Abstract
Hydroxychloroquine has been proposed for HIV treatment; however, little is known about its disposition in the lymphatic system, where replication takes place. Therefore, its distribution in lymphoid tissues (Peyer's patches and popliteal, submandibular, femoral, splenic, and prescapular lymph nodes) was evaluated and compared with that in blood. Results showed a high affinity of hydroxychloroquine for all of these tissues, with higher affinity for the splenic and submandibular lymph nodes, suggesting its potential use as a coadjuvant in HIV therapy.
TEXT
Hydroxychloroquine is a 4-aminoquinoline drug with more than 70 years of use as an antimalarial agent. Besides its antimalarial activity, it has well-documented efficacy and long-term safety in the therapy of autoimmune diseases like rheumatoid arthritis and systemic lupus erythematosus (1–3). The immunomodulatory effects of this agent are related to the reduction of inflammatory cytokine and IgG production, as well as down modulation of natural killer cell activity (4, 5). With regard to its in vivo anti-HIV-1 activity, it has been reported that hydroxychloroquine increases CD4+ T cell levels in HIV-infected patients who do not respond to antiretroviral therapy (6).
Although different studies have been performed to determine its blood/plasma distribution, as well as its pharmacokinetic parameters (7, 8), little is known about its disposition in lymphoid tissue; this distribution is important since lymph nodes are the major reservoir of HIV and the primary site of HIV replication (9). In a previous study, we found that in humans, hydroxychloroquine concentrations are higher in adenoid tissue than in plasma (10); therefore, the main objective of the present study was to determine the distribution of the drug in different lymphoid tissues by using the rabbit as an experimental model, taking into account that different studies have suggested that the rabbit immune system is more similar to the immune systems of primates and humans than is that of mice (11, 12, 13).
Healthy male New Zealand rabbits (Harlan, Mexico City, Mexico) 2 to 3 months old with a mean body weight of 2.4 kg were used. Each rabbit received a subcutaneous hydroxychloroquine (Sanofi Aventis) injection of 15 mg/kg of body weight. Blood samples were taken at 0, 10, 20, 30, 60, 120, 180, 240, 360, 480, and 840 min after drug administration (three animals per time point were used). Once the blood sample was taken, the animals were killed by cervical dislocation, cessation of circulation was confirmed, and tissue samples (Peyer's patches and popliteal, submandibular, femoral, prescapular, and splenic lymph nodes) were collected in preweighed vials on the same schedule as blood samples. The minced tissues were homogenized with 1 ml of 0.1 M phosphate buffer, pH 2.5. All samples were stored at −20 ± 1°C until use.
The study protocol complied with the Guide to the Care and Use of Experimental Animals and was approved by the Animal Ethics Committee of the Universidad Nacional Autónoma de México.
Hydroxychloroquine was assayed by high-performance liquid chromatography with a liquid extraction technique. Briefly, to 1 ml of blood or tissue homogenate, 2 ml of 0.4 N NaOH was added and the mixture was vortexed for 2 min; 8 ml of chloroform was added, and the mixture was vortexed for 1 min. Samples were centrifuged, the organic phase was transferred to an assay tube, and 1 ml of 0.1 M phosphate buffer, pH 5, was added. The aqueous phase was transferred, and 50 μl was injected into a Waters liquid chromatography system (Waters Corporation, MA). Analysis was performed on a Symmetry C18 analytical column (5 μm, 4.6 by 150 mm) at a flow rate of 0.5 ml/min with acetonitrile–0.01 M sodium dihydrogen phosphate buffer (pH 3) at 14:86 (vol/vol) as the mobile phase. The total recovery was 80 to 85%. The method was linear over a range of 200 to 5,000 ng/ml. The intraday coefficient of variation ranged from 3.8 to 7.2%. The limit of quantification was 120 ng/ml, and the limit of detection was 42.5 ng/ml.
Pharmacokinetic parameters were determined with the WinNonLin 5.0 program.
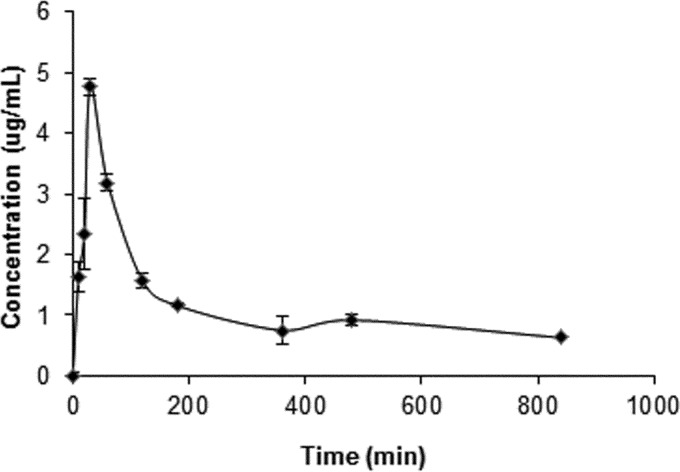
Considering that the drug concentrates in the cellular fraction of blood (7), our analysis was based on whole-blood determinations. The pharmacokinetic profile of hydroxychloroquine in whole blood is presented in Fig. 1. Our data show that the drug was rapidly absorbed after subcutaneous administration, with a time to maximum concentration of drug in blood of 30 min. The maximum concentration of drug in blood was 4.5 μg/ml. The half-life was 10.6 h, and the apparent clearance of the orally administered drug was 0.013 h−1.
FIG 1.
Mean blood hydroxychloroquine concentrations and standard deviations after subcutaneous drug administration (15 mg/kg) to rabbits (n = 3 per time point).
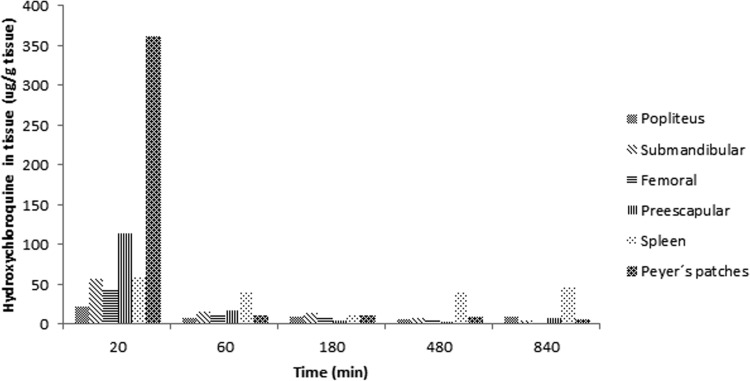
The biodistribution of hydroxychloroquine in lymphoid tissues is shown in Fig. 2. Hydroxychloroquine possesses two basic ionization sites with pKa values of 8.27 and 9.67. Although its permeability has not been evaluated, a high water-octanol partition coefficient of 3.85 has been reported (14). The results of the present study show that the drug is readily accumulated in lymphoid tissues, which could be related to the high partition coefficient. The lowest levels were found in femoral tissue, while spleen tissue showed the highest concentrations. In all of them, the maximum concentration was found at 20 to 30 min. Concentrations in the splenic lymph nodes were maintained for a longer time than in the other lymphoid tissues. These results were expected, considering that it has been reported that approximately 15% of the total lymphocytes in the body reside in the spleen, which is the largest reservoir in the body (15). The lymphoid tissue/blood drug concentration ratios determined are presented in Table 1. As in a previous study of humans (10), we found that hydroxychloroquine had a higher affinity for lymphoid tissues than for blood. Also, it has been shown that hydroxychloroquine and chloroquine do not accumulate in fatty tissues (16). This fact could be due to the amphiphilic properties of the drug, which allow its diffusion into the cellular lysosomal compartment, were the low pH induces its protonation and entrapment (17).
FIG 2.
Mean lymphoid tissue hydroxychloroquine concentrations after subcutaneous hydroxychloroquine administration (15 mg/kg) to rabbits (n = 3).
TABLE 1.
Tissue/blood hydroxychloroquine concentration ratios in rabbits
| Time (min) after drug administration | Ratio of hydroxychloroquine concn in following tissue to that in blood: |
|||||
|---|---|---|---|---|---|---|
| Popliteal lymph nodes | Submandibular lymph nodes | Femoral lymph nodes | Prescapular lymph nodes | Splenic lymph nodes | Peyer's patches | |
| 20 | 9.89 | 25.96 | 19.52 | 51.44 | 26.28 | 163.11 |
| 60 | 2.77 | 5.46 | 3.67 | 7.15 | 13.02 | 3.59 |
| 180 | 9.30 | 13.61 | 8.09 | 4.30 | 10.56 | 10.46 |
| 480 | 7.90 | 8.74 | 7.43 | 3.66 | 44.77 | 9.92 |
| 840 | 15.44 | 7.87 | 13.19 | 77.09 | 9.87 | |
Data on hydroxychloroquine efficacy against HIV are controversial. Some in vitro and in vivo studies have demonstrated only modest anti-HIV efficacy, as determined by viral burden measurements (6, 18, 19). Likewise, Paton et al. (20) found that in HIV-infected patients not taking antiretroviral therapy, the use of hydroxychloroquine not only reduced CD8 cell activation but resulted in a greater decline in the CD4 cell count and increased viral replication compared with a placebo. In contrast, Piconi et al. (6) evaluated the effect of hydroxychloroquine on HIV-infected patients treated with combined antiretroviral therapy. The patients were immunologic nonresponders with absolute CD4 cell counts of <200/ml. All of them received hydroxychloroquine at 400 mg/day for 6 months. The results showed induced immunomodulation, which was associated with increased percentages of circulating CD4+ T cells and was retained 2 months after therapy. The authors stated that hydroxychloroquine reduces lipopolysaccharide/T-like receptor-mediated immune activation.
It has been shown that the lymphatic system is involved in the dissemination of HIV in humans, and evidence indicates similar behavior in rabbits (21). The high levels of hydroxychloroquine found in all of the lymphoid tissues of rabbits suggest that the drug could be used as a coadjuvant in HIV therapy, particularly in patients who do not respond to antiretroviral therapy.
Footnotes
Published ahead of print 21 October 2013
REFERENCES
- 1.Momose Y, Arai S, Eto H, Kishimoto M, Okada M. 2013. Experience with the use of hydroxychloroquine for the treatment of lupus erythematosus. J. Dermatol. 40:94–97. 10.1111/1346-8138.12005 [DOI] [PubMed] [Google Scholar]
- 2.Tang C, Godfrey T, Stawell R, Nikpour M. 2012. Hydroxychloroquine in lupus: emerging evidence supporting multiple beneficial effects. Intern. Med. J. 42:968–978. 10.1111/j.1445-5994.2012.02886.x [DOI] [PubMed] [Google Scholar]
- 3.Clark P, Casas E, Tugwell P, Medina C, Gheno C, Tenorio G, Orozco JA. 1993. Hydroxychloroquine compared with placebo in rheumatoid arthritis. A randomized controlled trial. Ann. Intern. Med. 119:1067–1071 [DOI] [PubMed] [Google Scholar]
- 4.Tsokos GC. 1987. Immunomodulatory treatment in patients with rheumatic disease: mechanisms of action. Semin. Arthritis Rheum. 17:24–38. 10.1016/0049-0172(87)90014-X [DOI] [PubMed] [Google Scholar]
- 5.Fox RI. 1993. Mechanism of action of hydroxychloroquine as an antirheumatic drug. Semin. Arthritis Rheum. 23(2 Suppl 1):82–91 [DOI] [PubMed] [Google Scholar]
- 6.Piconi S, Parisotto S, Rizzardini G, Passerini S, Terzi R, Argenteri B, Meraviglia P, Capetti A, Biasin M, Trabattoni D, Clerici M. 2011. Hydroxychloroquine drastically reduces immune activation in HIV-infected, antiretroviral therapy-treated immunologic nonresponders. Blood 118:3263–3272. 10.1182/blood-2011-01-329060 [DOI] [PubMed] [Google Scholar]
- 7.Tett SE, Cutler DJ, Day R: Brown OG. 1988. A dose-ranging study of the pharmacokinetics of hydroxychloroquine following intravenous administration to healthy volunteers. Br. J. Clin. Pharmacol. 26:303–313. 10.1111/j.1365-2125.1988.tb05281.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carmichael SJ, Charles B, Tett SE. 2003. Population pharmacokinetics of hydroxychloroquine in patients with rheumatoid arthritis. Ther. Drug Monit. 25:671–681. 10.1097/00007691-200312000-00005 [DOI] [PubMed] [Google Scholar]
- 9.Cohen J. 2011. HIV/AIDS research. Tissue says blood is misleading, confusing HIV cure efforts. Science 334:1614. 10.1126/science.334.6063.1614 [DOI] [PubMed] [Google Scholar]
- 10.Aguirre-Cruz L, Torres KJ, Jung-Cook H, Fortuny C, Sánchez E, Soda-Mehry A, Sotelo J, Reyes-Terán G. 2010. Preferential concentration of hydroxychloroquine in adenoid tissue of HIV-infected subjects. AIDS Res. Hum. Retroviruses 26:339–342. 10.1089/aid.2009.0129 [DOI] [PubMed] [Google Scholar]
- 11.Graur D, Duret L, Gouy M. 1996. Phylogenetic position of the order Lagomorpha (rabbits, hares and allies). Nature 379:333–335. 10.1038/379333a0 [DOI] [PubMed] [Google Scholar]
- 12.Novacek MJ. 1996. Taxonomy. Where do rabbits and kin fit in? Nature 379:299–300 [DOI] [PubMed] [Google Scholar]
- 13.Pan R, Sampson JM, Chen Y, Vaine M, Wang S, Lu S, Kong XP. 2013. Rabbit anti-HIV-1 monoclonal antibodies raised by immunization can mimic the antigen-binding modes of antibodies derived from HIV-1 infected humans. J. Virol. 87:10221–10231. 10.1128/JVI.00843-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Warhurst D, Steele J, Adagu I, Craig J, Cullander C. 2003. Hydroxychloroquine is much less active than chloroquine against chloroquine-resistant Plasmodium falciparum, in agreement with its physicochemical properties. J. Antimicrob. Chemother. 52:188–193. 10.1093/jac/dkg319 [DOI] [PubMed] [Google Scholar]
- 15.Blum KS, Pabst R. 2007. Lymphocyte numbers and subsets in the human blood. Do they mirror the situation in all organs? Immunol. Lett. 108:45–51 http://dx.doi.org/10.1016/j.imlet.2006.10.009 [DOI] [PubMed] [Google Scholar]
- 16.Kuhn A, Ochsendorf F, Bonsmann G. 2010. Treatment of cutaneous lupus erythematosus. Lupus 19:1125–1136. 10.1177/0961203310370345 [DOI] [PubMed] [Google Scholar]
- 17.Ashfaq UA, Javed T, Rehman S, Nawaz Z, Riazuddin S. 2011. Lysosomotropic agents as HCV entry inhibitors. Virol. J. 8:163–168. 10.1186/1743-422X-8-163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sperber K, Louie M, Kraus T, Proner J, Sapira E, Lin S, Stecher V, Mayer L. 1995. Hydroxychloroquine treatment of patients with human immunodeficiency virus type 1. Clin. Ther. 17:622–636. 10.1016/0149-2918(95)80039-5 [DOI] [PubMed] [Google Scholar]
- 19.Sperber K, Kalb TH, Stecher VJ, Banerjee R, Mayer L. 1993. Inhibiton of human immunodeficiency virus type 1 replication by hydroxychloroquine in T cells and monocytes. AIDS Res. Hum. Retroviruses 9:91–98. 10.1089/aid.1993.9.91 [DOI] [PubMed] [Google Scholar]
- 20.Paton NI, Goodall RL, Dunn DT, Franzen S, Collaco-Moraes Y, Gazzard BG, Williams IG, Fisher MJ, Winston A, Fox J, Orkin C, Herieka EA, Ainsworth JG, Post FA, Wansbrough-Jones M, Kelleher P. 2012. Effects of hydroxychloroquine on immune activation and disease progression among HIV infected patients not receiving antiretroviral therapy: a randomized controlled trial. JAMA 308:353–361. 10.1001/jama.2012.6936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walder R, Kalvatchev Z, Pérez F, Garzaro D, Barrios M. 2001. Bovine immunodeficiency virus in experimentally infected rabbit: tropism for lymphoid and nonlymphoid tissues. Comp. Immunol. Microbiol. Infect. Dis. 24:1–20. 10.1016/S0147-9571(00)00010-2 [DOI] [PubMed] [Google Scholar]