Abstract
Ganciclovir is an antiviral agent that is frequently used in critically ill patients with cytomegalovirus (CMV) infections. Continuous venovenous hemodiafiltration (CVVHDF) is a common extracorporeal renal replacement therapy in intensive care unit patients. The aim of this study was to investigate the pharmacokinetics of ganciclovir in anuric patients undergoing CVVHDF. Population pharmacokinetic analysis was performed for nine critically ill patients with proven or suspected CMV infection who were undergoing CVVHDF. All patients received a single dose of ganciclovir at 5 mg/kg of body weight intravenously. Serum and ultradiafiltrate concentrations were assessed by high-performance liquid chromatography, and these data were used for pharmacokinetic analysis. Mean peak and trough prefilter ganciclovir concentrations were 11.8 ± 3.5 mg/liter and 2.4 ± 0.7 mg/liter, respectively. The pharmacokinetic parameters elimination half-life (24.2 ± 7.6 h), volume of distribution (81.2 ± 38.3 liters), sieving coefficient (0.76 ± 0.1), total clearance (2.7 ± 1.2 liters/h), and clearance of CVVHDF (1.5 ± 0.2 liters/h) were determined. Based on population pharmacokinetic simulations with respect to a target area under the curve (AUC) of 50 mg · h/liter and a trough level of 2 mg/liter, a ganciclovir dose of 2.5 mg/kg once daily seems to be adequate for anuric critically ill patients during CVVHDF.
INTRODUCTION
Ganciclovir is a prodrug nucleoside analogue that shows antiviral activity against members of the herpesvirus group and in particular against human cytomegalovirus (CMV) (1). It has a proven therapeutic effect in treatment of several CMV-related infections, such as retinitis, pneumonia, infections of the gastrointestinal tract, and infections of the nervous system, or prevention of CMV disease in patients with AIDS or an immunocompromised state following transplantation (1–3). The risk of CMV infection is increased in critically ill patients due to the requirement for mechanical ventilation, sepsis, immunodeficiency, transfusions, and renal failure (4).
Ganciclovir is excreted mainly by the kidneys and can be found almost unchanged in the urine with an elimination half-life of 2 to 4 h. Elimination is significantly prolonged in patients with renal impairment, and its clearance decreases linearly with diminishing creatinine clearance (5, 6). Therefore, a dosage reduction is required in these patients. In patients with normal renal function, a daily dosage of 10 mg/kg of body weight (BW) is recommended. Table 1 illustrates dosing recommendations for patients with impaired renal function adjusted by creatinine clearance, as described in the summary of product characteristics (7).
TABLE 1.
Manufacturer's dosage recommendation for patients with impaired renal function
| Creatinine clearance (ml/min) | Induction |
Maintenance |
||
|---|---|---|---|---|
| Dose (mg/kg) | Dosing interval (h) | Dose (mg/kg) | Dosing interval (h) | |
| ≥70 | 5.0 | 12 | 5.0 | 24 |
| 50–69 | 2.5 | 12 | 2.5 | 24 |
| 25–49 | 2.5 | 24 | 1.25 | 24 |
| 10–24 | 1.25 | 24 | 0.625 | 24 |
| <10 | 1.25 | 3 times per week following hemodialysis | 0.625 | 3 times per week following hemodialysis |
To date, no specific therapeutic exposure values for ganciclovir have been established (8). However, viremia suppression was reported with ganciclovir exposure (area under the curve [AUC]) of 40 to 50 mg · h/liter (9). Therefore, an AUC of >50 mg · h/liter has been used as the target exposure (10). In order to ensure deep tissue penetration and avoid underdosing in patients with life-threatening CMV infection, we analyzed both an AUC of >50 mg · h/liter and trough levels of 2 mg/liter. A specific exposure may reduce toxicity and maintains the therapeutic effect.
Continuous venovenous hemodiafiltration (CVVHDF) is a common form of extracorporeal renal replacement therapy in critically ill patients with renal failure. The elimination of any given drug by continuous renal replacement therapy (CRRT) is dependent on different factors such as specific properties of the membrane (pore size, filter surface area, adsorption, and filter material), characteristics of the CRRT technique used (blood flow rate and ultrafiltration rate), or properties of the drug (volume of distribution [V], molecular charge, molecular mass, and protein binding) (11, 12). The low molecular mass of ganciclovir (255.2 Da), high water solubility (3 mg/ml), and very low plasma protein binding (1 to 2%) are relevant factors in removal via CVVHDF (3, 7).
Pharmacokinetics (PK) of ganciclovir have been described in several studies (1, 13, 14). However, there is a lack of pharmacokinetic data for ganciclovir administered during CVVHDF. Accordingly, there is no specific dosage recommendation for patients undergoing CVVHDF. The aims of this study were to (i) investigate the pharmacokinetics of ganciclovir during CVVHDF in critically ill patients with suspected or proven CMV infection, (ii) find potential predictive factors for dose individualization, and (iii) establish a pharmacokinetic model of ganciclovir in order to evaluate different body weight-based dosage regimens (5 mg/kg/24 h, 2.5 mg/kg/24 h, and 1.5 mg/kg/12 h) and individualized dosing via target AUC to prevent under- or overexposure in patients with renal replacement therapy.
MATERIALS AND METHODS
Patient eligibility.
This was a prospective open-label study. All patients aged 18 years and older who were treated at an intensive care unit (ICU) and were prescribed ganciclovir as part of their required medical care due to suspected or proven CMV infection and who underwent CVVHDF for treatment of severe renal disease were eligible for this study. Exclusion criteria included an age of <18 years and extracorporeal therapy other than CVVHDF. This clinical trial was performed according to good clinical practice and the Helsinki Declaration. The study protocol was approved by the ethics committee of the Medical University of Vienna.
Medication.
All patients received 5 mg/kg ganciclovir in a 30-min infusion via a central venous line different from the one used for CVVHDF.
Sampling and storage.
Blood samples were drawn from the prefilter (arterial) and postfilter (venous) lines of the extracorporeal circuit at 0, 30, 60, 90, 180, 360, 480, and 1,440 min after finishing the infusion. Regarding tolerability and occurrence of hematological side effects, red blood cells (RBC), hemoglobin (HB), platelets (PLT) and white blood cells (WBC) were quantified on a daily basis during the treatment phase.
Ultradiafiltrate samples were taken from the outlet of the ultradiafiltrate compartment of the hemodiafilter at the corresponding times.
All samples were centrifuged immediately and stored at −70°C until assayed.
Continuous venovenous hemodiafiltration.
CVVHDF was performed by using an AN 69 HF hollow-fiber hemofilter (Prisma M100 Pre Set; Hospal Industrie, Meyzieu, France) with a membrane surface area of 0.9 m2. Dialyzers and lines were steam sterilized. The standard blood flow rate was 9 liters/h, the predilution volume was infused at a rate of 1 liter/h, and the dialysate rate was 1 liter/h, as described previously (15, 16). Net fluid balance was modified according to clinical requirements. No filter change occurred during the study period.
Sample assay.
The concentration of ganciclovir in serum and ultrafiltrate was measured by high-performance liquid chromatography (HPLC) using a Dionex UltiMate 3000 system (Dionex Corp., Sunnyvale, CA). Briefly, after the addition of 200 μl of methanol to 100 μl of serum or ultrafiltrate, the samples were centrifuged (5,000 × g for 5 min at 4°C), and 100 μl of the sample was injected onto a Hypersil BDS-C18 column (5 μm, 250- by 4.6-mm internal diameter [ID]; Thermo Fisher Scientific, Inc., Waltham, MA), preceded by a Hypersil BDS-C18 precolumn (5 μm, 10- by 4.6-mm ID) at a flow rate of 1 ml/min. Ganciclovir was monitored fluorimetrically at 278 nm (excitation) and 380 nm (emission). Mobile phase A consisted of potassium phosphate (50 mM [pH 3.0] with phosphoric acid) and heptanesulfonic acid (5 mM), and mobile phase B consisted of methanol. The mobile phase was filtered through a 0.45-μm filter (HVLP04700; Millipore, Vienna, Austria). The gradient ranged from 3% mobile phase B (0 min) to 14% at 30 min, was kept constant at 14% until 36 min, and finally was decreased linearly to 3% again at 37 min. The columns were allowed to reequilibrate for 13 min between runs. Linear calibration curves were performed from the peak areas of ganciclovir to the external standard by spiking drug-free human serum and ultrafiltrate with standard solutions of ganciclovir (final concentrations ranging from 0.005 μg to 10 μl/ml). For this method, the lower limit of quantification for ganciclovir was determined to be 5 ng/ml for ganciclovir in serum and ultrafiltrate. Intraday values for ganciclovir ranged from 4.1 to 8.0% and interday values ranged from 4.9 to 9.3% using ganciclovir concentrations of 0.01, 0.1, and 1 μg/ml.
Pharmacokinetic analysis.
Pharmacokinetic analysis was performed for all patients after they received a single dose of 5 mg/kg ganciclovir. The serum concentration-time curves of ganciclovir in plasma were adjusted to the data sets via nonlinear iterative least-square regression analysis. Curve modeling was performed by using the two-compartment PK model with the WinNonlin program (version 5.1; Scientific Consulting, USA). The following parameters were calculated: area under the concentration-time curve from 0 to 24 h (AUC0–24) using the linear trapezoidal rule, total clearance (CLtot), volume of distribution (V), distribution half-life (t1/2α), and elimination half-life (t1/2β). The sieving coefficient (S) was calculated as S = CUDF/CA. The clearance of hemodiafiltration (CLCVVHDF) was determined according to the formula CLCVVHDF = (CUDF/CA) × (QUF + QD) = (QUF + QD) × S, where CUDF is the concentration of ganciclovir in the ultradiafiltrate; CA and CV are the concentrations of ganciclovir in the prefilter (arterial) and postfilter (venous) lines of the extracorporeal circuit, respectively; and QUF and QD are the ultrafiltration rate and the dialyzation rate, respectively. Total removal (Retot) of the drug was calculated as Retot = (Cmax − Cmin)/Cmax × 100, where Cmax refers to the arterial peak serum concentration at the end of the first ganciclovir infusion and Cmin refers to the arterial trough serum concentration prior to the second infusion of ganciclovir. Removal of ganciclovir via hemodiafiltration (ReCVVHDF) was calculated as ReCVVHDF = CLCVVHDF/CLtot × 100.
Population pharmacokinetic model.
Population pharmacokinetic analysis of the arterial concentration-time data for single-dose ganciclovir was performed by using the nonlinear mixed-effects modeling software NONMEM, version 7.2 (Icon Development Solutions, Ellicott City, MD, USA). A two-compartmental model with linear elimination best described the structural model of the intravenous (i.v.) concentration-time data for ganciclovir. The following PK parameters were estimated from the model: total clearance (CLtot), volume of the first compartment (V1), volume of the second compartment (V2), and intercompartmental clearance (Q). Interindividual variability was estimated for all PK parameters by using an exponential error model. The residual error was described with a proportional error model. Covariates were not included in the model due to the small number of patients. Typical and individual PK parameters were estimated by using the “FOCE” method in NONMEM. Model evaluation was performed by using the objective function value, goodness-of-fit plots, standard errors, and visual predictive checks.
Model-based simulations.
With the final PK model, three different simulation scenarios were performed with the study population. The first scenario was simulation of ganciclovir courses 2 to 7 based on the dose resulting in a target AUC of 50 mg · h/liter. Therefore, the estimated individual clearance for each patient from the final PK model was multiplied with the target AUC, resulting in the next-course dose (individualized dose = AUCtarget × CLtot). The second scenario was simulation of courses 2 to 7 with a ganciclovir dose of 5 mg/kg, and the third scenario was simulation of courses 2 to 7 with 2.5 mg/kg for each patient. Simulations were performed separately for each patient, fixing the individual estimated PK parameters from the final PK model for the simulation of each patient's courses. Residual and interindividual variabilities were fixed to zero.
Monte Carlo simulation.
In addition, ganciclovir plasma levels were simulated in four populations of 1,000 patients by using a Monte Carlo approach. The simulated data sets comprised seven ganciclovir administrations. A weight of between 40 and 140 kg was randomly assigned to every simulated patient. Each population was characterized by a different dosing regimen (5 mg/kg/24 h, 2.5 mg/kg/24 h, 1.5 mg/kg/12 h, and target-AUC-adjusted dosing every 24 h).
Statistical analysis of PK parameters.
Correlation of body weight with total clearance and other PK values was performed by Spearman's correlation and expressed via Spearman's correlation coefficient. Two-sided P values of <0.05 were considered statistically significant.
RESULTS
Patients.
Nine intensive care unit patients with acute renal failure and proven or suspected CMV infection were included in this study. Detailed patient characteristics are listed in Table S1 in the supplemental material. All patients were anuric and had no additional diuresis. The mean age (± standard deviation [SD]) was 56 ± 9 years, and the mean body weight was 86 ± 25 kg. All patients were mechanically ventilated, and the mean SAPS II (severity of disease classification system) score was 62 ± 13. None of these patients received imipenem, mycophenolate, probenecid, tenofovir, or zidovudine, which are known to possibly enhance the toxic effects of ganciclovir (5, 17). None of these patients had a known hypersensitivity or intolerance to the substance ganciclovir.
Ganciclovir serum levels.
Ganciclovir was administered to all patients in a dosage of 5 mg/kg per day. The mean concentration time course of ganciclovir (levels drawn from the prefilter and postfilter lines of the extracorporeal circuit and from the ultradiafiltrate) is illustrated in Fig. 1. The mean peak plasma concentrations after infusion were 11.8 ± 3.5 mg/liter at the prefilter port and 10.9 ± 3 mg/liter at the postfilter port. Mean trough plasma concentrations were 2.4 ± 0.7 mg/liter at the prefilter port and 2.1 ± 0.7 mg/liter at the postfilter port. Detailed pharmacokinetics of ganciclovir are summarized in Table 2.
FIG 1.
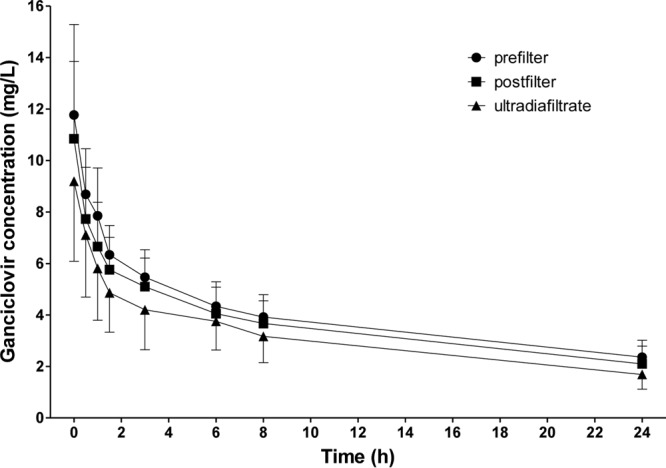
Serum ganciclovir levels drawn from the prefilter and postfilter lines of the extracorporeal circuit and from the ultradiafiltrate in anuric patients undergoing CVVHDF. Data are expressed as means ± SD (n = 9).
TABLE 2.
Pharmacokinetics of ganciclovir at a dosage of 5 mg/kg of body weighta
| Patient | Dose (mg) | AUC0–24 (mg · h/liter) | CLtot (liters/h) | CLCVVHDF (liters/h) | Retot (%) | ReCVVHDF (%) | V (liters) | t1/2α (h) | t1/2β (h) | S | C0/Cmax (mg/liter) | C0.5 (mg/liter) | C24/Cmin (mg/liter) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 375 | 104.30 | 1.76 | 1.22 | 65.72 | 69.32 | 63.11 | 0.60 | 25.19 | 0.61 | 8.78 | 7.19 | 3.01 |
| 2 | 425 | 103.90 | 2.48 | 1.82 | 775.12 | 73.39 | 64.99 | 0.51 | 25.68 | 0.91 | 10.57 | 8.83 | 2.63 |
| 3 | 575 | 87.96 | 4.51 | 1.76 | 88.26 | 39.02 | 94.88 | 0.54 | 19.84 | 0.88 | 16.01 | 10.17 | 1.88 |
| 4 | 200 | 75.48 | 1.16 | 1.34 | 65.38 | 115.52 | 50.31 | 0.82 | 30.13 | 0.67 | 8.06 | 6.32 | 2.79 |
| 5 | 375 | 117.40 | 1.63 | 1.72 | 80.92 | 105.52 | 57.48 | 0.55 | 24.52 | 0.86 | 17.03 | 11.18 | 3.25 |
| 6 | 600 | 67.83 | 4.19 | 1.38 | 82.17 | 32.94 | 167.40 | 0.85 | 27.69 | 0.69 | 10.09 | 7.01 | 1.90 |
| 7 | 500 | 87.18 | 3.20 | 1.26 | 84.96 | 39.38 | 100.40 | 0.57 | 21.72 | 0.63 | 14.83 | 9.61 | 2.23 |
| 8 | 375 | 86.64 | 1.78 | 1.60 | 68.35 | 89.89 | 90.63 | 1.69 | 35.21 | 0.80 | 7.74 | 7.37 | 2.45 |
| 9 | 400 | 97.43 | 3.67 | 1.66 | 91.24 | 45.23 | 41.79 | 0.44 | 7.89 | 0.83 | 12.79 | 10.54 | 1.12 |
| Mean ± SD | 92.01 ± 15.38 | 2.71 ± 1.22 | 1.53 ± 0.22 | 77.9 ± 9.74 | 67.8 ± 30.79 | 81.22 ± 38.30 | 0.73 ± 0.38 | 24.21 ± 7.61 | 0,76 ± 0,11 | 11.77 ± 3.52 | 8.69 ± 1.77 | 2.36 ± 0.66 |
AUC0–24, area under the curve from 0 to 24 h; CLtot, total clearance; CLCVVHDF, CVVHDF clearance; Retot, total removal; ReCVVHDF, removal via CVVHDF; V, volume of distribution; t1/2α, distribution half-life; t1/2β, elimination half-life; S, sieving coefficient; C0/Cmax, prefilter peak serum concentration directly after drug administration; C0.5, prefilter serum concentration 30 min after drug administration; C24/Cmin, prefilter trough serum concentration after 24 h.
Tolerability.
All patients tolerated the ganciclovir infusion (5 mg/kg/24 h) without any adverse reaction. Quantification of hematological parameters was assessed on a daily basis. RBC, HB, PLT, and WBC levels are shown in Table S2 in the supplemental material. We did not observe a decline of hematological parameters in these patients during therapy with ganciclovir.
Population pharmacokinetic model.
The PK model developed with NONMEM adequately described the concentration-time data of ganciclovir (model evaluation data not shown). The estimated population PK parameters CLtot, V1, V2, and Q are illustrated in Table 3. The individual PK parameters AUC, CLtot, and V estimated with NONMEM were comparable to those calculated with WinNonlin (see Table S3 in the supplemental material).
TABLE 3.
Ganciclovir population pharmacokinetic parameter estimates from population analysis with NONMEMa
| Parameter | Estimated value | Relative SE (%) | IIV (%) |
|---|---|---|---|
| CLtot (liters/h) | 2.2 | 20 | 61.5 |
| V1 (liters) | 32.4 | 11 | 33.6 |
| Q (liters/h) | 16.8 | 16 | 34.7 |
| V2 (liters) | 33.5 | 18 | 60.6 |
| Residual error | 0.0722 | 11 |
CLtot, total clearance; V1, volume of the first compartment; V2, volume of the second compartment; Q, intercompartmental clearance; IIV, interindividual variability.
A full covariate analysis was not performed due to the small number of patients. Because of inconsistent results in the literature regarding body weight (BW) as a covariate on CLtot, body weight was tested as a covariate on CLtot (10, 18). A significant correlation (P < 0.005) between BW and CLtot was found by using the following relation (with TVCLtot as the typical CLtot value and 86 as the mean patient body weight): CLtot = TVCLtot × (BW/86)1.68. However, due to the small number of patients and the associated uncertainty of this covariate for further simulations, we decided not to include BW in the model.
Model-based simulations.
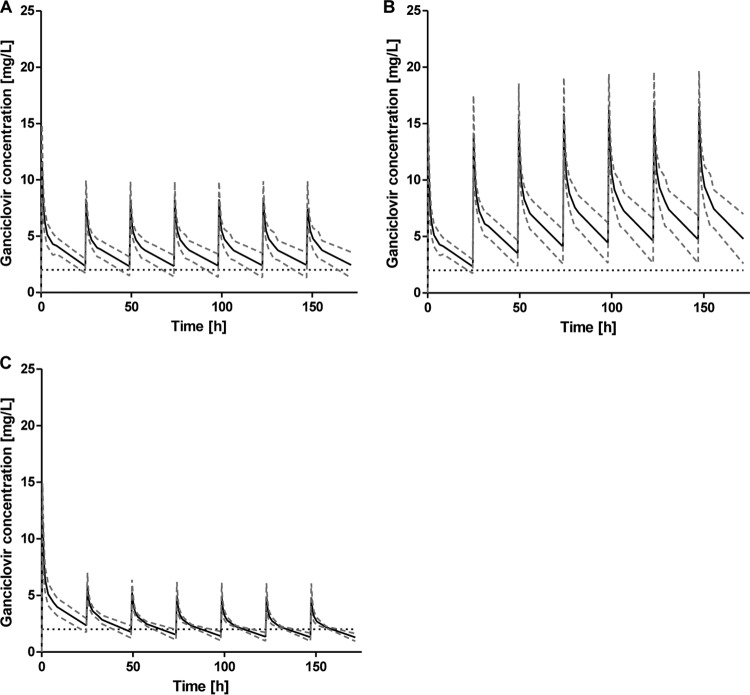
Simulations of arterial ganciclovir concentrations on an additional 6 days of dosing in the underlying nine patients showed that nearly every patient exceeded the anticipated trough level of 2 mg/liter with body weight-adjusted dosing (Fig. 2A and B), independent of the applied ganciclovir dose (5 or 2.5 mg/kg/24 h). In addition, AUC values of >50 mg · h/liter were achieved after each administration in all nine patients receiving ganciclovir doses of 5 and 2.5 mg/kg/24 h, with mean AUCs for the seven courses of 157.3 mg · h/liter and 91.4 mg · h/liter, respectively.
FIG 2.
Means ± standard deviations of the simulated concentration-time curves from the study population (n = 9) receiving a ganciclovir dose of 2.5 mg/kg/24 h (A) or 5 mg/kg/24 h (B) or an AUC-adjusted dose resulting in a target AUC of 50 mg · h/liter (C). The black dashed horizontal line represents the trough level of 2 mg/liter.
With the PK-adjusted dosing approach using a target AUC of 50 mg · h/liter, all nine patients reached the target AUC, and its interindividual variability could be reduced (Fig. 3), but trough concentrations systematically fell below the minimum value of 2 mg/liter (Fig. 2C). Only 15.9% of all ganciclovir administrations resulted in a trough concentration of >2 mg/liter.
FIG 3.
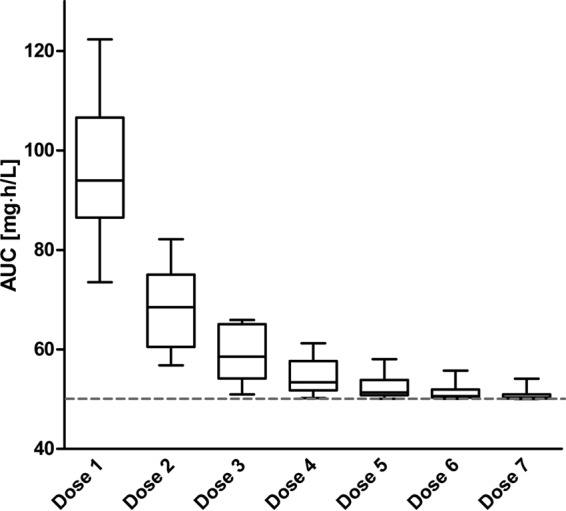
Box plots of ganciclovir AUCs (mg · h/liter) obtained with the AUC-adjusted simulated dosing of the study population. The gray dashed horizontal line represents the target AUC of 50 mg · h/liter. The median is indicated by the horizontal line, the bottom and top edges of the box represent the 25th and 75th percentiles of the AUC, and the whiskers represent the 2.5th and 97.5th percentiles.
Monte Carlo simulation.
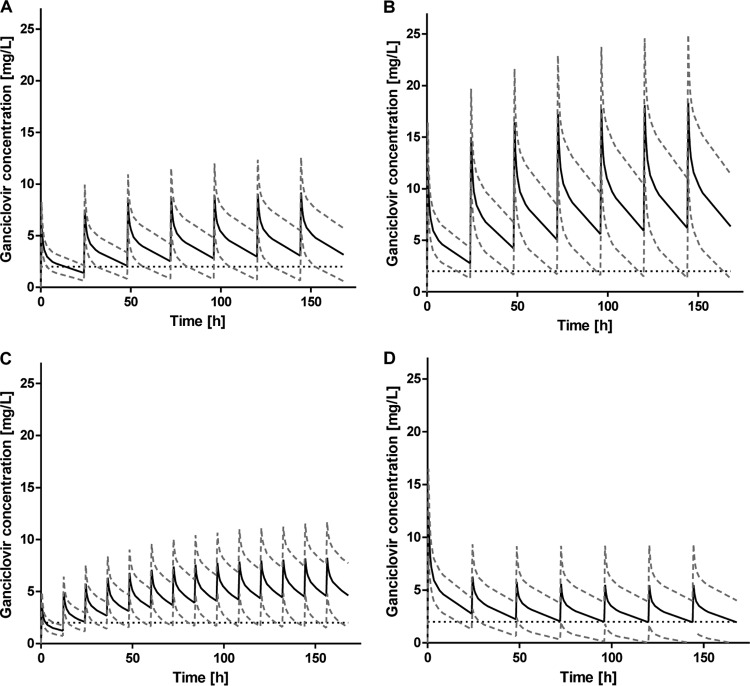
The results of the dosing scenarios in the study population could be confirmed in simulations of 1,000 new patients. Mean arterial ganciclovir concentrations for all patients exceeded the trough level of 2 mg/liter after the second ganciclovir infusion, independent of a ganciclovir dose of 5 mg/kg/24 h, 2.5 mg/kg/24 h, or 1.5 mg/kg/12 h (Fig. 4A to C). AUC values for the majority of the simulated patients, who received 5 mg/kg or 2.5 mg/kg of ganciclovir, passed the anticipated target AUC of 50 mg · h/liter (see Table S4 in the supplemental material). Thirty-seven percent of the patients receiving the lower dose of 1.5 mg/kg/12 h fell below the target AUC of 50 mg · h/liter at least once during the course of seven ganciclovir administrations.
FIG 4.
Means ± standard deviations of the simulated concentration-time curves from the simulated populations (n = 1,000) receiving a ganciclovir dose of 2.5 mg/kg/24 h (A), 5 mg/kg/24 h (B), or 1.5 mg/kg/12 h (C) or the AUC-adjusted dose resulting in a target AUC of 50 mg · h/liter (D). The black dashed horizontal line represents the trough level of 2 mg/liter.
As with the study population, AUC-adjusted dosing resulted in target AUC values for most of the simulated patients (data not shown), but often, the trough concentrations were below the threshold of 2 mg/liter. Trough levels were extremely low, almost reaching zero in the third course (Fig. 4D).
Statistical analysis of PK values.
Body weight correlated significantly with total clearance (r = 0.92; P < 0.005) and volume of distribution (r = 0.75; P < 0.05). Furthermore, body weight correlated with the minimal drug serum concentration (Cmin/C24) (r = 0.68; P < 0.05) but not with drug exposure by means of the AUC (P value was not significant).
DISCUSSION
Ganciclovir is an effective antiviral substance used for first-line treatment of CMV infections, which has a predominantly renal elimination (1, 3, 5, 19). Although ganciclovir is often used in routine clinical practice, pharmacokinetic data for critically ill patients undergoing CRRT are rare and inconsistent. To date, only two case reports of pharmacokinetic properties of ganciclovir in patients receiving CVVHDF have been published (20, 21). Table 4 summarizes available pharmacokinetic data on ganciclovir in patients undergoing CRRT. This is the first study investigating pharmacokinetics of ganciclovir in critically patients undergoing CVVHDF with modern high-flux membranes. Basic pharmacokinetic parameters were described by a two-compartment model. Furthermore, we performed three different dosing scenarios simulating seven ganciclovir courses (5 mg/kg, 2.5 mg/kg, and a target AUC of 50 mg · h/liter). Individualized dosing based on target AUC was calculated (AUCtarget multiplied by CLtot) as reported previously by Caldes et al. (10).
TABLE 4.
Pharmacokinetic characteristics of ganciclovir in different studiesa
| Reference | No. of patients | RRT | Dosage (mg/kg) | Cmax (mg/liter) | Cmin (mg/liter) | S | t1/2β (h) |
|---|---|---|---|---|---|---|---|
| 32 | 3 | CVVHD | 5/48 h | 16.1 | 5.5 | 0.75–0.95 | 18.6 |
| 34 | 3 | CVVHD | 5/48 h | 15.9–18.6 | 4.6–5.4 | 0.84 | 18.9 |
| 21 | 1 | CVVHDF | 5 | 20.3 | 8 | NA | 12.6 |
| 38 | 1 | HD | 5 | 20 | 1.5 | NA | NA |
| 20 | 1 | CVVHDF | 2.5/24 h | 5.7 | NA | NA | 14 |
| This study | 9 | CVVHDF | 5/24 h | 11.8 | 2.4 | 0.76 | 24.2 |
RRT, renal replacement therapy; Cmax, peak serum concentration; Cmin, trough serum concentration; S, sieving coefficient; t1/2β, elimination half-life; CVVHD, continuous venovenous hemodialysis; CVVHDF, continuous venovenous hemodiafiltration; HD, hemodialysis; NA, not applicable.
Actual dosing recommendations (7) refer to the renal function, adjusted by creatinine clearance calculated using the Cockcroft-Gault formula (22), but do not give guidance for anuric patients during CVVHDF at the intensive care unit. Extracorporeal elimination of a substance is influenced by several factors, such as CRRT calibration (blood flow rate and ultrafiltration rate) and properties of the membrane (pore size, filter surface area, adsorption, and filter material) (11, 12). Present-day synthetic dialyzer membranes, such as AN 69 acrylonitrile, as used in this study, have an increased drug removal in comparison to conventional (e.g., cuprophane) membranes (23). Furthermore, clearance of a substance may vary even between membranes of the same class (24, 25). In addition, pharmacokinetics in critically ill patients with severe sepsis or septic shock differ significantly from pharmacokinetics in healthy volunteers due to profound changes, such as capillary leakage, compromised tissue perfusion, changes in protein binding, pH, or an increase of total body water (26, 27).
No distinct dosing recommendations for ganciclovir in renal replacement patients have been described so far. A previous study used AUC levels of at least 50 mg · h/liter as the target exposure in solid-organ transplant patients (8–10). However, there are no data for any dosing recommendations ensuring effective drug levels during therapy. The IC50, the concentration of the drug that is required for 50% inhibition has been reported, depending on the strain, to range from 0.3 to 2.8 mg/liter (3, 8, 28). It is known that appropriate dosing of ganciclovir is important to avoid clinical inefficacy and possible development of resistance (29). However, the therapeutic range of the drug, especially the relationship between exposure, serum concentration, and clinical efficacy, has not been clearly defined (8). Significantly diminished tissue concentrations of antimicrobial drugs in comparison to their blood serum levels have been reported in several studies (30, 31). We aimed at AUC values of at least 50 mg · h/liter and a trough concentration of at least 2 mg/liter in the present study to avoid underdosing in these patients with life-threatening CMV infection.
Consistent with previous reports, serum concentrations in our study were elevated in comparison to those of patients with normal renal function (1, 3, 21, 32). Gando et al. reported elevated blood serum concentrations at the same dosage as we used (peak level of 11.8 mg/liter in our study, versus 20.3 mg/liter in the case report by Gando et al.) (21). However, this could be attributed to the different type of membrane (cellulose triacetate) that they used. McGloughlin et al. observed significantly lower drug levels (peak serum concentration of 5.7 mg/liter [trough concentration was not reported]) at a dosage of 2.5 mg/kg/24 h; however, considerably higher flow rates were used (20). The sieving coefficient was in the range described in other studies (1, 3, 10, 13, 33). The volume of distribution was elevated in comparison to that of non-critically ill patients, most likely due to additional fluid load (20, 34). The extracorporeal clearance reached approximately 55% of the total clearance in our study and was comparable to values reported previously (21, 34).
Estimated population pharmacokinetic parameters were comparable to those reported previously by Caldes et al. (10). However, the patients in our study had a lower total clearance.
All study patients reached an AUC above the target of 50 mg · h/liter in the simulated ganciclovir courses. Nearly all patients with body weight-adjusted dosing exceeded the target trough level. Interindividual variability of the AUC could be reduced in the individualized dosing approach by using the target AUC. However, trough concentrations systematically fell below the anticipated value of 2 mg/liter in this cohort. These findings were confirmed in a Monte Carlo simulation of 1,000 new patients. Solely, the lowest simulated dosage of 1.5 mg/kg/12 h did not result in adequate exposure by means of AUC.
Based on these findings, body weight-based dosing seems to be superior over AUC-adjusted dosing, as long as no clear clinically relevant AUC value is defined. With ganciclovir doses of 2.5 and 5 mg/kg/24 h, AUC values above 50 mg · h/liter and trough concentrations above 2 mg/liter could be achieved in the simulations of additional courses for most of the nine patients, whereas in the Monte Carlo simulations, AUC-based dosing led to extremely low ganciclovir trough levels (close to zero) in some patients (Fig. 4D). Therefore, with respect to a target AUC of 50 mg · h/liter and a ganciclovir trough level of 2 mg/liter, the majority of patients would be sufficiently dosed with 2.5 mg/kg/24 h.
Caldes et al., who also focused on target AUC, indicated that pharmacokinetic parameters, especially total clearance (CLtot), were not related to body weight and, therefore, that variability in renal function is more relevant than differences in body weight (10). In contrast to that assumption, we observed a significant correlation between body weight, total clearance, and volume of distribution in our anuric patients. However, it has to be mentioned that Caldes et al. estimated clearance based on a mean population body weight of 66.2 kg, whereas in the present Monte Carlo simulations, patients with a weight of between 40 and 140 kg were randomly generated. Furthermore, as the sieving coefficient (which was the same for everyone) is related to drug clearance, among patients undergoing CVVHDF, it may be assumed that CLtot and CLCVVHDF are closely correlated and, therefore, that weight-based dosing is not needed. However, we did not detect a significant correlation between CLtot and CLCVVHDF (see Fig. S1 in the supplemental material), which may be due to the large variability of CLtot in our data set. As at steady state, the product of the rate constant kss and the volume of distribution may be defined as the total body clearance (CLtot = kss × Vss) (35), large differences in the volume of distribution, due mostly to excessive fluid resuscitation (positive net fluid balance of up to 20 liters or more) in patients with severe septic shock, may be a possible explanation for interindividual differences in CLtot (36). Indeed, patient 6, who had a body weight of 120 kg, had about 3.7-fold-higher CLtot and also 3.3-fold-higher V values than patient 4 (4.19 liters and 167.4 liters, respectively, versus 1.6 liters and 50.3 liters, respectively), strongly supporting the effect of V on CLtot.
Adverse events of ganciclovir due to toxicity are primarily of a hematological nature, including anemia, thrombocytopenia, or leukopenia (3). Recently, it was reported that ganciclovir-associated cell toxicity is not solely dose dependent but also duration dependent (37). We did not observe any new hematological adverse events during treatment with ganciclovir in our patients (see Table S2 in the supplemental material). Higher serum levels and exposure may be accepted, especially in critically ill patients suffering from life-threatening infections, in order to achieve deep tissue penetration and a possibly shortened treatment duration.
Limitations of this study are the small number of patients and thus the lack of reliable covariates in the underlying pharmacokinetic model, e.g., covariates on clearance. However, this is a common number of patients in pharmacokinetic studies of critically ill patients with CRRT (15, 16, 24). Furthermore, we used one type of filter (AN 69 HF hollow-fiber hemofilter) and CVVHDF calibration (blood flow rate and ultrafiltration rate). Since this is a frequently used modern filter type with a commonly used blood flow rate, other filter types or flow rate conditions were not tested.
In conclusion, body weight dosing of ganciclovir was feasible in critically ill patients undergoing CVVHDF. Larger studies should be performed to confirm the optimal dosage, especially the impact of individualized dosing regimens. Based on our population pharmacokinetic simulations, with respect to a target area under the curve of 50 mg · h/liter and a trough level of 2 mg/liter, a daily ganciclovir dose of 2.5 mg/kg seems to be adequate for anuric patients during CVVHDF.
Supplementary Material
ACKNOWLEDGMENTS
No specific funding has been received to support this study. F.T. has received unrestricted research grants from Pfizer, Novartis, and Janssen. P.S. has received grants from Lilly and AstraZeneca. All other authors have no funding to declare.
We thank the nursing staff of ICU 13H1, Department of Internal Medicine 3, Medical University of Vienna, for their cooperation in this study.
Footnotes
Published ahead of print 21 October 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.00892-13.
REFERENCES
- 1.Crumpacker CS. 1996. Ganciclovir. N. Engl. J. Med. 335:721–729. 10.1056/NEJM199609053351007 [DOI] [PubMed] [Google Scholar]
- 2.Griffiths PD. 1997. Prophylaxis against CMV infection in transplant patients. J. Antimicrob. Chemother. 39:299–301. 10.1093/jac/39.3.299 [DOI] [PubMed] [Google Scholar]
- 3.McGavin JK, Goa KL. 2001. Ganciclovir: an update of its use in the prevention of cytomegalovirus infection and disease in transplant recipients. Drugs 61:1153–1183. 10.2165/00003495-200161080-00016 [DOI] [PubMed] [Google Scholar]
- 4.Osawa R, Singh N. 2009. Cytomegalovirus infection in critically ill patients: a systematic review. Crit. Care 13:R68. 10.1186/cc7875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faulds D, Heel RC. 1990. Ganciclovir. A review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy in cytomegalovirus infections. Drugs 39:597–638 [DOI] [PubMed] [Google Scholar]
- 6.Nevins TE, Dunn DL. 1992. Use of ganciclovir for cytomegalovirus infection. J. Am. Soc. Nephrol. 2(12 Suppl):S270–S273 [DOI] [PubMed] [Google Scholar]
- 7.Roche Laboratories, Inc. 2009. Cymevene (ganciclovir) package insert. Roche Laboratories, Inc, Basel, Switzerland [Google Scholar]
- 8.Scott JC, Partovi N, Ensom MH. 2004. Ganciclovir in solid organ transplant recipients: is there a role for clinical pharmacokinetic monitoring? Ther. Drug Monit. 26:68–77. 10.1097/00007691-200402000-00014 [DOI] [PubMed] [Google Scholar]
- 9.Wiltshire H, Paya CV, Pescovitz MD, Humar A, Dominguez E, Washburn K, Blumberg E, Alexander B, Freeman R, Heaton N, Zuideveld KP. 2005. Pharmacodynamics of oral ganciclovir and valganciclovir in solid organ transplant recipients. Transplantation 79:1477–1483. 10.1097/01.TP.0000164512.99703.AD [DOI] [PubMed] [Google Scholar]
- 10.Caldes A, Colom H, Armendariz Y, Garrido MJ, Troconiz IF, Gil-Vernet S, Lloberas N, Pou L, Peraire C, Grinyo JM. 2009. Population pharmacokinetics of ganciclovir after intravenous ganciclovir and oral valganciclovir administration in solid organ transplant patients infected with cytomegalovirus. Antimicrob. Agents Chemother. 53:4816–4824. 10.1128/AAC.00085-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ronco C, Bellomo R, Homel P, Brendolan A, Dan M, Piccinni P, La Greca G. 2000. Effects of different doses in continuous veno-venous haemofiltration on outcomes of acute renal failure: a prospective randomised trial. Lancet 356:26–30. 10.1016/S0140-6736(00)02430-2 [DOI] [PubMed] [Google Scholar]
- 12.Bohler J, Donauer J, Keller F. 1999. Pharmacokinetic principles during continuous renal replacement therapy: drugs and dosage. Kidney Int. Suppl. 1999:S24–S28 [PubMed] [Google Scholar]
- 13.Asano-Mori Y, Kanda Y, Oshima K, Watanabe T, Shoda E, Motokura T, Kurokawa M, Chiba S. 2006. Pharmacokinetics of ganciclovir in haematopoietic stem cell transplantation recipients with or without renal impairment. J. Antimicrob. Chemother. 57:1004–1007. 10.1093/jac/dkl089 [DOI] [PubMed] [Google Scholar]
- 14.Bailey TC, Trulock EP, Storch GA, Powderly WG. 1992. Ganciclovir for cytomegalovirus after heart transplantation. N. Engl. J. Med. 327:891 (Reply, 327:892-893.) [DOI] [PubMed] [Google Scholar]
- 15.Fuhrmann V, Schenk P, Jaeger W, Miksits M, Kneidinger N, Warszawska J, Holzinger U, Kitzberger R, Thalhammer F. 2007. Pharmacokinetics of voriconazole during continuous venovenous haemodiafiltration. J. Antimicrob. Chemother. 60:1085–1090. 10.1093/jac/dkm349 [DOI] [PubMed] [Google Scholar]
- 16.Fuhrmann V, Schenk P, Jaeger W, Ahmed S, Thalhammer F. 2004. Pharmacokinetics of moxifloxacin in patients undergoing continuous venovenous haemodiafiltration. J. Antimicrob. Chemother. 54:780–784. 10.1093/jac/dkh421 [DOI] [PubMed] [Google Scholar]
- 17.Studies of Ocular Complications of AIDS Research Group, AIDS Clinical Trials Group 1992. Mortality in patients with the acquired immunodeficiency syndrome treated with either foscarnet or ganciclovir for cytomegalovirus retinitis. N. Engl. J. Med. 326:213–220. 10.1056/NEJM199201233260401 [DOI] [PubMed] [Google Scholar]
- 18.Yuen GJ, Drusano GL, Fletcher C, Capparelli E, Connor JD, Lalezari JP, Drew L, Follansbee S, Busch D, Jacobson M, Spector SA, Squires K, Buhles W. 1995. Population differences in ganciclovir clearance as determined by nonlinear mixed-effects modelling. Antimicrob. Agents Chemother. 39:2350–2352. 10.1128/AAC.39.10.2350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Biron KK. 2006. Antiviral drugs for cytomegalovirus diseases. Antiviral Res. 71:154–163. 10.1016/j.antiviral.2006.05.002 [DOI] [PubMed] [Google Scholar]
- 20.McGloughlin S, Roberts JA, O'Donoghue S, Martin J, Briscoe S, Lipman J. 2011. Ganciclovir pharmacokinetics and suggested dosing in continuous venovenous haemodiafiltration. Int. J. Antimicrob. Agents 37:90–92. 10.1016/j.ijantimicag.2010.10.003 [DOI] [PubMed] [Google Scholar]
- 21.Gando S, Kameue T, Nanzaki S, Hayakawa T, Nakanishi Y. 1998. Pharmacokinetics and clearance of ganciclovir during continuous hemodiafiltration. Crit. Care Med. 26:184–187. 10.1097/00003246-199801000-00038 [DOI] [PubMed] [Google Scholar]
- 22.Cockcroft DW, Gault MH. 1976. Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41. 10.1159/000180580 [DOI] [PubMed] [Google Scholar]
- 23.Hudson JQ, Comstock TJ, Feldman GM. 2004. Evaluation of an in vitro dialysis system to predict drug removal. Nephrol. Dial. Transplant. 19:400–405. 10.1093/ndt/gfg550 [DOI] [PubMed] [Google Scholar]
- 24.Thalhammer F, Rosenkranz AR, Burgmann H, Graninger W, Hollenstein U, Schenk P, Thalhammer-Scherrer R, Traindl O, Horl WH, Breyer S. 1997. Single-dose pharmacokinetics of teicoplanin during hemodialysis therapy using high-flux polysulfone membranes. Wien. Klin. Wochenschr. 109:362–365 [PubMed] [Google Scholar]
- 25.Agarwal R, Cronin RE. 1994. Heterogeneity in gentamicin clearance between high-efficiency hemodialyzers. Am. J. Kidney Dis. 23:47–51 [DOI] [PubMed] [Google Scholar]
- 26.De Paepe P, Belpaire FM, Buylaert WA. 2002. Pharmacokinetic and pharmacodynamic considerations when treating patients with sepsis and septic shock. Clin. Pharmacokinet. 41:1135–1151. 10.2165/00003088-200241140-00002 [DOI] [PubMed] [Google Scholar]
- 27.Bugge JF. 2001. Pharmacokinetics and drug dosing adjustments during continuous venovenous hemofiltration or hemodiafiltration in critically ill patients. Acta Anaesthesiol. Scand. 45:929–934. 10.1034/j.1399-6576.2001.450802.x [DOI] [PubMed] [Google Scholar]
- 28.Plotkin SA, Drew WL, Felsenstein D, Hirsch MS. 1985. Sensitivity of clinical isolates of human cytomegalovirus to 9-(1,3-dihydroxy-2-propoxymethyl)guanine. J. Infect. Dis. 152:833–834. 10.1093/infdis/152.4.833 [DOI] [PubMed] [Google Scholar]
- 29.Emery VC, Griffiths PD. 2000. Prediction of cytomegalovirus load and resistance patterns after antiviral chemotherapy. Proc. Natl. Acad. Sci. U. S. A. 97:8039–8044. 10.1073/pnas.140123497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tomaselli F, Dittrich P, Maier A, Woltsche M, Matzi V, Pinter J, Nuhsbaumer S, Pinter H, Smolle J, Smolle-Juttner FM. 2003. Penetration of piperacillin and tazobactam into pneumonic human lung tissue measured by in vivo microdialysis. Br. J. Clin. Pharmacol. 55:620–624. 10.1046/j.1365-2125.2003.01797.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hutschala D, Skhirtladze K, Zuckermann A, Wisser W, Jaksch P, Mayer-Helm BX, Burgmann H, Wolner E, Muller M, Tschernko EM. 2005. In vivo measurement of levofloxacin penetration into lung tissue after cardiac surgery. Antimicrob. Agents Chemother. 49:5107–5111. 10.1128/AAC.49.12.5107-5111.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bastien O, Boulieu R, Bleyzac N, Estanove S. 1994. Clinical use of ganciclovir during renal failure and continuous hemodialysis. Intensive Care Med. 20:47–48. 10.1007/BF02425056 [DOI] [PubMed] [Google Scholar]
- 33.Fletcher C, Sawchuk R, Chinnock B, de Miranda P, Balfour HH., Jr 1986. Human pharmacokinetics of the antiviral drug DHPG. Clin. Pharmacol. Ther. 40:281–286. 10.1038/clpt.1986.177 [DOI] [PubMed] [Google Scholar]
- 34.Boulieu R, Bastien O, Bleyzac N. 1993. Pharmacokinetics of ganciclovir in heart transplant patients undergoing continuous venovenous hemodialysis. Ther. Drug Monit. 15:105–107. 10.1097/00007691-199304000-00006 [DOI] [PubMed] [Google Scholar]
- 35.Benet LZ, Galeazzi RL. 1979. Noncompartmental determination of the steady-state volume of distribution. J. Pharm. Sci. 68:1071–1074. 10.1002/jps.2600680845 [DOI] [PubMed] [Google Scholar]
- 36.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb S, Beale RJ, Vincent JL, Moreno R. 2013. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 39:165–228. 10.1007/s00134-012-2769-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Janoly-Dumenil A, Rouvet I, Bleyzac N, Bertrand Y, Aulagner G, Zabot MT. 2009. Effect of duration and intensity of ganciclovir exposure on lymphoblastoid cell toxicity. Antivir. Chem. Chemother. 19:257–262 [DOI] [PubMed] [Google Scholar]
- 38.Swan SK, Munar MY, Wigger MA, Bennett WM. 1991. Pharmacokinetics of ganciclovir in a patient undergoing hemodialysis. Am. J. Kidney Dis. 17:69–72 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.