Abstract
The efficacy of anidulafungin is driven by the area under the concentration-time curve (AUC)/MIC ratio. Patients in intensive care may be at risk for underexposure. In critically ill patients with an invasive Candida infection, the anidulafungin exposure and a possible correlation with disease severity or plasma protein levels were explored. Concentration-time curves were therefore obtained at steady state. Anidulafungin concentrations were measured with a validated liquid chromatography-tandem mass spectrometry (LC-MS/MS) method. The MIC values of the Candida species were determined with the Etest. The target AUC/MIC ratio was based on European Committee on Antimicrobial Susceptibility Testing (EUCAST) data. Twenty patients were included. The patients received a maintenance dose of 100 mg once daily after a loading dose of 200 mg on the first day. The mean (±standard deviation) AUC, maximum concentration of drug in plasma (Cmax), and minimum concentration of drug in plasma (Cmin) were 69.8 ± 24.1 mg · h/liter, 4.7 ± 1.4 mg/liter, and 2.2 ± 0.8 mg/liter, respectively. The MIC values of all cultured Candida species were below the EUCAST MIC breakpoints. The exposure to anidulafungin in relation to the MIC that was determined appeared sufficient in all patients. The anidulafungin exposure was low in our critically ill patients. However, combined with the low MICs of the isolated Candida strains, the lower exposure observed in comparison to the exposure in the general patient population resulted in favorable AUC/MIC ratios, based on EUCAST data. No correlation was observed between anidulafungin exposure and disease severity or plasma protein concentrations. In patients with less-susceptible Candida albicans or glabrata strains, we recommend considering determining the anidulafungin exposure to ensure adequate exposure. (This trial has been registered at ClinicalTrials.gov under registration no. NCT01047267.)
INTRODUCTION
Invasive candidiasis is a serious problem in intensive care unit (ICU) patients. From a retrospective matched case-control study in the United Kingdom between 2003 and 2007, the attributable mortality of candidiasis in ICU patients was estimated to be 28.3% (1).
One of the risk factors for mortality of patients with candidemia is inadequate antifungal therapy (2–4). For fluconazole, apart from delayed start of treatment (5), the area under the concentration-time curve (AUC) divided by the MIC has an impact on the mortality of patients with invasive Candida infections (6). Echinocandins are relatively new antifungal agents that are valuable for the treatment of patients with invasive candidiasis (7). Anidulafungin compared favorably with fluconazole in critically ill patients, particularly if given as empirical therapy (8). In a neutropenic murine disseminated candidiasis model, the AUC/MIC ratio of anidulafungin was a good predictor of efficacy (9, 10).
Anidulafungin has predictable pharmacokinetics in healthy volunteers; exposure is dose dependent and there is a low interindividual variability (11). In patients with fungal disease, anidulafungin clearance appeared to be approximately 30% higher in patients with invasive candidiasis than in patients with esophageal candidiasis. Patients with invasive candidiasis were more severely ill than patients with esophageal candidiasis (12). For caspofungin, the trough concentrations were more variable and correlated with albumin concentrations in surgical intensive care patients (13). However, at this time, there are limited data available on the pharmacokinetics of anidulafungin in critically ill patients (14).
The objective of this study was to determine the anidulafungin concentrations and exposure in critically ill patients and explore a possible correlation with disease severity or plasma protein levels.
MATERIALS AND METHODS
Patients and ethics.
This study was performed in the University Medical Center Groningen, the Netherlands, between June 2010 and November 2011. The trial was registered at ClinicalTrials.gov under registration no. NCT01047267. Patients were eligible for inclusion if they were at least 18 years old, admitted to an intensive care unit, and diagnosed with invasive candidiasis. Diagnosis of invasive candidiasis was based on the isolation of Candida species obtained from a sterile site. Patients were excluded if they were neutropenic or were allergic to echinocandins or any of the excipients of anidulafungin.
The study protocol was approved by the local institutional ethics committee. Written informed consent was obtained from the patient or the legal representative of the patient.
Study design and procedures.
Patients were treated with anidulafungin, starting with a loading dose of 200 mg on day one and continuing with a maintenance dose of 100 mg once daily.
On the first day of treatment, samples were drawn to measure the anidulafungin plasma concentrations at 3 and 12 h after the start of the 3-h infusion of the loading dose. A concentration-time curve was obtained at day 3 (±1 day) after the start of anidulafungin. Blood samples were taken just before administering anidulafungin and at 1.5, 2, 3, 4, 6, 8, 12, and 24 h after the start of the infusion. In addition, every 3 days during treatment, blood samples for anidulafungin trough concentrations were collected during ICU admission.
For each patient included, data were collected from the medical chart, including demographic data, medical history, and laboratory parameters. Total body water volumes were estimated using the method of Watson (15).
Predictive scoring systems were used to assess the disease severity. Disease severity scores have been developed to measure the disease severity and overall prognosis of patients, not to explain variability in pharmacokinetics, although they were successfully used earlier for this purpose (16–19). The scores that were calculated were the acute physiology and chronic health evaluation II (APACHE II) (20), logistic organ dysfunction system (LODS) (21), multiple organ dysfunction score (MODS) (22), organ dysfunctions and/or infection (ODIN) (23), simplified acute physiology score (SAPS II) (24), SAPS 3 (25), and sepsis-related organ failure assessment (SOFA) (26). All these different scores capture different aspects of disease severity; we calculated all of these scores to search for an appropriate disease severity score to correlate with anidulafungin exposure. The disease severity was assessed on the day that the concentration-time curve was obtained. On the same day, albumin and total protein concentrations in plasma were measured. Mortality was assessed at day 28 of treatment.
Anidulafungin pharmacokinetics.
Blood samples (4 ml) were drawn into Vacutainer tubes (Becton, Dickinson, Franklin Lakes, NJ). Plasma was separated and frozen at −80°C until it was processed. All samples were analyzed with a liquid chromatography-tandem mass spectrometry (LC-MS/MS) method that was validated according to the guidelines for bioanalytical method validation of the FDA (27). Sample preparation consisted of protein precipitation using aculeacin A as the internal standard. The method is accurate (bias ranging from −3.0 to 1.9%) and precise (within-run and between-run coefficients of variation of 2.2 to 7.7% and 1.6 to 9.0%, respectively). All calibration curves were linear over a range of 0.5 to 10.0 mg/liter for anidulafungin.
The AUC from 0 to 24 h (AUC0–24) was calculated using the log-linear trapezoidal rule with KINFIT (MWPharm 3.60; Mediware, the Netherlands) (28). The clearance (CL) was estimated by dividing the dose administered by the AUC0–24.
Microbiology.
MIC values were determined with the Etest (bioMérieux, Marcy l'Etoile, France). Etest assays were performed using RPMI glucose agar and inoculum density adjustment with a 0.5 McFarland standard. The plates were incubated at 35°C and were read after 24 h. If no growth was detected, the plates were incubated for another 24 h. The drug concentration shown on the Etest strip at the outer border of the elliptical inhibition halo was recorded as the MIC.
Pharmacokinetics/pharmacodynamics.
For the assessment of sufficient exposure, the calculated anidulafungin AUC in proportion to the MIC (AUC0–24 for the free, unbound fraction of drug [fAUC0–24]/MIC ratio) for each patient was compared with a target value based on European Committee on Antimicrobial Susceptibility Testing (EUCAST) data (29). Exposure for the general patient population of 110 mg · h/liter (11) is accepted as sufficient to treat infections with susceptible Candida species (29). A Candida species was considered susceptible if its MIC was not higher than the EUCAST clinical breakpoint. The free fraction of anidulafungin is assumed to be 1% (11). Therefore, the target value for Candida albicans [(110 mg · h/liter · 1%)/0.03 mg/liter] was 36.7. For Candida glabrata, the target value [(110 mg · h/liter · 1%)/0.06 mg/liter] was 18.3.
Statistics.
Based on a large variation in disease severity and the consequent expectation of a large variation in pharmacokinetics, a sample size of 18 patients is needed to detect a clinically relevant correlation of 60% with 80% power and significance level of α = 0.05 (two-sided). We planned to recruit 20 patients to cover a dropout rate of 10%.
The Mann-Whitney U test was used to assess whether the continuous data from two groups was significantly different. Correlations were determined with the Spearman correlation coefficient (rs) in SPSS version 20 (IBM, Armonk, NY).
Multiple linear regression analysis was performed using a backward elimination strategy, keeping variables with P values of <0.1 in the model. The variables that were included in the multiple linear regression analysis were gender (11), age (11), total body water (11), albumin (13), total bilirubin (30, 31), and the disease severity score with the lowest P value for the correlation.
RESULTS
Twenty patients were included; nine were female. Most patients were admitted after abdominal surgery with complications or severe abdominal infections. An overview of patient characteristics is shown in Table 1.
TABLE 1.
Patient characteristics
| Characteristic | Median value (IQRa) |
|---|---|
| Age (yr) | 71 (60–75) |
| Weight (kg) | 81 (72–102) |
| Height (cm) | 175 (165–181) |
| BMI (kg/m2) | 25.8 (23.4–36.4) |
| Total body water (liter) | 41 (35–45) |
| Underlying condition (no. of patients) | |
| Abdominal surgery | 12 |
| Pancreatitis | 3 |
| Infected prosthesis | 2 |
| Stomach perforation | 1 |
| Abdominal abscess | 1 |
| Pneumococcal sepsis | 1 |
| Diagnosis (no. of patients) | |
| Candidemia | 3 |
| Candida peritonitis | 13 |
| Candidemia and candida peritonitis | 4 |
IQR, interquartile range.
At day 28 after the start of treatment with anidulafungin, five of the patients were deceased. These patients died while on treatment with anidulafungin, at 4, 6, 7, 15, and 16 days after the start of treatment. All five patients died after withholding and withdrawing of therapy because of progressive multiple organ failure and lack of treatment options for the underlying disease. All patients suffered from infections when they died, but because no autopsies were performed, it remains uncertain whether they died because of infection or with infection and whether the Candida infection was still present.
Anidulafungin pharmacokinetics.
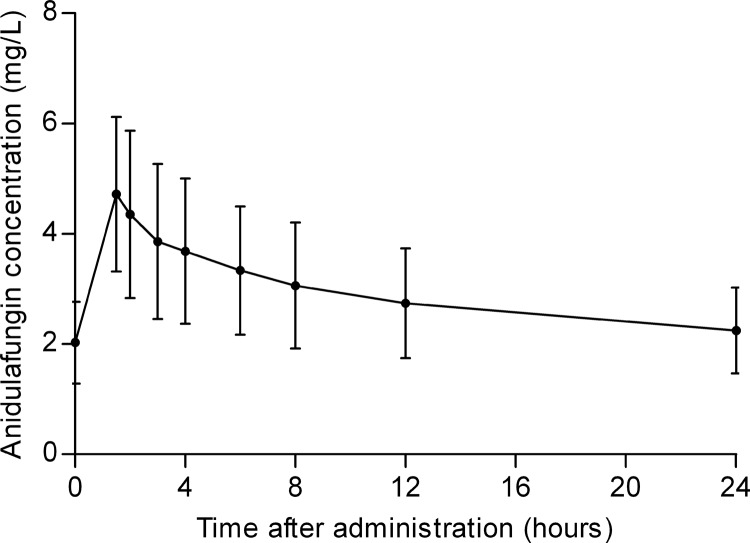
The mean concentration-time curve of anidulafungin with standard deviations (SD) is presented in Fig. 1. The mean (±SD) AUC0–24, maximum concentration of drug in plasma (Cmax), and minimum concentration of drug in plasma (Cmin) were 69.8 ± 24.1 mg · h/liter, 4.7 ± 1.4 mg/liter, and 2.2 ± 0.8 mg/liter, respectively. Both the anidulafungin Cmax and Cmin showed a significant correlation with the anidulafungin exposure (Cmax, rs = 0.854, P < 0.001; Cmin, rs = 0.884, P < 0.001). The mean (±SD) estimated clearance was 1.6 ± 0.6 liters/h.
FIG 1.
Mean anidulafungin concentration-time curve with standard deviations.
The anidulafungin trough concentrations ranged from 1.0 mg/liter to 4.7 mg/liter during treatment.
On the first day of treatment with anidulafungin, the mean (±SD) anidulafungin plasma concentration at the end of the infusion was 4.9 ± 1.4 mg/liter. Twelve hours after start of the infusion, the mean anidulafungin concentration was 2.3 ± 0.6 mg/liter. For logistical reasons, it was not possible to obtain samples from four patients on the first day of treatment.
Microbiology.
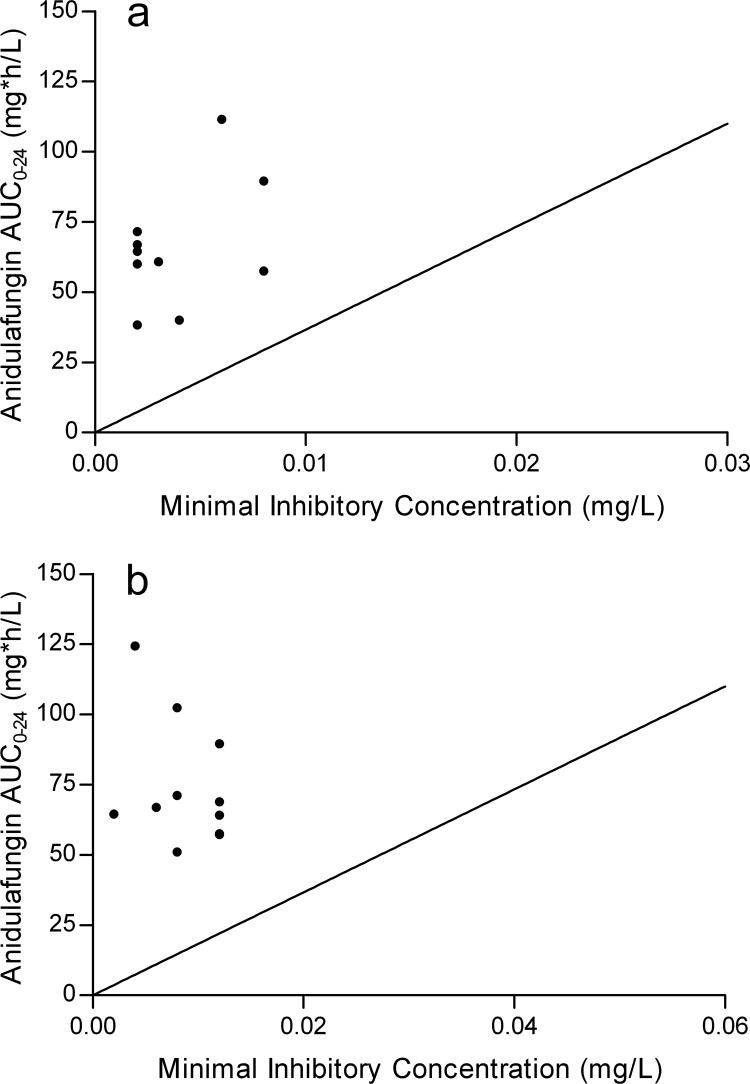
Blood cultures for Candida were positive in 7 patients, and for 17 patients, intra-abdominal fluid cultures were positive. In 13 patients, C. albicans was cultured, and in 11 patients, C. glabrata. No other Candida species were recovered. The MIC values for C. albicans ranged from <0.002 to 0.008 mg/liter, all below the EUCAST anidulafungin MIC breakpoint of 0.03 mg/liter. The MIC values for C. glabrata were also lower than the EUCAST MIC breakpoint of 0.06 mg/liter, ranging from 0.002 to 0.012 mg/liter. The distribution of the MICs can be seen in Fig. 2.
FIG 2.
AUC versus MIC for individual patients with C. albicans (a) or C. glabrata (b) infection. The line represents the ratio of the AUC of the general patient population and the EUCAST MIC breakpoint of the Candida species.
Pharmacokinetics/pharmacodynamics.
The mean (±SD) fAUC0–24/MIC ratio was 229 ± 105 for C. albicans and 118 ± 101 for C. glabrata and appeared to be above the target value for all patients based on EUCAST data. Figure 2 shows the AUC0-24 that was determined versus the MIC of the Candida species isolated.
Evaluation of possible variables of anidulafungin exposure.
Table 2 provides the median disease severity scores and data regarding a possible correlation with the anidulafungin exposure, reflected by the AUC0–24. None of the disease severity scores showed a significant correlation with the anidulafungin exposure.
TABLE 2.
Disease severity scores and correlation with anidulafungin exposure
| Score | Median (IQR) | Spearman correlation coefficient | P value |
|---|---|---|---|
| Apache II | 14 (11–15) | 0.078 | 0.743 |
| LODS | 5 (3–7) | 0.317 | 0.174 |
| MODS | 4 (3–8) | 0.374 | 0.105 |
| ODIN | 3 (2–4) | 0.150 | 0.527 |
| SAPS 2 | 41 (32–52) | 0.202 | 0.394 |
| SAPS 3 | 75 (63–78) | −0.094 | 0.695 |
| SOFA | 7 (4–9) | 0.153 | 0.520 |
The plasma protein concentrations were low in these patients. As expected, all 20 patients had albumin concentrations below the lower limit of normal (35 g/liter). The mean albumin concentration was 19 g/liter (range, 14 to 31 g/liter). The total protein concentration of most patients was also below the lower limit of normal (60 g/liter). Total protein concentrations ranged from 29 to 62 g/liter, with a mean of 46 g/liter. Neither albumin nor total protein concentration showed a significant correlation with the anidulafungin exposure (AUC0–24) (rs = 0.009, P = 0.677, and rs = 0.092, P = 0.700).
No significant correlation was observed between the anidulafungin exposure and the body weight of patients (rs = −0.282, P = 0.229). The correlation with total body water did not reach statistical significance (rs = −0.427, P = 0.061). A possible correlation between anidulafungin exposure and total bilirubin concentrations also could not be established (rs = 0.312, P = 0.181).
In the multiple linear regression analysis, total body water and bilirubin concentration showed a significant correlation with anidulafungin exposure (Table 3). The adjusted R square of the model is 0.345.
TABLE 3.
Multiple linear regression analysis for anidulafungin exposure
| Variable | β (95% CI)a | P value |
|---|---|---|
| Total body water | −2.566 (−4,192 to −0.941) | 0.004 |
| Total bilirubin | 0.232 (0.036 to 0.428) | 0.023 |
β, regression coefficient of the population; 95% CI, 95% confidence interval.
The deceased patients did not seem to have a different anidulafungin exposure than the surviving patients; the respective median exposures were 68.9 mg · h/liter (range, 64.2 to 89.5 mg · h/liter) and 60.8 mg · h/liter (range, 32.4 to 124.4 mg · h/liter).
DISCUSSION
We found low exposure to anidulafungin in our critically ill patients. Although the exposure was low, the MICs of the Candida species isolates were also low, and therefore, none of our patients received inadequate antifungal treatment. No correlation was observed between anidulafungin exposure and disease severity or plasma protein concentration in this group of critically ill patients.
An explanation for the lack of correlation could be that anidulafungin exposure is influenced slightly by several factors at the same time, making it difficult to correlate anidulafungin exposure with disease severity, plasma protein concentrations, or other contributing factors. Total body water, expected to be similar to the volume of distribution of anidulafungin (11), was also not significantly correlated to anidulafungin exposure. Based on studies with micafungin, which showed a reduced elimination clearance in the case of cholestatic hyperbilirubinemia (31) and a correlation of micafungin concentrations with total bilirubin (30), we explored a possible correlation between bilirubin concentrations and anidulafungin exposure. The expectation was that in patients with high bilirubin concentrations, the excretion of bilirubin and anidulafungin into bile would be decreased. No correlation could be established. As no correlation was observed between anidulafungin exposure and the individual contributing factors, a multiple linear regression analysis was performed. The multiple linear regression analysis provided a significant correlation between anidulafungin exposure and total body water and bilirubin concentrations. No significant correlations were observed between anidulafungin exposure and disease severity or plasma protein concentrations.
The group of patients is on the one hand a limitation and on the other hand a strength of this study. The limitation is that our group of patients was relatively homogeneous with respect to their critical illness and plasma protein concentrations. Possibly, this could be another explanation for the lack of correlation between anidulafungin exposure and disease severity or plasma protein concentrations. The strength of this group is that these are the critically ill patients that are in need of treatment with an echinocandin.
The low anidulafungin exposure in our group of patients compared to the exposure in the general patient population was expected based on previously published data (12), as anidulafungin clearance appeared to be approximately 30% higher in the more severely ill patients with invasive candidiasis than in patients with esophageal candidiasis. The anidulafungin exposure also appeared to be significantly lower (P = 0.045, Mann-Whitney U test) than was evidenced by earlier data from other intensive care patients, i.e, 69.8 ± 24.1 mg · h/liter versus 92.7 ± 38.0 mg · h/liter (14). From the data available for both groups, it appears that our patients were significantly older (P = 0.004), heavier (P = 0.001), and taller (P = 0.032) and had a higher total body water volume (P = 0.007). Further analysis is required to determine other factors causing the apparent difference.
The observed lower exposure appeared to be not clinically relevant for our patients. To assess the clinical relevance of the observed lower anidulafungin exposure, the fAUC0-24/MIC ratio was used because of the listed sample size and the complex pathology of critically ill patients. This could be advocated because the AUC/MIC ratio appeared to be a good predictor of anidulafungin efficacy (9, 10). We did not use the target values from these studies (9, 10) because the MICs used were measured with the CLSI method, whereas our MICs were determined with an Etest. MICs determined with an Etest are usually lower than those determined with the CLSI method (32) and were used in determining the EUCAST breakpoints; therefore, we used target values based on EUCAST data. The observed lower exposure combined with the low MICs of the Candida species that were isolated resulted in favorable fAUC0-24/MIC ratios in our patients.
Further research is necessary on factors that contribute to the variability of anidulafungin exposure, because total body water and bilirubin only partly explain the variability in anidulafungin exposure. This is necessary in order to anticipate a lower exposure in patients before starting treatment with anidulafungin, as the MIC of the pathogen is usually not available at that moment. Besides, validation of target values for the fAUC0-24/MIC ratio in clinical practice is needed. Investigation of limited sampling strategies for anidulafungin can be useful for future research and for specific clinical situations.
No correlation could be established between anidulafungin exposure and disease severity or plasma protein concentrations in this group of critically ill patients. In this population, we observed a lower anidulafungin exposure than in the general patient population. In patients infected with a susceptible Candida albicans or glabrata strain with a MIC well below the breakpoint, no problems are to be expected in the case of a lower exposure. However, in patients with less-susceptible Candida albicans or glabrata strains, a lower exposure can be a problem. If the MIC is high or unknown, we recommend considering determining the anidulafungin exposure to ensure that the exposure is adequate.
ACKNOWLEDGMENTS
We thank the staff of the Clinical and Hospital Pharmacy, Medical Microbiology, and Critical Care Departments in our hospital for their contribution to this study.
This investigator-initiated study was financially supported by Pfizer.
Footnotes
Published ahead of print 28 October 2013
REFERENCES
- 1.Hassan I, Powell G, Sidhu M, Hart WM, Denning DW. 2009. Excess mortality, length of stay and cost attributable to candidaemia. J. Infect. 59:360–365. 10.1016/j.jinf.2009.08.020 [DOI] [PubMed] [Google Scholar]
- 2.Almirante B, Rodriguez D, Park BJ, Cuenca-Estrella M, Planes AM, Almela M, Mensa J, Sanchez F, Ayats J, Gimenez M, Saballs P, Fridkin SK, Morgan J, Rodriguez-Tudela JL, Warnock DW, Pahissa A, Barcelona Candidemia Project Study Group 2005. Epidemiology and predictors of mortality in cases of Candida bloodstream infection: results from population-based surveillance, Barcelona, Spain, from 2002 to 2003. J. Clin. Microbiol. 43:1829–1835. 10.1128/JCM.43.4.1829-1835.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ibrahim EH, Sherman G, Ward S, Fraser VJ, Kollef MH. 2000. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest 118:146–155. 10.1378/chest.118.1.146 [DOI] [PubMed] [Google Scholar]
- 4.Tumbarello M, Posteraro B, Trecarichi EM, Fiori B, Rossi M, Porta R, de Gaetano Donati K, La Sorda M, Spanu T, Fadda G, Cauda R, Sanguinetti M. 2007. Biofilm production by Candida species and inadequate antifungal therapy as predictors of mortality for patients with candidemia. J. Clin. Microbiol. 45:1843–1850. 10.1128/JCM.00131-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garey KW, Rege M, Pai MP, Mingo DE, Suda KJ, Turpin RS, Bearden DT. 2006. Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: a multi-institutional study. Clin. Infect. Dis. 43:25–31. 10.1086/504810 [DOI] [PubMed] [Google Scholar]
- 6.Baddley JW, Patel M, Bhavnani SM, Moser SA, Andes DR. 2008. Association of fluconazole pharmacodynamics with mortality in patients with candidemia. Antimicrob. Agents Chemother. 52:3022–3028. 10.1128/AAC.00116-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pappas PG, Kauffman CA, Andes D, Benjamin DK, Jr, Calandra TF, Edwards JE, Jr, Filler SG, Fisher JF, Kullberg BJ, Ostrosky-Zeichner L, Reboli AC, Rex JH, Walsh TJ, Sobel JD, Infectious Diseases Society of America 2009. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 48:503–535. 10.1086/596757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kett DH, Shorr AF, Reboli AC, Reisman AL, Biswas P, Schlamm HT. 2011. Anidulafungin compared with fluconazole in severely ill patients with candidemia and other forms of invasive candidiasis: support for the 2009 IDSA treatment guidelines for candidiasis. Crit. Care 15:R253. 10.1186/cc10514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andes D, Diekema DJ, Pfaller MA, Bohrmuller J, Marchillo K, Lepak A. 2010. In vivo comparison of the pharmacodynamic targets for echinocandin drugs against Candida species. Antimicrob. Agents Chemother. 54:2497–2506. 10.1128/AAC.01584-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andes D, Diekema DJ, Pfaller MA, Prince RA, Marchillo K, Ashbeck J, Hou J. 2008. In vivo pharmacodynamic characterization of anidulafungin in a neutropenic murine candidiasis model. Antimicrob. Agents Chemother. 52:539–550. 10.1128/AAC.01061-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pfizer 2012. Ecalta: summary of product characteristics. Pfizer, New York, NY: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000788/WC500020673.pdf [Google Scholar]
- 12.Dowell JA, Knebel W, Ludden T, Stogniew M, Krause D, Henkel T. 2004. Population pharmacokinetic analysis of anidulafungin, an echinocandin antifungal. J. Clin. Pharmacol. 44:590–598. 10.1177/0091270004265644 [DOI] [PubMed] [Google Scholar]
- 13.Nguyen TH, Hoppe-Tichy T, Geiss HK, Rastall AC, Swoboda S, Schmidt J, Weigand MA. 2007. Factors influencing caspofungin plasma concentrations in patients of a surgical intensive care unit. J. Antimicrob. Chemother. 60:100–106. 10.1093/jac/dkm125 [DOI] [PubMed] [Google Scholar]
- 14.Liu P, Ruhnke M, Meersseman W, Paiva JA, Kantecki M, Damle B. 2013. Pharmacokinetics of anidulafungin in critically ill patients with candidaemia/invasive candidiasis. Antimicrob. Agents Chemother. 57:1672–1676. 10.1128/AAC.02139-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watson PE, Watson ID, Batt RD. 1980. Total body water volumes for adult males and females estimated from simple anthropometric measurements. Am. J. Clin. Nutr. 33:27–39 [DOI] [PubMed] [Google Scholar]
- 16.Tod M, Padoin C, Minozzi C, Cougnard J, Petitjean O. 1996. Population pharmacokinetic study of isepamicin with intensive care unit patients. Antimicrob. Agents Chemother. 40:983–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peeters MY, Bras LJ, DeJongh J, Wesselink RM, Aarts LP, Danhof M, Knibbe CA. 2008. Disease severity is a major determinant for the pharmacodynamics of propofol in critically ill patients. Clin. Pharmacol. Ther. 83:443–451. 10.1038/sj.clpt.6100309 [DOI] [PubMed] [Google Scholar]
- 18.van Zanten AR, Polderman KH, van Geijlswijk IM, van der Meer GY, Schouten MA, Girbes AR. 2008. Ciprofloxacin pharmacokinetics in critically ill patients: a prospective cohort study. J. Crit. Care 23:422–430. 10.1016/j.jcrc.2007.11.011 [DOI] [PubMed] [Google Scholar]
- 19.Beloeil H, Mazoit JX, Benhamou D, Duranteau J. 2005. Norepinephrine kinetics and dynamics in septic shock and trauma patients. Br. J. Anaesth. 95:782–788. 10.1093/bja/aei259 [DOI] [PubMed] [Google Scholar]
- 20.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. 1985. APACHE II: a severity of disease classification system. Crit. Care Med. 13:818–829 [PubMed] [Google Scholar]
- 21.Le Gall JR, Klar J, Lemeshow S, Saulnier F, Alberti C, Artigas A, Teres D. 1996. The logistic organ dysfunction system. A new way to assess organ dysfunction in the intensive care unit. ICU Scoring Group. JAMA 276:802–810 [DOI] [PubMed] [Google Scholar]
- 22.Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ. 1995. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit. Care Med. 23:1638–1652 [DOI] [PubMed] [Google Scholar]
- 23.Fagon JY, Chastre J, Novara A, Medioni P, Gibert C. 1993. Characterization of intensive care unit patients using a model based on the presence or absence of organ dysfunctions and/or infection: the ODIN model. Intensive Care Med. 19:137–144 [DOI] [PubMed] [Google Scholar]
- 24.Le Gall JR, Lemeshow S, Saulnier F. 1993. A new simplified acute physiology score (SAPS II) based on a European/North American multicenter study. JAMA 270:2957–2963 [DOI] [PubMed] [Google Scholar]
- 25.Moreno RP, Metnitz PG, Almeida E, Jordan B, Bauer P, Campos RA, Iapichino G, Edbrooke D, Capuzzo M, Le Gall JR, SAPS 3 Investigators 2005. SAPS 3—from evaluation of the patient to evaluation of the intensive care unit. Part 2. Development of a prognostic model for hospital mortality at ICU admission. Intensive Care Med. 31:1345–1355. 10.1007/s00134-005-2763-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, Reinhart CK, Suter PM, Thijs LG. 1996. The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 22:707–710 [DOI] [PubMed] [Google Scholar]
- 27.van Wanrooy MJ, Santoe RN, van der Elst KC, Wilmer CM, van Hateren K, Wessels AM, Greijdanus B, Alffenaar JW, Uges DR. 30 September 2013. Simultaneous quantification of anidulafungin and caspofungin in plasma by an accurate and simple liquid chromatography tandem mass-spectrometric method. Ther. Drug Monit. 10.1097/FTD.0b013e31829591a7 [DOI] [PubMed] [Google Scholar]
- 28.Proost JH, Meijer DK. 1992. MW/Pharm, an integrated software package for drug dosage regimen calculation and therapeutic drug monitoring. Comput. Biol. Med. 22:155–163 [DOI] [PubMed] [Google Scholar]
- 29.Arendrup MC, Rodriguez-Tudela JL, Lass-Florl C, Cuenca-Estrella M, Donnelly JP, Hope W, European Committee on Antimicrobial Susceptibility Testing—Subcommittee on Antifungal Susceptibility Testing (EUCAST-AFST) 2011. EUCAST technical note on anidulafungin. Clin. Microbiol. Infect. 17:E18–E20. 10.1111/j.1469-0691.2011.03647.x [DOI] [PubMed] [Google Scholar]
- 30.Muraki Y, Iwamoto T, Kagawa Y, Sakurai H, Usui M, Isaji S, Uemoto S, Okuda M. 2009. The impact of total bilirubin on plasma micafungin levels in living-donor liver transplantation recipients with severe liver dysfunction. Biol. Pharm. Bull. 32:750–754. 10.1248/bpb.32.750 [DOI] [PubMed] [Google Scholar]
- 31.Konishi H, Fukushima K, Sudo M, Sumi M, Minouchi T, Iga I, Shibata N, Takada K, Yamaji A. 2010. Reduced elimination clearance of micafungin in rats with cholestatic hyperbilirubinemia. Fundam. Clin. Pharmacol. 24:457–462. 10.1111/j.1472-8206.2009.00785.x [DOI] [PubMed] [Google Scholar]
- 32.Pfaller MA, Castanheira M, Diekema DJ, Messer SA, Moet GJ, Jones RN. 2010. Comparison of European Committee on Antimicrobial Susceptibility Testing (EUCAST) and Etest methods with the CLSI broth microdilution method for echinocandin susceptibility testing of Candida species. J. Clin. Microbiol. 48:1592–1599. 10.1128/JCM.02445-09 [DOI] [PMC free article] [PubMed] [Google Scholar]