Abstract
Pharmacodynamic profiling data of carbapenems for Acinetobacter spp. are sparse. This study aimed to determine the pharmacodynamic targets of carbapenems for Acinetobacter baumannii based on a range of percentages of the dosing interval in which free drug concentrations remained above the MIC (fT>MIC) in the neutropenic murine thigh infection model. fT>MIC values of 23.7%, 32.8%, and 47.5% resulted in stasis, 1-log reductions, and 2-log reductions in bacterial density after 24 h, respectively. The pharmacodynamic targets of carbapenems for A. baumannii demonstrated in vivo are similar to those of other Gram-negative bacteria.
TEXT
Acinetobacter baumannii, a Gram-negative bacillus with an impressive ability to acquire antimicrobial resistance, has emerged as an important and challenging pathogen in the current health care setting (1, 2). Carbapenems, the most potent of the beta-lactams, possess a broad spectrum of bactericidal activity against Gram-positive and Gram-negative bacteria, a characteristic that is desirable for empirical coverage in health care-associated infections (3). Previous studies have suggested that the pharmacodynamic targets for bacteriostatic and maximal bactericidal activity of carbapenems occur with an fT>MIC of ∼20 and ∼40%, respectively (4). While extensive work to define these targets has been done in Enterobacteriaceae and Pseudomonas aeruginosa (5–7), no data exist characterizing the pharmacodynamic targets for A. baumannii. Moreover, the breakpoints for carbapenems against Acinetobacter spp. were reassessed at recent Clinical and Laboratory Standards Institute (CLSI) workgroup meetings. However, requests for clinical or animal model data necessary to perform the evaluation rendered no response.
Despite the lack of robust data on their pharmacodynamics, the carbapenems remain an important therapeutic option for serious infections caused by A. baumannii (8). In the current study, we tested the efficacy of three carbapenems (doripenem, meropenem, and imipenem) in a neutropenic murine thigh infection model against A. baumannii isolates to establish a pharmacodynamic target for their antibacterial activity.
Commercially available doripenem (Ortho-McNeil-Janssen Pharmaceuticals Inc., Raritan, NJ), meropenem (Hospira Inc., Lake Forest, IL), and imipenem-cilastatin (Merck & Co., Inc., Whitehouse Station, NJ) were used for all in vivo analyses. Vials were reconstituted and diluted to the appropriate concentrations according to the manufacturer's instruction. Dosing solutions were stored refrigerated until the time of use and were discarded after 24 h.
Fourteen clinical A. baumannii isolates were used for the in vivo studies. Doripenem, meropenem, and imipenem MICs were determined in triplicate by broth microdilution in accordance with CLSI guidelines (9). Isolates were maintained in double-strength skim milk (BD Biosciences, Sparks, MD) at −80°C. Each isolate was subcultured twice on Trypticase soy agar with 5% sheep blood (BD Biosciences) prior to use.
The protocol was reviewed and approved by the Hartford Hospital Institutional Animal Care and Use Committee. The well-described murine neutropenic thigh infection model was used to determine efficacy (10). Pathogen-free, female ICR mice weighing approximately 20 to 22 g were acquired from Harlan Sprague Dawley, Inc. (Indianapolis, IN), and utilized throughout these experiments. Animals were provided food and water ad libitum. Mice were rendered neutropenic with intraperitoneal injections of 100 and 150 mg cyclophosphamide (Cytoxan; Bristol-Myers Squibb, Princeton, NJ)/kg of body weight, given 1 and 4 days prior to inoculation, respectively. Three days prior to inoculation, mice were given a single 5-mg/kg intraperitoneal injection of uranyl nitrate. This produces a predictable degree of renal impairment to slow drug clearance (11). Two hours prior to the initiation of antimicrobial therapy, each thigh was inoculated intramuscularly with a 0.1-ml solution containing approximately 107 CFU of test isolate.
Beginning 2 h after inoculation, groups of three mice were administered humanized dosing regimens of either 500 mg doripenem intravenously (i.v.) every 8 h as a 1-h or 4-h infusion, 1 g meropenem i.v. every 8 h as a 1-h infusion, or standard imipenem dosing of 55 mg/kg every 8 h using pharmacokinetic data derived from ICR mice in the neutropenic thigh model, as previously developed and validated by our group (6, 7, 12). All therapies were administered over a 24-h period. Doses were administered as 0.2-ml subcutaneous injections. Control animals (three per group) were administered normal saline at the same volume, route, and frequency as the treatment regimen with the most doses per interval. Groups of three untreated control mice were euthanized by CO2 exposure, followed by cervical dislocation just prior to the initiation of therapy (0 h). All other treatment and control mice were sacrificed 24 h after the initiation of therapy. Mice that did not survive to 24 h were harvested at the time of expiration. Following sacrifice, thighs were removed and homogenized individually in 5 ml of normal saline. Serial dilutions of thigh homogenate were plated onto Trypticase soy agar with 5% sheep blood for determination of bacterial density. Efficacy, defined as the change in bacterial density, was calculated as the change in log10 CFU obtained for carbapenem-treated mice after 24 h. A sigmoid dose-effect model, derived from the Hill equation, was used to calculate fT>MIC values of static, 1-log, and 2-log kill for the composite data set.
The phenotypic profiles and respective fT>MIC values for the 14 Acinetobacter baumannii isolates evaluated are listed in Table 1. The carbapenem MICs for the isolates ranged from 0.06 to 16 μg/ml, 0.13 to 32 μg/ml, and 0.13 to 32 μg/ml against doripenem, meropenem, and imipenem, respectively. A total of 14 control and 38 active treatment groups were evaluated. The mean (±standard deviation) bacterial density for 0-h control mice at the start of dosing was 5.92 ± 0.35 log10 CFU per thigh, which increased to 9.21 ± 0.70 log10 CFU after 24 h. The mice that received treatment all survived to 24 h, while six control mice failed to survive to 24 h. Bacterial densities were similar to those in mice that survived to 24 h and were included in the data analysis. A robust relationship between antibacterial effect and fT>MIC (R2 = 0.83) was observed against A. baumannii (Fig. 1). fT>MIC values of ≤20% showed minimal activity, with increases in bacterial density for 9 of 10 treatments. An fT>MIC of >38% produced reductions in thigh bacterial burden of ≥1 log10 CFU in 22 of 23 treatments. Target fT>MIC values needed to achieve a static effect and 1- and 2-log10 CFU reductions for A. baumannii are 23.67%, 32.82%, and 47.53%, respectively (R2 = 0.83). While the current study was designed to be analyzed as a composite data set, when individual agents were evaluated, similar 1-log kill targets (31 to 42% fT>MIC) and maximum killing (∼4-log reductions) were observed.
TABLE 1.
Phenotypic susceptibility profiles and corresponding fT>MIC of Acinetobacter baumannii isolates utilized in the efficacy studies of carbapenemsa
| A. baumannii isolate no. | MIC (mg/liter) |
% fT>MIC |
|||||
|---|---|---|---|---|---|---|---|
| DOR | MEM | IPM | DOR 1 h | DOR 4 h | MEM | IPM | |
| 12-13 | 0.06 | 0.13 | 0.13 | 100 | ND | 85 | 70 |
| 1-30 | 0.5 | 0.5 | 0.5 | 75 | ND | 75 | ND |
| 1-50 | 0.5 | 2 | 0.5 | 75 | ND | 58 | 55 |
| 14-12 | 1 | 2 | 1 | 55 | 82.5 | ND | 50 |
| 2-48 | 1 | 2 | 1 | 55 | ND | ND | 50 |
| 12-26 | 2 | 2 | 2 | 42.5 | 70 | 58 | 42.5 |
| 2-73 | 2 | 4 | 0.5 | 42.5 | 70 | 45 | 55 |
| 12-9 | 4 | 8 | 4 | 30 | 52.5 | ND | 37.5 |
| 12-6 | 8 | 8 | 8 | 20 | ND | 30 | 30 |
| 12-17 | 8 | 8 | 8 | 20 | ND | ND | 30 |
| 1-11 | 8 | 16 | 4 | 20 | ND | 18 | ND |
| 14-6 | 8 | 16 | 16 | 20 | ND | 18 | 25 |
| 1-51 | 16 | 16 | 2 | 10 | ND | 18 | ND |
| 4-12 | 16 | 32 | 32 | 10 | ND | ND | 17.5 |
“ND” represents regimens that were not tested against that particular isolate. DOR, doripenem; IPM, imipenem-cilastatin; MEM, meropenem.
FIG 1.
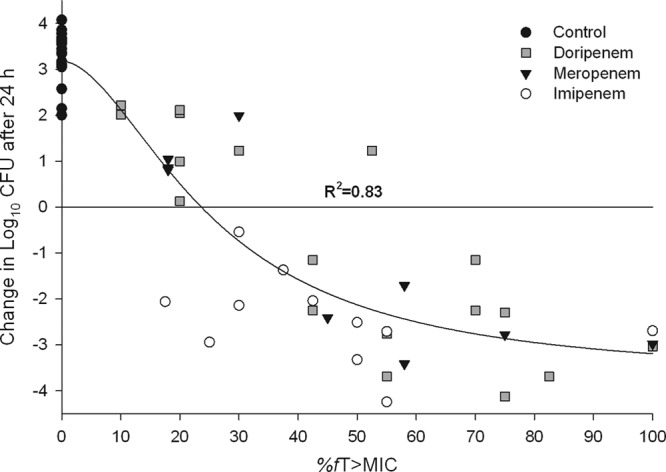
Composite assessment of carbapenem antibacterial effect versus fT>MIC for 14 A. baumannii isolates in the neutropenic thigh infection model. Symbols represent mean data.
With the unceasing progression of drug resistance, particularly among Gram-negative bacteria, carbapenems are becoming increasingly utilized to treat severely ill patients with nosocomial infections. However, there is a paucity of published data describing the pharmacodynamic parameters required for bacteriostatic and bactericidal effects of carbapenems for Acinetobacter spp. to provide guidance for optimal dosing regimen design. In the current study, carbapenems (doripenem, meropenem, and imipenem) were found to require fT>MIC values for stasis, 1-log reductions, and 2-log reductions similar to those observed with carbapenems in previous animal infection models against other Gram-negative pathogens (3–7, 13). These data identify fT>MIC targets of carbapenems for bacteriostatic (24%) and bactericidal (33 to 48%) activity against Acinetobacter spp. and provide guidance for breakpoint setting authorities, such as CLSI and EUCAST.
ACKNOWLEDGMENTS
We thank Mary Anne Banevicius, Henry Christensen, Jennifer Hull, Jami Jain, Lucinda Lamb, Debora Santini, Wonhee So, Christina Sutherland, and Pamela Tessier for their assistance with the animal experimentation and in vitro testing.
This study was supported internally by the Center for Anti-Infective Research and Development (Hartford, CT).
The authors report no financial disclosure relevant to this study.
Footnotes
Published ahead of print 28 October 2013
REFERENCES
- 1.Maragakis LL, Perl TM. 2008. Acinetobacter baumannii: epidemiology, antimicrobial resistance, and treatment options. Clin. Infect. Dis. 46:1254–1263. 10.1086/529198 [DOI] [PubMed] [Google Scholar]
- 2.Peleg AY, Seifert H, Paterson DL. 2008. Acinetobacter baumannii: emergence of a successful pathogen. Clin. Microbiol. Rev. 21:538–582. 10.1128/CMR.00058-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhanel GG, Wiebe R, Dilay L, Thomson K, Rubinstein E, Hoban DJ, Noreddin AM, Karlowsky JA. 2007. Comparative review of the carbapenems. Drugs 67:1027–1052. 10.2165/00003495-200767070-00006 [DOI] [PubMed] [Google Scholar]
- 4.Craig WA, Ebert S, Watanabe Y. 1993. Differences in time above the MIC (T>MIC) required for efficacy of beta-lactams in animal infection models, abstr 86. Prog. Abstr. 35th Intersci. Conf. Antimicrob. Agents Chemother., San Francisco, CA American Society for Microbiology, Washington, DC [Google Scholar]
- 5.Drusano GL. 2003. Prevention of resistance: a goal for dose selection of antimicrobial agents. Clin. Infect. Dis. 36(Suppl 1):S42–S50. 10.1086/344653 [DOI] [PubMed] [Google Scholar]
- 6.DeRyke CA, Banevicius MA, Fan HW, Nicolau DP. 2007. Bactericidal activities of meropenem and ertapenem against extended-spectrum-β-lactamase-producing Escherichia coli and Klebsiella pneumoniae in a neutropenic mouse thigh model. Antimicrob. Agents Chemother. 51:1481–1486. 10.1128/AAC.00752-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ong CT, Tessier PR, Li C, Nightingale CH, Nicolau DP. 2007. Comparative in vivo efficacy of meropenem, imipenem, and cefepime against Pseudomonas aeruginosa expressing MexA-MexB-OprM efflux pumps. Diagn. Microbiol. Infect. Dis. 57:153–161. 10.1016/j.diagmicrobio.2006.06.014 [DOI] [PubMed] [Google Scholar]
- 8.Fishbain J, Peleg AY. 2010. Treatment of Acinetobacter infections. Clin. Infect. Dis. 51:79–84. 10.1086/653120 [DOI] [PubMed] [Google Scholar]
- 9.Clinical and Laboratory Standards Institute 2013. Methods for dilution antimicrobial susceptibility test for bacteria that grow aerobically; approved standard—. 9th ed. CLSI publication M07-A9. Clinical Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 10.Crandon JL, Nicolau DP. Human simulated studies of aztreonam and aztreonam-avibactam to evaluate activity against challenging Gram-negative organisms, including metallo-β-lactamase producers. Antimicrob. Agents Chemother. 57:3299–3306. 10.1128/AAC.01989-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andes D, Craig WA. 1998. In vivo activities of amoxicillin and amoxicillin-clavulanate against Streptococcus pneumoniae: application to breakpoint determinations. Antimicrob. Agents Chemother. 42:2375–2379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim A, Banevicius MA, Nicolau DP. 2008. In vivo pharmacodynamic profiling of doripenem against Pseudomonas aeruginosa by simulating human exposures. Antimicrob. Agents Chemother. 52:2497–2502. 10.1128/AAC.01252-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Craig WA. 1998. Pharmacokinetics and pharmacodynamics of antibiotics in mice and men. Clin. Infect. Dis. 26:1–12. 10.1086/516284 [DOI] [PubMed] [Google Scholar]


