Abstract
Pentavalent antimonial drugs such as meglumine antimoniate (Glucantime [Glu; Sanofi-Aventis, São Paulo, Brazil]) produce severe side effects, including cardiotoxicity and hepatotoxicity, during the treatment of leishmaniasis. We evaluated the role of residual Sb(III) in the hepatotoxicity of meglumine antimoniate, as well as the protective effect of the antioxidant ascorbic acid (AA) during antimonial chemotherapy in a murine model of visceral leishmaniasis. BALB/c mice infected with Leishmania infantum were treated intraperitoneally at 80 mg of Sb/kg/day with commercial meglumine antimoniate (Glu) or a synthetic meglumine antimoniate with lower Sb(III) level (MA), in association or not with AA (15 mg/kg/day), for a 20-day period. Control groups received saline or saline plus AA. Livers were evaluated for hepatocytes histological alterations, peroxidase activity, and apoptosis. Increased proportions of swollen and apoptotic hepatocytes were observed in animals treated with Glu compared to animals treated with saline or MA. The peroxidase activity was also enhanced in the liver of animals that received Glu. Cotreatment with AA reduced the extent of histological changes, the apoptotic index, and the peroxidase activity to levels corresponding to the control group. Moreover, the association with AA did not affect the hepatic uptake of Sb and the ability of Glu to reduce the liver and spleen parasite loads in infected mice. In conclusion, our data supports the use of pentavalent antimonials with low residue of Sb(III) and the association of pentavalent antimonials with AA, as effective strategies to reduce side effects in antimonial therapy.
INTRODUCTION
At the beginning of the last century, Gaspar Vianna, pioneer researcher in the treatment of leishmaniasis, reported the efficacy of antimony(III) potassium tartrate (tartar emetic) for treatment of leishmaniasis (1). The less toxic pentavalent antimony [Sb(V)] complexes, including meglumine antimoniate (Glucantime [Glu]), were introduced for the therapy of leishmaniasis from the 1940s. Even though pentavalent antimonials are still the first-line drugs in several countries against all forms of leishmaniasis, their use in the clinical setting has several limitations (2, 3). These compounds have to be given parenterally, daily, for at least 3 weeks (typically, 20 mg of Sb/kg/day for 20 to 30 days). Antimony therapy is often accompanied by local pain during intramuscular injection and by severe side effects that include cardiotoxicity, pancreatitis, hepatotoxicity, and nephrotoxicity (4–8). As consequences, careful medical supervision is required, and compliance problems are common.
The mechanisms involved in the toxicity of pentavalent antimonials are not fully elucidated. However, it is generally accepted that Sb(III), either present as residue in pentavalent antimonials (9) or produced in the tissues through reduction (10), may be responsible for their side effects and antileishmanial action (11). Studies of the mechanism of cytotoxicity of the trivalent tartar emetic drug suggest that Sb(III) compromises thiol homeostasis through depletion of intracellular glutathione and inhibition of glutathione reductase (12, 13). Then, Sb(III) enhances oxidative stress and leads to apoptosis through increase of reactive oxygen species (ROS) (13–15). The toxicity of some batches of pentavalent antimonial drug has also been attributed to contamination with As and Pb (16).
Recently, two new synthetic processes were proposed for meglumine antimoniate (17). One of these methods used KSb(OH)6 as a source of antimony, instead of SbCl5 in the commercial process for Glu. This resulted in a more simple method and a final product that exhibited lower in vitro cytotoxicity, compared to Glu (18). It has been suggested that this synthetic meglumine antimoniate may be less toxic in vivo than Glu (11).
Ascorbic acid (AA), known as vitamin C, is an important antioxidant and ROS scavenger which is associated to arsenicals in the treatment of cancer (19–21). Among its several benefits, AA can increase the cytotoxicity of As(III) against multidrug-resistant cells, presumably through reduction of glutathione intracellular concentration (22, 23). AA was also found to reduce the toxicity of arsenicals in rats through the inhibition of oxidative stress (24, 25). Considering the possible role of Sb(III) in the toxicity of pentavalent antimonials and the chemical similarities between Sb(III) and As(III), we hypothesized that AA may reduce the toxicity of pentavalent antimonials during chemotherapy of leishmaniasis.
In the present study, the use of a pentavalent antimonial drug with reduced Sb(III) content and the association of these drugs with AA were investigated as novel strategies for improved therapy of leishmaniasis. More specifically, commercial and synthetic forms of meglumine antimoniate differing in their amount of Sb(III) were compared for their hepatotoxicity and efficacy in a murine model of visceral leishmaniasis (VL). AA also was evaluated for its hepatoprotective effect during pentavalent antimonial chemotherapy and its interference with the drug antileishmanial efficacy.
MATERIALS AND METHODS
Materials.
A commercial sample of meglumine antimoniate (Glucantime [Glu]; 5-ml vials at 300 mg/ml, batch 6050 [Sanofi-Aventis, São Paulo, Brazil]) was obtained from the Brazilian Ministry of Health. Ascorbic acid (5-ml vials at concentration of 100 mg/ml; batch 10080785) was obtained from Hypofarma (Ribeirão das Neves, MG, Brazil). N-Methyl-d-glucamine (NMG; 98%), bromopyrogallol red (BPR), and antimony(III) potassium tartrate (tartar emetic) were obtained from Aldrich Chemical Co. (Milwaukee, WI). Potassium hexahydroxoantimoniate [KSb(OH)6] was obtained from Fluka Chemie GmbH (Switzerland). Synthetic meglumine antimoniate (MA) was obtained from the reaction of NMG with KSb(OH)6, as previously described (17). The total antimony concentration in MA and Glu, as determined by atomic absorption spectroscopy, was ca. 30% (wt/wt).
Trace metal analysis in pentavalent antimonials.
The traces of Pb and As were assayed in the antimonial drugs using an Perkin-Elmer NexION 300 ICP-MS apparatus. The procedures used to determine Sb(III) were described in detail previously (9, 18). The photometric method is based on the specific interaction of Sb(III) with the chromogen BPR. The absorbance of BPR at 560 nm decreases proportionally to the amount of Sb(III) in the analyte solution, as a consequence of the formation of a 1:1 BPR-Sb(III) complex. Briefly, 0.5 ml of analyte solution was prepared from 0.1 ml of 0.1 M phosphate, 0.01 ml of 5% (wt/vol) tartrate, 0.05 ml of 350 μM BPR solution in a 1:1 water-ethanol (vol/vol) mixture and 0.34 ml of water. The pH was then adjusted to 6.8 by using sodium hydroxide. The absorbance was registered at 560 nm before (Ao) and after (Am) adding 5 μl of the sample to be analyzed. For each experiment, a calibration curve was established by using tartar emetic as the source of Sb(III) and plotting the difference in the absorbance (Ao − Am) as a function of Sb(III) concentration.
The voltammetric method is based on anodic stripping voltammetry at a gold wire electrode and was described in detail recently (9). The detection technique was a square wave in 1 mM HCl using a deposition potential of −0.6 V (versus Ag/AgCl/KCl at 3 M). Prior to the detection, the Glu solution was diluted in alkaline conditions (10 mM NaOH) to avoid the loss of Sb(III). Overall, Glu was diluted 107-fold. Similarly, MA powder was first dissolved in 10 mM NaOH to give a solution of 6.1 μg/ml before being diluted further 500 times in the voltammetric cell. In both cases (MA powder and Glu), determination was made by the method of standard additions.
Animals and parasites.
BALB/c mice (female, 4 to 6 weeks old, 18 to 22 g) were obtained from the Centro de Pesquisas René Rachou, Fundação Oswaldo Cruz-Fiocruz (CPqRR; Belo Horizonte, MG, Brazil). Free access was allowed to standard diet and tap water was supplied ad libitum. Leishmania infantum promastigotes of strain MHOM/BR/70/BH46, originally obtained from the CPqRR, were maintained in vitro at 22 ± 1°C in Schneider's medium supplemented with 10% bovine fetal serum by serial subcultures after every 48 to 72 h.
Infection of animals and treatment protocol.
BALB/c mice were inoculated through the tail vein with 2 × 107 of L. infantum promastigotes/200 μl, obtained after 8 days of culture growth. Infected animals were divided into six groups (n = 7), and treatment was initiated 7 days after infection with the following preparations given daily intraperitoneally (i.p.) for 20 days: (i) Glu group, Glu at 80 mg of Sb/kg/day; (ii) MA group, MA at 80 mg of Sb/kg/day; (iii) Glu+AA group, Glu at 80 mg of Sb/kg/day and ascorbic acid at 15 mg/kg/day given 15 min before antimonial drug; (iv) MA+AA group, MA at 80 mg of Sb/kg/day and ascorbic acid at 15 mg/kg/day; (v) AA group, ascorbic acid at 15 mg/kg/day; and (vi) saline group, isotonic saline.
The choice of AA dose was based on the 250-mg/kg dose given daily by oral route which promoted hepatoprotection in rats exposed for 30 days to arsenite trioxide (24). Conversion of the oral dose to an i.p. dose was made, considering that an oral dose of 250 mg/kg would produce a peak plasma concentration close to that of an i.p. dose of 8 mg/kg in rats (26), which in turn would be equivalent to 16 mg/kg in mice.
Animals were sacrificed on day 30 postinfection by cervical dislocation after ketamine-xylazine anesthesia. Experiments were carried out according to the institutional guidelines for the care and use of laboratory animals and received approval from the Ethics Committee in Animal Experimentation of the CPqRR (protocol L-0024/8).
Determination of parasite burden.
Three days after the interruption of treatment, the number of viable parasites at the site of infection was determined using the quantitative limiting dilution assay (27) with the following modifications. Briefly, organs were weighed and fragmented, and a tissue homogenate was obtained in 1 ml of Schneider's medium supplemented with 10% bovine fetal serum and 1 ml of a solution containing 100 U of penicillin/ml and 100 mg of streptomycin/ml at pH 7.0. Each tissue homogenate was serially diluted (10-fold) into 96-well flat-bottom microtiter plates (Nunclon; Nunc). Samples, in triplicate, were incubated at 26°C for 10 days. The wells containing motile promastigotes were identified with an inverted microscope (Axiovert 25; Zeiss), and the parasite burden was determined from the highest dilution at which promastigotes had grown after 10 days of incubation, i.e., the parasite burden = 10highest dilution. Data are presented as the parasite burden per milligrams of organ.
Determination of antimony in the liver.
Antimony was determined in the liver as previously described (28) using graphite furnace atomic absorption spectroscopy (Analyst AA600; Perkin-Elmer). The livers were recovered, homogenized, and digested with nitric acid in a dry block MA 4004 (Marconi, Sao Paolo, Brazil). The analytical method for the determination of Sb in the liver was validated and showed suitable levels of precision (coefficient of variation < 5%), accuracy (80 to 120% analyte recovery), and linearity (range, 10 to 180 μg of Sb/liter). The quantification limit of the analytical method was 0.93 μg of Sb/g of wet organ.
Microscopic alterations.
Heart, liver, kidney, pancreas, and spleen specimens were fixed in 10% buffered formalin solution. After 24 h, the fragments were put in 70% alcohol, dehydrated in graded ethanol crescent solutions to pure ethanol, and embedded in paraffin for routine histological protocol. Then, 6-μm tissue sections were obtained using a microtome and stained by hematoxylin and eosin (H&E) for general histological study (29). The periodic acid-Schiff (PAS) technique was used for the identification of glycogen in the liver section (30).
The liver parenchyma were evaluated under optical microscopy in order to compare the main structural damage among the different treatments. Ten fields of each mouse slide were randomly analyzed using ×40 and ×10 objective lenses. The images were segmented, and the averages of the light areas were evaluated. The images were obtained by digital camera (Q-Color 3; Olympus) attached to Olympus BX41 microscope and processed using the software Image Pro-Plus.
Peroxidase activity in liver.
The peroxidase activity was measured as a marker of oxidative stress using the 3,3′-diaminobenzidine (DAB) technique (31). Ten fields of each slide were analyzed using ×60 and ×10 objective lenses in order to obtain relative labeling average for each treatment (32, 33). Images were obtained using the equipment and software described above.
Apoptosis in the liver.
Apoptotic cells were first detected by the observation of cell morphology changes and counted to estimate the apoptotic event, as previously described (34, 35). The analysis was performed on scanned images with a ×40 panchromatic objective lens, selecting the central regions, and avoiding necrosis areas, using the software Sigma Scan Pro 5 imaging program. Apoptosis was further evaluated semiquantitatively by the TUNEL (terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling) method (36) in paraffin-embedded sections mounted on glass slides using a commercial kit (FragEL DNA fragmentation detection kit; Colorimetric-Calbiochem), according to the manufacturer's instructions.
Cells were considered positive when they showed strong nuclear staining, recognized by the presence of dark brown lumps and at least one of the following morphological criteria: (i) anoikis (cell shrinkage with loss of adhesion with adjacent cells), (ii) nuclear condensation (condensed chromatin, sometimes partially permeating the nuclear membrane forming crescent figures), (iii) fragmentation of the nuclear and cytoplasmic membrane (without karyorrhexis or rupture), or (iv) formation of apoptotic bodies resulting from cell fractionation. Positive hepatocytes were then counted blindly, and results were expressed as a percentage of the total hepatocytes, excluding the surrounding massive necrosis lesion. The apoptotic index, i.e., the total number of cells with apoptosis/total number of cells × 100, was determined in 15 fields per slide.
Statistical analysis.
Comparison of the results obtained between different experimental groups was performed using Kruskal-Wallis test (followed by Dunn's multiple comparison test) or Mann-Whitney test in the case of data with non-normal distribution. Comparison between normally distributed data was performed by one-way analysis of variance (ANOVA) with the Bonferroni post test. Differences with P values of <0.05 were considered statistically significant.
RESULTS
Sb(III) content in pentavalent antimonial compounds.
Two different forms of meglumine antimoniate were evaluated: the commercial form (Glu) and meglumine antimoniate (MA) synthesized from KSb(OH)6 (17). Trace metal analysis did not show detectable levels of Pb (<2.5 μg/g) or As (<1.5 μg/g) in either Glu or MA. On the other hand, residual Sb(III) could be detected by two different methods: a recently developed voltammetric method (9) and a photometric method based on the specific interaction of Sb(III) with BPR chromogen (18). Although Glu and MA have similar total Sb levels (30% [wt/wt]), Glu had a higher content of Sb(III) compared to MA. Interestingly, Sb(III) showed much higher availability in the voltammetric than in the photometric assay. According to the voltammetric method, Glu and MA showed, respectively, 24.1% ± 0.4% and 16.3% ± 0.7% of Sb(III) in relation to total Sb. In agreement with our previous report (18), the photometric method showed Sb(III) proportion of 0.068% ± 0.002% in Glu and did not detect significant level of Sb(III) in MA (proportion < 0.007%).
Antileishmanial activity and hepatotoxicity of pentavalent antimonial compounds in a murine model of visceral leishmaniasis.
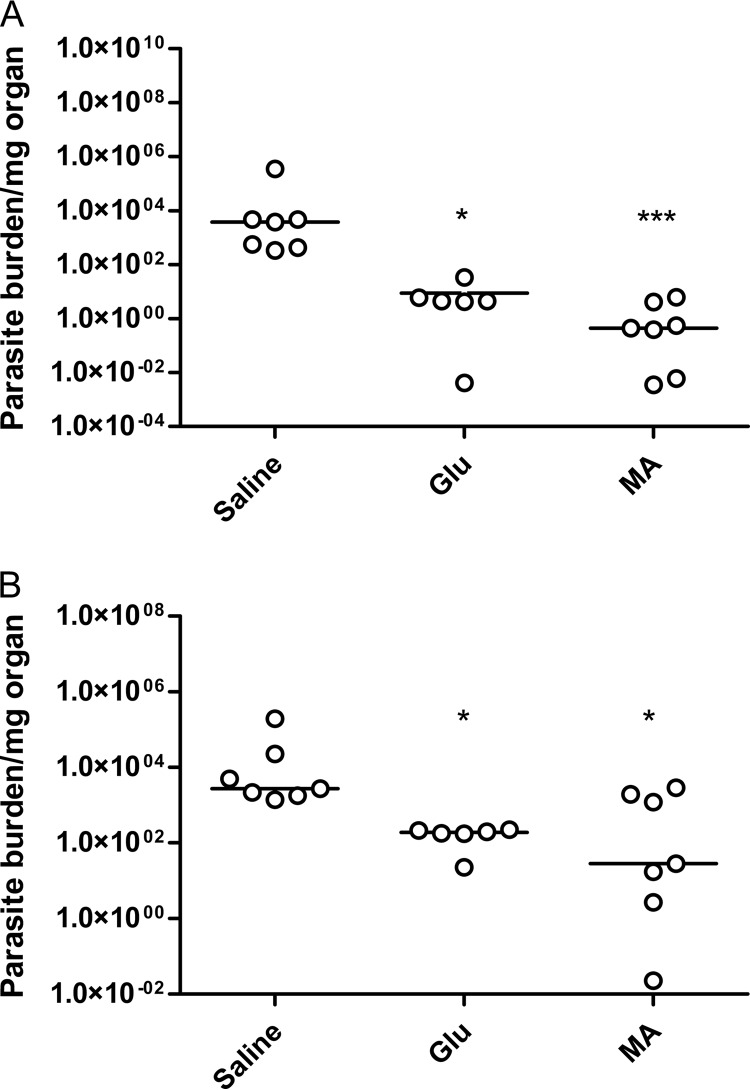
BALB/c mice experimentally infected with L. infantum underwent chemotherapy with Glu or MA at the same dose of total antimony of 80 mg/(kg day) for 20 days by the i.p. route. Three days after the end of treatment, the animals were sacrificed, and the parasite burdens were evaluated in the liver and spleen. As shown in Fig. 1, significant reductions of liver and spleen parasite loads were observed in the groups treated with both antimonial drugs compared to the control group treated with saline. No significant difference in parasite load was encountered between Glu and MA groups. In accordance with this result, similar concentrations of Sb were found in the livers of mice after treatment with Glu and MA (4.2 ± 1.0 versus 4.9 ± 1.0 μg/g of wet liver).
FIG 1.
Parasite loads in the livers (A) and spleens (B) of BALB/c mice infected with L. infantum after 20 days of treatment with commercial (Glu) or synthetic meglumine antimoniate (MA) (80 mg of Sb/kg/day) or saline by the i.p. route. The data are shown as dot plots, and lines correspond to the median of each group (n = 6 to 7). *, P < 0.05 (Kruskal-Wallis, followed by Dunn's multiple-comparison post test).
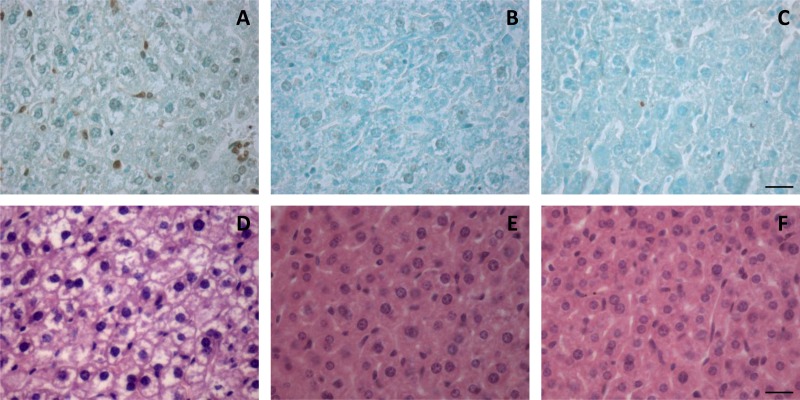
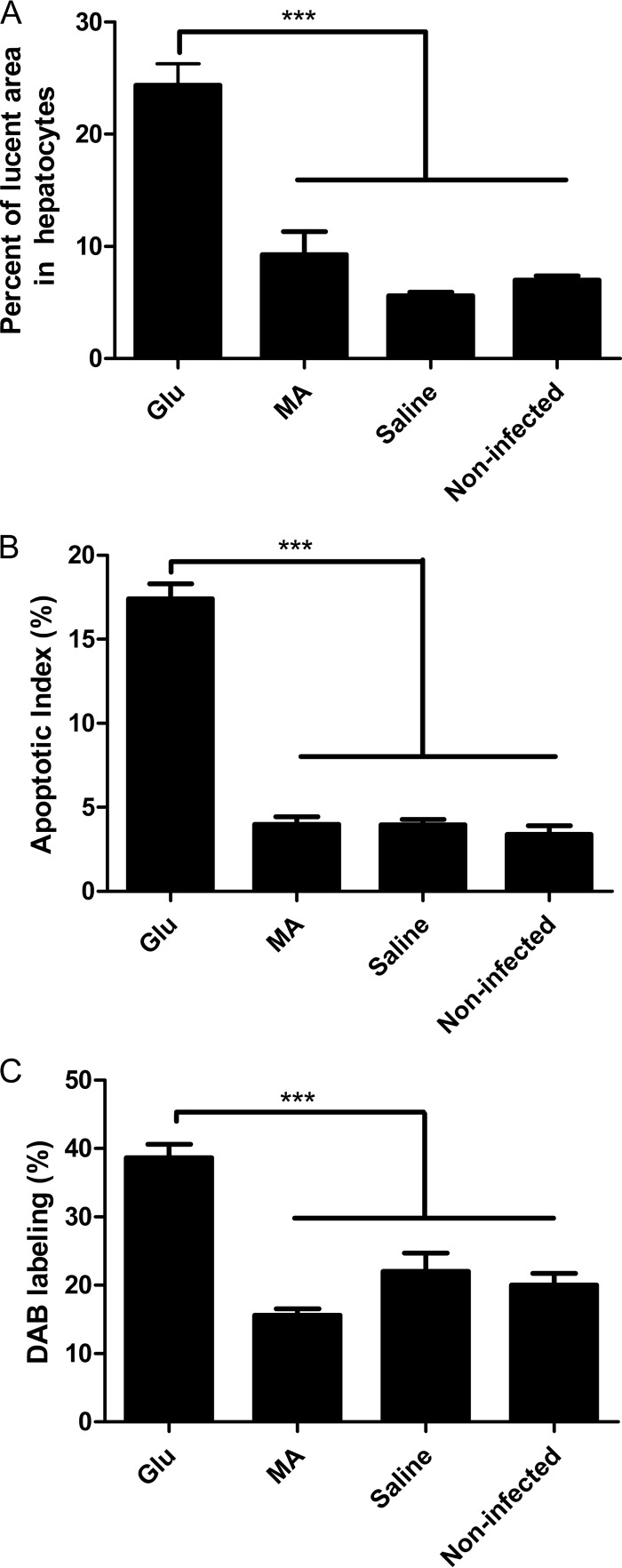
The hearts, kidneys, pancreases, spleens, and livers were evaluated for histopathological changes after treatment, and comparison was also made between infected and noninfected control groups. Heart, kidney, pancreas, and spleen samples did not show significant histological changes upon treatment with Glu or MA (data not shown). On the other hand, histopathological changes were observed in the liver. As illustrated in Fig. 2D, the hepatocytes of the Glu group showed vacuolization and granulosity in contrast to those of control and MA groups. Figure 3A shows the result of quantification of lucent areas, indicating a significant increase after treatment with Glu. Since PAS evaluation was negative (data not shown), this change could be attributed to alteration in water homeostasis, which may be caused by free radicals. Further evidence of hepatotoxicity in Glu group was obtained from the increase of TUNEL-positive hepatocytes (Fig. 2A) and of the apoptotic index (Fig. 3B).
FIG 2.
Histopathology (D to F) and apoptosis (A to C) analysis in the livers of L. infantum-infected BALB/c mice treated as follows: A and D, Glu, given i.p. at 80 mg of Sb/kg/day; B and E, MA, given i.p. at 80 mg of Sb/kg/day; or C and F, saline, given i.p. Histology was evaluated by H&E staining. Apoptosis was assessed by TUNEL. Cells were considered positive when they showed a strong nuclear staining recognized by the presence of dark brown lumps and morphological changes characteristic of apoptosis (see Materials and Methods for details). Bar, 20 μm.
FIG 3.
Effect of treatment of L. infantum-infected BALB/c mice with commercial (Glu) or synthetic (MA) meglumine antimoniate using different liver parameters: A, lucent area in hepatocytes; B, apoptotic index of hepatocytes; and C, peroxidase activity. Mice were treated with Glu or MA (80 mg of Sb/kg/day) or saline for 20 days by the i.p. route. Noninfected mice receiving saline were also used as controls. The data are means ± the standard errors of the mean (SEM; n = 3 to 7). ***, P < 0.001 (one-way ANOVA with Bonferroni post test).
It is noteworthy that the group treated with MA, in contrast to Glu group, did not show significant histological changes and increases in apoptotic activity (Fig. 2B, 2E, and 3B) compared to the control. These data suggest that Sb(III) present at a higher level in Glu is responsible for the hepatotoxicity.
In accordance with this model, treatment with Glu, but not MA, significantly increased the peroxidase activity in the hepatic tissue, as evidenced by the greater level of conversion of DAB substrate (Fig. 3C). These data suggest an increased oxidative stress in the liver upon treatment with Glu.
Hepatoprotective effect of ascorbic acid in mice treated with Glu.
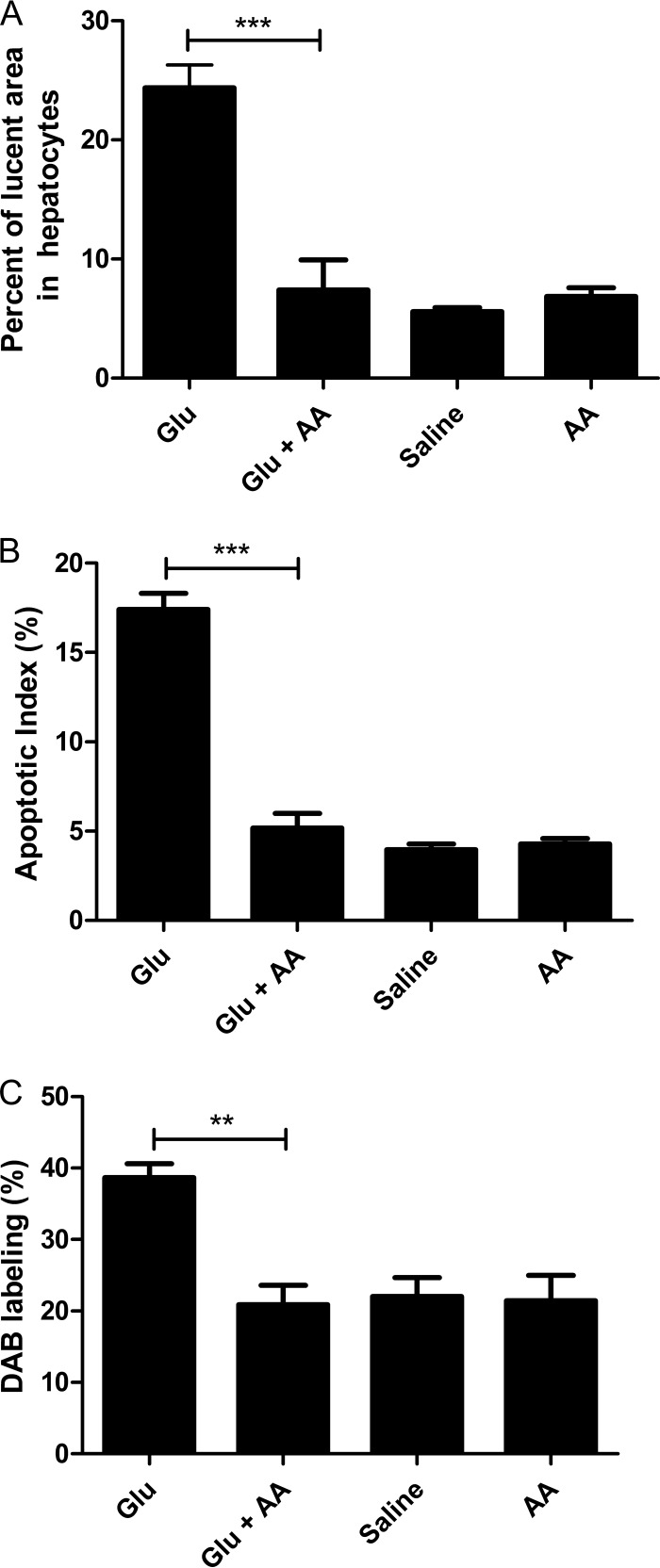
AA was evaluated in association with Glu for its ability to reduce hepatotoxicity in the murine model of VL. An experimental group received Glu at 80 mg of Sb/kg plus AA at 15 mg/kg daily for 20 days, and comparison was made with experimental groups treated with either Glu, AA, or saline. As shown in Fig. 4, cotreatment with AA (the Glu+AA group) reduced the lucent area of hepatocytes (Fig. 4A) and the apoptotic index (Fig. 4B) to an extent similar to that observed in saline-treated animals. The reduction of oxidative stress following cotreatment with AA was supported by the decrease of peroxidase activity compared to treatment with Glu alone (Fig. 4C).
FIG 4.
Influence of AA on the liver parameters of L. infantum-infected BALB/c mice treated or not to with commercial meglumine antimoniate (Glu). The liver parameters measured included the percentage of lucent area in the hepatocytes (A), the apoptotic index of the hepatocytes (B), and the peroxidase activity (C). Mice were treated with Glu (80 mg/kg/day), AA (15 mg/kg/day), Glu+AA, or saline alone for 20 days by the i.p. route. Data are means ± the SEM (n = 3 to 7). ***, P < 0.001; **, P < 0.01 (one-way ANOVA with Bonferroni post test).
In order to verify that the reduced histological alterations in Glu+AA group were not due to a lower uptake of Sb in the liver, the amount of Sb in the liver was compared between the Glu and the Glu+AA groups. A similar value of ∼4 μg of Sb/g of wet tissue was found in both groups.
Interference of ascorbic acid with the antileishmanial efficacy of Glu.
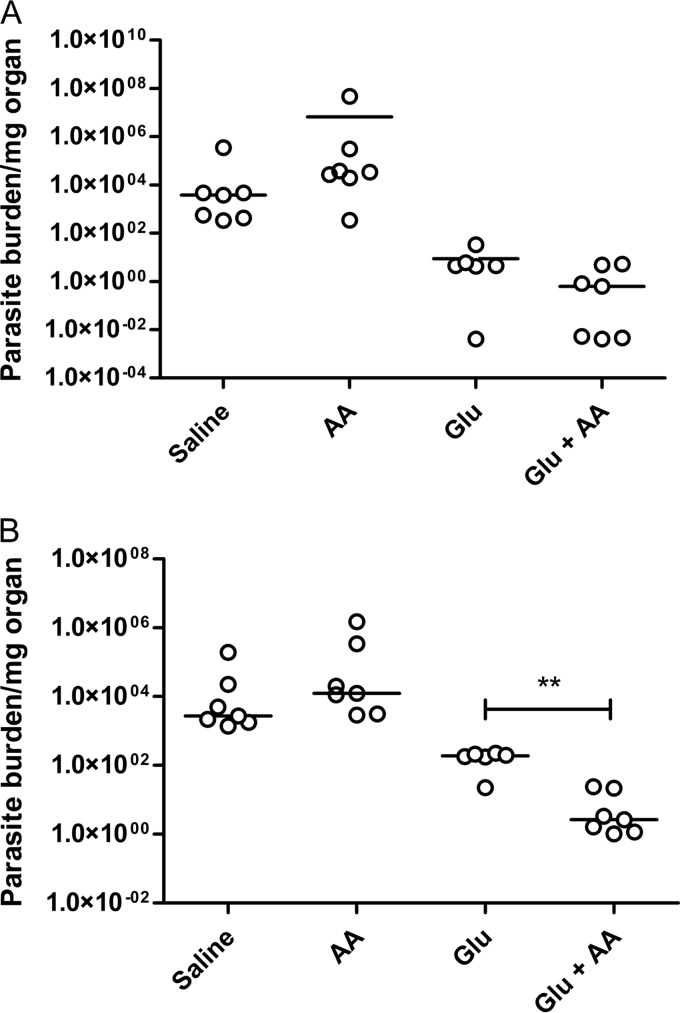
Since antimonial drugs seem to exert their activity against Leishmania parasite through the induction of oxidative stress (12, 37, 38), it was also important to evaluate the level of interference of the antioxidant AA on the efficacy of Glu. As shown in Fig. 5, the extent of parasite suppression in the liver did not differ significantly between the Glu+AA and the Glu groups. On the other hand, the association of AA with Glu improved the efficacy of the antimonial drug with regard to parasite suppression in the spleen. Thus, cotreatment with AA slightly increased the effectiveness of Glu in this VL model. In the case of MA, cotreatment with AA had no significant effect on the parasite suppressions in both the liver and the spleen (data not shown).
FIG 5.
Parasite loads in the liver (A) and spleen (B) in BALB/c mice infected with L. infantum after 20 days of treatment with Glu (80 mg of Sb/kg/day) in the presence or absence of AA (15 mg/kg/day) by the i.p. route. The data are shown as dot plots, and lines correspond to the median of each group (n = 6 to 7). **, P < 0.01 (Mann-Whitney test).
DISCUSSION
The present study reports for the first time a murine model of Glu-induced hepatotoxicity, based on histopathological changes and the increase of apoptotic index of hepatocytes. The observed changes are consistent with previous reports of histological alterations in the liver of nonhuman primate (39), elevation of serum level of hepatic enzymes (2–4), and hepatic failure (5) during pentavalent antimonial chemotherapy. The fact that among the different organs evaluated only the liver showed significant histopathological changes may be explained by the pharmacokinetics of meglumine antimoniate leading to elevated concentration of Sb in the liver (40).
Considering that toxic As and Pb metals were not detected by ICP-MS in the antimonial drugs and that MA with lower Sb(III) residue generated virtually no hepatotoxicity, one can reasonably assume that the hepatotoxicity of Glu is due to residual Sb(III) and its higher bioavailability from this antimonial compound. The apparently lower bioavailability of Sb(III) from MA is consistent with our previous study (18) showing a much lower cytotoxicity of this compound against a tumor cell line compared to Glu.
The proposal that residual Sb(III) in Glu promoted hepatotoxicity is consistent with the high dose of Sb(III) administered, which corresponds to ∼20 mg of Sb(III) per kg of body weight, based on 24% proportion of antimony under the trivalent form. Indeed, previous study of the subchronic toxicity of antimony(III) potassium tartrate in mice by the i.p. route revealed hepatocellular necrosis following 12 daily doses of 18 mg of Sb(III)/kg (41).
The discrepancy between Sb(III) concentrations determined by the different analytical methods is probably due to a combination of several experimental and/or chemical factors that promote a relatively strong complexation of Sb(III) to the meglumine antimoniate matrix in the photometric assay compared to the voltammetric assay: a higher pH value (pH 6.8 versus 3.2 for voltammetry), a much lower dilution factor for the BPR method (102 versus 107), and a higher ionic strength (20 versus 1 mM). These parameters (pH, dilution factor, and ionic strength) were all previously shown to have a significant influence on the level of Sb(III) determined in Glu (9). In that study, the amount of Sb(III) detected was shown to decrease markedly at higher pH, presumably because of pH-induced change in the chemical equilibrium of antimony complex(es) in solution. In addition, although the sample was diluted up to 108-fold at neutral pH (∼ 6), Sb(III) was still present as a complex, highlighting the strong complexation constant between Sb(III) and meglumine/meglumine antimoniate ligand (9). In the present work, the BPR assay is based on the competition between BPR and the meglumine/meglumine antimoniate ligand for Sb(III) binding. Thus, the much lower concentrations of Sb(III) detected by this method may be attributed to a relatively high pH value (6.8) and low dilution factor, which favored strong complexation of Sb(III) with the meglumine/meglumine antimoniate ligand and consequently decreased the amount of Sb(III)-BPR complex.
The fact that the amount of Sb(III) detected by the photometric method is at least 10-fold higher in Glu than MA, whereas it is only 1.5-fold higher by voltammetry, indicates that the pH and dilution conditions of the photometric assay promoted the release of Sb(III) more effectively from Glu than MA. This suggests that Sb(III) is more readily available from Glu than MA at neutral pH. A possible explanation is that the high-affinity ligand for Sb(III) may be saturated in Glu but not in MA.
The induction of peroxidase activity in the livers of mice treated with Glu is also in agreement with the role of Sb(III) in the mechanism of toxicity of this pentavalent antimonial drug (11, 18). Indeed, the interference of Sb(III) with the thiol homeostasis (13) results in the increase of ROS production, oxidative stress, and the induction of peroxidase activity and apoptosis (42, 43).
It is generally accepted that pentavalent antimonials are prodrugs that suffer reduction in vivo from Sb(V) to Sb(III) (10, 11). However, the recent observation that Glu contains high amounts of residual Sb(III) (9) led us to propose an alternative model, in which meglumine antimoniate may act as molecular carrier of Sb(III) and release it specifically in the acidic intracellular compartment where Leishmania parasite resides. Both models assume that Sb(III) is the final active form against the parasite, resulting in depletion of intracellular reduced thiols and enhanced susceptibility of the parasite to oxidative stress (12). Considering that Sb(III) mediates both the antileishmanial action and the toxicity of pentavalent antimonials, one may wonder why MA is active but apparently not toxic. A possible explanation for this is the selectivity of Sb(III) release that may occur in the acidic parasitophorous vacuoles of macrophages but to a lesser extent in the hepatocytes. This interpretation is also consistent with the finding that pentavalent antimonials are active against axenic amastigotes grown at pH 5.5 but relatively inactive against Leishmania promastigotes grown at pH 7 (12).
The demonstration that cotreatment with AA protects the liver against pentavalent antimonial drug toxicity is an important contribution of the present work. This effect may be attributed to the ability of AA to act as secondary defense against oxidative stress. Although a similar effect of AA was previously reported in the case of As(III)-based drug (24, 25), this is the first demonstration of a beneficial effect of AA for a metalloid drug in the pentavalent state. These data strongly suggest that cotreatment with AA may be able to reduce side effects in antimonial chemotherapy. Even though the demonstration of the protective effect of AA is limited to the liver, we expect that in future studies this effect will be extended to other critical organs such as the heart, pancreas, and kidney.
Surprisingly, AA was found to improve the antileishmanial efficacy of Glu. As a possible explanation, AA may reduce the intracellular thiol concentration of Leishmania parasite, as previously reported in multidrug-resistant tumor cells (21, 22), turning the antimonial drug more active (44). One should also consider that AA may exert either an antioxidant or a pro-oxidant activity, depending on the concentration and experimental conditions (45). It is noteworthy that the dose of AA used in our study was ∼10-fold lower than the effective dose of AA in the treatment of multiple myeloma in combination with arsenic trioxide, which exploits AA pro-oxidant activity (19, 20). Thus, we suggest here the coexistence two opposite actions of AA that may take place in distinct cellular compartments: an antioxidant action reducing the drug side effects and a pro-oxidant action enhancing the drug efficacy. In conclusion, our data support the use of pentavalent antimonials with low amount of Sb(III) and the association of pentavalent antimonials with AA as effective strategies for minimizing the side effects mediated by oxidative stress during antimonial therapy.
ACKNOWLEDGMENTS
We acknowledge the Brazilian agencies CNPq (303046/2009 and a studentship), FAPEMIG (PPM-00382-11, REDE 40/11, and CBB-APQ-01123-09), and CAPES (2447/2009 and a studentship) for financial support. F.F. and C.D. are recipients of a research fellowship from CNPq.
Footnotes
Published ahead of print 4 November 2013
REFERENCES
- 1.Vianna G. 1912. Tratamento da leishmaniose tegumentar por injeções intravenosas de tártaro emético. Ann. 7th Cong. Bras. Med. Cirurg. 4:426–428 [Google Scholar]
- 2.Marsden PD. 1985. Pentavalent antimonials: old drugs for new diseases. Rev. Soc. Bras. Med. Trop. 18:187–198. 10.1590/S0037-86821985000300011 [DOI] [Google Scholar]
- 3.Berman JD. 1997. Human leishmaniasis: clinical, diagnostic, and chemotherapeutic developments in the last 10 years. Clin. Infect. Dis. 24:684–703. 10.1093/clind/24.4.684 [DOI] [PubMed] [Google Scholar]
- 4.Hepburn NC, Siddique I, Howie AF, Beckett GJ, Hayes PC. 1994. Hepatotoxicity of sodium stibogluconate in leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 88:453–455. 10.1016/0035-9203(94)90432-4 [DOI] [PubMed] [Google Scholar]
- 5.Oliveira AL, Brustoloni YM, Fernandes TD, Dorval ME, Cunha RV, Bóia MN. 2009. Severe adverse reactions to meglumine antimoniate in the treatment of visceral leishmaniasis: a report of 13 cases in the southwestern region of Brazil. Trop. Doct. 39:180–182. 10.1258/td.2008.080369 [DOI] [PubMed] [Google Scholar]
- 6.Oliveira LF, Schubach AO, Martins MM, Passos SL, Oliveira RV, Marzochi MC, Andrade CA. 2011. Systematic review of the adverse effects of cutaneous leishmaniasis treatment in the New World. Acta Trop. 118:87–96. 10.1016/j.actatropica.2011.02.007 [DOI] [PubMed] [Google Scholar]
- 7.Mlika RB, Hamida MB, Hammami H, Jannet SB, Badri T, Fenniche S, Mokhtar I. 2012. Should we continue to indicate meglumine antimoniate as first-line treatment for cutaneous leishmaniasis in Tunisia. Dermat. Ther. 25:615–618. 10.1111/j.1529-8019.2012.01522.x [DOI] [PubMed] [Google Scholar]
- 8.Wise ES, Armstrong MS, Watson J, Lockwood DN. 2012. Monitoring toxicity associated with parenteral sodium stibogluconate in the day-case management of returned travelers with New World cutaneous leishmaniasis. PLoS Negl. Trop. Dis. 6:e1688. 10.1371/journal.pntd.0001688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salaün P, Frézard F. 2013. Unexpectedly high levels of antimony (III) in the pentavalent antimonial drug Glucantime: insights from a new voltammetric approach. Anal. Bioanal. Chem. 405:5201–5214. 10.1007/s00216-013-6947-5 [DOI] [PubMed] [Google Scholar]
- 10.Goodwin LG, Page JE. 1943. A study of the excretion of organic antimonials using a polarographic procedure. Biochem. J. 37:198–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frézard F, Demicheli C, Ribeiro RR. 2009. Pentavalent antimonials: new perspectives for old drugs. Molecules 14:2317–2336. 10.3390/molecules14072317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wyllie S, Cunningham ML, Fairlamb AH. 2004. Dual action of antimonial drugs on thiol redox metabolism in the human pathogen Leishmania donovani. J. Biol. Chem. 279:39925–39932. 10.1074/jbc.M405635200 [DOI] [PubMed] [Google Scholar]
- 13.Wyllie S, Fairlamb AH. 2006. Differential toxicity of antimonial compounds and their effects on glutathione homeostasis in a human leukaemia monocyte cell line. Biochem. Pharmacol. 71:257–267. 10.1016/j.bcp.2005.10.043 [DOI] [PubMed] [Google Scholar]
- 14.Timerstein MA, Plews PI, Walker CV, Woolery MD, Wey HE, Toraason MA. 1995. Antimony induces oxidative-stress and toxicity in cultured cardiac myocytes. Toxicol. Appl. Pharmacol. 130:41–47. 10.1006/taap.1995.1006 [DOI] [PubMed] [Google Scholar]
- 15.Pulido MD, Parrish AR. 2003. Metal-induced apoptosis: mechanisms. Mutat. Res. 533:227–241. 10.1016/j.mrfmmm.2003.07.015 [DOI] [PubMed] [Google Scholar]
- 16.Silva-Junior JB. 2001. Relatório técnico antimoniato de meglumina. Rev. Soc. Bras. Med. Trop. 34:103–105. 10.1590/S0037-86822001000100017 [DOI] [PubMed] [Google Scholar]
- 17.Demicheli C, Ochoa R, Lula IS, Gozzo FC, Eberlin MN, Frézard F. 2003. Pentavalent organoantimonial derivatives: two simple and efficient synthetic methods for meglumine antimonate. Appl. Organomet. Chem. 17:226–231. 10.1002/aoc.425 [DOI] [Google Scholar]
- 18.Dzamitika SA, Falcão CAB, Oliveira FB, Marbeuf C, Garnier-Suillerot A, Demicheli C, Rossi-Bergmann B, Frézard F. 2006. Role of residual Sb(III) in meglumine antimoniate cytotoxicity and MRP1-mediated resistance. Chem. Biol. Interact. 160:217–224. 10.1016/j.cbi.2006.01.008 [DOI] [PubMed] [Google Scholar]
- 19.González MJ, Miranda-Massari JR, Mora EM, Guzmán A, Riordan NH, Riordan HD, Casciari JJ, Jackson JA, Román-Franco A. 2005. Orthomolecular oncology review: ascorbic acid and cancer 25 years later. Integ. Cancer Ther. 4:32–44. 10.1177/1534735404273861 [DOI] [PubMed] [Google Scholar]
- 20.Chen Q, Espey MG, Krishna MC, Mitchell JB, Corpe CP, Buettner GR, Shacter E, Levine M. 2005. Pharmacologic ascorbic acid concentrations selectively kill cancer cells: action as a pro-drug to deliver hydrogen peroxide to tissues. Proc. Natl. Acad. Sci. U. S. A. 102:13604–13609. 10.1073/pnas.0506390102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fromberg A, Gutsch D, Schulze D, Vollbracht C, Weiss G, Czubayko F, Aigner A. 2011. Ascorbate exerts anti-proliferative effects through cell cycle inhibition and sensitizes tumor cells toward cytostatic drugs. Cancer Chemother. Pharmacol. 67:1157–1166. 10.1007/s00280-010-1418-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bahlis NJ, McCafferty-Grad J, Jordan-McMurry I, Neil J, Reis I, Kharfan-Dabaja M, Eckman J, Goodman M, Fernandez HF, Boise LH, Lee KP. 2002. Feasibility and correlates of arsenic trioxide combined with ascorbic acid-mediated depletion of intracellular glutathione for the treatment of relapsed/refractory multiple myeloma. Clin. Cancer Res. 8:3658–3668 [PubMed] [Google Scholar]
- 23.Qazilbash MH, Saliba RM, Nieto Y, Parikh G, Pelosini M, Khan FB, Jones RB, Hosing C, Mendoza F, Weber DM, Wang M, Popat U, Alousi A, Anderlini P, Champlin RE, Giralt S. 2008. Arsenic trioxide with ascorbic acid and high-dose melphalan: results of a phase II randomized trial. Biol. Blood Marrow Transplant. 14:1401–1407. 10.1016/j.bbmt.2008.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh S, Rana SVS. 2007. Amelioration of arsenic toxicity by l-ascorbic acid in laboratory rats. J. Environ. Biol. 28:377–384 [PubMed] [Google Scholar]
- 25.Singh S, Rana SVS. 2007. Protective effect of ascorbic acid against oxidative stress induced by inorganic arsenic in liver and kidney of rat. Indian J. Exp. Biol. 45:371–375 [PubMed] [Google Scholar]
- 26.Chen Q, Espey MG, Sun AY, Lee J-H, Krishna MC, Shacter E, Choyke PL, Pooput C, Kirk KL, Buettner GR, Levine M. 2007. Ascorbate in pharmacologic concentrations selectively generates ascorbate radical and hydrogen peroxide in extracellular fluid in vivo. Proc. Natl. Acad. Sci. U. S. A. 104:8749–8754. 10.1073/pnas.0702854104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Titus RG, Marchand M, Boon T, Louis JA. 1985. A limiting dilution assay for quantifying Leishmania major in tissues of infected mice. Parasite Immunol. 7:545–555. 10.1111/j.1365-3024.1985.tb00098.x [DOI] [PubMed] [Google Scholar]
- 28.Schettini DA, Ribeiro RR, Demicheli C, Rocha OGF, Melo MN, Michalick MSM, Frézard F. 2006. Improved targeting of antimony to the bone marrow of dogs using liposomes of reduced size. Int. J. Pharm. 315:140–147. 10.1016/j.ijpharm.2006.01.048 [DOI] [PubMed] [Google Scholar]
- 29.Luna LG. 1968. Production, p 38–39 In Luna LG. (ed), Manual of histologic staining methods of the Armed Forces Institute of Pathology, Blakiston Division, 3rd ed. McGraw-Hill Book Co, New York, NY [Google Scholar]
- 30.Trott JR. 1961. An evaluation of methods commonly used for the fixation and staining of glycogen. J. Histochem. Cytochem. 9:703–710. 10.1177/9.6.703 [DOI] [PubMed] [Google Scholar]
- 31.Angermuller S, Fahimi HD. 1983. Selective staining of cell organelles in rat liver with 3,3′-diaminobenzidine. J. Histochem. Cytochem. 31:230–232. 10.1177/31.1A_Suppl.6186726 [DOI] [PubMed] [Google Scholar]
- 32.Seedorf U, Raabe M, Ellinghaus P, Kannenberg F, Fobker M, Engel T, Denis S, Wouters F, Wirtz KWA, Wanders RJA, Maeda N, Assmann G. 1998. Defective peroxisomal catabolism of branched fatty acyl coenzyme A in mice lacking the sterol carrier protein-2/sterol carrier protein-x gene function. Genes Dev. 12:1189–1201. 10.1101/gad.12.8.1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beier K. 1992. Light microscopic morphometric analysis of peroxisomes by automatic image analysis: advantages of immunostaining over the alkaline DAB method. J. Histochem. Cytochem. 40:115–121. 10.1177/40.1.1370307 [DOI] [PubMed] [Google Scholar]
- 34.Soini Y, Paakko P, Lehto VP. 1998. Histopathological evaluation of apoptosis in cancer. Am. J. Pathol. 153:1041–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ribeiro MC, Coutinho LMB, Hilbi A. 2004. The role of apoptosis, cell proliferation index, bcl-2, and p53 in glioblastoma prognosis. Arq. Neuropsiquiatr. 62:262–270. 10.1590/S0004-282X2004000200014 [DOI] [PubMed] [Google Scholar]
- 36.Gavrieli Y, Sherman Y, Ben-Sasson SA. 1992. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J. Cell Biol. 119:493–501. 10.1083/jcb.119.3.493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sudhandiran G, Shaha C. 2003. Antimonial-induced increase in intracellular Ca2+ through non-selective cation channels in the host and the parasite is responsible for apoptosis of intracellular Leishmania donovani amastigotes. J. Biol. Chem. 278:25120–25132. 10.1074/jbc.M301975200 [DOI] [PubMed] [Google Scholar]
- 38.Basu JM, Mookerjee A, Sen P, Bhaumik S, Sen P, Banerjee S, Naskar K, Choudhuri SK, Saha B, Raha S, Roy S. 2006. Sodium antimony gluconate induces generation of reactive oxygen species and nitric oxide via phosphoinositide 3-kinase and mitogen-activated protein kinase activation in Leishmania donovani-infected macrophages. Antimicrob. Agents Chemother. 50:1788–1797. 10.1128/AAC.50.5.1788-1797.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grimaldi GJr, Porozzi R, Friedrich K, Teva A, Marchevsky RS, Vieria F, Miekeley N, Paumgartten FJR. 2010. Comparative efficacies of two antimony regimens to treat Leishmania braziliensis induced cutaneous leishmaniasis in rhesus macaques (Macaca mulatta). Antimicrob. Agents Chemother. 54:502–505. 10.1128/AAC.00858-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carter KC, Baillie AJ, Alexander J, Dolan TF. 1988. The therapeutic effect of sodium stibogluconate in BALB/c mice infected with Leishmania donovani is organ dependent. J. Pharm. Pharmacol. 40:370–373. 10.1111/j.2042-7158.1988.tb05271.x [DOI] [PubMed] [Google Scholar]
- 41.Lynch BS, Capen CC, Nestmann ER, Veenstra G, Dey JA. 1999. Review of subchronic/chronic toxicity of antimony potassium tartrate. Regul. Toxicol. Pharmacol. 30:9–17. 10.1006/rtph.1999.1312 [DOI] [PubMed] [Google Scholar]
- 42.Lecureur V, Le Thiec A, Le Meur A, Amiot L, Drenou B, Bernard M, Lamy T, Fauchet R, Fardel O. 2002. Potassium antimonyl tartrate induces caspase- and reactive oxygen species-dependent apoptosis in lymphoid tumoral cells. Br. J. Haematol. 119:608–615. 10.1046/j.1365-2141.2002.03863.x [DOI] [PubMed] [Google Scholar]
- 43.Lösler S, Schlief S, Kneifel C, Thiel E, Schrezenmeier H, Rojewski MT. 2009. Antimony trioxide- and arsenic-trioxide-induced apoptosis in myelogenic and lymphatic cell lines, recruitment of caspases, and loss of mitochondrial membrane potential are enhanced by modulators of the cellular glutathione redox system. Ann. Hematol. 88:1047–1058. 10.1007/s00277-009-0736-4 [DOI] [PubMed] [Google Scholar]
- 44.Goyeneche-Patino DA, Valderrama L, Walker J, Saravia NG. 2008. Antimony resistance and trypanothione in experimentally selected and clinical strains of Leishmania panamensis. Antimicrob. Agents Chemother. 52:4503–4506. 10.1128/AAC.01075-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bijur GN, Ariza ME, Hitchcock CL, Williams MV. 1997. Antimutagenic and promutagenic activity of ascorbic acid during oxidative stress. Environ. Mol. Mutagen. 30:339–345. [DOI] [PubMed] [Google Scholar]