Abstract
Candida parapsilosis isolates occasionally display resistance in vitro to echinocandins and cause breakthrough infections to echinocandins. The degree of the in vivo cross-resistance among echinocandins and the fitness loss associated with caspofungin (CAS) resistance of C. parapsilosis are not well studied. We compared the activities of CAS and anidulafungin (ANF), each given at 2 dosing schedules (high dose or low dose) in a nonneutropenic mouse model of invasive candidiasis (IC) caused by ANF-susceptible isolates of C. parapsilosis with different degrees of susceptibility to CAS (CAS resistant [CAS-R], MIC, >16 mg/liter; CAS intermediate [CAS-I], MIC, 4 mg/liter; and CAS susceptible [CAS-S], MIC, 2 mg/liter). We analyzed tissue fungal burden, histopathology, and weight loss patterns. Increasing CAS resistance was associated with reduced virulence of C. parapsilosis isolates (mortality rates for CAS-S versus CAS-I versus CAS-R, 100% versus 11.1% versus 0%, respectively; P < 0.001). High doses of either echinocandin were active against infection with the CAS-I isolate when assessed by fungal burden reduction and weight gain. In contrast to CAS-S and CAS-I isolates, there was no reduction in fungal burden in mice infected with the CAS-R isolate following treatment with either echinocandin, each given at a high or low dose. Nevertheless, mice infected with the CAS-R isolate had reduced disease severity following echinocandin treatment, suggesting that echinocandins have activity in vivo, even against echinocandin-resistant strains. A complex interplay of residual echinocandin activity, decreased virulence, and/or fitness of isolates with altered cell wall and possible immunomodulatory effects can be encountered in vivo during infection with CAS-resistant C. parapsilosis isolates.
INTRODUCTION
Candida parapsilosis is one of the most common causes of invasive candidiasis (IC) worldwide (1, 2). Over the past 2 decades, the incidence of infection by C. parapsilosis has increased (1, 3), especially in critically ill neonates and in patients hospitalized in intensive care units (ICUs) (4–8). Overall morbidity and mortality are less in C. parapsilosis IC, due to its lower virulence, than in Candida albicans (9, 10).
The introduction of echinocandins, a class of antifungal agents that act by blocking the cell wall polysaccharide β-glucan synthesis, is considered a significant advance in the management of Candida infections (11). Specifically, echinocandins, including caspofungin (CAS), micafungin (MICA), and anidulafungin (ANF), have limited toxicity in patients and possess broad-spectrum fungicidal activity against azole-resistant Candida species, making these agents the preferred first-line therapy for many forms of IC (12). Nonetheless, echinocandins demonstrate reduced in vitro activity against C. parapsilosis (1, 12–15), and failures of echinocandin treatment have been reported in C. parapsilosis IC (16, 17). In particular, clinical failure due to CAS-resistant (CAS-R) isolates has been recently reported in patients receiving CAS (18, 19). Furthermore, a rise in the incidence of C. parapsilosis infections, which has been partially attributed to the increased use of CAS, has been noted in some cancer centers and the ICU setting (17–19). Overall, the emergence of echinocandin-resistant C. parapsilosis infections raises concerns about their optimal management.
ANF retains activity in vitro against most CAS- and MICA-resistant C. parapsilosis isolates and is recommended for C. parapsilosis IC treatment (20–22). However, the in vivo implications of differential in vitro activities of the various echinocandins against C. parapsilosis are not known, and the degree of the in vivo cross-resistance and the fitness loss associated with CAS resistance have not been systematically studied for C. parapsilosis.
The purpose of the present study was to establish a clinically relevant mouse model of invasive C. parapsilosis infection in immunocompetent mice and to compare the in vivo activities of ANF and CAS in experimental candidemia caused by C. parapsilosis isolates that are sensitive in vitro to ANF, showing different degrees of susceptibility to CAS.
MATERIALS AND METHODS
Susceptibility testing.
Antifungal susceptibility testing was performed by the broth microdilution method in duplicate according to the Clinical and Laboratory Standards Institute (CLSI M27-A3) guidelines for yeasts in RPMI growth medium (23, 24). The plates were incubated at 37°C for 24 h, and the MICs (in mg/liter) of all isolates were determined as the concentration causing a >50% reduction in growth.
Candida parapsilosis isolates.
The C. parapsilosis isolates used in this study are listed in Table S1 in the supplemental material. Two well-characterized genotypically identical Candida parapsilosis clinical isolates (isolates 35176 and 35177) associated with prosthetic valve endocarditis breakthrough to CAS were obtained from the Infectious Diseases Laboratory at the Wayne State University at Detroit, MI (19), and one clinical isolate (20447.040) was obtained from St. Luke's Episcopal Hospital of Houston, TX. All three isolates were sensitive to ANF (MIC, 1 to 2 mg/liter). Two isolates displayed reduced CAS susceptibility (CAS-S) (CAS-R, MIC, >16 mg/liter; CAS intermediate [CAS-I], MIC, 4 mg/liter), and the third one displayed susceptibility to CAS (MIC, 2 mg/liter). Yeast cells were stored at −80°C in 10% glycerol. Before each experiment, isolates were transferred to Sabouraud dextrose agar (SDA) and incubated at 37°C for 48 h. One morphologically identical colony was subcultured twice into Sabouraud dextrose broth and placed on a rotating incubator at 37°C overnight. Yeast cells were collected by centrifugation, washed twice with phosphate-buffered saline (PBS), and finally counted with a hemocytometer. The inoculum was adjusted to ×10x/ml using progressive dilution in PBS. For verification of the infecting Candida inocula, we plated appropriate dilutions in yeast agar glucose (YAG) plates and counted the CFU after 24 h of incubation.
Antifungal agents.
The clinical formulations of anidulafungin (Ecalta; Pfizer Pharmaceuticals, Athens, Greece) and caspofungin (Cancidas; MSD Pharmaceuticals, Athens, Greece) were used for all experiments. For in vivo experiments, antifungals were reconstituted according to the manufacturer's instructions, while for susceptibility testing, antifungals were reconstituted according to the CLSI M27-A3 directions (23); anidulafungin was dissolved in 1% dimethyl sulfoxide (DMSO) (Sigma), while caspofungin was diluted in sterile water. Stock solutions of each antifungal agent (25 mg/ml and 5 mg/ml, respectively) were stored at −70°C.
Animals.
Female 6- to 8-week-old BALB/c mice (The Foundation for Research and Technology—Hellas [FORTH], Heraklion, Crete, Greece) weighing 18 to 22 g each were used for all experiments. Mice were housed (5 per cage) in presterilized filter-top cages and provided with food, water, and bedding in the biohazard isolation suite at the University of Crete animal care facilities. Mice had access to food and water ad libitum. All procedures were performed in accordance with the highest standards for humane handling, care, and treatment of research animals and were approved by the University of Crete Animal Care and Use Committee.
Murine infection model.
Initial experiments were performed to define the optimal C. parapsilosis inoculum for studying antifungal activity (inoculum range of 5 × 107 to 2 × 108 total yeast cells per mouse). Groups of 6- to 8-week-old female BALB/c mice (n = 10 per group unless stated otherwise) were infected with each of the three C. parapsilosis isolates, via injection of yeast cells dissolved in 100 μl of PBS into the lateral tail veins. Mice were observed three times daily until day 14 after infection for assessment of cumulative survival. The body weight of each mouse was recorded daily for 14 days after infection. Moribund mice were euthanized and recorded as having died the following day.
Treatment endpoints.
The efficacy of respective echinocandin regimens was analyzed by 3 endpoints in independent experiments: (i) tissue fungal burden studies, including analysis of the rates or target organ clearance of C. parapsilosis; (ii) analysis of weight loss patterns; and (iii) histopathology.
The kinetics of fungal burden reduction in target organs (kidney, spleen, and liver) infected by C. parapsilosis strains was analyzed by euthanizing animals at 12, 24, 48, 72, and 120 h after infection and counting the number of colonies per gram of organ tissue. A total of 15 mice were used for each experiment on the kinetics of the fungal burden.
Starting 6 h after infection with each of C. parapsilosis isolate, intraperitoneal (i.p.) administration of each echinocandin (CAS or ANF) in two doses (1 and 10 mg/kg of body weight) or DMSO (control group) was performed once daily for 7 days or until the death of the animal. Such doses have been previously used in either neutropenic or immunocompetent murine models of invasive candidiasis and reflect human regimens (22, 25). Specifically, kidney, spleen, and liver were aseptically removed and weighed in selected mice (n = 6) euthanized on day 2 after infection. The target organs were homogenized in 1 ml PBS with a tissue homogenizer (Polytron dispensing and mixing technology PT 1600 E; Kinematica, AG, Luzern, Switzerland) for determination of fungal burden by plating serial dilutions onto the surface of Sabouraud dextrose agar with 5% (wt/vol) chloramphenicol plates, incubated at 37°C for 48 h. Colonies were counted, and the number of CFU per gram of organ tissue was calculated for each animal. Results were analyzed and expressed as the mean fungal burden ± standard deviation (SD) of two independent experiments.
Antifungal efficacy was also evaluated by analysis of weight loss in infected mice treated with antifungal agents. In different experiments, 6 h after infection with each C. parapsilosis strain, mice (n = 5 per group) received i.p. injection of each antifungal agent (CAS and ANF) in two doses (1 and 10 mg/kg) or DMSO (control group). Each treatment group comprised five mice.
To further evaluate differences in fungal burden of the organs in mice infected with CAS-S, CAS-I, or CAS-R C. parapsilosis isolates, histopathological examination of the kidneys was performed in selected mice (n = 3) euthanized on day 2 after infection and treated as described above. Briefly, kidneys of mice were processed, stored in 10% formaldehyde, and embedded in paraffin wax for histopathological examination. Matched sections were then stained with hematoxylin and eosin and periodic acid-Schiff stain (PAS) and examined in a blinded fashion by light microscopy. The degree of histopathological findings was scored from − to ++ as follows: −, absence of blastospores or filamentous form in kidney sections; +, sparse (≤3 yeast cells) and/or few foci of yeast cells or filamentous form (<2 foci) in kidney sections; and ++, abundant (≥2 foci) and/or large (≥3 yeast cells) foci of yeast cells or filamentous form in kidney sections.
Statistical analysis.
All graphic data are expressed as means ± SD and were statistically analyzed by analysis of variance (ANOVA) with Dunnett's posttest for multiple comparisons, using computer Prism software (Prism 5; GraphPad Software, Inc., San Diego, CA). Survival curves were estimated by the Kaplan-Meier method and compared by the Mantel-Cox (log rank) test. A P value of <0.05 is considered statistically significant.
RESULTS
Establishment of a C. parapsilosis mouse model simulating hematogenous infection.
C. parapsilosis typically afflicts immunocompetent patients and is characterized by reduced mortality compared to other Candida spp. Thus, we attempted to establish a physiologically relevant model with low mortality rates in nonneutropenic mice. Pilot experiments using a range of C. parapsilosis inocula (106 to 2 × 108 yeast cells per mouse) demonstrated that for the CAS-I and CAS-R isolates, the optimal inoculum was 1.8 × 108 yeast cells per mouse, while for the CAS-S isolate, the optimal inoculum was 1.3 × 108 yeast cells per mouse. These inocula resulted in a subacute infection with mortality rates of 10 to 30% (Fig. 1a), which are close to those of C. parapsilosis infection in humans (12).
FIG 1.
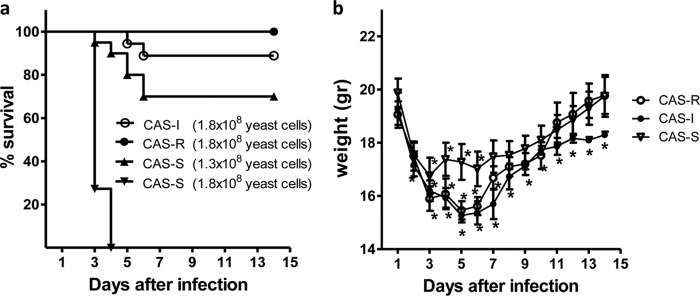
Establishment of the experimental mouse model. (a) Survival of untreated mice infected with the three CAS-S (1.3 × 108; n = 20 mice, 2 independent experiments; and 1.8 × 108 yeast cells/mouse; n = 12 mice, 2 independent experiments), CAS-I (1.8 × 108 yeast cells/mouse; n = 18 mice, 2 independent experiments) and CAS-R (1.8 × 108 yeast cells/mouse; n = 10 mice, 2 independent experiments) C. parapsilosis isolates. (b) Weight loss of untreated mice infected with the three C. parapsilosis isolates (n = 5 mice for each group, 1 independent experiment). Error bars represent the mean ± standard deviation (SD); *, P < 0.05 (for differences in weight compared to day 0), by ANOVA with Dunnett's posttest for multiple comparisons. -S, sensitive; -R, resistant; -I, intermediate sensitive.
Despite the relatively low mortality rates, infection with the standardized inocula resulted in proliferative fungal growth in all organs tested, characterized by an increase in fungal load and histopathological evidence of filamentation of C. parapsilosis in the affected organs (Fig. 2). Specifically, the kinetics of fungal growth by assessment of the number of CFU/g of organ tissue showed that the peak of infection occurred on day 2, with a significant decrease in fungal load starting on day 3 of infection (Fig. 2). In addition, measurement of weight loss of infected mice, an established marker of disease severity in C. albicans models (9, 26, 27), correlated with disease progression in our C. parapsilosis model. All infected mice lost >25% of their baseline weight by day 5 of infection (P < 0.05); evidence that infected but untreated mice suffered high morbidity and disease severity, with recovery starting on day 6 after infection (Fig. 1b).
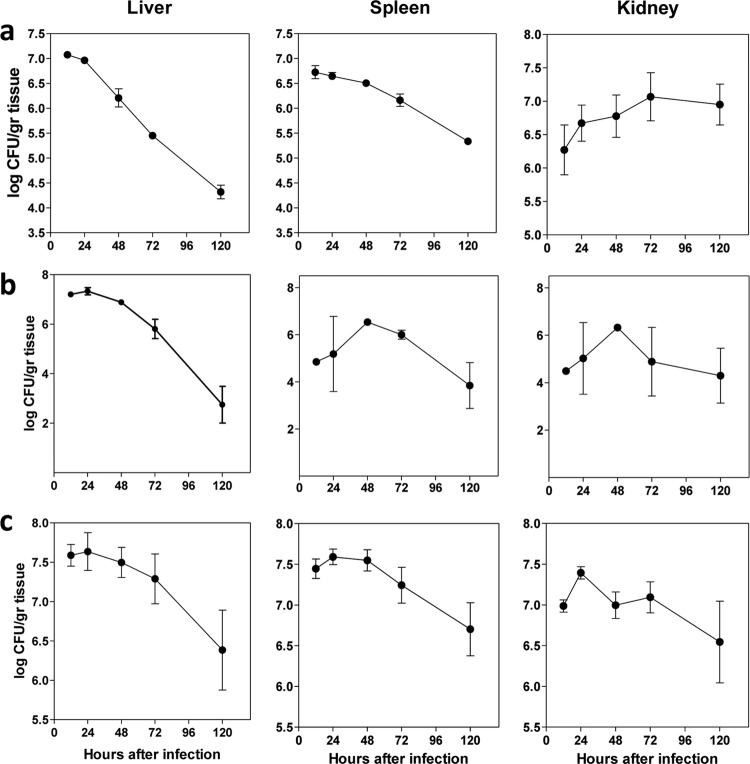
FIG 2.
Kinetics of fungal burden in different organs suggestive of proliferative growth of C. parapsilosis showing a peak on day 2 and rapid clearance of the infection by the host following day 3. Shown are the kinetics of fungal burdens (log CFU/g organ tissue) in different organs (spleen, liver, and kidney) in untreated mice infected with (a) CAS-S, (b) CAS-I, or (c) CAS-R C. parapsilosis isolates (n = 3 mice for each time point, 1 independent experiment).
The C. parapsilosis CAS-S strain is more virulent than C. parapsilosis strains with reduced susceptibility to CAS in the mouse model.
After establishing the mouse model, we compared the virulence levels of three different C. parapsilosis isolates in the model with different degrees of susceptibility to caspofungin. The CAS-S isolate was more virulent than the other two isolates, with a mortality rate of 100% (P < 0.001) (Fig. 1a). There was a trend toward reduced mortality following infection with the CAS-R isolate compared to the CAS-I isolate (mortality rate of 11.1% versus 0%, respectively; P = 0.45).
Echinocandins possess significant activity against CAS-S and CAS-I C. parapsilosis isolates in vivo.
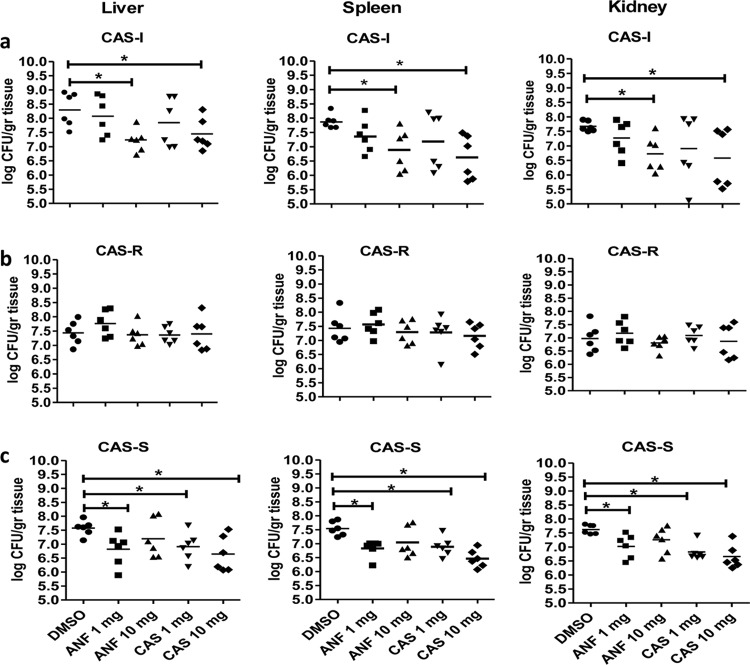
Next, we compared the in vivo activities of CAS and ANF against those of CAS-S and CAS-I strains in the infection model. Both agents exhibited significant and comparable activities against the CAS-I and CAS-S isolates, as evidenced by significant reduction in mean fungal burden in all affected organs, an effect more pronounced with the administration of higher doses than that with lower doses of both antifungals (Fig. 3a and c).
FIG 3.
Echinocandin treatment is associated with significant reduction in tissue fungal burden in mice infected with CAS-S and CAS-I C. parapsilosis isolates. Fungal burden (log CFU/g organ tissue) in different organs (spleen, liver, and kidney) of mice on day 2 of infection with the CAS-I (a), CAS-R (b), or CAS-S (c) C. parapsilosis isolate and treated with either DMSO (control) or different doses of echinocandins (CAS or ANF). Six mice were used for each group (5 groups [30 mice total]). Results were analyzed as the mean fungal burden ± standard deviation of two independent experiments. Each symbol represents an independent experiment, and error bars represent the mean. *, P < 0.05 for control versus treatment groups by ANOVA with Dunnett's posttest for multiple comparisons. CAS, caspofungin; ANF, anidulafungin; -S, sensitive; -R, resistant; -I, intermediate sensitive. Horizontal bars show the statistically significant differences between controls and treatment groups.
Infection with the CAS-I isolate resulted in a significantly higher kidney mean fungal burden in untreated mice than that those in mice treated with either echinocandin at high doses by day +2 of infection: kidney reduction for high-dose CAS versus control (mean ± SD), 1.091 ± 0.417 log CFU/g (P = 0.026); high-dose ANF, 0.943 ± 0.256 log CFU/g (P = 0.004). Additionally, weight loss was less pronounced in mice infected by the CAS-I isolate following CAS or ANF treatment: weight loss for high-dose CAS versus control (mean ± SD), 14.7% ± 2.0% (P = 0.09), and weight loss for high-dose ANF versus control, 13.1% ± 12.3% (P = 0.02) (Fig. 4a).
FIG 4.
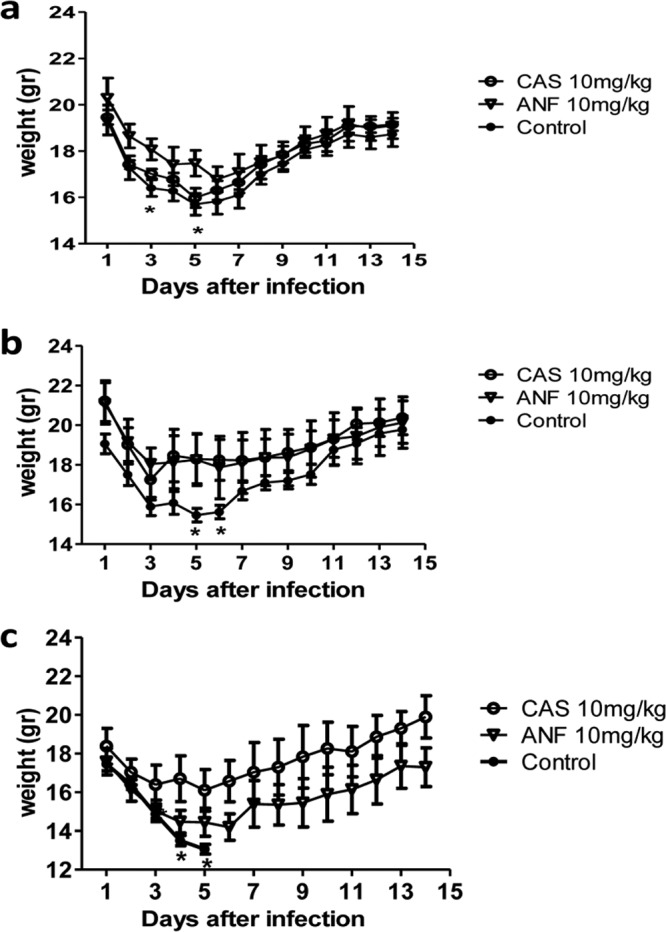
Decreased weight loss in infected mice following CAS or ANF administration in IC caused by all three C. parapsilosis isolates. Weight loss was measured in mice treated with each echinocandin (CAS or ANF) or DMSO (control), and infected with (a) CAS-I, (b) CAS-R, and (c) CAS-S C. parapsilosis isolate (n = 5 mice per group; 1 independent experiment). Error bars represent the mean ± standard deviation (SD). *, P < 0.05 for control versus treatment groups by ANOVA with Dunnett's posttest for multiple comparisons. CAS, caspofungin; ANF, anidulafungin; -S, sensitive; -R, resistant; -I, intermediate sensitive.
In animals infected with the CAS-S strain, high and low doses of CAS or the low dose of ANF treatment resulted in lower organ fungal burdens than those in controls. However, at higher doses of ANF, a paradoxical effect was evident that seemed to vary, depending on the echinocandin and the site of infection (Fig. 3c). Specifically, the mean fungal burden in the kidneys of DMSO-treated mice (control) was significantly higher than that in mice treated with high-dose CAS or low-dose ANF on day 2 after infection: kidney for high-dose CAS versus control (mean ± SD), 0.959 ± 0.183 log CFU/g (P = 0,0004) and that for low-dose ANF, 0.601 ± 0.185 log CFU/g (P = 0.008). However, mice treated with high-dose ANF had no significant reduction in mean fungal burden compared to that in DMSO-treated mice (control): kidney reduction for high-dose ANF versus control (mean ± SD), 0.364 ± 0.339 log CFU/g (P = 0.096). The paradoxical effect of high-dose ANF in mice infected with CAS-S C. parapsilosis was confirmed in an additional experiment (data not shown). There was a considerable variability in fungal burdens of mice infected by the CAS-I C. parapsilosis isolates, which were treated with CAS, which precluded the assessment of the CAS paradoxical effect in this particular experimental system. The amount of weight loss was smaller in mice infected by the CAS-S C. parapsilosis following high-dose CAS or ANF treatment, while all mice of the control group lost substantial weight and died on day 5 after infection (Fig. 4c).
Treatments with either CAS or ANF are less effective against the CAS-R C. parapsilosis strain.
In animals infected with the CAS-R strain, neither echinocandin was effective at reducing the mean fungal burden by day +2 (Fig. 3b). However, antifungal treatment still accelerated animal recovery and resulted in less severe disease, as evidenced by patterns of weight loss versus controls. In the CAS-R-infected and DMSO-treated control animals, the weight loss for high-dose CAS versus control (mean ± SD) was 10.4% ± 4.4% (P = 0.044), and that for high-dose ANF versus the control was 11.6% ± 6.7% (P = 0.038) (Fig. 4b).
Histopathological analysis of tissue fungal burden.
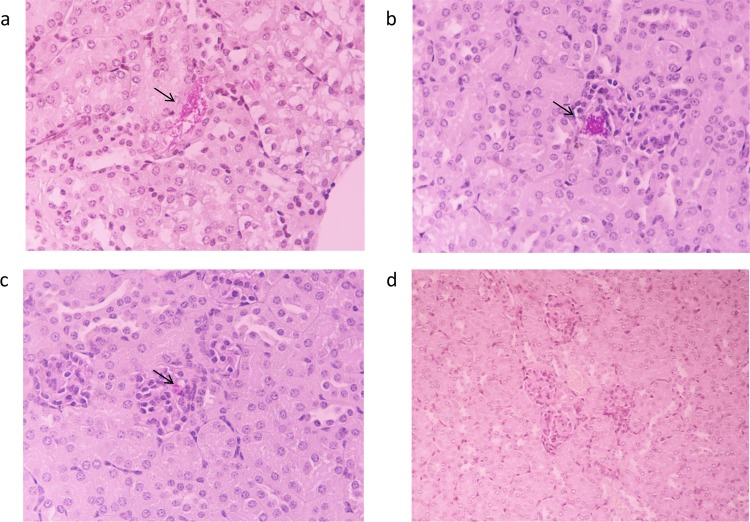
Consistent with the results of fungal burden in all affected organs of echinocandin- and DMSO-treated mice, kidney histological sections revealed differences in fungal burdens between the three C. parapsilosis strains on day 2 after infection (Table 1). Sections in control animals infected with either of three strains showed the presence of scattered foci of yeast cells, occasionally coexisting with filamentous structures in the glomeruli, whereas the tubuli appeared normal (Fig. 5a and b). In contrast, in mice infected with either CAS-I or CAS-S isolates and treated with 10 mg/kg of CAS or 10 mg/kg of ANF, there was significant reduction or absence of yeast cells and filamentous forms in kidney sections (Fig. 5c and d). In particular, mice infected with the CAS-S isolate and treated with a low dose of ANF demonstrated fewer yeast cells in the kidneys than infected mice treated with a higher dose of ANF. Regarding the CAS-R isolate, kidney sections obtained from echinocandin-treated mice revealed no significant reduction in the number of yeast cells compared to that in controls (Table 1). Differences in histopathology scores between the different treatment groups are shown in Table 1.
TABLE 1.
Histopathological analysis of tissue fungal burden
| Mouse group and cell form | Histopathology for C. parapsilosis isolatea |
||
|---|---|---|---|
| CAS-I | CAS-R | CAS-S | |
| Control DMSO-treated mice | |||
| Yeast cells | ++ | ++ | ++ |
| Filamentous form | + | − | − |
| Caspofungin-treated (high dose) mice | |||
| Yeast cells | − | ++ | − |
| Filamentous form | − | − | − |
| Anidulafungin-treated (high dose) mice | |||
| Yeast cells | + | ++ | ++ |
| Filamentous form | + | − | − |
Shown are the differences in histopathology scores in kidneys of mice infected with each of three C. parapsilosis isolates between the different treatment groups. The degree of histopathological findings was scored from − to ++: −, absence of yeast cells or filamentous form in kidney sections; +, sparse (≤3 yeast cells) and/or few foci of yeast cells or filamentous form (<2 foci) in kidney sections; ++, abundant (≥2 foci) and/or large (≥3 yeast cells) foci of yeast cells or filamentous form in kidney sections.
FIG 5.
Representative PAS-stained kidney sections (on day 2 of infection) from mice infected with C. parapsilosis isolates. (a) Tubuli appear normal, whereas blastospores and hyphae are present in glomeruli of nontreated mice. Original magnification, ×400. (b) Presence of yeast cells in a glomerulus of untreated or echinocandin-treated mice. Original magnification, ×400. (c) Fewer yeast cells or smaller foci of yeast cells in a glomerulus of echinocandin-treated mice. Original magnification, ×400. (d) Absence of yeast cells or hyphae in kidney sections of echinocandin-treated mice. Original magnification, ×400. n = 3 mice per group, 1 independent experiment.
DISCUSSION
In this work, we established a reproducible model of C. parapsilosis IC in immunocompetent mice. This model mimics the pathobiology of hematogenous infection in humans and allows comparisons of antifungal drug activity based on differences in fungal burden and weight loss of infected mice.
In previous C. parapsilosis mouse models, chemotherapy-induced neutropenia or administration of corticosteroids was used in order to achieve a reproducible infection, in part because of the attenuated virulence of this yeast compared to other Candida species (22, 28–31). In the present study, immunocompetent mice were used, since this type of infection in humans mostly occurs in nonneutropenic adults, especially in patients requiring prolonged use of central venous or peritoneal dialysis catheters or indwelling devices or receiving parenteral nutrition (1, 5, 19) due to the affinity of C. parapsilosis to foreign materials.
We specifically utilized an inoculum of C. parapsilosis that resulted in subacute infection and relatively low mortality (10 to 30%), similar to the course of infection observed with C. parapsilosis in humans. C. parapsilosis fungemia mortality ranges from 4% to 45% (32–35). Trofa et al. reported average crude mortality rates of 28.5%, compared to that of 44.8% for C. albicans, confirming C. parapsilosis's decreased virulence (1). Due to the relatively low mortality rates associated with C. parapsilosis fungemia in humans, as well as in the present model, fungal burden in the affected organs (CFU/g) instead of mortality rates was measured as an accurate marker of infection severity. In addition, weight loss was used as a composite marker of morbidity that depends on both fungal virulence and the host's inflammatory immune response. Several studies have used this marker for disease severity in mice with IC or other type of infection (9, 26, 27). Notably, fungal clearance is observed in control mice relatively early following infection with C. parapsilosis in our model, so it was difficult to assess the therapeutic effect of antifungal therapy based only on fungal burden. For that reason, we used weight loss assessment as another dynamic measure of antifungal drug efficacy that was assessed on a daily basis. It is plausible that discrepancies between weight loss and fungal burden reduction reflect the limitations of our model.
We also assessed the in vivo activity of CAS versus that of ANF against isolates of C. parapsilosis displaying different degrees of susceptibility to CAS. Regarding the CAS-R strain, we found that, although there was no difference in fungal burdens between the untreated and echinocandin-treated mice, there was less weight loss and more rapid weight gain in the latter group. Regarding the CAS-I strain, only high doses of either CAS or ANF were effective in reducing the fungal burden and attenuating weight loss compared to the case in untreated mice. Finally, regarding the CAS-S strain, it was more virulent than either CAS-I or CAS-R strains. In the CAS-S strain infection, treatment with either CAS or ANF at a low dose achieved lower organ fungal burdens than those in controls, while the echinocandin-treated groups gained weight more rapidly than the DMSO-treated mice. Similarly, the comparative activity of CAS versus ANF as reflected by tissue fungal burden (CFU/g) and the degree of weight loss showed that mice treated with echinocandins had more rapid weight gain than controls in all isolates tested.
Echinocandins possess reduced in vitro activity against C. parapsilosis (2, 12–15), and clinical failures due to CAS-R isolates have been reported recently (16, 17), especially in patients receiving CAS (18, 19). The reduced susceptibility of C. parapsilosis to echinocandins has been related to polymorphisms in the FSK1 gene (16, 36, 37). Other studies suggested that interspecies differences in CAS susceptibility result from the combination of structural differences in the cell wall components, a reduced affinity for the glucan synthase protein complex, or variations in the regulatory network of this complex (28, 38). Also, the unique mitochondrial respiratory network of this yeast may play an important role in its decreased susceptibility to echinocandins (39). However, the lack of a molecular mechanism of resistance in the clinical strains used in our study is a limitation of our study. The optimal management of the infections caused by CAS-R C. parapsilosis is unclear. In vitro studies have demonstrated that ANF retains in vitro activity against most CAS-R isolates (20–22). However, in the present study, it was demonstrated that CAS and ANF possess comparable activities against infections caused by all isolates tested.
The present data highlight the discrepancy between tissue fungal burden assessment and weight loss and the morbidity in our experimental model of CAS-R C. parapsilosis infection. Specifically, echinocandin treatment resulted in less morbidity, despite the lack of reduction in tissue fungal burden. To complicate things further, the CAS-nonsusceptible isolate had attenuated virulence compared to the CAS-S isolate. It is plausible that the switch to enhanced chitin cell wall biosynthesis, resulting in altered glucan-host effector immune cell interactions, might account for these differences (40). Finally, an interesting finding of the present study was the observation of a paradoxical effect that seems to differ according to the type of echinocandin and the infected site. Several studies have described paradoxical effects for other echinocandins, especially for CAS (41–47).
In conclusion, our findings demonstrate that there is no straightforward in vitro or in vivo correlation of echinocandin activity against C. parapsilosis displaying nonsusceptibility to CAS. Specific endpoints of echinocandin treatment response, including markers of morbidity (organ fungal burden versus weight loss), showed limited concordance with in vitro susceptibility. A complex interplay of pharmacokinetics (48), relative potency of echinocandin activity (48), fitness loss (49), paradoxical effects, and immunomodulatory effects (50) that differ according to site of infection can be encountered in vivo during infection with CAS-resistant C. parapsilosis isolates. Further studies will be needed for comparison of C. parapsilosis infection to those caused by C. albicans strains with different degrees of susceptibility to echinocandins. Experimentation with immunosuppressed murine models or models with catheter-associated C. parasilosis fungemia would also add additional relevance.
Supplementary Material
ACKNOWLEDGMENTS
We thank J. Vazquez (Wayne State University School of Medicine) for providing the C. parapsilosis isolates and Sofia Maraki (University Hospital of Heraklion) for valuable help.
This study was supported by an educational grant from Pfizer, Pharmaceuticals, Athens, Greece (WS839508). D. Kontoyiannis acknowledges the Frances King Black Endowed Professorship.
D.P.K. has received research support and honoraria from Pfizer, Astellas Pharma US, Inc., and Merck and Co., Inc. R.E.L. has received research support from Merck & Co., Inc., and Astellas, Inc. All other authors report no conflicts of interest.
Footnotes
Published ahead of print 21 October 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.01025-13.
REFERENCES
- 1.Trofa D, Gácser A, Nosanchuk JD. 2008. Candida parapsilosis, an emerging fungal pathogen. Clin. Microbiol. Rev. 21:606–625. 10.1128/CMR.00013-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pfaller MA, Diekema DJ. 2007. Epidemiology of invasive candidiasis: a persistent public health problem. Clin. Microbiol. Rev. 20:133–163. 10.1128/CMR.00029-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Asbeck EC, Clemons KV, Stevens DA. 2009. Candida parapsilosis: a review of its epidemiology, pathogenesis, clinical aspects, typing and antimicrobial susceptibility. Crit. Rev. Microbiol. 35:283–309. 10.3109/10408410903213393 [DOI] [PubMed] [Google Scholar]
- 4.Kuhn DM, Mikherjee PK, Clark TA, Pujol C, Chandra J, Hajjeh RA, Warnock DW, Soil DR, Ghannoum MA. 2004. Candida parapsilosis characterization in an outbreak setting. Emerg. Infect. Dis. 10:1074–1081. 10.3201/eid1006.030873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wenzel RP, Gennings C. 2005. Bloodstream infections due to Candida species in the intensive care unit: identifying especially high-risk patients to determine prevention strategies. Clin. Infect. Dis. 41(Suppl 6):S389–S393. 10.1086/430923 [DOI] [PubMed] [Google Scholar]
- 6.Levy I, Rubin LG, Vasishtha S, Tucci V, Sood SK. 1998. Emergence of Candida parapsilosis as the predominant species causing candidemia in children. Clin. Infect. Dis. 26:1086–1088. 10.1086/520277 [DOI] [PubMed] [Google Scholar]
- 7.Sarvikivi E, Lyytikäinen O, Soll DR, Pujol C, Pfaller MA, Richardson M, Koukila-Kähkölä P, Luukkainen P, Saxén H. 2005. Emergence of fluconazole resistance in a Candida parapsilosis strain that caused infections in a neonatal intensive care unit. J. Clin. Microbiol. 43:2729–2735. 10.1128/JCM.43.6.2729-2735.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lupetti A, Tavanti A, Davini P, Ghelardi E, Corsini V, Merusi I, Boldrini A, Campa M, Senesi S. 2002. Horizontal transmission of Candida parapsilosis candidemia in a neonatal intensive care unit. J. Clin. Microbiol. 40:2363–2369. 10.1128/JCM.40.7.2363-2369.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arendrup M, Horn T, Frimodt-Møller N. 2002. In vivo pathogenicity of eight medically relevant Candida species in an animal model. Infection 30:286–291. 10.1007/s15010-002-2131-0 [DOI] [PubMed] [Google Scholar]
- 10.Pappas PG, Rex JH, Lee J, Hamill RJ, Larsen RA, Powderly W, Kauffman CA, Hyslop N, Mangino JE, Chapman S, Horowitz HW, Edwards JE, Dismukes WE, Mycoses Study Group NIAID 2003. A prospective observational study of candidemia: epidemiology, therapy, and influences on mortality in hospitalized adult and pediatric patients. Clin. Infect. Dis. 37:634–643. 10.1086/376906 [DOI] [PubMed] [Google Scholar]
- 11.Wiederhold NP, Lewis RE. 2003. The echinocandin antifungals: an overview of the pharmacology, spectrum and clinical efficacy. Expert Opin. Invest. Drugs 12:1313–1333. 10.1517/13543784.12.8.1313 [DOI] [PubMed] [Google Scholar]
- 12.Pappas PG, Kauffman CA, Andes D, Benjamin DK, Jr, Calandra TF, Edwards JE, Jr, Filler SG, Fisher JF, Kullberg BJ, Ostrosky-Zeichner L, Reboli AC, Rex JH, Walsh TJ, Sobel JD, Infectious Diseases Society of America 2009. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 48:503–535. 10.1086/596757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cantón E, Espinel-Ingroff A, Pemán J, del Castillo L. 2010. In vitro fungicidal activities of echinocandins against Candida metapsilosis, C. orthopsilosis, and C. parapsilosis evaluated by time-kill studies. Antimicrob. Agents Chemother. 54:2194–2197. 10.1128/AAC.01538-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Channoum MA, Chen A, Buhari M, Chadra J, Mukherjee PK, Baxa D, Golembieski A, Vazquez JA. 2009. Differential in vitro activity of anidulafungin, caspofungin, and micafungin against Candida parapsilosis isolates recovered from a burn unit. Clin. Microbiol. Infect. 15:274–279. 10.1111/j.1469-0691.2008.02660.x [DOI] [PubMed] [Google Scholar]
- 15.Pfaller MA, Boyken L, Hollis RJ, Kroeger J, Messer SA, Tendolkar S, Diekema DJ. 2008. In vitro susceptibility of invasive isolates of Candida spp. to anidulafungin, caspofungin, and micafungin: six years of global surveillance. J. Clin. Microbiol. 46:150–156. 10.1128/JCM.01901-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun HY, Singh N. 2010. Characterisation of breakthrough invasive mycoses in echinocandin recipients: an evidence-based review. Int. J. Antimicrob. Agents 35:211–218. 10.1016/j.ijantimicag.2009.09.020 [DOI] [PubMed] [Google Scholar]
- 17.Sipsas NV, Lewis RE, Tarrand J, Hachem R, Rolston KV, Raad II, Kontoyiannis DP. 2009. Candidemia in patients with hematologic malignancies in the era of new antifungal agents (2001–2007): stable incidence but changing epidemiology of a still frequently lethal infection. Cancer 115:4745–4752. 10.1002/cncr.24507 [DOI] [PubMed] [Google Scholar]
- 18.Cheung C, Guo Y, Gialanella P, Feldmesser M. 2006. Development of candidemia on caspofungin therapy: a case report. Infection 34:345–348. 10.1007/s15010-006-5613-7 [DOI] [PubMed] [Google Scholar]
- 19.Moudgal V, Little T, Boikov D, Vazquez JA. 2005. Multiechinocandin- and multiazole-resistant Candida parapsilosis isolates serially obtained during therapy for prosthetic valve endocarditis. Antimicrob. Agents Chemother. 49:767–769. 10.1128/AAC.49.2.767-769.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pfaller MA, Diekema DJ, Andes D, Arendrup MC, Brown SD, Lockhart SR, Motyl M, Perlin DS, CLSI Subcommittee for Antifungal Testing 2011. Clinical breakpoints for the echinocandins and Candida revisited: integration of molecular, clinical, and microbiological data to arrive at species-specific interpretive criteria. Drug Resist. Updat. 14:164–176. 10.1016/j.drup.2011.01.004 [DOI] [PubMed] [Google Scholar]
- 21.Pfaller MA, Diekema DJ, Boyken L, Messer SA, Tendolkar S, Hollis RJ, Goldstein BP. 2005. Effectiveness of anidulafungin in eradicating Candida species in invasive candidiasis. Antimicrob. Agents Chemother. 49:4795–4797. 10.1128/AAC.49.11.4795-4797.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salas V, Pastor FJ, Calvo E, Mayayo E, Quindós G, Carrillo AJ, Guarro J. 2011. Anidulafungin in treatment of experimental invasive infection by Candida parapsilosis: in vitro activity, (1→3)-β-d-glucan and mannan serum levels, histopathological findings, and in vivo efficacy. Antimicrob. Agents Chemother. 55:4985–4989. 10.1128/AAC.00500-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clinical and Laboratory Standards Institute 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts, 3rd ed. Approved standard M27-A3 CLSI, Wayne, PA [Google Scholar]
- 24.Pfaller MA, Diekema DJ. 2012. Progress in antifungal susceptibility testing of Candida spp. by use of Clinical and Laboratory Standards Institute broth microdilution methods, 2010 to 2012. J. Clin. Microbiol. 50:2846–2856. 10.1128/JCM.00937-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wiederhold NP, Najvar LK, Bocanegra RA, Kirkpatrick WR, Patterson TF. 2011. Caspofungin dose escalation for invasive candidiasis due to resistant Candida albicans. Antimicrob. Agents Chemother. 55:3254–3260. 10.1128/AAC.01750-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spellberg B, Ibrahim AS, Edwards JE, Jr, Filler SG. 2005. Mice with disseminated candidiasis die of progressive sepsis. J. Infect. Dis. 192:336–343. 10.1086/430952 [DOI] [PubMed] [Google Scholar]
- 27.Trammell RA, Toth LA. 2011. Markers for predicting death as an outcome for mice used in infectious disease research. Comp. Med. 61:492–498 http://aalas.publisher.ingentaconnect.com/content/aalas/cm/2011/00000061/00000006/art00002# [PMC free article] [PubMed] [Google Scholar]
- 28.Barchiesi F, Spreghini E, Tomassetti S, Della Vittoria A, Arzeni D, Manso E, Scalise G. 2006. Effects of caspofungin against Candida guilliermondii and Candida parapsilosis. Antimicrob. Agents Chemother. 50:2719–2727. 10.1128/AAC.00111-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barchiesi F, Spreghini E, Tomassetti S, Giannini D, Scalise G. 2007. Caspofungin in combination with amphotericin B against Candida parapsilosis. Antimicrob. Agents Chemother. 51:941–945. 10.1128/AAC.00880-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Földi R, Kovács R, Gesztelyi R, Kardos G, Berényi R, Juhász B, Szilágyi J, Mózes J, Majoros L. 2012. Comparison of in vitro and vivo efficacy of caspofungin against Candida parapsilosis, C. orthopsilosis, C. metapsilosis and C. albicans. Mycopathologia 174:311–318. 10.1007/s11046-012-9554-7 [DOI] [PubMed] [Google Scholar]
- 31.Szilágyi J, Földi R, Gesztelyi R, Bayegan S, Kardos G, Juhász B, Majoros L. 2012. Comparison of the kidney fungal burden in experimental disseminated candidiasis by species of the Candida parapsilosis complex treated with fluconazole, amphotericin B and caspofungin in a temporarily neutropenic murine model. Chemotherapy 58:159–164. 10.1159/000337088 [DOI] [PubMed] [Google Scholar]
- 32.Brito LR, Guimarães T, Nucci M, Rosas RC, Paula Almeida L, Da Matta DA, Colombo AL. 2006. Clinical and microbiological aspects of candidemia due to Candida parapsilosis in Brazilian tertiary care hospitals. Med. Mycol. 44:261–266. 10.1080/13693780500421476 [DOI] [PubMed] [Google Scholar]
- 33.Colombo AL, Nucci M, Park BJ, Nouér SA, Arthington-Skaggs B, da Matta DA, Warnock D, Morgan J, Brazilian Network Candidemia Study 2006. Epidemiology of candidemia in Brazil: a nationwide sentinel surveillance of candidemia in eleven medical centers. J. Clin. Microbiol. 44:2816–2823. 10.1128/JCM.00773-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gudlaugsson O, Gillespie S, Lee K, Vande Berg J, Hu J, Messer S, Herwaldt L, Pfaller M, Diekema D. 2003. Attributable mortality of nosocomial candidemia, revisited. Clin. Infect. Dis. 37:1172–1177. 10.1086/378745 [DOI] [PubMed] [Google Scholar]
- 35.Kossoff EH, Buescher ES, Karlowicz MG. 1998. Candidemia in a neonatal intensive care unit: trends during fifteen years and clinical features of 111 cases. Pediatr. Infect. Dis. J. 17:504–508. 10.1097/00006454-199806000-00014 [DOI] [PubMed] [Google Scholar]
- 36.Perlin DS. 2007. Resistance to echinocandin-class antifungal drugs. Drug Resist. Updat. 10:121–130. 10.1016/j.drup.2007.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garcia-Effron G, Katiyar SK, Park S, Edlind TD, Perlin DS. 2008. A naturally occurring proline-to-alanine amino acid change in Fks1p in Candida parapsilosis, Candida orthopsilosis, and Candida metapsilosis accounts for reduced echinocandin susceptibility. Antimicrob. Agents Chemother. 52:2305–2312. 10.1128/AAC.00262-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Asbeck E, Clemons KV, Martinez M, Tong AJ, Stevens DA. 2008. Significant differences in drug susceptibility among species in the Candida parapsilosis group. Diagn. Microbiol. Infect. Dis. 62:106–109. 10.1016/j.diagmicrobio.2008.04.019 [DOI] [PubMed] [Google Scholar]
- 39.Chamilos G, Lewis RE, Kontoyiannis DP. 2006. Inhibition of Candida parapsilosis mitochondrial respiratory pathways enhances susceptibility to caspofungin. Antimicrob. Agents Chemother. 50:744–747. 10.1128/AAC.50.2.744-747.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ben-Ami R, Garcia-Effron G, Lewis RE, Gamarra S, Leventakos K, Perlin DS, Kontoyiannis DP. 2011. Fitness and virulence costs of Candida albicans FKS1 hot spot mutations associated with echinocandin resistance. J. Infect. Dis. 204:626–635. 10.1093/infdis/jir351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Asbeck EC, Hoepelman AI, Scharringa J, Verhoef J. 2009. The echinocandin caspofungin impairs the innate immune mechanism against Candida parapsilosis. Int. J. Antimicrob. Agents 33:21–26. 10.1016/j.ijantimicag.2008.06.013 [DOI] [PubMed] [Google Scholar]
- 42.Stevens DA, Espiritu M, Parmar R. 2004. Paradoxical effect of caspofungin: reduced activity against Candida albicans at high drug concentrations. Antimicrob. Agents Chemother. 48:3407–3411. 10.1128/AAC.48.9.3407-3411.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Torres-Rodriguez JM, Carrillo-Muñoz A, Gallach-Bau C, Madrenys N. 1989. Susceptibility of Candida species to cilofungin (LY-121019). Mycoses 32:316–318 [DOI] [PubMed] [Google Scholar]
- 44.Chamilos G, Lewis RE, Albert N, Kontoyiannis DP. 2007. Paradoxical effect of echinocandins across Candida species in vitro: evidence for echinocandin-specific and Candida species-related differences. Antimicrob. Agents Chemother. 51:2257–2259. 10.1128/AAC.00095-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clemons KV, Espiritu M, Parmar R, Stevens DA. 2006. Assessment of the paradoxical effect of caspofungin in therapy of candidiasis. Antimicrob. Agents Chemother. 50:1293–1297. 10.1128/AAC.50.4.1293-1297.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fleischhacker M, Radecke C, Schulz B, Ruhnke M. 2008. Paradoxical growth effects of the echinocandins caspofungin and micafungin, but not of anidulafungin, on clinical isolates of Candida albicans and C. dubliniensis. Eur. J. Clin. Microbiol. Infect. Dis. 27:127–131. 10.1007/s10096-007-0411-4 [DOI] [PubMed] [Google Scholar]
- 47.Stevens DA, White TC, Perlin DS, Selitrennikoff CP. 2005. Studies of the paradoxical effect of caspofungin at high drug concentrations. Diagn. Microbiol. Infect. Dis. 51:173–178. 10.1016/j.diagmicrobio.2004.10.006 [DOI] [PubMed] [Google Scholar]
- 48.Andes D, Diekema DJ, Pfaller MA, Bohrmuller J, Marchillo K, Lepak A. 2010. In vivo comparison of the pharmacodynamic targets for echinocandin drugs against Candida species. Antimicrob. Agents Chemother. 54:2497–2506. 10.1128/AAC.01584-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ben-Ami R, Kontoyiannis DP. 2012. Resistance to echinocandins comes at a cost: the impact of FKS1 hotspot mutations on Candida albicans fitness and virulence. Virulence 3:95–97. 10.4161/viru.3.1.18886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ben-Ami R, Lewis RE, Kontoyiannis DP. 2008. Immunocompromised hosts: immunopharmacology of modern antifungals. Clin. Infect. Dis. 47:226–235. 10.1086/589290 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.