Abstract
Sulfadoxine-pyrimethamine has never been recommended for the treatment of Plasmodium vivax malaria as the parasite is intrinsically resistant to pyrimethamine. The combination was introduced as a promising agent to treat Plasmodium falciparum malaria in many countries but was withdrawn after a few years due to development and spread of resistant strains. Presently, sulfadoxine-pyrimethamine is used as a partner drug of artemisinin-based combination therapy to treat uncomplicated falciparum malaria, and a combination of artesunate-sulfadoxine-pyrimethamine is currently in use in India. In countries like India, where both P. vivax and P. falciparum are equally prevalent, some proportion of P. vivax bacteria is exposed to sulfadoxine-pyrimethamine due to misdiagnosis and mixed infections. As reports on the in vivo therapeutic efficacy of sulfadoxine-pyrimethamine in P. vivax are rare, the study of mutations in the marker genes P. vivax dhfr (pvdhfr) and pvdhps is important for predicting drug selection pressure and sulfadoxine-pyrimethamine resistance monitoring. We studied the prevalence of point mutations and haplotypes of both the genes in 80 P. vivax isolates collected from urban Kolkata, India, by the DNA sequencing method. Point mutation rates in both the genes were low. The double mutant pvdhfr A15N50R58N117I173 (mutations are in boldface) and the single mutant pvdhps genotype S382G383K512A553V585 were more prevalent, while 35% of the isolates harbored the wild-type genotype. The triple mutant ANRNI-SGKAV was found in 29.9% isolates. No quintuple mutant genotype was recorded. The P. vivax parasites in urban Kolkata may still be susceptible to sulfadoxine-pyrimethamine. Hence, a combination of antimalarial drugs like artesunate-sulfadoxine-pyrimethamine introduced for P. falciparum infection might be effective in P. vivax infection also. Study of the therapeutic efficacy of this combination in P. vivax is thus strongly suggested. (The study protocol was registered in the Clinical Trial Registry-India [CTRI] of the Indian Council of Medical Research under registration number CTRI/2011/09/002031.)
INTRODUCTION
The burden of malaria caused by Plasmodium vivax has been greatly underappreciated both in terms of its clinical spectrum and incidence of disease (1). Plasmodium vivax is a widely distributed human malarial parasite, with an estimated population of 2.49 billion people globally living at risk of infection in 2010 (2), of which 1.13 billion are from India (3).
In most parts of the world chloroquine (CQ) remains the first line of treatment for patients with vivax malaria. In India, CQ was replaced by artemisinin-based combination therapy (ACT), a combination of artesunate plus sulfadoxine-pyrimethamine (AS+SP), in 2009 for Plasmodium falciparum malaria, but in the case of vivax malaria, CQ remains the first-line agent along with primaquine (PQ) for 14 days.
In countries like India where two different species of human malarial parasites are equally prevalent, correct diagnosis is essential for treatment. But the mixed infections cannot be easily differentiated from monoinfection on the basis of either microscopic examination and/or clinical symptoms. Therefore, some proportion of the P. vivax population is often inadvertently exposed to SP selection pressure, and this might have caused the selection of SP-resistant alleles in P. vivax isolates. It has been considered that P. vivax is intrinsically resistant to pyrimethamine (4), but a high therapeutic efficacy of SP has also been reported (5).
The two components of SP target dihydrofolate reductase (dhfr) and dihydropteroate synthase (dhps) of the folate biosynthesis pathway of the malarial parasite. Point mutations within the genes that encode these enzymes are the primary causes of resistance to the drugs. Several mutations in drug resistance markers have been identified in P. vivax. Most of these genes had initially been identified and analyzed in P. falciparum (6–10), and orthologous genes have been determined in P. vivax. The mutations at positions 15, 50, 58, 117, and 173 in the P. vivax dhfr (pvdhfr) gene showing homology to codons 16, 51, 59, 108, and 164, respectively, which are the five key positions in the P. falciparum dhfr (pfdhfr) gene, were determined (11). Similarly, five mutations at codons 382, 383, 512, 553, and 585 in the pvdhps gene corresponding to codons 436, 437, 540, 581, and 613 of the homologous gene pfdhps have already been identified (12, 13).
Different studies of P. vivax parasites in different areas of malaria endemicity showed that mutations in the pvdhfr gene at codons 57, 58, 61, 117, and 173 (14, 15) and in the pvdhps gene at codons 382, 383, 512, 553, and 585 might be involved in antifolate resistance clinically (16–18).
The prevalence of pvdhfr and pvdhps mutations has been studied in many regions in Southeast Asia, including Indonesia, Papua New Guinea, Sri Lanka, and Pakistan; but such information from India is scarce (19–22), and no data are available from this part of the country.
The present study was designed to determine the prevalence of polymorphism in the pvdhfr and pvdhps genes among the field isolates of urban Kolkata.
(This research was conducted by S. Ganguly in partial fulfillment of the requirements for a Ph.D. from the West Bengal University of Health Sciences, Kolkata, India.)
MATERIALS AND METHODS
Parasite isolates.
Parasite isolates were collected during a therapeutic efficacy study of chloroquine either alone or in combination with 14 days of primaquine treatment in P. vivax malaria in the urban population of Kolkata, India, from December 2011 to June 2012. That was a randomized, double-arm, open-label, interventional trial for evaluation of the clinical and parasitological responses of CQ and CQ plus PQ (CQ+PQ) for treatment of uncomplicated P. vivax malaria based on the 2009 WHO therapeutic-efficacy protocol (23). The protocol of the study was reviewed and approved by the Institutional Ethics Committee of the Kolkata School of Tropical Medicine and was registered in the Clinical Trial Registry-India (CTRI) of the Indian Council of Medical Research under registration number CTRI/2011/09/002031. A total of 250 P. vivax-positive patients confirmed by microscopy and PCR were recruited and randomized into two study arms: 125 patients in each arm (CQ and CQ+PQ). Among these, 205 patients completed 42 days of follow-up, and all cases were classified as adequate clinical and parasitological response (ACPR) (24). A total of 80 isolates (30% of the total isolates) were included in the present study to determine the prevalence of polymorphisms in marker genes associated with antifolate drugs.
Preparation of DNA template from blood samples.
Genomic DNA of P. vivax from all blood samples collected in EDTA-coated vials was extracted using a QIAamp DNA Blood Kit (Qiagen, Hilden, Germany), according to the manufacturer's instructions, with the modification that the incubation time with proteinase K was increased to 20 min at 56°C to improve the yield of the extraction. Extracted parasite genomic DNA of all the samples was preserved at −20°C, and an aliquot was used as the DNA source for further study.
PCR amplification and sequencing of pvdhfr and pvdhps genes.
Fragments of the pvdhfr and pvdhps genes spanning codons 15, 50, 58, 117, and 173 and codons 382, 383, 512, 553, and 585, respectively, were amplified by seminested and nested PCR amplification strategies as described earlier (16, 17) with some minor modifications. All amplification reactions were carried out in a final volume of 50 μl. The oligonucleotide primers and PCR conditions are summarized in Table 1.
TABLE 1.
Primers and profiles used for PCR amplification of the pvdhfr and pvdhps genes
| Gene | Primer name | Primer sequence (5′–3′) | Mg+2 concn (mM) | PCR program |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Denaturation |
Annealing |
Elongation |
No. of cycles |
||||||||
| Temp (°C) | Time (min) | Temp (°C) | Time (min) | Temp (°C) | Time (min) | 1st PCR | 2nd PCR | ||||
| pvdhfr | VDT-OF | ATGGAGGACCTTTCAGATGTATTTGACATT | 2 | 94 | 1 | 60 | 2 | 72 | 2 | 25 | |
| VDT-OR | GGCGGCCATCTCCATGGTTATTTTATCGTG | ||||||||||
| VDT-OF | ATGGAGGACCTTTCAGATGTATTTGACATT | 94 | 1 | 62 | 2 | 72 | 2 | 30 | |||
| VDF-NR | TCACACGGGTAGGCGCCGTTGATCCTCGTG | ||||||||||
| pvdhps | PVDHPS-OF | ATTCCAGAGTATAAGCACAGCACATTTGAG | 3 | 94 | 2 | 60 | 2 | 72 | 1 | 25 | |
| PVDHPS-OR | CTAAGGTTG ATGTATCCTTGTGAGCACATC | ||||||||||
| PVDHPS-NF | AATGGCAAGTGATGGGGCGAGCGTGATTGA | 94 | 2 | 52 | 2 | 72 | 1 | 30 | |||
| PVDHPS-NR | CAGTCTGCACTCCCCGATGGCCGCGCCACC | ||||||||||
Sequencing and analysis.
The quality and concentration of PCR products were ascertained by agarose gel electrophoresis. The products were subsequently purified using a commercial kit (QIAquick PCR Purification Kit; Qiagen) and used as the templates for sequencing. Sequencing was outsourced from Chromous Biotech Pvt., Ltd., Bangaluru, India. Sequencing reactions were carried out with an ABI Prism BigDye Terminator Cycle Sequencing Ready Reaction Kit on a 3730 XL genetic analyzer (PerkinElmer, Branchburg, NJ, USA).The sequences were analyzed using the free software Bioedit Sequence Alignment Editor, version 7.0.5.2 (www.mbio.ncsu.edu/bioedit/bioedit.html). The sequences were then aligned using the online sequence alignment tool ClustalW (available at www.ebi.ac.uk/clustalw).
Data interpretation.
Single nucleotide polymorphisms in individual codons, both synonymous and nonsynonymous, were noted. Five important codons, 15, 50, 58, 117, and 173, in the pvdhfr gene were analyzed for genotype study. The wild-type pvdhfr genotype was defined as residues A15N50S58S117I173, while the mutant pvdhfr genotype was defined as having at least one amino acid change in the corresponding residues.
Similarly, five important codons, 382, 383, 512, 553, and 585, in the pvdhps gene were analyzed for genotype study. The wild-type pvdhps genotype was defined as S382A383K512A553V585 while the mutant pvdhps genotype was defined as one with at least one amino acid change in the corresponding residues.
RESULTS
Mutation analysis in the pvdhfr and pvdhps genes.
The pvdhfr gene was successfully amplified and sequenced in 70 isolates. Sequences were aligned and compared with the wild-type reference sequence (PlasmoDB accession no. PVX_089950) to detect point mutations. Polymorphisms were observed at 10 different codons, of which five were synonymous and five were nonsynonymous.
Among nonsynonymous mutants, S58R and S117N were more prevalent (40.6% and 39.1%, respectively). Genotype analysis of five codons of pvdhfr (15, 50, 58, 117, and 173) revealed that most of the isolates (57.1%; 40/70) had the wild-type sequence A15N50S58S117I173. The double mutant genotype A15N50R58N117I173 (mutations in boldface) was found in 38.6% (27/70) of the isolates. Two different single mutant genotypes, A15N50R58S117I173 and A15N50S58N117I173, were observed in two isolates and one isolate, respectively (Table 2). The only allelic variant in respect to tandem repeat with deletion of the sequence GDNTSG (18 bp; nucleotides 265 to 282) was found in only two isolates.
TABLE 2.
Mutation profile of the pvdhfr and pvdhps genes in study isolates
| Gene and mutation and/or genotypea | Mutation frequency |
|
|---|---|---|
| No. of isolates with the mutation (%) | 95% CIb | |
| pvdhfr (n = 70) | ||
| Nonsynonymous mutations | ||
| S58R | 29 (41.4) | 29.86–52.94 |
| S97N | 9 (12.8) | 4.97–20.63 |
| S117N | 28 (40) | 28.52–51.48 |
| D156N | 2 (2.9) | 0–6.83 |
| A183E | 2 (2.9) | 0–6.83 |
| Synonymous mutations | ||
| G38G | 10 (14.3) | 6.1–22.5 |
| Y49Y | 2 (2.9) | 0–6.83 |
| Y69Y | 8 (11.4) | 3.95–18.85 |
| E119E | 2 (2.9) | 0–6.83 |
| G174G | 3 (4.3) | 0–9.05 |
| Genotype | ||
| Wild type | ||
| A15 N50 S58 S117 I173 | 40 (57.1) | 45.51–68.69 |
| Single mutants | ||
| A15 N50 R58 S117 I173 | 2 (2.9) | 0–6.83 |
| A15 N50 S58 N117 I173 | 1 (1.4) | 0–4.15 |
| Double mutant | ||
| A15 N50 R58 N117 I173 | 27 (38.6) | 27.2–50.0 |
| pvdhps (n = 78) | ||
| Nonsynonymous mutations | ||
| E380V | 1 (1.3) | 0–3.8 |
| A383G | 47 (60.3) | 49.44–71.16 |
| V401F | 1 (1.3) | 0–3.8 |
| D459A | 3 (3.8) | 0–8.04 |
| A553G | 3 (3.8) | 0–8.04 |
| Synonymous mutations | ||
| G484G | 1 (1.3) | 0–3.8 |
| R487R | 1 (1.3) | 0–3.8 |
| L600L | 2 (2.6) | 0–6.13 |
| Genotype | ||
| Wild Type | ||
| S382 A383 K512 A553 V585 | 30 (38.4) | 27.61–49.19 |
| Single mutants | ||
| S382 G383 K512 A553 V585 | 45 (57.7) | 46.74–68.66 |
| S382 A383 K512 G553 V585 | 1 (1.3) | 0–3.8 |
| Double mutant | ||
| S382 G383 K512 G553 V585 | 2 (2.6) | 0–6.13 |
Mutations in the genotypes are shown in boldface. n, number of isolates.
CI, confidence interval.
The pvdhps gene was successfully amplified and sequenced in 78 isolates. Sequences were aligned and compared with the wild-type reference sequence (PlasmoDB accession number PVX_123230) to detect point mutations. Polymorphisms were observed at eight different codons, of which three were synonymous and five were nonsynonymous. Among nonsynonymous mutants A383G was most prevalent (60.36%; 47/78). Genotype analysis of five codons of pvdhps (382, 383, 512, 553, and 585) revealed that 38.5% (30/78) of the isolates were wild-type S382A383K512A553V585. Two different single mutant genotypes were observed, the S382G383K512A553V585 genotype (mutation in boldface) in 57.7% (45/78) of the cases and S382A383K512G553V585 in one isolate only. The double mutant genotype S382G383K512G553V585 was found in 2.5% (2/78) of the isolates (Table 2).
Mutations analysis in the pvdhfr-pvdhps genes.
The pvdhfr-pvdhps genes were sequenced in 67 isolates. Wild-type ANSSI-SAKAV was the most prevalent sequence (34.8%). The triple mutant ANRNI-SGKAV (mutations in boldface) was found in 29.9% of the isolates. Two types of double mutant haplotype were found: ANRNI-SAKAV in 6% of the isolates and ANSSI-SGKGV in 1.45% of the cases. Two types of single mutant haplotype were found: ANSSI-SGKAV was in 23.9% of the isolates, and ANRSI-SAKAV was found in 3% of the cases. A quadruple mutant haplotype, the ANRNI-SGKGV haplotype, was found in only 1.45% of the cases (Fig. 1).
FIG 1.
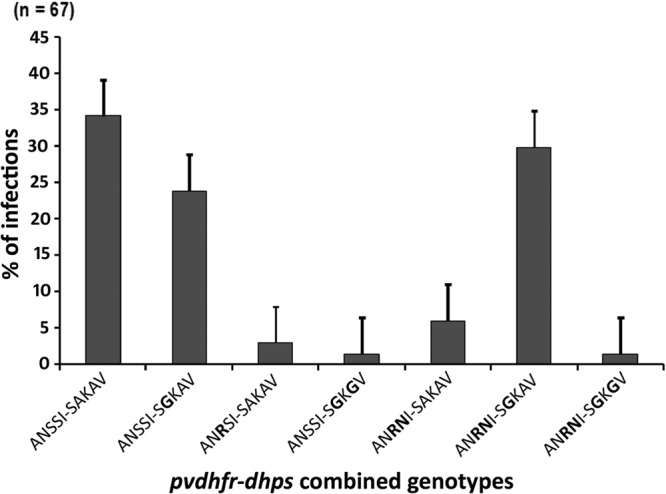
Prevalence of the pvdhfr-pvdhps combined genotypes in study population (n = 67) (mutations in boldface).
DISCUSSION
Mutations in the pvdhfr and pvdhps genes have been found to be associated with antifolate drug resistance (25, 26). Several reports on in vitro drug assays and clinical therapeutic assessments generally agree with this hypothesis (27–29), suggesting that genotyping of molecular markers may provide valuable information about the trends of SP resistance in P. vivax. Mutant 117N alone could increase the 50% inhibitory concentration (IC50) of pyrimethamine by more than 80 times, and a combination of S58R and S117N increases resistance to this drug 400 times more than the wild type (28). In the present study, we observed both the mutations in 40% of the isolates. But we did not find mutations at codons I13L, F57L, T61M, P33L, S93H, S117T, I172V, and I173L which have been reported from different parts of the country by various workers (19–22). It has been hypothesized that the S117N mutation is the first step in the drug resistance selection process, and S117T has been strongly associated with SP resistance (30) in areas with extensive use of SP (31, 32), but the mutation was not found in the present study. A high risk of therapeutic failure of SP is significantly associated with the quadruple mutant allele L57R58M61T11 (17, 28). No triple or quadruple mutation in pvdhfr was detected in the parasite population prevailing in the study area, which is unlike the findings made by other investigators from India (19, 21, 22).
Mutations in codons 382, 383, 512, and 553 were more prevalent in areas with extensive use of SP than in those with low SP use (33). In the present study, we recorded five nonsynonymous mutations in the pvdhps gene, among which A383G and A553G are the two key codons related to sulfadoxine resistance. The double mutant S382G383K512G553V585 genotype (mutations in boldface) has been found to be directly related to sulfadoxine resistance (32) which was noted in only two isolates.
The present study showed that resistance-conferring mutations were found in two codons of pvdhfr and two codons of the pvdhps gene. The wild-type genotype was recorded in a large proportion of the isolates. As we did not study the therapeutic efficacy of this combination in P. vivax malaria, it is not possible to correlate the mutation genotype of pvdhfr and pvdhps with efficacy outcome. In the study area, SP alone has never been recommended by the National Drug Policy of India for the treatment of vivax malaria but was in use for chloroquine-resistant falciparum malaria. SP has been used as a partner drug with artesunate for the treatment of P. falciparum malaria since 2009, so a certain proportion of the P. vivax population has been exposed to this antifolate drug due to a wrong diagnosis and also in the case of mixed infection, resulting in a selection pressure which might be reflected in the observed mutation pattern in the pvdhfr and pvdhps genes. Reports on polymorphisms in pvdhfr and pvdhps are not available from the present study area before the introduction of AS+SP, so the data could not be compared. We reported a high prevalence of quadruple and quintuple mutations in the pfdhfr-pfdhps genes in field isolates of P. falciparum from the same state (34) where SP was introduced for the treatment of chloroquine-resistant falciparum malaria in 2002, but no such data are available from urban Kolkata. The types and rates of multiple mutations of the pvdhfr-pvdhps genotypes are lower than those found in other parts of the country (21, 22). The results suggest that the P. vivax parasites in urban Kolkata may still be susceptible to SP. Hence, any combination of antimalarial drug with antifolates like AS+SP that have been introduced for P. falciparum might be effective in P. vivax malaria also. A study of the therapeutic efficacy of this combination in P. vivax malaria is thus strongly suggested.
ACKNOWLEDGMENTS
We are grateful to the Department of Health and Family Welfare, Government of West Bengal, India, for funding the project.
We are grateful to Nandita Basu, the Director, Kolkata School of Tropical Medicine, for her continuous support, encouragement, and kind permission to publish the data.
A.K.M. and S.G. conceived and designed the study protocol; S.G., P.S, and M.C performed PCR and sequencing analysis and interpretation of data; A.K.M. and S.G drafted the manuscript. All authors read and approved the final manuscript.
Footnotes
Published ahead of print 21 October 2013
REFERENCES
- 1.Price RN, Tjitra E, Guerra CA, Yeung S, White NJ, Anstey NM. 2007. Vivax malaria: neglected and not benign. Am. J. Trop. Med. Hyg. 77:79–87 [PMC free article] [PubMed] [Google Scholar]
- 2.Gething PW, Elyazar IRF, Moyes CM, Smith DL, Battle KE, Guerra CA, Patil AP, Tatem AJ, Howes RE, Myers MF, George DB, Horby P, Wertheim HFL, Price RN, Mueller I, Baird JK, Hay SI. 2012. A long neglected world malaria map: Plasmodium vivax endemicity in 2010. PLoS Negl. Trop. Dis. 6:e1814. 10.1371/journal.pntd.0001814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Battle KE, Gething WG, Elyazar IRF, Moyes CL, Sinka ME, Howes RE, Guerra CA, Price RN, Baird JK, Hay SI. 2012. The global public health significance of Plasmodium vivax. Adv. Parasitol. 80:1–94. 10.1016/B978-0-12-397900-1.00001-3 [DOI] [PubMed] [Google Scholar]
- 4.Young MD, Burgess RW. 1959. Pyrimethamine resistance in Plasmodium vivax malaria. Bull. World Health Org. 20:27–36 [PMC free article] [PubMed] [Google Scholar]
- 5.Leslie T, Mayan MI, Hasan MA, Safi MH, Klinkenberg E, Whitty CJ, Rowland M. 2007. Sulfadoxine-pyrimethamine, chlorproguanil-dapsone, or chloroquine for the treatment of Plasmodium vivax malaria in Afghanistan and Pakistan: a randomized controlled trial. JAMA 297:2201–2209. 10.1001/jama.297.20.2201 [DOI] [PubMed] [Google Scholar]
- 6.Kublin JG, Dzinjalamala FK, Kamwendo DD, Malkin EM, Cortese JF, Martino LM, Mukadam RA, Rogerson SJ, Lescano AG, Molyneux ME, Winstanley PA, Chimpeni P, Taylor TE, Plowe CV. 2002. Molecular markers for failure of sulfadoxine-pyrimethamine and chlorproguanil-dapsone treatment of Plasmodium falciparum malaria. J. Infect. Dis. 185:380–388. 10.1086/338566 [DOI] [PubMed] [Google Scholar]
- 7.Basco LK, Tahar R, Ringwald P. 1998. Molecular basis of in vivo resistance to sulfadoxine-pyrimethamine in African adult patients infected with Plasmodium falciparum malaria parasites. Antimicrob. Agents Chemother. 42:1811–1814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Happi CT, Gbotosho GO, Folarin OA, Akinboye DO, Yusuf BO, Ebong OO, Sowunmi A, Kyle DE, Milhous W, Wirth DF, Oduola AM. 2005. Polymorphisms in Plasmodium falciparum dhfr and dhps genes and age related in vivo sulfadoxine-pyrimethamine resistance in malaria-infected patients from Nigeria. Acta Trop. 95:183–193. 10.1016/j.actatropica.2005.06.015 [DOI] [PubMed] [Google Scholar]
- 9.Triglia T, Menting JG, Wilson C, Cowman AF. 1997. Mutations in dihydropteroate synthase are responsible for sulfone and sulfonamide resistance in Plasmodium falciparum. Proc. Natl. Acad. Sci. U. S. A. 94:13944–13949. 10.1073/pnas.94.25.13944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vinayak S, Alam MT, Mixson-Hayden T, McCollum AM, Sem R, Shah NK, Lim P, Muth S, Rogers WO, Fandeur T, Barnwell JW, Escalante AA, Wongsrichanalai C, Ariey F, Meshnick SR, Udhayakumar V. 2010. Origin and Evolution of Sulfadoxine Resistant Plasmodium falciparum. PLoS Pathog. 6:e1000830. 10.1371/journal.ppat.1000830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eldin de Pecoulas P, Basco LK, Tahar R, Ouatas T, Mazabraud A. 1998. Analysis of the Plasmodium vivax dihydrofolate reductase-thymidylate synthase gene sequences. Gene 211:177–185. 10.1016/S0378-1119(98)00118-8 [DOI] [PubMed] [Google Scholar]
- 12.Triglia T, Wang P, Sims PF, Hyde JE, Cowman AF. 1998. Allelic exchange at the endogenous genomic locus in Plasmodium falciparum proves the role of dihydropteroate synthase in sulfadoxine-resistant malaria. EMBO J. 17:3807–3815. 10.1093/emboj/17.14.3807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Triglia T, Cowman AF. 1994. Primary structure and expression of the dihydropteroate synthetase gene of Plasmodium falciparum. Proc. Natl. Acad. Sci. U. S. A. 91:7149–7153. 10.1073/pnas.91.15.7149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Imwong M, Pukrittayakamee S, Renia L, Letourneur F, Charlieu JP, Leartsakulpanich U, Looareesuwan S, White NJ, Snounou G. 2003. Novel point mutations in the dihydrofolate reductase gene of Plasmodium vivax: evidence for sequential selection by drug pressure. Antimicrob. Agents Chemother. 47:1514–1521. 10.1128/AAC.47.5.1514-1521.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barnadas C, Ratsimbasoa A, Tichit M, Bouchier C, Jahevitra M, Picot S, Ménard D. 2008. Plasmodium vivax resistance to chloroquine in Madagascar: clinical efficacy and polymorphisms in pvmdr1 and pvcrt-o genes. Antimicrob. Agents Chemother. 52:4233–4240. 10.1128/AAC.00578-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Imwong M, Pukrittayakamee S, Cheng Q, Moore C, Looareesuwan S, Snounou G, White NJ, Day NP. 2005. Limited polymorphism in the dihydropteroate synthetase gene (dhps) of Plasmodium vivax isolates from Thailand. Antimicrob. Agents. Chemother. 49:4393–4395. 10.1128/AAC.49.10.4393-4395.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imwong M, Pukrittakayamee S, Looareesuwan S, Pasvol G, Poirriez J, White NJ, Snounou G. 2001. Association of genetic mutations in Plasmodium vivax dhfr with resistance to sulfadoxine-pyrimethamine: geographical and clinical correlates. Antimicrob. Agents Chemother. 45:3122–3127. 10.1128/AAC.45.11.3122-3127.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hawkins VN, Joshi H, Rungsihirunrat K, Na-Bangchang K, Sibley CH. 2007. Antifolates can have a role in the treatment of Plasmodium vivax. Trends Parasitol. 23:213–222. 10.1016/j.pt.2007.03.002 [DOI] [PubMed] [Google Scholar]
- 19.Kaur S, Prajapati SK, Kalyanaraman K, Mohmmed A, Joshi H, Chauhan VS. 2006. Plasmodium vivax dihydrofolate reductase point mutations from the Indian subcontinent. Acta Trop. 97:174–180. 10.1016/j.actatropica.2005.10.003 [DOI] [PubMed] [Google Scholar]
- 20.Valecha N, Joshi H, Eapen A, Ravinderan J, Kumar A, Prajapati SK, Ringwald P. 2006. Therapeutic efficacy of chloroquine in Plasmodium vivax from areas with different epidemiological patterns in India and their pvdhfr gene mutation pattern. Trans. R. Soc. Trop. Med. Hyg. 100:831–837. 10.1016/j.trstmh.2005.11.012 [DOI] [PubMed] [Google Scholar]
- 21.Prajapati SK, Joshi H, Dev V, Dua VK. 2011. Molecular epidemiology of Plasmodium vivax anti-folate resistance in India. Malar. J. 10:102. 10.1186/1475-2875-10-102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alam MT, Bora H, Bharti PK, Saifi MA, Das MK, Dev Kumar VA, Singh N, Dash AP, Das B, Wajihullah, Sharma YD. 2007. Similar trends of pyrimethamine resistance-associated mutations in Plasmodium vivax and P. falciparum. Antimicrob. Agents. Chemother. 51:857–863. 10.1128/AAC.01200-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization 2009. Methods for surveillance of antimalarial drug efficacy. World Health Organization, Geneva, Switzerland [Google Scholar]
- 24.Ganguly S, Saha P, Guha SK, Das S, Bera DK, Biswas A, Kundu PK, Saha B, Ray K, Maji AK. 2013. In vivo therapeutic efficacy of chloroquine alone or in combination with primaquine in vivax malaria in Kolkata, West Bengal, India and polymorphism in pvmdr1 and pvcrt-o genes. Antimicrob. Agents. Chemother. 57:1246–1251. 10.1128/AAC.02050-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marfurt J, de Monbrison F, Brega S, Barbollat L, Müller I, Sie A, Goroti M, Reeder JC, Beck HP, Picot S, Genton B. 2008. Molecular markers of in vivo Plasmodium vivax resistance to amodiaquine plus sulfadoxine-pyrimethamine: mutations in pvdhfr and pvmdr1. J. Infect. Dis. 198:409–417. 10.1086/589882 [DOI] [PubMed] [Google Scholar]
- 26.Sá JM, Nomura T, Neves J, Baird JK, Wellems TE, del Portillo HA. 2005. Plasmodium vivax: allele variants of the mdr1 gene do not associate with chloroquine resistance among isolates from Brazil, Papua, and monkey-adapted strains. Exp. Parasitol. 109:256–259. 10.1016/j.exppara.2004.12.005 [DOI] [PubMed] [Google Scholar]
- 27.Hastings MD, Maguire JD, Bangs MJ, Zimmerman PA, Reeder JC, Baird JK, Sibley CH. 2005. Novel Plasmodium vivax dhfr alleles from the Indonesian Archipelago and Papua New Guinea: association with pyrimethamine resistance determined by a Saccharomyces cerevisiae expression system. Antimicrob. Agents Chemother. 49:733–740. 10.1128/AAC.49.2.733-740.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hastings MD, Porter KM, Maguire JD, Susanti I, Kania W, Bangs MJ, Sibley CH, Baird JK. 2004. Dihydrofolate reductase mutations in Plasmodium vivax from Indonesia and therapeutic response to sulfadoxine plus pyrimethamine. J. Infect. Dis. 189:744–750. 10.1086/381397 [DOI] [PubMed] [Google Scholar]
- 29.Rungsihirunrat K, Na-Bangchang K, Hawkins VN, Mungthin M, Sibley CH. 2007. Sensitivity to antifolates and genetic analysis of Plasmodium vivax isolates from Thailand. Am. J. Trop. Med. Hyg. 76:1057–1065 [PubMed] [Google Scholar]
- 30.Brega S, de Monbrison F, Severini C, Udomsangpetch R, Sutanto I, Ruckert P, Peyron F, Picot S. 2004. Real-time PCR for dihydrofolate reductase gene single-nucleotide polymorphisms in Plasmodium vivax isolates. Antimicrob. Agents Chemother. 48:2581–2587. 10.1128/AAC.48.7.2581-2587.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Korsinczky M, Fischer K, Chen N, Baker J, Rieckmann K, Cheng Q. 2004. Sulfadoxine resistance in Plasmodium vivax is associated with a specific amino acid in dihydropteroate synthase at the putative sulfadoxine-binding site. Antimicrob. Agents Chemother. 48:2214–2222. 10.1128/AAC.48.6.2214-2222.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu F, Lim CS, Nam DH, Kim K, Lin K, Kim TS, Lee HW, Chen JH, Wang Y, Sattabongkot J, Han ET. 2010. Mutations in the antifolate-resistance-associated genes dihydrofolate reductase and dihydropteroate synthase in Plasmodium vivax isolates from malaria-endemic countries. Am. J. Trop. Med. Hyg. 83:474–479. 10.4269/ajtmh.2010.10-0004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rungsihirunrat K, Sibley CH, Mungthin M, Na-Bangchang K. 2008. Geographical distribution of amino acid mutations in Plasmodium vivax DHFR and DHPS from malaria endemic areas of Thailand. Am. J. Trop. Med. Hyg. 78:462–467 [PubMed] [Google Scholar]
- 34.Saha P, Guha SK, Das S, Mullick S, Ganguly S, Biswas A, Bera DK, Chattopadhyay G, Das M, Kundu PK, Ray K, Maji AK. 2012. Comparative efficacy of artemisinin combination therapies (ACTs) in P. falciparum malaria and polymorphism of pfATPase6, pfcrt, pfdhfr and pfdhps genes in tea gardens of Jalpaiguri district, India. Antimicrob. Agents Chemother. 56:2511–2517. 10.1128/AAC.05388-11 [DOI] [PMC free article] [PubMed] [Google Scholar]


