohilum rostratum fungal meningitis associated with contaminated corticosteroids (15). Lutsar et al. previously reported that in a group of 14 matched CSF and plasma samples collected from patients with CNS fungal infections, the median voriconazole CSF/plasma ratio was 0.46 and ranged from 0.22 to 1.0 (34). A similar ratio was found in our 82 matched CSF and bloodstream samples, where the median ratio was 0.52. It is important to note that there are no clinical data correlating voriconazole CSF concentrations with response in patients with fungal CNS infections.
Members of our group have briefly reported this laboratory's experience with posaconazole bloodstream concentrations (35). In that report, 202 posaconazole concentrations were measured, and 70% were <0.7 μg/ml. Here we expand upon our experience and provide information on 1,548 posaconazole bloodstream concentrations. Of note, concentrations within the serum or plasma were more heavily distributed toward the lower end of the range of measured concentrations, and high percentages were below levels previously reported to be associated with prophylactic (58.4% were <0.7 μg/ml) or treatment (81.1% were <25 μg/ml) efficacy (4, 6). Others have also reported that a large percentage of patients may be achieving low bloodstream concentrations of posaconazole (3, 36). Several factors may influence the bioavailability of the currently available oral suspension, including the use of agents that raise gastric pH or promote gastric motility, and nausea and vomiting (37, 38). New formulations of posaconazole are currently under development, including both a solid oral tablet and an intravenous formulation. Higher and more consistent posaconazole bloodstream concentrations have been reported for these new formulations, which have the potential to improve patient outcomes with this agent (39–41).
The exact threshold associated with clinical efficacy with posaconazole is unknown. As prophylaxis, studies have suggested that a concentration of 0.5 or 0.7 μg/ml be used as the target level. Jang et al. reviewed the exposure-response relationship from two large, prospective, multicenter trials that evaluated posaconazole as primary prophylaxis in neutropenic patients undergoing chemotherapy for acute leukemia or myelodysplastic syndrome and those with severe graft-versus-host disease (4). Using clinical failure as the endpoint, the primary composite efficacy endpoint in these two trials, there was a clear relationship between posaconazole levels and clinical outcomes, and the incidence of breakthrough invasive fungal infections was higher for those patients with average steady-state concentrations below 0.7 μg/ml. Other studies have suggested that a level of 0.5 μg/ml may be a suitable threshold for prophylaxis. In a retrospective review of 17 cardiothoracic transplant recipients in which posaconazole therapeutic-drug monitoring was performed, Shields et al. reported that 3 of 6 patients with consistent plasma concentrations above 0.5 μg/ml had a favorable response, while 8 of 8 patients with levels at or below this threshold failed therapy. Three patients were excluded from the outcome analysis, as therapy was discontinued due to potential hepatotoxicity. Of the 14 remaining patients, 9 received posaconazole as primary or secondary prophylaxis, and 5 received it as primary treatment of a fungal infection. It is noteworthy that 2 of the 3 patients with a favorable response had levels of >1.55 μg/ml. A recent retrospective, multicenter study of 86 patients for whom posaconazole therapeutic-drug monitoring was performed reported similar results (3). The median level in those who received posaconazole as prophylaxis and had a breakthrough invasive fungal infection (0.289 μg/ml) was significantly lower than that in those who did not (0.485 μg/ml). Posaconazole concentrations were also lower in the 4 patients who failed treatment for an indication other than neutropenic fever (0.436 μg/ml) than in those who had a successful outcome (0.955 μg/ml). The need for higher levels for the treatment of invasive fungal infections was suggested in one study that used posaconazole as salvage therapy for invasive aspergillosis (6). Using a quartile analysis, Walsh et al. reported that response rates were highest among patients in the highest posaconazole quartile (75%, with a median level of 1.25 μg/ml). However, it must be noted that not all studies have found a correlation between posaconazole concentrations and response (42, 43). Overall, this agent appears to be well tolerated, with an adverse-effect profile similar to that of fluconazole (9). In addition, there does not appear to be a clear relationship between exposure and adverse effects with the currently available formulation (3, 4, 36). It is currently unknown if this will change with the availability of new posaconazole formulations that result in higher and more consistent bloodstream levels.
As discussed above, general target concentrations for voriconazole and posaconazole have been evaluated clinically. However, for both agents, the pharmacokinetic/pharmacodynamic parameters associated with in vivo efficacy have been more specifically defined (44–49). Therefore, the goal concentrations of these agents could be individualized in order to optimize responses while avoiding toxicities based on the denominator of the pharmacokinetic/pharmacodynamic equation (i.e., the in vitro MIC of the antifungal against the organism). However, in many instances of invasive mold infections, the organism responsible for causing disease is not able to be isolated for susceptibility testing. It is of interest that the bloodstream levels of voriconazole and posaconazole measured in samples received from pediatric institutions appeared to be lower than all levels taken together. The clinical implications of this are unknown, but it suggests that therapeutic-drug monitoring studies in pediatric patients are warranted.
Similar to what was observed for voriconazole and posaconazole, itraconazole concentrations in this study, as measured by both bioassay and HPLC, were highly variable and tended to be more heavily distributed toward the lower end of the concentration range. In addition, many were below the lower limit of quantification by either assay. Low and variable concentrations of this triazole have been previously reported (50–52). The literature regarding therapeutic-drug monitoring of itraconazole is mixed, as some studies have shown a relationship between itraconazole bloodstream concentrations and clinical outcome, while others have not. In a nonrandomized prospective study in patients with hematologic disease with severe neutropenia who received itraconazole prophylaxis, invasive fungal infections occurred more frequently in patients in which levels were <0.25 μg/ml for >2 weeks (19). Others have reported that this level may be too low and that a higher concentration may be needed for prophylactic efficacy. Glasmacher et al. reported that in hematologic malignancy patients who developed invasive fungal infections while on itraconazole prophylaxis, the percentage of days with levels of ≥0.5 μg/ml was significantly lower than for patients without breakthrough infections (48% versus 100%) (18). In addition, patients with fatal invasive fungal infections had lower median itraconazole levels immediately before the occurrence of the infection than did those with nonfatal mycoses. While those two studies reported an association between itraconazole concentrations and prophylactic efficacy, other studies have not found such a relationship (52, 53). Although most studies have evaluated itraconazole concentrations when used as prophylaxis, an early clinical trial did suggest that levels might also be important when this agent is used as treatment. Denning et al. reported that itraconazole concentrations were higher in those who had complete responses against invasive aspergillosis and stable disease than in those who failed treatment (50). However, these differences were not statistically significant.
As noted above, both bioassays and analytical assays (e.g., HPLC and liquid chromatography/mass spectrometry [LC/MS]) are available for the determination of itraconazole concentrations, and this may confound the picture regarding therapeutic-drug monitoring of this agent. As various factors can influence the results of the bioassays, the results obtained by this method and the analytical assays are discordant (54, 55). Bioassays are unable to distinguish between itraconazole and the active metabolite hydroxyitraconazole, and concentrations measured by this method range between 2 and 10 times higher than itraconazole concentrations determined by analytical means (24, 56). Several factors may contribute to this, including the indicator organism and type of medium used for the assay. While it was previously reported that the hydroxyitraconazole-to-itraconazole ratio is approximately 2:1 (57), our results show that this can be highly variable. It has also been reported that itraconazole and hydroxyitraconazole are equipotent (54, 56); thus, the results from analytical assays could be reported as a bioactive level by summing the two concentrations. However, in a large in vitro analysis of the activity of itraconazole and hydroxyitraconazole that used 50% inhibition of growth turbidity as the endpoint for both yeast and molds, variable potency was observed between these two agents against Candida glabrata and dimorphic pathogens (54). Against C. glabrata, itraconazole MICs were 3 or more dilutions lower than those of itraconazole against 26.7% of the isolates tested. Although most studies that evaluated associations between itraconazole levels and therapeutic responses used HPLC to measure concentrations, one study utilized a bioassay in order to explore the concentration-toxicity relationship. Lestner et al. reported that an itraconazole level of 17.1 μg/ml was an appropriate threshold for therapeutic-drug monitoring, as concentrations above this were associated with significant toxicity (23). This included clinical features associated with congestive heart failure. Due to the discordance between itraconazole levels measured by a bioassay and those measured by analytical assays, it is difficult to translate the concentration-toxicity relationship when levels are measured by HPLC or LC/MS.
One limitation of the current study is that in many cases, we do not have access to other information, including patient outcomes as well as the doses that were administered. Thus, we cannot evaluate relationships between exposure of these agents and either clinical response or toxicity. In addition, we are unable to comment on the high levels that were observed in some samples with each agent. However, our collective experience is similar to those reported from individual centers and in studies involving a few institutions. For each agent, marked variability was observed. The range of concentrations was wide, but with each drug, levels were more heavily distributed toward the lower end of the respective concentration ranges, and this did not appear to change markedly over time. Furthermore, with each agent, many of the samples had concentrations that were below the lower limit of quantification for our assays, which also fall below threshold levels reported in the literature to be associated with the clinical response, either as prophylaxis or as treatment, with these antifungals.
ACKNOWLEDGMENTS
N.P.W. has received research support from Merck, Astellas, bioMérieux, Viamet, Medicis, Merz, and F2G and has served on advisory boards for Merck, Astellas, Toyama, and Viamet.
Footnotes
Published ahead of print 4 November 2013
REFERENCES
- 1.Pascual A, Calandra T, Bolay S, Buclin T, Bille J, Marchetti O. 2008. Voriconazole therapeutic drug monitoring in patients with invasive mycoses improves efficacy and safety outcomes. Clin. Infect. Dis. 46:201–211. 10.1086/524669 [DOI] [PubMed] [Google Scholar]
- 2.Smith J, Safdar N, Knasinski V, Simmons W, Bhavnani SM, Ambrose PG, Andes D. 2006. Voriconazole therapeutic drug monitoring. Antimicrob. Agents Chemother. 50:1570–1572. 10.1128/AAC.50.4.1570-1572.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dolton MJ, Ray JE, Chen SC, Ng K, Pont L, McLachlan AJ. 2012. Multicenter study of posaconazole therapeutic drug monitoring: exposure-response relationship and factors affecting concentration. Antimicrob. Agents Chemother. 56:5503–5510. 10.1128/AAC.00802-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jang SH, Colangelo PM, Gobburu JV. 2010. Exposure-response of posaconazole used for prophylaxis against invasive fungal infections: evaluating the need to adjust doses based on drug concentrations in plasma. Clin. Pharmacol. Ther. 88:115–119. 10.1038/clpt.2010.64 [DOI] [PubMed] [Google Scholar]
- 5.Park WB, Kim NH, Kim KH, Lee SH, Nam WS, Yoon SH, Song KH, Choe PG, Kim NJ, Jang IJ, Oh MD, Yu KS. 2012. The effect of therapeutic drug monitoring on safety and efficacy of voriconazole in invasive fungal infections: a randomized controlled trial. Clin. Infect. Dis. 55:1080–1087. 10.1093/cid/cis599 [DOI] [PubMed] [Google Scholar]
- 6.Walsh TJ, Raad I, Patterson TF, Chandrasekar P, Donowitz GR, Graybill R, Greene RE, Hachem R, Hadley S, Herbrecht R, Langston A, Louie A, Ribaud P, Segal BH, Stevens DA, van Burik JA, White CS, Corcoran G, Gogate J, Krishna G, Pedicone L, Hardalo C, Perfect JR. 2007. Treatment of invasive aspergillosis with posaconazole in patients who are refractory to or intolerant of conventional therapy: an externally controlled trial. Clin. Infect. Dis. 44:2–12. 10.1086/508774 [DOI] [PubMed] [Google Scholar]
- 7.Trifilio S, Ortiz R, Pennick G, Verma A, Pi J, Stosor V, Zembower T, Mehta J. 2005. Voriconazole therapeutic drug monitoring in allogeneic hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 35:509–513. 10.1038/sj.bmt.1704828 [DOI] [PubMed] [Google Scholar]
- 8.Trifilio S, Pennick G, Pi J, Zook J, Golf M, Kaniecki K, Singhal S, Williams S, Winter J, Tallman M, Gordon L, Frankfurt O, Evens A, Mehta J. 2007. Monitoring plasma voriconazole levels may be necessary to avoid subtherapeutic levels in hematopoietic stem cell transplant recipients. Cancer 109:1532–1535. 10.1002/cncr.22568 [DOI] [PubMed] [Google Scholar]
- 9.Cornely OA, Maertens J, Winston DJ, Perfect J, Ullmann AJ, Walsh TJ, Helfgott D, Holowiecki J, Stockelberg D, Goh YT, Petrini M, Hardalo C, Suresh R, Angulo-Gonzalez D. 2007. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N. Engl. J. Med. 356:348–359. 10.1056/NEJMoa061094 [DOI] [PubMed] [Google Scholar]
- 10.Ullmann AJ, Lipton JH, Vesole DH, Chandrasekar P, Langston A, Tarantolo SR, Greinix H, Morais de Azevedo W, Reddy V, Boparai N, Pedicone L, Patino H, Durrant S. 2007. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N. Engl. J. Med. 356:335–347. 10.1056/NEJMoa061098 [DOI] [PubMed] [Google Scholar]
- 11.Pascual A, Csajka C, Buclin T, Bolay S, Bille J, Calandra T, Marchetti O. 2012. Challenging recommended oral and intravenous voriconazole doses for improved efficacy and safety: population pharmacokinetics-based analysis of adult patients with invasive fungal infections. Clin. Infect. Dis. 55:381–390. 10.1093/cid/cis437 [DOI] [PubMed] [Google Scholar]
- 12.Tan K, Brayshaw N, Tomaszewski K, Troke P, Wood N. 2006. Investigation of the potential relationships between plasma voriconazole concentrations and visual adverse events or liver function test abnormalities. J. Clin. Pharmacol. 46:235–243. 10.1177/0091270005283837 [DOI] [PubMed] [Google Scholar]
- 13.Denning DW, Ribaud P, Milpied N, Caillot D, Herbrecht R, Thiel E, Haas A, Ruhnke M, Lode H. 2002. Efficacy and safety of voriconazole in the treatment of acute invasive aspergillosis. Clin. Infect. Dis. 34:563–571. 10.1086/324620 [DOI] [PubMed] [Google Scholar]
- 14.Kerkering TM, Grifasi ML, Baffoe-Bonnie AW, Bansal E, Garner DC, Smith JA, Demicco DD, Schleupner CJ, Aldoghaither RA, Savaliya VA. 2013. Early clinical observations in prospectively followed patients with fungal meningitis related to contaminated epidural steroid injections. Ann. Intern. Med. 158:154–161. 10.7326/0003-4819-158-3-201302050-00568 [DOI] [PubMed] [Google Scholar]
- 15.Kauffman CA, Pappas PG, Patterson TF. 2013. Fungal infections associated with contaminated methylprednisolone injections. N. Engl. J. Med. 368:2495–2500. 10.1056/NEJMra1212617 [DOI] [PubMed] [Google Scholar]
- 16.Kauffman CA, Frame PT. 1977. Bone marrow toxicity associated with 5-fluorocytosine therapy. Antimicrob. Agents Chemother. 11:244–247. 10.1128/AAC.11.2.244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stamm AM, Diasio RB, Dismukes WE, Shadomy S, Cloud GA, Bowles CA, Karam GH, Espinel-Ingroff A. 1987. Toxicity of amphotericin B plus flucytosine in 194 patients with cryptococcal meningitis. Am. J. Med. 83:236–242. 10.1016/0002-9343(87)90691-7 [DOI] [PubMed] [Google Scholar]
- 18.Glasmacher A, Hahn C, Leutner C, Molitor E, Wardelmann E, Losem C, Sauerbruch T, Marklein G, Schmidt-Wolf IG. 1999. Breakthrough invasive fungal infections in neutropenic patients after prophylaxis with itraconazole. Mycoses 42:443–451. 10.1046/j.1439-0507.1999.00505.x [DOI] [PubMed] [Google Scholar]
- 19.Boogaerts MA, Verhoef GE, Zachee P, Demuynck H, Verbist L, De Beule K. 1989. Antifungal prophylaxis with itraconazole in prolonged neutropenia: correlation with plasma levels. Mycoses 32(Suppl 1):103–108 [DOI] [PubMed] [Google Scholar]
- 20.Lewis RE. 2008. What is the “therapeutic range” for voriconazole? Clin. Infect. Dis. 46:212–214. 10.1086/524670 [DOI] [PubMed] [Google Scholar]
- 21.Pennick GJ, Clark M, Sutton DA, Rinaldi MG. 2003. Development and validation of a high-performance liquid chromatography assay for voriconazole. Antimicrob. Agents Chemother. 47:2348–2350. 10.1128/AAC.47.7.2348-2350.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bodet CA, III, Jorgensen JH, Drutz DJ. 1985. Simplified bioassay method for measurement of flucytosine or ketoconazole. J. Clin. Microbiol. 22:157–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lestner JM, Roberts SA, Moore CB, Howard SJ, Denning DW, Hope WW. 2009. Toxicodynamics of itraconazole: implications for therapeutic drug monitoring. Clin. Infect. Dis. 49:928–930. 10.1086/605499 [DOI] [PubMed] [Google Scholar]
- 24.Summers KK, Hardin TC, Gore SJ, Graybill JR. 1997. Therapeutic drug monitoring of systemic antifungal therapy. J. Antimicrob. Chemother. 40:753–764. 10.1093/jac/40.6.753 [DOI] [PubMed] [Google Scholar]
- 25.Andes D. 2013. Optimizing antifungal choice and administration. Curr. Med. Res. Opin. 29(Suppl 4):13–18. 10.1185/03007995.2012.761135 [DOI] [PubMed] [Google Scholar]
- 26.Andes D, Pascual A, Marchetti O. 2009. Antifungal therapeutic drug monitoring: established and emerging indications. Antimicrob. Agents Chemother. 53:24–34. 10.1128/AAC.00705-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith J, Andes D. 2008. Therapeutic drug monitoring of antifungals: pharmacokinetic and pharmacodynamic considerations. Ther. Drug Monit. 30:167–172. 10.1097/FTD.0b013e318167d0e0 [DOI] [PubMed] [Google Scholar]
- 28.Imhof A, Schaer DJ, Schanz U, Schwarz U. 2006. Neurological adverse events to voriconazole: evidence for therapeutic drug monitoring. Swiss Med. Wkly. 136:739–742 http://www.smw.ch/docs/pdf200x/2006/45/smw-11547.pdf [DOI] [PubMed] [Google Scholar]
- 29.Troke PF, Hockey HP, Hope WW. 2011. Observational study of the clinical efficacy of voriconazole and its relationship to plasma concentrations in patients. Antimicrob. Agents Chemother. 55:4782–4788. 10.1128/AAC.01083-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suzuki Y, Tokimatsu I, Sato Y, Kawasaki K, Sato Y, Goto T, Hashinaga K, Itoh H, Hiramatsu K, Kadota JI. 2013. Association of sustained high plasma trough concentration of voriconazole with the incidence of hepatotoxicity. Clin. Chim. Acta 424:119–122. 10.1016/j.cca.2013.05.025 [DOI] [PubMed] [Google Scholar]
- 31.Mulanovich V, Lewis RE, Raad II, Kontoyiannis DP. 2007. Random plasma concentrations of voriconazole decline over time. J. Infect. 55:e129–e130. 10.1016/j.jinf.2007.07.019 [DOI] [PubMed] [Google Scholar]
- 32.Roffey SJ, Cole S, Comby P, Gibson D, Jezequel SG, Nedderman AN, Smith DA, Walker DK, Wood N. 2003. The disposition of voriconazole in mouse, rat, rabbit, guinea pig, dog, and human. Drug Metab. Dispos. 31:731–741. 10.1124/dmd.31.6.731 [DOI] [PubMed] [Google Scholar]
- 33.Moriyama B, Elinoff J, Danner RL, Gea-Banacloche J, Pennick G, Rinaldi MG, Walsh TJ. 2009. Accelerated metabolism of voriconazole and its partial reversal by cimetidine. Antimicrob. Agents Chemother. 53:1712–1714. 10.1128/AAC.01221-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lutsar I, Roffey S, Troke P. 2003. Voriconazole concentrations in the cerebrospinal fluid and brain tissue of guinea pigs and immunocompromised patients. Clin. Infect. Dis. 37:728–732. 10.1086/377131 [DOI] [PubMed] [Google Scholar]
- 35.Thompson GR, III, Rinaldi MG, Pennick G, Dorsey SA, Patterson TF, Lewis JS., II 2009. Posaconazole therapeutic drug monitoring: a reference laboratory experience. Antimicrob. Agents Chemother. 53:2223–2224. 10.1128/AAC.00240-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shields RK, Clancy CJ, Vadnerkar A, Kwak EJ, Silveira FP, Massih RC, Pilewski JM, Crespo M, Toyoda Y, Bhama JK, Bermudez C, Nguyen MH. 2011. Posaconazole serum concentrations among cardiothoracic transplant recipients: factors impacting trough levels and correlation with clinical response to therapy. Antimicrob. Agents Chemother. 55:1308–1311. 10.1128/AAC.01325-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krishna G, Martinho M, Chandrasekar P, Ullmann AJ, Patino H. 2007. Pharmacokinetics of oral posaconazole in allogeneic hematopoietic stem cell transplant recipients with graft-versus-host disease. Pharmacotherapy 27:1627–1636. 10.1592/phco.27.12.1627 [DOI] [PubMed] [Google Scholar]
- 38.Krishna G, Moton A, Ma L, Medlock MM, McLeod J. 2009. Pharmacokinetics and absorption of posaconazole oral suspension under various gastric conditions in healthy volunteers. Antimicrob. Agents Chemother. 53:958–966. 10.1128/AAC.01034-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krishna G, Ma L, Martinho M, O'Mara E. 2012. Single-dose phase I study to evaluate the pharmacokinetics of posaconazole in new tablet and capsule formulations relative to oral suspension. Antimicrob. Agents Chemother. 56:4196–4201. 10.1128/AAC.00222-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krishna G, Ma L, Martinho M, Preston RA, O'Mara E. 2012. A new solid oral tablet formulation of posaconazole: a randomized clinical trial to investigate rising single- and multiple-dose pharmacokinetics and safety in healthy volunteers. J. Antimicrob. Chemother. 67:2725–2730. 10.1093/jac/dks268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maertens J, Cornely O, Ullmann A, Heinz W, Krishna G, Caceres M, Kartsonis N. 2012. Phase 1B study of the pharmacokinetics and safety of posaconazole IV in patients at risk for invasive fungal infection, abstr A-1946a. Abstr 52nd Intersci. Conf. Antimicrob. Agents Chemother., San Francisco, CA [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Felton TW, Baxter C, Moore CB, Roberts SA, Hope WW, Denning DW. 2010. Efficacy and safety of posaconazole for chronic pulmonary aspergillosis. Clin. Infect. Dis. 51:1383–1391. 10.1086/657306 [DOI] [PubMed] [Google Scholar]
- 43.Catanzaro A, Cloud GA, Stevens DA, Levine BE, Williams PL, Johnson RH, Rendon A, Mirels LF, Lutz JE, Holloway M, Galgiani JN. 2007. Safety, tolerance, and efficacy of posaconazole therapy in patients with nonmeningeal disseminated or chronic pulmonary coccidioidomycosis. Clin. Infect. Dis. 45:562–568. 10.1086/519937 [DOI] [PubMed] [Google Scholar]
- 44.Mavridou E, Bruggemann RJ, Melchers WJ, Mouton JW, Verweij PE. 2010. Efficacy of posaconazole against three clinical Aspergillus fumigatus isolates with mutations in the cyp51A gene. Antimicrob. Agents Chemother. 54:860–865. 10.1128/AAC.00931-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mavridou E, Bruggemann RJ, Melchers WJ, Verweij PE, Mouton JW. 2010. Impact of cyp51A mutations on the pharmacokinetic and pharmacodynamic properties of voriconazole in a murine model of disseminated aspergillosis. Antimicrob. Agents Chemother. 54:4758–4764. 10.1128/AAC.00606-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Howard SJ, Lestner JM, Sharp A, Gregson L, Goodwin J, Slater J, Majithiya JB, Warn PA, Hope WW. 2011. Pharmacokinetics and pharmacodynamics of posaconazole for invasive pulmonary aspergillosis: clinical implications for antifungal therapy. J. Infect. Dis. 203:1324–1332. 10.1093/infdis/jir023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Andes D, Marchillo K, Conklin R, Krishna G, Ezzet F, Cacciapuoti A, Loebenberg D. 2004. Pharmacodynamics of a new triazole, posaconazole, in a murine model of disseminated candidiasis. Antimicrob. Agents Chemother. 48:137–142. 10.1128/AAC.48.1.137-142.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Andes D, Marchillo K, Stamstad T, Conklin R. 2003. In vivo pharmacokinetics and pharmacodynamics of a new triazole, voriconazole, in a murine candidiasis model. Antimicrob. Agents Chemother. 47:3165–3169. 10.1128/AAC.47.10.3165-3169.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lepak AJ, Marchillo K, Vanhecker J, Andes DR. 2013. Posaconazole pharmacodynamic target determination against wild-type and Cyp51 mutant isolates of Aspergillus fumigatus in an in vivo model of invasive pulmonary aspergillosis. Antimicrob. Agents Chemother. 57:579–585. 10.1128/AAC.01279-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Denning DW, Lee JY, Hostetler JS, Pappas P, Kauffman CA, Dewsnup DH, Galgiani JN, Graybill JR, Sugar AM, Catanzaro A, Gallis H, Perfect JR, Dockery B, Dismukes WE, Stevens DA. 1994. NIAID Mycoses Study Group multicenter trial of oral itraconazole therapy for invasive aspergillosis. Am. J. Med. 97:135–144. 10.1016/0002-9343(94)90023-X [DOI] [PubMed] [Google Scholar]
- 51.Glasmacher A, Molitor E, Hahn C, Bomba K, Ewig S, Leutner C, Wardelmann E, Schmidt-Wolf IG, Mezger J, Marklein G, Sauerbruch T. 1998. Antifungal prophylaxis with itraconazole in neutropenic patients with acute leukaemia. Leukemia 12:1338–1343. 10.1038/sj.leu.2401137 [DOI] [PubMed] [Google Scholar]
- 52.Kageyama S, Masuya M, Tanaka I, Oka K, Morita K, Tamaki S, Tsuji K, Katayama N, Sugimoto H, Kagawa Y, Kojima M, Shiku H. 1999. Plasma concentration of itraconazole and its antifungal prophylactic efficacy in patients with neutropenia after chemotherapy for acute leukemia. J. Infect. Chemother. 5:213–216. 10.1007/s101560050038 [DOI] [PubMed] [Google Scholar]
- 53.Nucci M, Biasoli I, Akiti T, Silveira F, Solza C, Barreiros G, Spector N, Derossi A, Pulcheri W. 2000. A double-blind, randomized, placebo-controlled trial of itraconazole capsules as antifungal prophylaxis for neutropenic patients. Clin. Infect. Dis. 30:300–305. 10.1086/313654 [DOI] [PubMed] [Google Scholar]
- 54.Odds FC, Bossche HV. 2000. Antifungal activity of itraconazole compared with hydroxy-itraconazole in vitro. J. Antimicrob. Chemother. 45:371–373. 10.1093/jac/45.3.371 [DOI] [PubMed] [Google Scholar]
- 55.Odds FC, Dupont B, Rinaldi MG, Stevens DA, Warnock DW, Woestenborghs R. 1999. Bioassays for itraconazole blood levels: an interlaboratory collaborative study. J. Antimicrob. Chemother. 43:723–727. 10.1093/jac/43.5.723 [DOI] [PubMed] [Google Scholar]
- 56.Hostetler JS, Heykants J, Clemons KV, Woestenborghs R, Hanson LH, Stevens DA. 1993. Discrepancies in bioassay and chromatography determinations explained by metabolism of itraconazole to hydroxyitraconazole: studies of interpatient variations in concentrations. Antimicrob. Agents Chemother. 37:2224–2227. 10.1128/AAC.37.10.2224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Conte JE, Jr, Golden JA, Kipps J, McIver M, Zurlinden E. 2004. Intrapulmonary pharmacokinetics and pharmacodynamics of itraconazole and 14-hydroxyitraconazole at steady state. Antimicrob. Agents Chemother. 48:3823–3827. 10.1128/AAC.48.10.3823-3827.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]