Abstract
Data on chloroquine (CQ)-resistant Plasmodium vivax in Latin America is limited, even with the current research efforts to sustain an efficient malaria control program in all these countries where P. vivax is endemic and where malaria still is a major public health issue. This study estimated in vivo CQ resistance in patients with uncomplicated P. vivax malaria, with use of CQ and primaquine simultaneously, in the Brazilian Amazon. Of a total of 135 enrolled subjects who accomplished the 28-day follow-up, parasitological failure was observed in 7 (5.2%) patients, in whom plasma CQ and desethylchloroquine (DCQ) concentrations were above 100 ng/dl. Univariate analysis showed that previous exposure to malaria and a higher initial mean parasitemia were associated with resistance but not with age or gender. In the multivariate analysis, only high initial parasitemia remained significant. Hemoglobin levels were similar at the beginning of the follow-up and were not associated with parasitemia. However, at day 3 and day 7, hemoglobin levels were significantly lower in patients presenting CQ resistance. The P. vivax dhfr (pvdhfr), pvmrp1, pvmdr1, and pvdhps gene mutations were not related to resistance in this small sample. P. vivax CQ resistance is already a problem in the Brazilian Amazon, which could be to some extent associated with the simultaneous report of anemia triggered by this parasite, a common complication of the disease in most of the areas of endemicity.
INTRODUCTION
Plasmodium vivax is the most geographically spread malaria-related species (1) and was responsible in 2010 for 363,948 cases of malaria in the Americas (231,618 cases were reported in Brazil) (2). Chloroquine (CQ) has been the well-tolerated therapy of choice for the treatment of acute vivax malaria since 1946 (3). The drug clears fever and parasitemia within 72 h of the first dose and is rapidly absorbed and slowly eliminated, principally as the parent drug and as the metabolite desethylchloroquine (DCQ) in a proportion of roughly 3:1 (4). The plasma half-life is about 50 h, and therapeutic levels against vivax malaria persist in blood until days 21 to 35 after the start of treatment (5). CQ-resistant P. vivax favors recurrence due to recrudescence, which means the reappearance of parasitemia from asexual blood-stage parasites following blood schizonticidal therapy (6). Ideally the confirmation of in vivo CQ resistance (CR) requires the determination of the levels of the drug and its major active metabolite in the blood.
P. vivax CR was first described in 1989, when Australians expatriated from Papua New Guinea failed routine treatment (7). Since that pioneer report, P. vivax CR has been demonstrated especially in East Asia (Indonesia, Malaysia, Myanmar, Thailand, Vietnam, and Philippines) (6). In South America, there is already evidence of the phenomenon, but not many data are available.
After the first evidence of failure of combined CQ and primaquine (PQ) therapy for P. vivax malaria acquired by Canadian travelers in Guyana (8), some studies carried out in different regions did not detected recurrent parasitemia within 28 days (9–11) or 30 days (12). In Brazil, the first clinical evidence was reported in 2000 in Manaus (13). In Colombia, P. vivax CR was described in three cases among 27 subjects (14), and in Peru four cases were confirmed among 177 subjects (15). In 2007, the proper 28-day follow-up of 109 patients with P. vivax prescribed only CQ (PQ prescription was postponed to day 28) led to the confirmation of 10.1% resistance after plasmatic CQ dosage (16).
PQ is routinely used as an hypnozoiticidal drug despite having schizonticidal activity against P. vivax by itself (17). There is some in vitro evidence of synergy between primaquine and CQ against P. falciparum schizonts (18). However, there is no evidence of synergy between these two drugs against P. vivax asexual blood stages. Actually, the available evidence shows that treatment efficacy was not significantly different at the 28-day follow-up between the CQ monotherapy group and the group receiving CQ with PQ for 14 days in patients with uncomplicated vivax malaria (19). However, these data refer only to strains from Asia and cannot be easily extrapolated to Latin America.
Curiously, the same areas reporting P. vivax CR also have identified severe disease attributed to this species (20). The rationale of a more prolonged parasitemia due to drug resistance explains especially severe anemia; however, to date no evidence of individual patients well characterized for P. vivax CR evolving with anemia exists, because in most of these areas, CQ is no longer used.
In this study, we have estimated in vivo CQ resistance in patients with uncomplicated P. vivax from the western Brazilian Amazon with use of CQ (standard dose of 25 mg/kg over the first 3 days) and PQ (0.5 mg/kg/day over the first 7 days), as well as the hemoglobin level dynamics over the follow-up period in both resistant and sensitive groups.
MATERIALS AND METHODS
Study site and patients.
The study was performed from December 2007 to July 2008 at the Fundação de Medicina Tropical Dr. Heitor Vieira Dourado (FMT-HVD), which reports 20% of all the malaria cases in Manaus (03°06′S, 60°01′W). Patients living in the urban or periurban areas of this city with uncomplicated P. vivax malaria confirmed by a thick blood smear (TBS) and PCR a posteriori (21) were randomly selected in the outpatient clinics, and epidemiological and clinical history was fully obtained. Parasite densities were estimated by experienced microscopists by counting the number of parasites in 100 leukocytes in high-magnification fields and using the individual number of leukocytes/mm3 from the full blood count. The study included patients of both sexes, aged 12 to 60 years, presenting a blood parasite density of 250 to 100,000 parasites/ml and an axillary temperature of ≥37.5°C or history of fever in the last 48 h. Exclusion criteria were the use of antimalarials in the previous 60 days, refusal to be followed up for 28 days, and any clinical complication.
Treatment.
Patients received daily supervised treatment with 25 mg/kg of CQ phosphate (Farmanguinhos) over a 3-day period (10 mg/kg on day 0 and 7.5 mg/kg on days 1 and 2) associated with PQ (Farmanguinhos) over a 7-day period at the dosage of 0.5 mg/kg per day. The same batches of drugs were used in the study. Patients who vomited the first dose within 30 min after drug ingestion under observation were retreated with a similar dose.
Patient follow-up and resistance definition.
Participants were evaluated on days 0, 1, 2, 3, 7, 14, and 28 (active surveillance) and, if they felt ill, at any time during the follow-up period (passive surveillance). A full blood count was performed only on day 0, and in every evaluation, determination of hemoglobin in venous blood (using a portable HemoCue photometer [Anglholm, Sweden]), TBS, and PCR were performed. CQ and DCQ plasma levels were determined only in cases of parasitological failure (22). Three aliquots of 100 μl of whole blood from day-of-recurrence (DR) samples were spotted onto filter paper for later analysis by high-performance liquid chromatography (HPLC) to determine the levels of CQ and DCQ as previously described (23, 24). Resistance was defined as peripheral parasitemia in the presence of a CQ and DCQ blood level sum exceeding the minimal effective concentration of 100 ng/ml. Resistant cases were further treated with oral arthemeter/lumefantrine for 3 days.
Molecular characterization.
Samples from all patients with P. vivax CR and a from a subsample (the same number selected randomly) of patients without P. vivax CR were subjected to molecular characterization of a few candidate genes, mostly nonexplored in the literature, which could be putative markers of CQ resistance. Extraction of whole DNA was carried out using a QIAamp DNA Minikit (Qiagen, Germany) according to the manufacturer's protocol. The PCR primers and different reaction conditions used to amplify P. vivax dhfr (pvdhfr), pvmrp1, pvmdr1, and pvdhps gene sequences were as previously described (Table 1). Briefly, after initial denaturation at 94°C for 2 min, the samples were subjected to 35 cycles (94°C for 1 min more, 58°C for 30 s, and 72°C for 1 min), with a final extension at 72°C for 10 min. For each fragment, PCR products were visualized by 1% agarose gel electrophoresis and staining with ethidium bromide to confirm a single band. The DNA concentration was measured with a NanoDrop 2000 instrument (Thermo Scientific). Sequencing reactions were carried out using an ABI 3130xl Genetic Analyzer (Applied Biosystems, USA) as specified by the manufacturer's protocol.
TABLE 1.
Oligonucleotide primers used for PCR amplification and DNA sequencing of P. vivax genes
| Gene | Chromosome | Primer | Sequence (5′ → 3′) | bp | Mutations sought | Reference(s) |
|---|---|---|---|---|---|---|
| pvdhfr | 5 | PV1F | CAGTGAAGGGACAAAGAATGAACC | 560 | S58R/S117N/I173L | 42, 43 |
| PV1R | ACTCGGGGAAGAAGACGTCAC | |||||
| pvmrp1 | 2 | PV5F | CATATCGGGAAAAAGCGTAATTAACG | 523 | L1282I/Y1393D/G1419A/V1478I/H1586Y | 44 |
| PV5R | CTTCGATTGGTCTATGGCTGGTG | |||||
| PV6F | TCGAGAACGTATTCGTCAGTTATAAG | 497 | ||||
| PV6R | GTTGCTCGAAAGGTTAGCCTTTC | |||||
| pvmdr1 | 10 | PV7F | GCCATGTTCATTTCTGAGACGCTG | 337 | M908L/L958M | 36, 45 |
| PV7R | TCGCTCTGATGGCAAACACTC | |||||
| pvdhps | 14 | PV8F | TTTTAAAGTACATTGAGCAAATCGTG | 200 | S382C/A383G | 42, 43 |
| PV8R | CTGATCACTTGTGTGGTTTATGTG | |||||
| PV9F | GCGGTTTATTTGTCGATCCTGTG | 244 | ||||
| PV9R | TTTTTCCTGGCATCACTTGCTG |
Statistical analysis.
The estimation of possible factors associated with P. vivax CR was assessed in both univariate and multivariate (logistic regression) analyses. The interaction term between resistance status and day of follow-up was assessed by random-effects linear regression. Mean parasitemia levels throughout the follow-up were compared by using the Mann-Whitney U test. Fisher's test was employed to evaluate the association between point mutations and CQ resistance. Correlation between hemoglobin and parasitemia on day 0 was evaluated through the Spearman test. A P value of <0.05 was considered significant for all the analyses. Analysis was performed using SPSS software for Windows (version 16; SPSS, Inc., Chicago, IL).
Ethics.
This study was approved by the ethics committee of the FMT-HVD, and informed consent was obtained from all patients.
RESULTS
Of a total of 154 patients included, 135 fulfilled the follow-up. Thirteen subjects voluntarily withdrew from the 28-day follow-up, and six were excluded because they were diagnosed with mixed P. vivax-P. falciparum malaria on day 0 (confirmed by PCR performed a posteriori). Demographic and clinical characteristics of the enrolled patients are presented in Table 2.
TABLE 2.
Demographic and clinical features of the study participants on admittance and before treatment
| Parameter | Value |
|---|---|
| Sample size | 135 |
| Mean age, yr (SD) | 35.9 (12.5) |
| No. (%) male | 102 (75.5) |
| Wt, kg (SD) | 72.1 (14.1) |
| Mean body temp, °C (SD) | 36.7 (1.1) |
| No. (%) with axillary temp > 37.5°C | 33 (24.4) |
| Parasite geometric mean per mm3 (SD) | 3.720 (4.136) |
| No. (%) with presence of gametocytes at microscopy | 132 (97.8) |
| Mean hemoglobin, g/dl (SD) | 13.4 (1.7) |
| No. (%) with anemiaa | 27 (20.0) |
Hemoglobin level of <12 g/dl for females and <13 g/dl for males (according to WHO criteria).
Parasitological failure was observed in 7/135 patients (5.2%; 95% confidence interval, 0.2 to 10.5). Blood CQ/DCQ concentrations in these seven patients were all above the minimal effective concentration (>100 ng/ml).
On day 3, mean blood levels of CQ/DCQ were lower in patients carrying resistant parasites (1,069.8 ng/ml) than in patients carrying sensitive ones (3,311.1 ng/ml) (P = 0.049), even when the drug plasma concentration was more than 10 times higher than the effective levels in the first group. On days 7, 14, and 28, drug levels were not significantly different between patients carrying resistant and susceptible P. vivax parasites (Table 3).
TABLE 3.
Average blood levels of chloroquine plus desethylchloroquine in patients carrying resistant and susceptible P. vivax parasites
| Day of follow-up | CQ-DCQ level(ng/ml, mean ± SD) in blood of patients carrying: |
P value | |
|---|---|---|---|
| Resistant isolates | Susceptible isolates | ||
| 3 | 1,069.8 ± 810.6 | 3,311.1 ± 2,361.8 | 0.049 |
| 7 | 1.321.5 ± 922.6 | 1,914.2 ± 1,700.0 | 0.463 |
| 14 | 1,044.3 ± 667.6 | 1,351.4 ± 1,763.0 | 0.679 |
| 28 | 647.4 ± 227.2 | 783.8 ± 530.0 | 0.543 |
In the univariate analysis, previous exposure to malaria and a higher initial parasitemia mean were associated with treatment failure resistance (Table 4). In the multivariate analysis, only high initial parasitemia remained associated with CQ resistance, independently of age and gender.
TABLE 4.
Univariate and multivariate (logistic regression) analyses of factors associated with treatment resistance in patients with uncomplicated Plasmodium vivax malaria under supervised treatment with chloroquine plus primaquine
| Factor | Univariate analysis |
Logistic regression analysis |
||||||
|---|---|---|---|---|---|---|---|---|
| Odds ratio | 95% Confidence interval |
P valuea | Adjusted odds ratio | 95% Confidence interval |
P value | |||
| Lower | Upper | Lower | Upper | |||||
| Anemiab | 0.61 | 0.11 | 3.35 | 0.568 | 1.47 | 0.24 | 8.99 | 0.674 |
| History of 2–5 previous malaria episodes | 6.15 | 1.14 | 33.12 | 0.016 | 4.83 | 0.86 | 27.23 | 0.074 |
| Asexual parasitemia > 5,000 | 7.50 | 1.39 | 40.56 | 0.008 | 5.83 | 1.04 | 32.77 | 0.045 |
| Constant | 0.01 | 0.000 | ||||||
Boldface indicates significance.
Hemoglobin level of <12 g/dl for females and <13 g/dl for males.
Baseline parasitemia on day 0 was significantly higher in resistant than in sensitive cases. In the following days, however, parasitemia was approximately the same in both groups, until day 28, when recrudescences were observed in all seven resistant cases (late parasitological failures) (Fig. 1A). Hemoglobin levels were similar in the beginning of the follow-up and were not correlated with parasitemia (Fig. 1B). From this point, hemoglobin remained relatively constant for the sensitive group but decreased in the resistant patients. On days 3 and 7, hemoglobin levels were significantly lower in patients presenting CQ resistance. After day 14, however, hemoglobin levels improved in this group, reaching levels similar to those in the sensitive group (Fig. 2).
FIG 1.
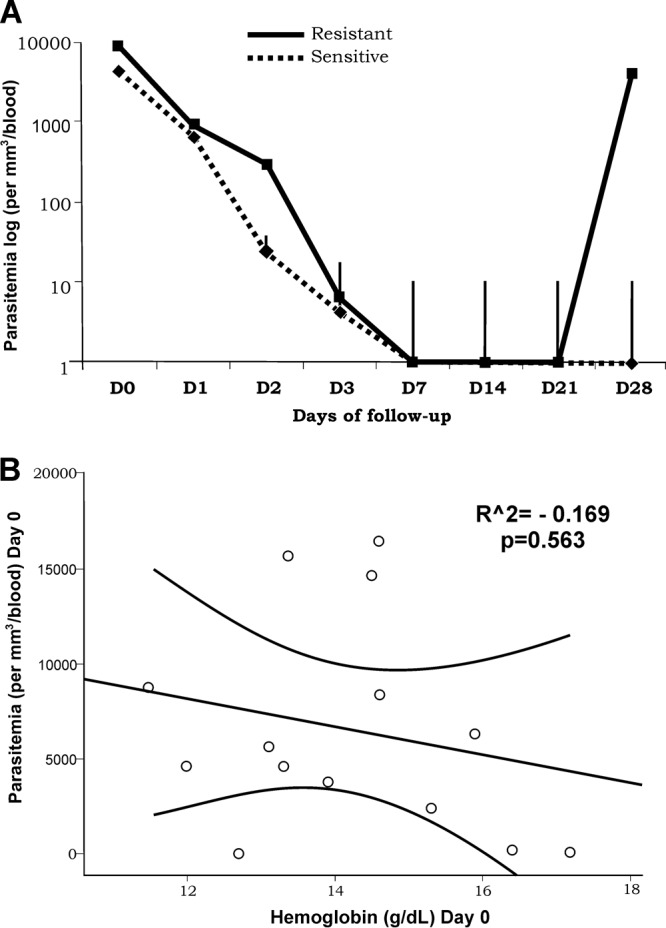
Parasitemia levels (presented as means with error bars) throughout the follow-up according to resistance status (A) and correlation between hemoglobin and parasitemia on day 0 (B). *, statistically significant.
FIG 2.
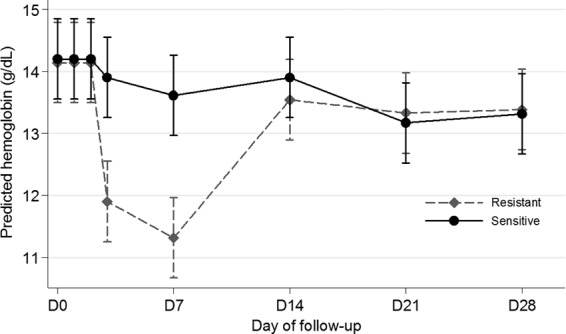
Hemoglobin levels during the follow-up according to resistance status.
The pvdhfr, pvmrp1, pvmdr1, and pvdhps gene mutations studied were not related to CQ resistance (Table 5).
TABLE 5.
Frequency of polymorphisms in the pvdhfr, pvmrp1, pvmdr1, and pvdhps genes for sensitive and resistant samples
| Gene and polymorphism | No. of isolates/total (%) |
P | |
|---|---|---|---|
| Resistant | Susceptible | ||
| pvdhfr | |||
| 58 | 6/7 (85.7) | 3/7 (42.8) | 0.266 |
| 117 | 6/7 (85.7) | 7/7 (100) | 1.000 |
| 173 | 3/7 (42.8) | 2/7 (28.5) | 1.000 |
| pvmrp1 | |||
| 1282 | 7/7 (100) | 7/7 (100) | |
| 1393 | 7/7 (100) | 7/7 (100) | |
| 1419 | 4/7 (57.1) | 2/7 (28.5) | 0.592 |
| 1478 | 7/7 (100) | 7/7 (100) | |
| 1586 | 1/7 (14.2) | 2/7 (28.5) | 1.000 |
| pvmdr1 | |||
| 908 | 0/7 (0) | 2/7 (28.5) | 0.067 |
| 958 | 7/7 (100) | 7/7 (100) | |
| pvdhps | |||
| 205 | 5/7 (71.4) | 6/7 (85.7) | 1.000 |
| 382 | 5/7 (71.4) | 5/7 (71.4) | 1.000 |
| 383 | 2/7 (28.5) | 5/7 (71.4) | 0.286 |
DISCUSSION
CQ and PQ are still the drugs of choice to treat vivax malaria in many areas of endemicity, including Brazil. A previous report from a study in the Brazilian Amazon demonstrated that CQ failure in P. vivax infection was around 10.1% (16). In this study, the in vivo CQ resistance rate in patients with uncomplicated P. vivax malaria using CQ and PQ simultaneously was 5.2%, with a proper dosage of blood levels of CQ and DCQ. It has been claimed that the concomitant use of PQ and CQ could result in a synergistic effect against asexual forms of P. vivax, as has been shown for P. falciparum parasites (18). However, the in vivo data available for P. vivax show that the parasitological responses to CQ and to CQ plus PQ are quite similar (19). Consequently, the possibility that such synergism results in the underreporting of CQ resistance in areas where PQ is systematically used should be examined. The findings presented here suggest that treatment efficacy was not substantially improved by the concurrent administration of PQ in terms of reducing therapeutic failure (the confidence interval ranged from 0.2 to 10.5% and therefore included the 10.1% frequency found in a study performed in Manaus years ago, in which CQ was used by itself and PQ was postponed until day 28).
There is a paucity of available data regarding risk factors associated with P. vivax CR. In a clinical trial carried out in Thailand, children <5 years old were at greater risk of recurrent P. vivax infection than older patients, probably due to less natural immunity against malaria in this age group (25). The present study included patients aged 12 to 60 years, following World Health Organization guidelines (22), and no association with mean age was found. In this work, clearance occurred with a similar time profile in both sensitive and resistant groups. Gender and baseline values of weight, temperature, hemoglobin levels, and platelet counts also were not associated with resistance. In Afghanistan, a lower initial hemoglobin concentration was independently associated with recurrence on day 56 (26). There is a previous report of body temperature of ≥38°C as a factor contributing to delay in parasite clearance in uncomplicated falciparum malaria in children (27).
In this work, only higher parasitemia on day 0 was an independent risk factor for P. vivax CR. Some authors found that high baseline parasitemia density may be a predictor of CQ resistance in patients presenting falciparum malaria (27, 28). It is known that laboratory strains of P. falciparum (29) and P. vivax (30) have a marked variability in growth rates, with CQ-resistant isolates growing faster than CQ-susceptible isolates.
Significantly lower hemoglobin levels were observed in patients harboring CQ-resistant P. vivax on days 3 and 7, suggesting that CQ resistance could be an important anemia-triggering factor during malarial episodes. However, after a temporary parasite clearance, hemoglobin increases to a level similar to that observed for sensitive parasite carriers. This short effect of resistant parasites upon hemoglobin levels could be explained by the late parasitological failures seen in all patients from the study. In areas with more severe and widespread P. vivax CR, one could hypothesize that the impact could be more evident. Although higher parasitemia was demonstrated for resistant patients on day 0, the parasite loads became similar on day 3, showing that parasitemia alone should not explain the hemoglobin decrease from day 0 to day 7 in the resistant group, unless initial parasitemia in the bone marrow milieu plays a role in worsening of anemia (31).
The simultaneous occurrence of severe vivax disease and CQ resistance in some countries has raised the question of a possible association between severity and resistance (32). CQ resistance actually has been reported from Brazil almost at the same time as clinical severity. Our data showed a link between CQ resistance and a malaria complication represented here by hemoglobin decrease, but it is difficult to predict how this phenomenon contributes to severity and anemia in areas where malaria is endemic from a long time perspective. Previous evidence showed that a lower initial hemoglobin concentration was independently associated with recurrence of vivax malaria treated with CQ (26), suggesting a similar relationship between drug resistance and decreased hemoglobin during malaria episodes. As we found that high parasite densities were concurrent with P. vivax CQ resistance, the possibility arises that the highly CQ-resistant isolates found in Papua may be more pathogenic to the host, in agreement with previous observations for falciparum malaria in Papua (33). When Fernandez-Becerra et al. (34) compared a patient with severe vivax malaria to another patient with uncomplicated malaria, they found higher levels of both pvmdr1 and pvcrt-o gene expression in the former patient.
With respect to molecular genotyping, we did not find any molecular marker related to CQ resistance. Actually, no trustworthy molecular marker for P. vivax CR has been identified so far (1, 6). Molecular characterization of P. vivax isolates carried out previously in Brazil has shown that the sequences of the pvcrt-o and pvmdr1 genes were not associated with CQ resistance (35, 36).
Evidence is growing that P. vivax may infect many more people than has been appreciated (37). Evidence also points to this supposedly benign parasite causing a spectrum of severe and life-threatening syndromes (38, 39). P. vivax CR may be rising in the Brazilian Amazon, and probably this fact is contributing to the increase in complicated vivax malaria. To maintain an efficient malaria control program, drug resistance surveillance assays must be conducted on a regular basis to assess antimalarial efficacy and to ensure that the information is available to policy makers. The Amazon Network for the Surveillance of Antimalarial Drug Resistance (RAVREDA), funded in 2001, was definitely a pivotal initiative that contributed to expanding the knowledge on P. vivax CR in Latin America.
Despite the association shown, it is important to notice that no causal relationship could be definitely established between P. vivax CR and anemia in the present work, especially due to the limitation of the small sample size. Moreover, both phenomena still have unclear pathogenesis. Anemia, for instance, seems to present multifactor determinants (40), with rosetting being one of the recently described mechanisms (41). Further studies from other areas of endemicity in which other causes of anemia are well characterized may contribute to the better understanding of the association between P. vivax CR and anemia.
ACKNOWLEDGMENTS
This study was funded partially by the Amazon Network for Monitoring Antimalarial Drug Resistance (RAVREDA), supported by the Brazilian Ministry of Health, the Pan-American Health Organization, and USAID's Amazon Malaria Initiative, and also by the PRONEX Malaria Network, funded by the Brazilian Ministry of Science and Technology (MCT), the National Council for Scientific and Technological Development (CNPq), the Brazilian Ministry of Health (DECIT/SCTIE/MS), and the Research Support Foundation of Amazonas (FAPEAM) (grant 555.666/2009-3). M.V.G.L. has a level 2 productivity fellowship from CNPq, and R.C.R.-L. had a fellowship from CNPq (grant number 575788/2008-9).
Footnotes
Published ahead of print 28 October 2013
REFERENCES
- 1.Mueller I, Galinski MR, Baird JK, Carlton JM, Kochar DK, Alonso PL, del Portillo HA. 2009. Key gaps in the knowledge of Plasmodium vivax, a neglected human malaria parasite. Lancet Infect. Dis. 9:555–566. 10.1016/S1473-3099(09)70177-X [DOI] [PubMed] [Google Scholar]
- 2.WHO 2012. World malaria report 2012 WHO, Geneva, Switzerland [Google Scholar]
- 3.Most H, London IM, et al. 1946. Chloroquine for treatment of acute attacks of vivax malaria. JAMA 131:963–967. 10.1001/jama.1946.02870290013005 [DOI] [PubMed] [Google Scholar]
- 4.McChesney EW, Fasco MJ, Banks WF., Jr 1967. The metabolism of chloroquine in man during and after repeated oral dosage. J. Pharmacol. Exp. Ther. 158:323–331 [PubMed] [Google Scholar]
- 5.Coatney GR, Ruhe DS, et al. 1949. Studies in human malaria; the protective and therapeutic action of chloroquine (SN 7618) against St. Elizabeth strain vivax malaria. Am. J. Hyg. (Lond.) 49:49–59 [PubMed] [Google Scholar]
- 6.Baird JK. 2004. Chloroquine resistance in Plasmodium vivax. Antimicrob. Agents Chemother. 48:4075–4083. 10.1128/AAC.48.11.4075-4083.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rieckmann KH, Davis DR, Hutton DC. 1989. Plasmodium vivax resistance to chloroquine? Lancet ii:1183–1184 [DOI] [PubMed] [Google Scholar]
- 8.Phillips EJ, Keystone JS, Kain KC. 1996. Failure of combined chloroquine and high-dose primaquine therapy for Plasmodium vivax malaria acquired in Guyana, South America. Clin. Infect. Dis. 23:1171–1173. 10.1093/clinids/23.5.1171 [DOI] [PubMed] [Google Scholar]
- 9.Baird JK, Tiwari T, Martin GJ, Tamminga CL, Prout TM, Tjaden J, Bravet PP, Rawlins S, Ferrel M, Carucci D, Hoffman SL. 2002. Chloroquine for the treatment of uncomplicated malaria in Guyana. Ann. Trop. Med. Parasitol. 96:339–348. 10.1179/000349802125001023 [DOI] [PubMed] [Google Scholar]
- 10.Castillo CM, Osorio LE, Palma GI. 2002. Assessment of therapeutic response of Plasmodium vivax and Plasmodium falciparum to chloroquine in a malaria transmission free area in Colombia. Mem. Inst. Oswaldo Cruz 97:559–562. 10.1590/S0074-02762002000400020 [DOI] [PubMed] [Google Scholar]
- 11.Villalobos-Salcedo JM, Tada MS, Kimura E, Menezes MJ, Pereira da Silva LH. 2000. In-vivo sensitivity of Plasmodium vivax isolates from Rondônia (western Amazon region, Brazil) to regimens including chloroquine and primaquine. Ann. Trop. Med. Parasitol. 94:749–758. 10.1080/00034980020027960 [DOI] [PubMed] [Google Scholar]
- 12.Machado RL, de Figuereido Filho AF, Calvosa VS, Figueredo MC, Nascimento JM, Povoa MM. 2003. Correlation between Plasmodium vivax variants in Belém, Para State, Brazil and symptoms and clearance of parasitemia. Braz J. Infect. Dis. 7:175–177. 10.1590/S1413-86702003000300002 [DOI] [PubMed] [Google Scholar]
- 13.Alecrim MG, Alecrim W, Macedo V. 1999. Plasmodium vivax resistance to chloroquine (R2) and mefloquine (R3) in Brazilian Amazon region. Rev. Soc. Bras. Med. Trop. 32:67–68 [DOI] [PubMed] [Google Scholar]
- 14.Soto J, Toledo J, Gutierrez P, Luzz M, Llinas N, Cedeno N, Dunne M, Berman J. 2001. Plasmodium vivax clinically resistant to chloroquine in Colombia. Am. J. Trop. Med. Hyg. 65:90–93 [DOI] [PubMed] [Google Scholar]
- 15.Ruebush TK, II, Zegarra J, Cairo J, Andersen EM, Green M, Pillai DR, Marquino W, Huilca M, Arevalo E, Garcia C, Solary L, Kain KC. 2003. Chloroquine-resistant Plasmodium vivax malaria in Peru. Am. J. Trop. Med. Hyg. 69:548–552 [PubMed] [Google Scholar]
- 16.Santana Filho FS, Arcanjo AR, Chehuan YM, Costa MR, Martinez-Espinosa FE, Vieira JL, Barbosa MG, Alecrim WD, Alecrim MG. 2007. Chloroquine-resistant Plasmodium vivax, Brazilian Amazon. Emerg. Infect. Dis. 13:1125–1126. 10.3201/eid1307.061386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pukrittayakamee S, Imwong M, Looareesuwan S, White NJ. 2004. Therapeutic responses to antimalarial and antibacterial drugs in vivax malaria. Acta Trop. 89:351–356. 10.1016/j.actatropica.2003.10.012 [DOI] [PubMed] [Google Scholar]
- 18.Bray PG, Martin RE, Tilley L, Ward SA, Kirk K, Fidock DA. 2005. Defining the role of PfCRT in Plasmodium falciparum chloroquine resistance. Mol. Microbiol. 56:323–333. 10.1111/j.1365-2958.2005.04556.x [DOI] [PubMed] [Google Scholar]
- 19.Naing C, Aung K, Win DK, Wah MJ. 2010. Efficacy and safety of chloroquine for treatment in patients with uncomplicated Plasmodium vivax infections in endemic countries. Trans. R. Soc. Trop. Med. Hyg. 104:695–705. 10.1016/j.trstmh.2010.08.009 [DOI] [PubMed] [Google Scholar]
- 20.Tjitra E, Anstey NM, Sugiarto P, Warikar N, Kenangalem E, Karyana M, Lampah DA, Price RN. 2008. Multidrug-resistant Plasmodium vivax associated with severe and fatal malaria: a prospective study in Papua, Indonesia. PLoS Med. 5:e128. 10.1371/journal.pmed.0050128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Snounou G, Viriyakosol S, Jarra W, Thaithong S, Brown KN. 1993. Identification of the four human malaria parasite species in field samples by the polymerase chain reaction and detection of a high prevalence of mixed infections. Mol. Biochem. Parasitol. 58:283–292. 10.1016/0166-6851(93)90050-8 [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization 2006. Guidelines for the treatment of malaria. World Health Organization, Geneva, Switzerland [Google Scholar]
- 23.Dua VK, Kar PK, Gupta NC, Sharma VP. 1999. Determination of chloroquine and desethylchloroquine in plasma and blood cells of Plasmodium vivax malaria cases using liquid chromatography. J. Pharm. Biomed. Anal. 21:199–205. 10.1016/S0731-7085(99)00097-7 [DOI] [PubMed] [Google Scholar]
- 24.Patchen LC, Mount DL, Schwartz IK, Churchill FC. 1983. Analysis of filter-paper-absorbed, finger-stick blood samples for chloroquine and its major metabolite using high-performance liquid chromatography with fluorescence detection. J. Chromatogr. 278:81–89 [DOI] [PubMed] [Google Scholar]
- 25.Phyo AP, Lwin KM, Price RN, Ashley EA, Russell B, Sriprawat K, Lindegardh N, Singhasivanon P, White NJ, Nosten F. 2011. Dihydroartemisinin-piperaquine versus chloroquine in the treatment of Plasmodium vivax malaria in Thailand: a randomized controlled trial. Clin. Infect. Dis. 53:977–984. 10.1093/cid/cir631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Awab GR, Pukrittayakamee S, Imwong M, Dondorp AM, Woodrow CJ, Lee SJ, Day NP, Singhasivanon P, White NJ, Kaker F. 2010. Dihydroartemisinin-piperaquine versus chloroquine to treat vivax malaria in Afghanistan: an open randomized, non-inferiority trial. Malar. J. 9:105. 10.1186/1475-2875-9-105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sowunmi A, Adewoye EO, Gbotsho GO, Happi CT, Sijuade A, Folarin OA, Okuboyejo TM, Michael OS. 2010. Factors contributing to delay in parasite clearance in uncomplicated falciparum malaria in children. Malar. J. 9:53. 10.1186/1475-2875-9-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hess FI, Iannuzzi A, Leafasia J, Cowdrey D, Nothdurft HD, Von Sonnenburg F, Loscher T, Rieckmann KH. 1996. Risk factors of chloroquine resistance in Plasmodium falciparum malaria. Acta Trop. 61:293–306. 10.1016/0001-706X(96)00011-3 [DOI] [PubMed] [Google Scholar]
- 29.Reilly HB, Wang H, Steuter JA, Marx AM, Ferdig MT. 2007. Quantitative dissection of clone-specific growth rates in cultured malaria parasites. Int. J. Parasitol. 37:1599–1607. 10.1016/j.ijpara.2007.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Russell B, Chalfein F, Prasetyorini B, Kenangalem E, Piera K, Suwanarusk R, Brockman A, Prayoga P, Sugiarto P, Cheng Q, Tjitra E, Anstey NM, Price RN. 2008. Determinants of in vitro drug susceptibility testing of Plasmodium vivax. Antimicrob. Agents Chemother. 52:1040–1045. 10.1128/AAC.01334-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ru YX, Mao BY, Zhang FK, Pang TX, Zhao SX, Liu JH, Wickramasinghe SN. 2009. Invasion of erythroblasts by Pasmodium vivax: a new mechanism contributing to malarial anemia. Ultrastruct. Pathol. 33:236–242. 10.3109/01913120903251643 [DOI] [PubMed] [Google Scholar]
- 32.Price RN, Douglas NM, Anstey NM. 2009. New developments in Plasmodium vivax malaria: severe disease and the rise of chloroquine resistance. Curr. Opin. Infect. Dis. 22:430–435. 10.1097/QCO.0b013e32832f14c1 [DOI] [PubMed] [Google Scholar]
- 33.Chotivanich K, Udomsangpetch R, Simpson JA, Newton P, Pukrittayakamee S, Looareesuwan S, White NJ. 2000. Parasite multiplication potential and the severity of falciparum malaria. J. Infect. Dis. 181:1206–1209. 10.1086/315353 [DOI] [PubMed] [Google Scholar]
- 34.Fernandez-Becerra C, Pinazo MJ, Gonzalez A, Alonso PL, del Portillo HA, Gascon J. 2009. Increased expression levels of the pvcrt-o and pvmdr1 genes in a patient with severe Plasmodium vivax malaria. Malar. J. 8:55. 10.1186/1475-2875-8-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sa JM, Nomura T, Neves J, Baird JK, Wellems TE, del Portillo HA. 2005. Plasmodium vivax: allele variants of the mdr1 gene do not associate with chloroquine resistance among isolates from Brazil, Papua, and monkey-adapted strains. Exp. Parasitol. 109:256–259. 10.1016/j.exppara.2004.12.005 [DOI] [PubMed] [Google Scholar]
- 36.Orjuela-Sanchez P, Santana Filho FS, Machado-Lima A, Chehuan YF, Costa MR, Alecrim MG, del Portillo HA. 2009. Analysis of single-nucleotide polymorphisms in the crt-o and mdr1 genes of Plasmodium vivax among chloroquine-resistant isolates from the Brazilian Amazon region. Antimicrob. Agents Chemother. 53:3561–3564. 10.1128/AAC.00004-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gething PW, Elyazar IR, Moyes CL, Smith DL, Battle KE, Guerra CA, Patil AP, Tatem AJ, Howes RE, Myers MF, George DB, Horby P, Wertheim HF, Price RN, Mueller I, Baird JK, Hay SI. 2012. A long neglected world malaria map: Plasmodium vivax endemicity in 2010. PLoS Negl. Trop. Dis. 6:e1814. 10.1371/journal.pntd.0001814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alexandre MA, Ferreira CO, Siqueira AM, Magalhaes BL, Mourao MP, Lacerda MV, Alecrim M. 2010. Severe Plasmodium vivax malaria, Brazilian Amazon. Emerg. Infect. Dis. 16:1611–1614. 10.3201/eid1610.100685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lacerda MV, Fragoso SC, Alecrim MG, Alexandre MA, Magalhaes BM, Siqueira AM, Ferreira LC, Araujo JR, Mourao MP, Ferrer M, Castillo P, Martin-Jaular L, Fernandez-Becerra C, Del Portillo H, Ordi J, Alonso PL, Bassat Q. 2012. Postmortem characterization of patients with clinical diagnosis of Plasmodium vivax malaria: to what extent does this parasite kill? Clin. Infect. Dis. 55:e67–74. 10.1093/cid/cis615 [DOI] [PubMed] [Google Scholar]
- 40.Douglas NM, Anstey NM, Buffet PA, Poespoprodjo JR, Yeo TW, White NJ, Price RN. 2012. The anaemia of Plasmodium vivax malaria. Malar. J. 11:135. 10.1186/1475-2875-11-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marin-Menendez A, Bardaji A, Martinez-Espinosa FE, Botto-Menezes C, Lacerda MV, Ortiz J, Cistero P, Piqueras M, Felger I, Mueller I, Ordi J, del Portillo H, Menendez C, Wahlgren M, Mayor A. 2013. Rosetting in Plasmodium vivax: a cytoadhesion phenotype associated with anaemia. PLoS Negl. Trop. Dis. 7:e2155. 10.1371/journal.pntd.0002155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barnadas C, Musset L, Legrand E, Tichit M, Briolant S, Fusai T, Rogier C, Bouchier C, Picot S, Menard D. 2009. High prevalence and fixation of Plasmodium vivax dhfr/dhps mutations related to sulfadoxine/pyrimethamine resistance in French Guiana. Am. J. Trop. Med. Hyg. 81:19–22 [PubMed] [Google Scholar]
- 43.Hawkins VN, Joshi H, Rungsihirunrat K, Na-Bangchang K, Sibley CH. 2007. Antifolates can have a role in the treatment of Plasmodium vivax. Trends Parasitol. 23:213–222. 10.1016/j.pt.2007.03.002 [DOI] [PubMed] [Google Scholar]
- 44.Raj DK, Mu J, Jiang H, Kabat J, Singh S, Sullivan M, Fay MP, McCutchan TF, Su XZ. 2009. Disruption of a Plasmodium falciparum multidrug resistance-associated protein (PfMRP) alters its fitness and transport of antimalarial drugs and glutathione. J. Biol. Chem. 284:7687–7696. 10.1074/jbc.M806944200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barnadas C, Ratsimbasoa A, Tichit M, Bouchier C, Jahevitra M, Picot S, Menard D. 2008. Plasmodium vivax resistance to chloroquine in Madagascar: clinical efficacy and polymorphisms in pvmdr1 and pvcrt-o genes. Antimicrob. Agents Chemother. 52:4233–4240. 10.1128/AAC.00578-08 [DOI] [PMC free article] [PubMed] [Google Scholar]


