Abstract
Among 119 echinocandin non-wild-type (non-WT) Candida glabrata strains from two global surveys, mutations in fks hot spots (HSs) were detected in 28 (from 7 countries and 8 U.S. states): 24 strains (85.7%) had non-WT MICs for micafungin, 22 (78.6%) for anidulafungin, and 25 (89.3%) for caspofungin. The most common FKS substitutions among non-WT strains were at positions F659 (n = 7) and S663 (n = 7). Three isolates displaying WT MIC results had F625Y, L630I, and D632Y substitutions or non-HS mutations. Mutations that have been reported to decrease the echinocandin binding to the 1,3-β-d-glucan synthase were categorized as resistant by applying the new CLSI breakpoint criteria for all three echinocandins.
TEXT
Candida glabrata is a leading human fungal pathogen that causes life-threatening infections and poses a challenge to antifungal therapeutic options due to its refractory susceptibility to the azole agents and the ability to develop resistance to both azoles and the echinocandins (1, 2). Echinocandin resistance in C. glabrata is usually caused by the acquisition of point mutations in hot spot (HS) regions of the fks gene encoding the 1,3-β-d-glucan synthase (GS), the target of the echinocandin class (3). Furthermore, it is thought that the haploid nature of C. glabrata makes it particularly adept at acquiring resistance mutations.
Due to the spectrum and fungicidal potency against Candida spp., the echinocandin agents (anidulafungin, caspofungin, and micafungin) are recommended as first-line therapy for most patients with invasive candidiasis, followed by de-escalation to fluconazole, as directed by the susceptibility of the infecting organism. Resistance to the systemically active echinocandin and azole antifungal agents is distinctly uncommon among bloodstream infection isolates of C. albicans, C. tropicalis, and C. parapsilosis; however, the emergence of resistance to both classes of antifungal agents is now evident for C. glabrata (4), suggesting that this species requires greater monitoring for resistance. Furthermore, a 10-year study conducted in a single U.S. medical center found that the presence of an elevated caspofungin MIC (>0.12 μg/ml) or micafungin MIC (>0.06 μg/ml) served as a sensitive screen for the presence of clinically significant mutations in the fks gene (2).
In the present study, we evaluated all C. glabrata strains collected during two global surveillance programs from 2001 to 2011 that displayed MIC values above the epidemiologic cutoff values (ECVs) or had non-wild-type (non-WT) MICs (5, 6) for one or more of the echinocandins upon initial testing. Isolates were retested by the reference broth microdilution (BMD) method (7, 8) and were evaluated for the presence of fks mutations.
A total of 119 C. glabrata isolates were selected for analysis and were equally distributed among the two surveillance studies and throughout the years tested. These were all isolates that upon initial testing displayed non-WT MIC values for one or more of the echinocandins (micafungin, 26 isolates with non-WT MICs; caspofungin, 83 isolates; anidulafungin, 67 isolates). All 119 isolates were retrieved from storage and tested against three echinocandins to confirm the antifungal susceptibility pattern using the CLSI BMD according to the M27-A3 guideline (7). Results were read visually after a 24-h incubation as the lowest concentration producing prominent inhibition (≥50%) of growth relative to that of the drug-free control for the echinocandins (anidulafungin, caspofungin, and micafungin) and three azoles (fluconazole, posaconazole, and voriconazole). Quality control (QC) was ensured using recognized QC strains Candida krusei ATCC 6258 and C. parapsilosis ATCC 22019, and ranges were within those published in CLSI guideline M27-S4 (8).
Additionally, all isolates were characterized regarding the presence or absence of mutations in the HS regions of the fks1, fks2, and fks3 genes as described earlier (3, 9). In a subset of 22 isolates, the complete fks1 and fks2 genes were sequenced (a list of oligonucleotides used is available from the author).
Repeat susceptibility testing results revealed that 31 (26.0%), 23 (19.3%), and 39 (32.8%) isolates had MIC values that were greater than the respective ECVs (5) for micafungin (ECV, 0.03 μg/ml), anidulafungin (ECV, 0.25 μg/ml), and caspofungin (ECV, 0.12 μg/ml), respectively. Whereas the original MIC values were considered to be non-WT for at least one of the agents, results were lower upon retesting. The vast majority of the changes in MIC results generated upon repeat testing showed MIC results that were 1 or 2 doubling dilutions lower for isolates previously categorized as susceptible or intermediate. Notably, isolates for which MIC values were >1 μg/ml (categorized as resistant) to any echinocandin remained resistant upon repeat testing. The differences between initial MIC results and repeat results were noted equally for the two programs and could be associated with variability in testing and/or reagents or with the difficult of precisely establishing a 50% MIC endpoint.
Mutations on the HS regions of the 1,3-β-d-GS-encoding genes were detected among 28 strains (Table 1). A total of 14 strains (51.8% of the strains carrying mutations) had substitutions in positions S663 and F659 (seven each) of FKS2 HS1, leading to three different substitution types for each locus (Table 1). The most common mutations were S629P on FKS1 HS1 (four strains) and S663P on FKS2 HS1 (four strains). One isolate displayed a substitution on FKS2 HS2 (P1371S), and no mutations were detected for fks1 HS2 or the fks3 gene compared to the echinocandin-susceptible C. glabrata ATCC MYA-2950 sequence. Isolates carrying mutations on fks HSs were detected in seven countries and were more common in the United States (15 strains; eight states) and Germany (5 strains). Eleven (39.3%) of these isolates harboring fks HS mutations were also resistant to fluconazole (Table 1).
TABLE 1.
FKS hot spot alterations in isolates of Candida glabrataa
| FKS alterationb | Yr | Sourcec | MIC (μg/ml) |
|||
|---|---|---|---|---|---|---|
| Micafungin | Anidulafungin | Caspofungin | Fluconazole | |||
| FKS1 HS1 | ||||||
| F625Y | 2010 | Texas | 0.03 | 0.25 | 0.12 | 16 |
| F625S | 2011 | Australia | 0.12 | 1 | 0.5 | 128 |
| S629P | 2008 | Ohio | 1 | 2 | 16 | 128 |
| S629P | 2008 | Washington | 0.5 | 2 | 16 | 32 |
| S629P | 2011 | Louisiana | 4 | 2 | 16 | 128 |
| S629P, R631S | 2008 | Indiana | 16 | 4 | 2 | 8 |
| L630I | 2009 | Massachusetts | 0.015 | 0.03 | 0.03 | 4 |
| D632Y | 2008 | Washington | 0.015 | 0.25 | 0.12 | 128 |
| FKS2 HS1 | ||||||
| F659S | 2006 | Germany | 0.06 | 0.5 | 0.5 | 16 |
| F659S | 2007 | Indiana | 0.06 | 0.25 | 0.25 | 16 |
| F659S | 2009 | Germany | 0.06 | 1 | 0.5 | 128 |
| F659V | 2003 | Japan | 0.25 | 1 | 8 | 2 |
| F659V | 2006 | Virginia | 0.12 | 1 | 2 | 128 |
| F659Y | 2005 | Ohio | 0.12 | 1 | 1 | 4 |
| F659Y | 2011 | Canada | 0.25 | 1 | 2 | 16 |
| L662W | 2004 | Japan | 0.5 | 2 | 1 | 64 |
| L662W | 2010 | Germany | 0.25 | 1 | 0.5 | 2 |
| S663P | 2008 | Germany | 0.25 | 1 | 1 | 64 |
| S663P | 2008 | Indiana | 4 | 4 | 16 | 4 |
| S663P | 2011 | Australia | 2 | 2 | 2 | 128 |
| S663P | 2011 | Greece | 2 | 4 | 4 | 16 |
| S663F | 2008 | Washington | 0.12 | 0.5 | 0.25 | 4 |
| S663F | 2010 | Ohio | 0.12 | 0.5 | 0.25 | 4 |
| S663Y | 2011 | Indiana | 0.5 | 1 | 0.5 | 8 |
| L664R | 2004 | Spain | 0.25 | 1 | 0.25 | 128 |
| L664R | 2004 | Spain | 0.25 | 1 | 0.5 | 64 |
| D666Y | 2005 | Germany | 0.06 | 0.12 | 0.25 | 8 |
| FKS2 HS2 | ||||||
| P1371S | 2011 | New York | 0.03 | 0.12 | 0.25 | 4 |
Strains were collected from 2001 to 2011 in two global surveillance studies.
Amino acid substitutions of FKS1 HS2 or FKS3 HS1 were not detected.
The U.S. state or country is given.
Among 22 strains selected for the full sequencing of the fks1 and fks2 genes, 13 had non-HS mutations (Table 2), four of which also harbored HS mutations.
TABLE 2.
C. glabrata strains displaying non-HS FKS alterationsa
| Isolate | Yr | Source | MIC (μg/ml) |
FKS alterationb |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| FKS1 HS1 | FKS2 HS1 | FKS2 HS2 | Non-HS |
||||||||
| Micafungin | Anidulafungin | Caspofungin | Fluconazole | FKS1 | FKS2 | ||||||
| 46228A | 2011 | South Africa | 0.015 | 0.06 | 0.25 | 128 | Neg | Neg | Neg | Y1279F | T926P |
| 49889F | 2011 | Canada | 0.015 | 0.03 | 0.25 | 32 | Neg | Neg | Neg | Neg | G680S |
| 10729J | 2004 | United Kingdom | 0.015 | 0.06 | 0.25 | 1 | Neg | Neg | Neg | L195F | Neg |
| 10685J | 2004 | Spain | 0.015 | 0.06 | 0.25 | 32 | Neg | Neg | Neg | Neg | F384Y |
| 10702J | 2005 | Spain | 0.015 | 0.12 | 0.25 | 16 | Neg | Neg | Neg | A995T | Neg |
| 10728J | 2002 | Spain | 0.015 | 0.06 | 0.25 | 1 | Neg | Neg | Neg | D140Y, A1749V | Neg |
| 44075F | 2011 | Washington | 0.03 | 0.06 | 0.12 | 32 | Neg | Neg | Neg | Neg | T926P |
| 17823F | 2011 | Michigan | 0.06 | 0.12 | 0.06 | 128 | Neg | Neg | Neg | Neg | A450T |
| 10708J | 2005 | Poland | 0.06 | 0.25 | 0.25 | 16 | Neg | Neg | Neg | A1749V | Neg |
| 760F | 2008 | Washington | 0.12 | 0.5 | 0.25 | 4 | Neg | S663F | Neg | Neg | T926P |
| 37706F | 2011 | United States | 0.5 | 1 | 0.5 | 8 | Neg | S663Y | Neg | Neg | G680S |
| 10700J | 2004 | Japan | 0.5 | 2 | 1 | 64 | Neg | L662W | Neg | Neg | E78D, T926P |
| 49079F | 2011 | Australia | 2 | 2 | 2 | >128 | Neg | S663P | Neg | Neg | T926P |
Strains were collected from 2001 to 2011 in two global surveillance studies.
Neg, Negative.
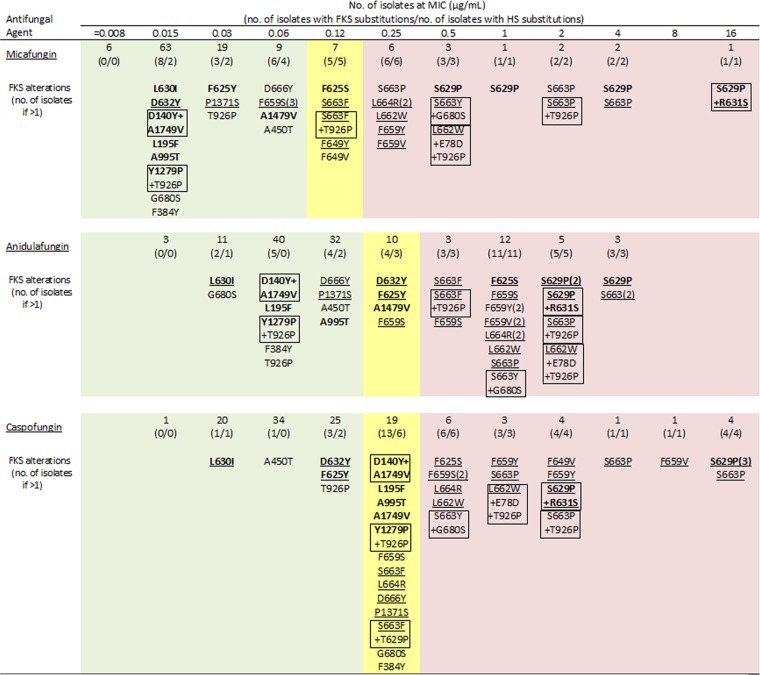
Seven isolates had more than one amino acid substitution: two strains had two different non-HS alterations; four strains had one HS plus one or two non-HS substitutions, and one strain had two HS mutations leading to S629P and R631S alterations (Fig. 1). The isolate carrying two HS alterations had a micafungin MIC of 16 μg/ml, an anidulafungin MIC of 2 μg/ml, and a caspofungin MIC of 4 μg/ml. Of the isolates displaying one or two non-HS substitutions, all had micafungin MIC values of ≤0.12 μg/ml and were categorized as susceptible using revised CLSI breakpoints (Fig. 1). Eight strains carrying a non-HS substitution had MIC values of 0.25 μg/ml for caspofungin and were categorized as intermediate using revised breakpoints (Fig. 1).
FIG 1.
MIC distribution for echinocandins tested against 119 C. glabrata strains and FKS alterations at each MIC value as tested using the CLSI reference method. FKS1 alterations are in boldface, and hot spot (HS) alterations are underlined. Combinations of amino acid substitutions in a single strain are boxed. MIC values corresponding to the CLSI breakpoint criteria are highlighted in green for susceptible, yellow for intermediate, and light red for resistant.
Among 82 isolates showing no FKS alterations, only one was categorized as resistant to anidulafungin and caspofungin (MIC, 1 μg/ml for both), but this isolate was susceptible to micafungin (MIC, 0.06 μg/ml). One, seven, and five isolates harboring no fks mutations had a MIC that was greater than the ECV for anidulafungin, caspofungin, and micafungin, respectively.
All isolates categorized as micafungin intermediate (5 strains) or resistant (15; MIC, >0.12 μg/ml) displayed HS substitutions, and the highest MIC values were achieved by the presence of two HS alterations (Fig. 1). Among the isolates categorized as resistant (MIC, >0.25 μg/ml) to anidulafungin (23 isolates) and to caspofungin (19 isolates), 22 (95.2%) and 19 (100.0%), respectively, harbored HS substitutions. Eight echinocandin-susceptible strains displayed HS substitutions; however, these specific mutations were not detected in intermediate or resistant strains. This is consistent with the results recently published demonstrating that isolates displaying low micafungin MIC values respond to treatment with this echinocandin regardless of the presence of mutations (10). Mutations detected for echinocandin-susceptible strains should be further studied to clarify their role in clinical outcomes.
This study highlights a possible issue with the echinocandin MIC testing and suggests that isolates for which MIC values range from 0.03 to 0.25 μg/ml for any of the echinocandins may show poor reproducibility upon repeat reference testing. Furthermore, reference strains currently used for QC, and recommended by CLSI and EUCAST guidelines (8, 11), may not be optimal to monitor echinocandin susceptibility testing of C. glabrata.
Among 119 C. glabrata strains from this 10-year worldwide collection, FKS alterations were detected in 37 strains (31.1%) tested with elevated MIC values; 28 of these strains had HS substitutions. Our results suggest that the current CLSI breakpoints (8) for C. glabrata and all three echinocandins are appropriate for the differentiation of populations carrying HS and non-HS alterations, including those that are documented to generate poor clinical responses (2, 12, 13). Although caspofungin seemed more sensitive in detecting fks mutations, micafungin provided better differentiation of those isolates with clinically important amino acid substitutions (categorized as resistant) from those harboring non-HS alterations (categorized as susceptible or intermediate) or no HS substitutions (categorized as susceptible) (2, 12, 13).
Finally, the lack of reproducibility of caspofungin MIC test results due to the variability in reagent preparation and quality, as reported by Arendrup et al. (14), could lead to the selection of micafungin test results as a better predictor for echinocandin resistance in C. glabrata.
ACKNOWLEDGMENTS
The global antifungal surveillance programs which served as the source of data used in the development of the manuscript were supported in part by Pfizer, Inc., and by Astellas, Inc. An educational grant from Astellas was received to perform this study.
We acknowledge the excellent technical assistance of R. Dietrich and S. E. Farrell in performing the susceptibility testing and sequencing analysis and S. Benning in the preparation of the manuscript.
JMI Laboratories, Inc., has received research and educational grants from 2011 to 2013 from Aires, American Proficiency Institute (API), Anacor, Astellas, AstraZeneca, Bayer, bioMérieux, Cempra, Cerexa, Contrafect, Cubist, Dipexium, Furiex, GlaxoSmithKline, Johnson & Johnson (J&J), LegoChem Biosciences, Inc., Meiji Seika Kaisha, Merck, Nabriva, Novartis, Pfizer, PPD Therapeutics, Premier Research Group, Rempex, Rib-X Pharmaceuticals, Seachaid, Shionogi, The Medicines Co., Theravance, and ThermoFisher Scientific. Some JMI employees are advisors/consultants for Astellas, Cubist, Pfizer, Cempra, Cerexa-Forest, J&J, and Theravance. In regard to speakers bureaus and stock options, we have none to declare.
D. Diekema has received research funding from Cerexa, bioMérieux, Pfizer, T2 biosystems, and PurThread, Inc.; no speakers bureau or consultant/advisor work was performed.
Footnotes
Published ahead of print 14 October 2013
REFERENCES
- 1.Katiyar SK, Alastruey-Izquierdo A, Healey KR, Johnson ME, Perlin DS, Edlind TD. 2012. Fks1 and Fks2 are functionally redundant but differentially regulated in Candida glabrata: implications for echinocandin resistance. Antimicrob. Agents Chemother. 56:6304–6309. 10.1128/AAC.00813-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexander BD, Johnson MD, Pfeiffer CD, Jimenez-Ortigosa C, Catania J, Booker R, Castanheira M, Messer SA, Perlin DS, Pfaller MA. 2013. Increasing echinocandin resistance in Candida glabrata: clinical failure correlates with presence of FKS mutations and elevated minimum inhibitory concentrations. Clin. Infect. Dis. 56:1724–1732. 10.1093/cid/cit136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castanheira M, Woosley LN, Pfaller MA, Diekema DJ, Messer SA, Jones RN. 2010. Low prevalence of fks1 hotspot 1 mutations in a worldwide collection of Candida spp. Antimicrob. Agents Chemother. 54:2655–2659. 10.1128/AAC.01711-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ostrosky-Zeichner L. 2013. Candida glabrata and FKS mutations: witnessing the emergence of the true multidrug-resistant Candida. Clin. Infect. Dis. 56:1733–1734. 10.1093/cid/cit140 [DOI] [PubMed] [Google Scholar]
- 5.Pfaller MA, Castanheira M, Diekema DJ, Messer SA, Jones RN. 2011. Triazole and echinocandin wild-type MIC distributions with epidemiological cutoff values for six uncommon species of Candida. J. Clin. Microbiol. 49:3800–3804. 10.1128/JCM.05047-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turnidge J, Kahlmeter G, Kronvall G. 2006. Statistical characterisation of bacterial wild-type MIC value distributions and the determination of epidemiological cut-off values. Clin. Microbiol. Infect. 12:418–425. 10.1111/j.1469-0691.2006.01377.x [DOI] [PubMed] [Google Scholar]
- 7.Clinical and Laboratory Standards Institute 2008. M27-A3. Reference method for broth dilution antifungal susceptibility testing of yeasts, 3rd ed. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 8.Clinical and Laboratory Standards Institute 2012. M27-S4 Reference method for broth dilution antifungal susceptibility testing of yeasts, 4th informational supplement Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 9.Healey KR, Katiyar SK, Castanheira M, Pfaller MA, Edlind TD. 2011. Candida glabrata mutants demonstrating paradoxical caspofungin reduced susceptibility but micafungin increased susceptibility. Antimicrob. Agents Chemother. 55:3947–3949. 10.1128/AAC.00044-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lepak A, Castanheira M, Diekema D, Pfaller M, Andes D. 2012. Optimizing echinocandin dosing and susceptibility breakpoint determination via in vivo pharmacodynamic evaluation against Candida glabrata with and without fks mutations. Antimicrob. Agents Chemother. 56:5875–5882. 10.1128/AAC.01102-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arendrup MC, Cuenca-Estrella M, Lass-Florl C. 2012. EUCAST technical note on the EUCAST definitive document EDef 7.2: method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for yeasts EDef 7.2 (EUCAST-AFST). Clin. Microbiol. Infect. 18:E246–E247. 10.1111/j.1469-0691.2012.03880.x [DOI] [PubMed] [Google Scholar]
- 12.Arendrup MC, Perlin DS, Jensen RH, Howard SJ, Goodwin J, Hope W. 2012. Differential in vivo activity of anidulafungin, caspofungin, and micafungin against C. glabrata with and without FKS resistance mutations. Antimicrob. Agents Chemother. 56:2435–2442. 10.1128/AAC.06369-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perlin DS. 2007. Resistance to echinocandin-class antifungal drugs. Drug Resist. Updat. 10:121–130. 10.1016/j.drup.2007.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arendrup MC, Rodriguez-Tudela JL, Park S, Garcia-Effron G, Delmas G, Cuenca-Estrella M, Gomez-Lopez A, Perlin DS. 2011. Echinocandin susceptibility testing of Candida spp. using EUCAST EDef 7.1 and CLSI M27-A3 standard procedures: analysis of the influence of bovine serum albumin supplementation, storage time, and drug lots. Antimicrob. Agents Chemother. 55:1580–1587. 10.1128/AAC.01364-10 [DOI] [PMC free article] [PubMed] [Google Scholar]