Abstract
Aggregatibacter actinomycetemcomitans, a periodontopathogen, has been associated with several systemic diseases. Herein, we report the protective effect of human lactoferrin (hLF) during A. actinomycetemcomitans bacteremia in lactoferrin knockout (LFKO−/−) mice. The prophylactic, concurrent, and therapeutic intravenous (i.v.) administrations of hLF significantly cleared the bacteria from blood and organs. Nevertheless, all modes of hLF administration significantly decreased the concentrations of serum proinflammatory cytokines, such as interferon gamma (IFN-γ), tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), IL-6, IL-10, and IL-12p70. Additionally, hLF administration significantly decreased hepatic and splenic proinflammatory cytokine expression levels compared to those in the non-hLF-treated group. Furthermore, administration of hLF decreased the serum C-reactive protein level, inducible nitric oxide synthase (iNOS) and myeloperoxidase (MPO) gene expression levels in liver and spleen. hLF treatment has also resulted in a 6-fold decrease in spleen weight with the migration of typical inflammatory cells in infected mice as a result of decreased inflammatory response. These results reveal that hLF protects against A. actinomycetemcomitans bacteremia, as indicated by rapid bacterial clearance and decreased host proinflammatory mediators.
INTRODUCTION
Aggregatibacter actinomycetemcomitans is the most extensively reported organism found in cases of periodontitis, a chronic inflammatory disease that results in bone resorption, destruction of the connective tissues, and eventual bone loss during later stages of the disease. Oral bacteria very often have the potential to enter the bloodstream as a result of minor trauma, including typical daily activities, such as brushing teeth and certain invasive medical procedures (1). Therefore, it has been recommended that prophylactic antibiotics be prescribed before medical procedures to limit bacteremia and eventual endocarditis (2).
The immune system of the host normally protects the body from potentially harmful environmental stimuli by recognizing and responding with multiple immunological reactions. While controlling antigenic stimuli, human lactoferrin (hLF), a major defense protein of the innate immune system, also exerts direct first-line defense through its significant impact on the development of adaptive immune responses. The level of the LF is elevated severalfold during infection against the antigenic stimuli (3, 4). LF has been isolated primarily from human milk and is secreted by glandular epithelial cells. It is also expressed by immune cells and is notably detected in the secondary granules of neutrophils from which it is released during the inflammatory process. LF also has profound modulatory action on the adaptive immune system by promoting the maturation of T-cell precursors into competent helper cells and the differentiation of immature B cells into efficient antigen-presenting cells (3).
There have been many studies demonstrating the protective effect of LF and LF-derived peptides against in vivo infections in wild-type mouse models (Table 1) (5–10). Nonetheless, there is no information about the exclusive protective role of hLF during bacteremia in the absence of endogenous mouse lactoferrin (mLF). We also demonstrated in our previous study that LF knockout (LFKO−/−) mice had a higher alveolar bone loss with increased expression of proinflammatory cytokines as well as chemokines during oral infection with A. actinomycetemcomitans. We also reported that the oral infection of A. actinomycetemcomitans resulted in a higher level of systemic dissemination in LFKO−/− mice than in wild-type mice (11). Furthermore, oral pathogens have the potential to cause frequent bacteremia, and it is our interest to establish a model for LF to control bacteremia in the absence of mLF. Therefore, in the present study, we examined experimental A. actinomycetemcomitans bacteremia and the eventual patterns of antimicrobial and immunomodulatory activities with respect to hLF treatment in the LFKO−/− mouse model.
TABLE 1.
In vivo studies of the effects of LF in different infection models
| LF administration | Source(s) | Target | Reference |
|---|---|---|---|
| Oral | |||
| 20 mg/kg | bLF protein | Entamoeba histolytica | 36 |
| 20–25 mg/mouse | bLF protein | Mycobacterium tuberculosis | 37 |
| 5 or 100 mg/mouse | bLF protein | Salmonella enterica serovar Typhimurium | 38 |
| 10 mg/kg | hLF protein | Listeria monocytogenes | 39 |
| 62.5 mg/mouse | bLF protein | Influenza virus | 40 |
| 0.5 g/kg, 7 days | bLF protein | Candida albicans | 41 |
| 0.5–2%, 7 days | bLF protein | Fecal microflora | 5 |
| 2%, 7 days | bLF protein | Clostridium | 6 |
| 2 mg/dose | hLF protein peptide | Colitis | 27 |
| 200 mg/kg rats | bLF protein | Colitis | 42 |
| 2%, 12 days | bLF protein | IBD | 24 |
| i.v. | |||
| 100 mg/ml, 2 or 12 days | hLF and bLF | LPS | 19 |
| 10 mg, 2 h, 24 h | bLF protein | Escherichia coli | 35 |
| 10 mg/mouse | bLF protein | E. coli | 43 |
| 0.5 mg/mouse | bLF protein | E. coli | 44 |
| 250 μg/body wt | hLF protein | LPS | 45 |
| 10 mg/mouse | hLF and bLF protein | E. coli | 50 |
| 10 mg/g body wt | bLF protein | E. coli | 47 |
| 10 mg/mouse | bLF protein | Listeria monocytogenes | 46 |
| 10 mg/mouse | bLF protein | E. coli | 48 |
| Other | |||
| Oral and i.v., 0.04 to 40 mg/kg | hLF peptide | S. aureus | 49 |
| Ileal, 2 mg/loop | bLF protein | Cholera toxin | 48 |
| Oral and i.v., 100 mg/ml | hLF peptide | S. aureus | 10 |
MATERIALS AND METHODS
Mice.
The experimental groups comprised of 7- to 8-week-old male LFKO−/− mice (12), a generous gift from Orla Conneely (Department of Molecular and Cellular Biology, Baylor College of Medicine, Houston, TX) and David Briles (Department of Microbiology, University of Alabama, Birmingham, AL). Mice colonies were bred and maintained in the transgenic animal facility of Rutgers School of Dental Medicine, Newark, NJ. The experimental protocol was approved by the campus Institutional Animal Care and Use Committee (IACUC) of the Rutgers School of Dental Medicine, Newark, NJ.
Bacterial strain, infection, and preparation of hLF.
A. actinomycetemcomitans CU1000NRif (where “N” represents nalidixic acid resistant and Rif represents rifampin resistant), a spontaneous rifampin-resistant strain, was used as reported earlier (13). The bacterial count was determined quantitatively, and the bacteria were resuspended to 1 × 107 CFU per 0.1 ml of phosphate-buffered saline (PBS) and intravenously (i.v.) injected into the tail vein. The stock solution of low endotoxin-free apo-lactoferrin (85 to 90% purity) (Sigma-Aldrich, St. Louis, MO), prepared in pyrogen-free PBS and filter sterilized using 0·2-μm-pore Acrodisc filters, was used for i.v. injection or oral feeding without further purification (Gelman Sciences, Ann Arbor, MI). To determine the optimal antimicrobial concentration of hLF against A. actinomycetemcomitans, we used 100 to 500 μg/g of mouse body weight. For all other further experiments, 300 μg/g mouse body weight was used.
In vivo experimental design.
The effect of hLF was determined in seven different sets of experiments in LFKO−/− mice, which include the following groups: (i) sham-infected control mice i.v. injected with PBS (control), (ii) mice i.v. injected with hLF (300 μg/g body weight) (hLF only), (iii) mice orally fed with hLF (300 μg/g body weight) with a micropipette one time per day for 3 days and A. actinomycetemcomitans i.v. injected (oral feeding), (iv) mice i.v. injected with A. actinomycetemcomitans (bacteria only), (v) mice i.v. injected with hLF and with A. actinomycetemcomitans i.v. injected 2 h later (prophylactic), (vi) mice i.v. injected with A. actinomycetemcomitans and hLF at the same time (concurrent), and (vii) mice i.v. injected with A. actinomycetemcomitans and then i.v. injected with hLF 2 h later (therapeutic).
Determination of the viable levels.
To determine the effective concentration of hLF against bacterial clearance in the blood, different concentration ranging from 100 to 500 μg/g of body weight were tested. The blood samples were collected at different time intervals by cardiac puncture, and serially diluted samples were plated on A. actinomycetemcomitans growth medium (AAGM) plates supplemented with rifampin (24 μg/ml). Plates were incubated at 37°C in a 5% CO2 incubator for 48 h, and the numbers of colonies enumerated were expressed as CFU/ml of blood. To determine the bacterial counts in the organs (brain, heart, kidney, liver, lungs, and spleen), the organs were aseptically removed from the mice and placed in 1 ml of PBS in 50-ml Kendall tissue homogenizers (Tyco Healthcare Group, Mansfield, MA). Following homogenization of the tissues, serially diluted samples were plated on AAGM plates, as described previously. In total, three independent experiments representing three biological replicates were performed, and data were statistically analyzed using one-way analysis of variance (ANOVA) (13).
CBC.
Heparinized blood was obtained by retro-orbital phlebotomy under anesthesia, and the complete blood count (CBC) was determined by using the automated H1 Technicon system. (Antech Diagnostics, New Hyde Park, NY).
Serum analysis.
The bioavailability of hLF in mouse serum samples was analyzed using the sandwich enzyme-linked immunosorbent assay (ELISA), as described previously (11). The serum proinflammatory cytokines were analyzed by Milliplex mouse cytokine/chemokine custom multicytokine detection, and the C-reactive protein (CRP) levels were analyzed using mouse acute-phase kit (Millipore Corporation, Billerica, MA). The Luminex 100 system was used to acquire the results, and MILLIPLEX Analyst software (VigeneTech, Carlisle, MA) was used to analyze the results. Acquisition and analysis have been optimized for multiple parameter measurements as described in the manual.
Real-time RT-PCR gene expression analysis.
The extraction of total RNA was carried out using an RNeasy minikit (Qiagen, Inc., Valencia, CA). Real-time reverse transcription (RT)-PCR analysis was carried out according to the manufacturer's protocol (high-capacity cDNA reverse transcription kit with Fast SYBR green master mix; ABI, Foster City, CA). The primer sequences and conditions for the hepatic and splenic cytokine expression levels were followed as reported earlier (11). The primers used for the expression analysis of the inducible nitric oxide synthase (iNOS) and myeloperoxidase (MPO) are given in Table 2. Results are the means ± standard errors of the means (SEM) of the duplicate experiments of three independent samples.
TABLE 2.
List of primers used in this study
| Target | Primer sequence (5′→3′) | Annealing temp (°C)a | Amplicon size (bp) |
|---|---|---|---|
| β-Actin | |||
| Sense | ATGTTTGAGACCTTCAACA | 56 | 495 |
| Antisense | CACGTCAGACTTCATGATGG | ||
| iNOS | |||
| Sense | CAGCTGGGCTGTACAAACCTT | 56 | 94 |
| Antisense | CATTGGAAGTGAAGCGTTTCG | ||
| MPO | |||
| Sense | GGAAGGAGACCTAGAGGTTGG | 58 | 127 |
| Antisense | TAGCACAGGAAGGCCAATG |
Annealing temperature for PCR.
Histopathology of spleen.
At 48 h postinfection, aseptic collection of spleen was performed as described above. The tissues were fixed in 4% paraformaldehyde for 48 h. Tissue sections were routinely processed, embedded in paraffin, mounted on slides, and stained with hematoxylin and eosin (H&E) (14). Tissue sections were observed under an Olympus light microscope at ×1,000 magnification.
Statistical analysis.
Significance of differences between the groups was calculated using Student's t test. Values of P < 0.05 were considered statistically significant. Continuous variables were compared by pairwise t test for two independent samples. Bonferroni correction was used to protect against multiple comparison. Nonlinear regression was used to determine a best-fit curve for the ELISA standard curve and to generate an R2 value. The R2 values of the standard curves for these assays were generally greater than 0.95, with most assays having an R2 value of 0.99. Assays with standard curves with R2 values of <0.9 were repeated. Correlation analysis of two variables was carried out with JMP software SAS 9.1 (SAS, Cary, NC).
RESULTS
The antimicrobial activity of hLF in the blood revealed that a prophylactic injection dose of 300 μg of body weight was optimal for bacterial clearance. Therefore, for further experiments, 300 μg was used (Fig. 1). Prophylactic, concurrent, and therapeutic i.v. administrations of hLF revealed that the bacteria were rapidly cleared from the blood compared to the case in the group administered bacteria only (Fig. 2). Analysis of the bacteria from various organs revealed that i.v. administration of hLF had significantly cleared the bacteria compared to the case in the non-hLF-treated group. However, the oral feeding of hLF did not show a significant decrease in the bacterial count compared to case in the bacterium-only group. Comparison of the modes of administration of hLF revealed that the concurrent administration was effective against A. actinomycetemcomitans compared to the non-hLF-treated mouse group (Fig. 2).
FIG 1.
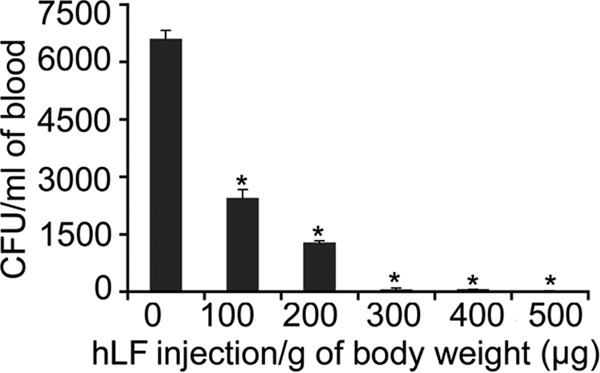
(A) Determination of the hLF concentration against circulating A. actinomycetemcomitans in LFKO−/− mice. Mice (n = 4) were injected with hLF (100 to 500 μg/g of body weight) 2 h before injection of A. actinomycetemcomitans (1 × 107). Bacterial counts in the blood after 6 h are expressed as mean CFU/ml of blood. Asterisks indicate significance (P < 0.05) of differences in the bacterial counts between hLF-treated and untreated groups, as calculated from duplicates by Student's t test.
FIG 2.
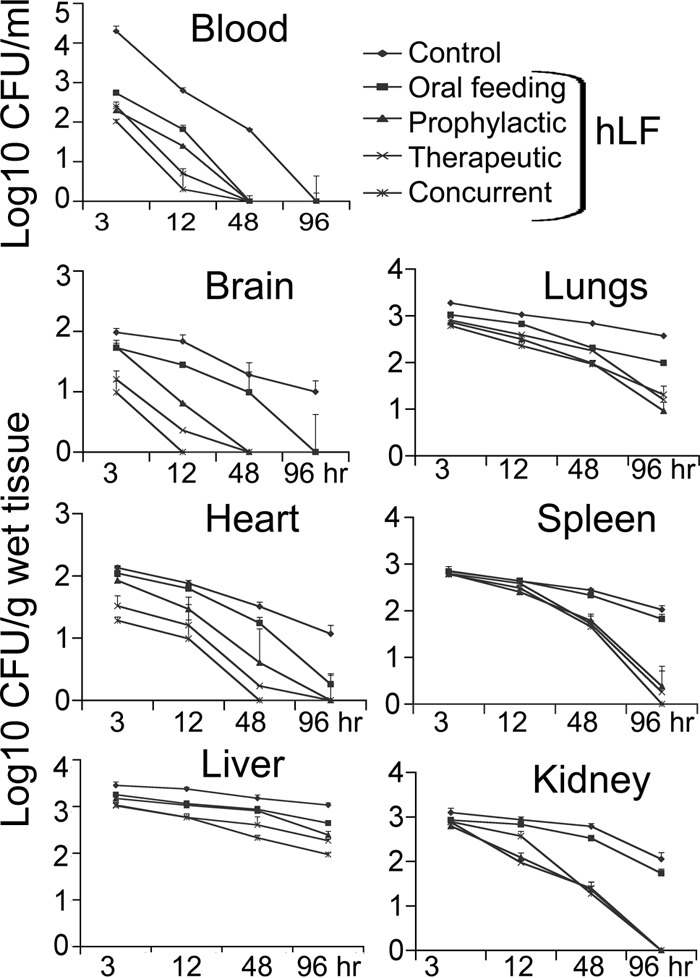
Time course of A. actinomycetemcomitans clearance by hLF in blood and different organs of LFKO−/− mice during bacteremia. Experimental groups (n = 8) were i.v. injected with 0.1 ml of A. actinomycetemcomitans (CFU/ml) or hLF (300 μg/g of body weight). The amounts of bacteria from blood and different organs are expressed as mean CFU/ml blood or CFU/g of tissue wet weight. Asterisks indicate statistical significance (P < 0.05) of differences between the groups, as calculated by Student's t test. The data shown are from the mean of triplicate experiments.
Next, the ELISA analysis of the residual hLF in the serum samples showed a significant decrease in the hLF concentrations up to 96 h in all groups tested. Among the groups, concurrent administration had the smallest amount of residual hLF (P = 0.002) compared to the amounts in the other groups. However, oral feeding of hLF prior to A. actinomycetemcomitans bacteremia did not show detectable hLF in the serum samples (Fig. 3).
FIG 3.
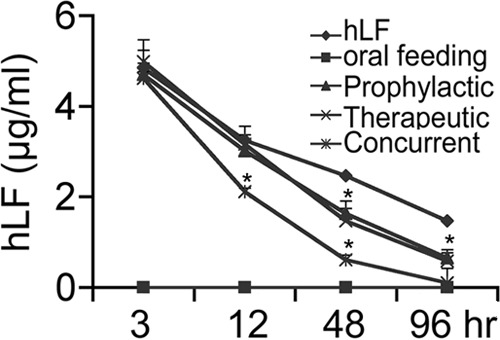
Bioavailability of hLF in the serum samples of LFKO−/− mice. At different time intervals, blood was collected from mice (n = 5) injected with hLF (300 μg/g of body weight), with or without A. actinomycetemcomitans injection. The amount of hLF in the serum was assessed by ELISA in duplicate experiments for each mouse, and the data are presented as means ± SEM. Asterisks indicate significant difference (P < 0.05) between infection and the hLF-injected group.
The efficacy of hLF on the inhibition of A. actinomycetemcomitans-induced serum proinflammatory mediators was analyzed (Table 3). The experimental bacteremia significantly increased the serum cytokine levels, including those of interferon gamma (IFN-γ) (P = 0.008), tumor necrosis factor alpha (TNF-α) (P = 0.01), interleukin-1β (IL-1β) (P = 000), IL-6 (P = 0.05), IL-10 (P = 0.05), and IL-12p70 (P = 0.04) at 48 h postinfection compared to the PBS/hLF-only control group. Analysis of the results indicates a significant decrease in IFN-γ, TNF-α, IL-1β, and IL-6 levels in the prophylactic, concurrent, and therapeutic hLF-administered groups. However, there was a higher IL-10 concentration (238 ± 12.3 pg/ml) in the concurrent group, and there were slightly elevated concentrations of both IL-10 and IL12p70 in the group given prophylactic administration compared to the group given therapeutic administration of hLF. Similarly, prophylactic oral feeding of hLF also exhibited higher levels of IL-10 (136 ± 15.6 pg/ml) and IL-12p70 (106 ± 31.5 pg/ml). Analysis of the cytokine release in the serum revealed that the IL-6 level was the maximum concentration (34,312 ± 713 pg/ml) of all cytokines tested in this study, followed by IFN-γ (Table 3). Subsequent analysis of proinflammatory cytokine gene expression in the spleen and liver indicated that the concurrent and therapeutic hLF administrations resulted in significant reduction compared to the result in the non-hLF-treated group. However, prophylactic hLF administration did not show a significant decrease in A. actinomycetemcomitans-induced expression of the IL-10 and IL-12 genes. There was no significant decrease in the levels of IL-6, IL-10, and IL-12 observed in the group treated with oral feeding of hLF (Fig. 4).
TABLE 3.
Evaluation of the effects of hLF on serum proinflammatory mediators during A. actinomycetemcomitans bacteremia
| hLF treatment | Concn (pg/ml) of serum inflammatory mediator after treatmenta |
|||||
|---|---|---|---|---|---|---|
| IFN-γ | TNF-α | IL-1β | IL-6 | IL-10 | IL-12p70 | |
| PBS | 29.5 ± 2.1 | 16.5 ± 2.1 | 15.0 ± 1.4 | 27.5 ± 2.1 | 6.4 ± 4.0 | 21.0 ± 1.4 |
| hLF only | 36.0 ± 1.4 | 22.5 ± 0.7 | 18.0 ± 0.0 | 82.0 ± 2.8 | 6.3 ± 1.9 | 26.0 ± 1.4 |
| Bacteria | 1,517 ± 200 | 493 ± 83.4 | 903 ± 115.3 | 34,312 ± 713 | 490 ± 34.0 | 861 ± 89.8 |
| hLF oral feeding | 48.1 ± 0.8* | 6.4 ± 1.3* | 46.72 ± 6.5* | 618 ± 86.3* | 136 ± 15.6* | 106 ± 31.5* |
| Prophylactic | 48.5 ± 2.1* | 20.5 ± 0.7* | 18.0 ± 0.0* | 143.0 ± 4.2* | 32.5 ± 2.8* | 33.5 ± 2.1* |
| Therapeutic | 0.0 ± 0.0* | 2.6 ± 1.2* | 5.7 ± 1.2* | 2.0 ± 1.2* | 4.1 ± 2.0* | 17.1 ± 9.8* |
| Concurrent | 4.2 ± 2.2* | 7.3 ± 1.6* | 7.2 ± 0.6* | 778 ± 12.3* | 238 ± 12.3* | 13.2 ± 0.6* |
Serum samples were collected at 48 h postinfection from each group, and the significance (*, P < 0.05) of differences in the reduction of the proinflammatory cytokines analyzed during bacteremia was determined. Different modes of hLF administration (300 μg/g body weight) were analyzed for significant differences from the non-hLF-treated groups.
FIG 4.
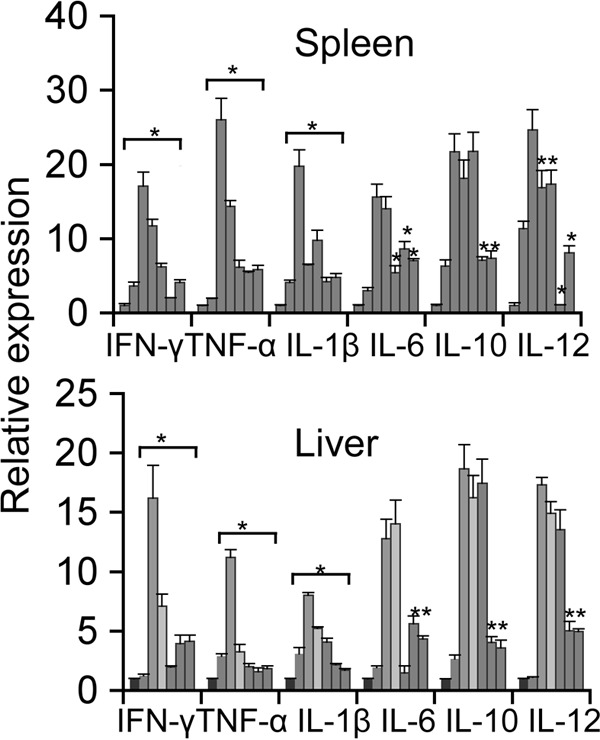
Real-time PCR analysis of splenic and liver cytokine mRNA expression. Shown are relative levels of expression of cytokines in the (i) sham-infected control, (ii) hLF-injected, (iii) A. actinomycetemcomitans-injected, and (iv) hLF-pretreated A. actinomycetemcomitans-injected groups. Results for the (i) control, (ii) hLF-alone, (iii) bacterium-alone, (iv) hLF oral feeding, (v) prophylactic, (vi) therapeutic, and (vii) concurrent treatment groups were calculated by the cycle threshold (2−ΔΔCT) method using β-actin as an internal control. Results are shown as mean fold change ± SEM over uninfected controls. Asterisks indicate statistically significant difference compared to uninfected controls (P < 0.05), as calculated by Student's t test.
Analysis of spleen weights in A. actinomycetemcomitans-infected mice after 96 h revealed that there was a significant increase (381 mg) in the spleen weights compared to those in hLF-treated infected mice (5.12 mg; P = 0.03). All hLF treatment groups were shown to have a significant decrease in their splenic weight compared to the bacterium-only group. However, there was no significant decrease in the splenic weight observed in the oral feeding group (Table 4). Analysis of the morphological features of the spleen in infected mice revealed the presence of small inflammatory foci with a central area of necrosis. A. actinomycetemcomitans infection led to the migration of different cell populations to the cordal space in the red pulp and also invasion of the white pulp (Fig. 5).
TABLE 4.
Change in spleen weight during A. actinomycetemcomitans bacteremia
| LFKO−/− mouse treatment group | Spleen wt (mg)a |
|---|---|
| LFKO−/− control | 58.6 ± 3.17 |
| hLF alone | 63.5 ± 0.76 |
| Bacteria only | 381.2 ± 3.32* (P < 0.003) |
| hLF oral feeding | 259 ± 12.31 |
| Prophylactic | 74.3 ± 7.20* (P < 0.03) |
| Therapeutic | 81.3 ± 5.5* (P < 0.001) |
| Concurrent | 68.8 ± 8.97* (P < 0.04) |
Spleens of mice (n = 8) were dissected 96 h postinfection, and their weights were measured. Values are presented as means ± SEM. *, significant (P < 0.05) difference from the control and/or the hLF treatment group, as calculated by Student's t test.
FIG 5.
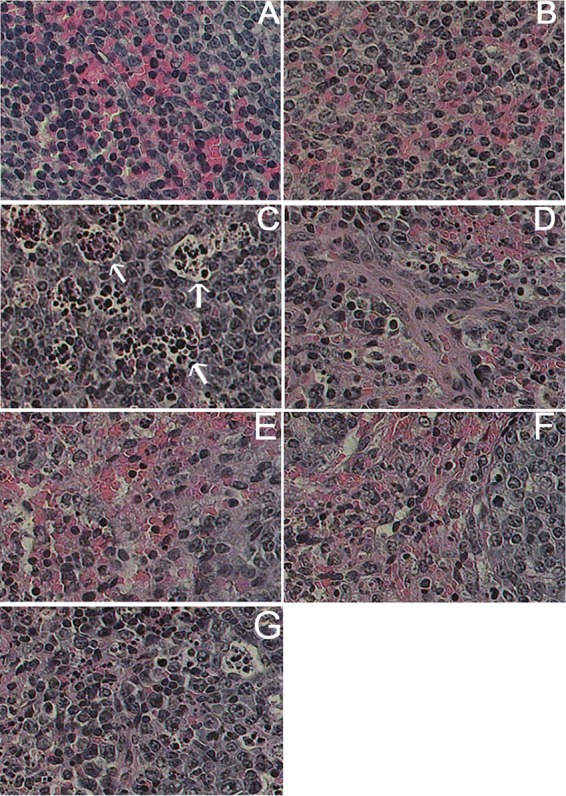
Histopathological analysis of spleen during A. actinomycetemcomitans bacteremic conditions treated with hLF. Histopathology of LFKO−/− mouse spleen. Arrows show the morphological changes in the spleen section. Shown are results for the following treatment groups: (A) PBS, (B) hLF only, C) bacteria only, (D) oral feeding of hLF, (E) prophylactic hLF i.v. administration, (F) concurrent hLF i.v. administration, and (G) therapeutic hLF i.v. administration.
Analysis of the CRP levels revealed that there was a significant elevation in the bacterium-only group compared to the sham-infected group. Mice treated with hLF alone did not show any increase in CRP level. The prophylactic (P = 0.07), therapeutic (P = 0.002), and concurrent (P = 0.003) hLF administrations significantly reduced the A. actinomycetemcomitans-induced serum CRP levels compared to those in the bacterium-only group (Fig. 6). Real-time RT-PCR analysis of the other acute-phase reactions, such as iNOS and MPO mRNA expression in the spleen, revealed that all treatment groups showed a significant decrease. Nonetheless, the hLF oral feeding did not show a significant decrease in expression in the liver tissue (Fig. 7).
FIG 6.
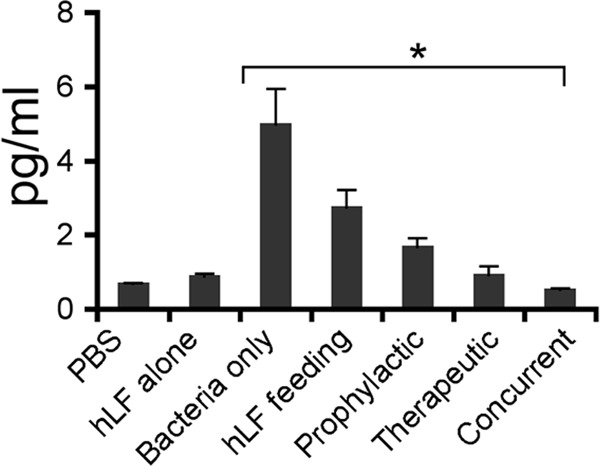
Assessment of CRP levels in hLF-treated A. actinomycetemcomitans bacteremic mice. Serum samples were collected at 48 h postinfection in each group and analyzed for CRP levels in duplicates. The data presented are means ± SEM. The asterisk indicates significance (P < 0.05) compared to the non-hLF-treated group, as calculated by Student's t test.
FIG 7.
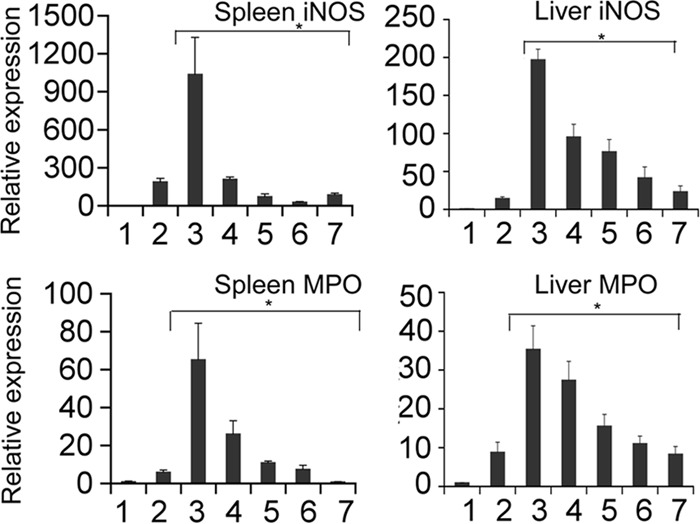
Acute-phase iNOS and MPO gene expression during A. actinomycetemcomitans bacteremia in hLF-treated mice. The gene expression of iNOS and MPO from spleen and liver was calculated by using β-actin as an internal control. Results are shown as mean fold change ± SEM over uninfected controls. Asterisks indicate statistically significant difference compared to the uninfected control (P < 0.05), as calculated by Student's t test. The bars represent the following treatment groups: 1, control; 2, hLF alone; 3, bacteria alone; 4, hLF oral feeding; 5, prophylactic; 6, therapeutic; and 7, concurrent.
In order to analyze the changes in the complete blood count during A. actinomycetemcomitans infection and hLF administrations, we examined the percentage of peripheral blood. The total lymphocyte count was slightly decreased during infection in hLF-treated or nontreated infected mice. There were increases in neutrophil counts in the hLF-treated (21% ± 1.13%) and bacterium-only (22% ± 0.71%) groups, as well as in the prophylactic group (15.72% ± 6.19%). There was no significant change in the oral feeding, therapeutic, and concurrent hLF-administered groups. Furthermore, there were no differences seen in the cell counts of monocytes, eosinophils, and basophils among any groups of mice (Table 5).
TABLE 5.
Peripheral blood count during A. actinomycetemcomitans infection
| LFKO−/− mouse treatment group | Mean ± SEM % of: |
||||
|---|---|---|---|---|---|
| Lymphocytes | Neutrophils | Monocytes | Eosinophils | Basophils | |
| Control | 72.5 ± 2.72 | 11.5 ± 0.85 | 3.4 ± 0.41 | 2.5 ± 0.26 | 1.5 ± 0.26 |
| hLF alone | 70.6 ± 3.16 | 21 ± 1.13* | 2.1 ± 0.52 | 6.4 ± 2.3 | 1 ± 0.63 |
| Bacteria only | 75 ± 0.2 | 22 ± 0.71* | 3.1 ± 1.12 | 2.4 ± 1.89 | 1 ± 0.42 |
| hLF feeding | 73 ± 9.31 | 11 ± 5.21 | 2 ± 0.93 | 3 ± 1.86 | 1 ± 0.50 |
| Prophylactic | 72.5 ± 5.79 | 15.72 ± 6.19* | 3.41 ± 2.17 | 2.5 ± 1.3 | 1.06 ± 0.36 |
| Therapeutic | 67 ± 10.54 | 26.0 ± 9.8 | 3 ± 7.2 | 2 ± 5.7 | 1 ± 1.09 |
| Concurrent | 67 ± 10.13 | 27.87 ± 6.12 | 3 ± 1.98 | 2.3 ± 1.97 | 1.5 ± 1.21 |
Mice (n = 8) were used around 7 weeks of age at the time of analysis. *, significant difference (P < 0.05) from the control and/or hLF treatment group.
DISCUSSION
Recent studies have suggested that the Gram-negative periodontopathogen A. actinomycetemcomitans can easily enter into the bloodstream through routine daily procedures, such as teeth brushing and chewing food. Also, the invasiveness of this strain increases the potential risk of systemic diseases, including infective endocarditis (15, 16), atherosclerosis (17), and brain abscesses (18). More recently, increasing attention has been paid to the innate immunity in combating pathogen infection. LF represents one of the elements of the innate immunity system (19) and is abundant in secretory fluids of mammals and secondary granules of neutrophils (20). LF is considered an important mediator in host defense against various environmental insults. LF is also considered a first-line defense protein in protection against a multitude of microbial infections and prevention of systemic inflammation (21).
The present study also showed that i.v. administration of hLF rapidly cleared the bacteria from blood and various organs. There have been several reports demonstrating the protective role of both hLF and bovine lactoferrin (bLF) against systemic diseases and bacteremia in mouse models showing bacterial clearance, diminishing levels of lipopolysaccharide (LPS), or reduction in bacterial induced inflammatory cytokines (Table 1). Indeed, LF is well documented as having direct antimicrobial activity, including an iron-dependent bacteriostatic property and non-iron-dependent bactericidal action on LPS-bearing Gram-negative bacteria (22). While suppressing microbial growth, LF also directly exerts its first-line defense activity with significant impact through development of adaptive immune responses. LF, indeed, has a profound modulatory action on the adaptive immune system (23). It has also been reported that the oral feeding of LF in a rat model exerts a protective effect against colitis via modulation of the immune system and correction of cytokine imbalance (6). In contrast, LF oral feeding did not protect the mice against inflammatory bowel disease (IBD) (24).
It has been shown that LF can also function as an anti-inflammatory factor in that it regulates the production of inflammatory cytokines in a manner similar to that of other anti-inflammatory cytokines (23). In addition, to being a potential member of first-line host defense; in vitro and in vivo studies suggest that this anti-inflammatory activities may be due to inhibition of several cytokines, including TNF-α and IL-1β, that are key mediators of inflammatory response (19, 25). It has also been reported that the levels of IL-6 and IL-10 were apparent when hLF was administered as a prophylactic prior to LPS challenge (Table 1). The present study also demonstrates that the injection of hLF greatly reduce the levels of A. actinomycetemcomitans-induced cytokines. However, a previous report on activated murine leukocytes revealed that the levels of IL-10 and IL-12 are increased during hLF treatment in vitro. At this point, with the available information, it is difficult to explain the immunomodulatory role of hLF on these cytokines. Further investigation is required to elucidate the underlying mechanism of hLF administration on IL-10 and IL-12 in LFKO−/− mice.
It has been reported that hLF has the ability to increase neutrophil migration in peripheral blood and in organs (19). Also, it was demonstrated that the induction of IL-6 and TNF-α by i.v. hLF injection could contribute to the increased mobilization of neutrophils from the bone marrow (26). The results of this study also show increased IL-6 and TNF-α expression and thus may contribute to bacterial clearance. It has also been shown that bLF can stimulate phagocytic activity of human neutrophils during infection (20). The anti-infective effect of hLF against A. actinomycetemcomitans may be multifactorial. Since antimicrobial and anti-inflammatory activities have been described for hLF in vitro, it is possible that hLF exerts both of these properties in our infection model. In vivo studies have shown that hLF downregulates dextran sulfate-induced IL-1β production (27). Thus, the anti-inflammatory capacity of hLF in our model could be a consequence of either its antibacterial activity, downregulation of inflammatory cytokines, or both.
We also noticed that hLF has the ability to inhibit LPS-induced acute-phase responses, such as serum CRP levels as well as iNOS and MPO mRNA expression in spleen (28, 29). It has been reported that hLF treatment can decrease the serum CRP levels in many systemic diseases (30). The present study documents the decrease of splenic and hepatic immune responses and iNOS and MPO expression. Similarly, in a previous report, A. actinomycetemcomitans LPS stimulation resulted in higher levels of splenic immune responses (31). Morphological and histological observations of spleen have shown strong evidence that hLF treatment can decrease the levels of inflammatory responses compared to those in hLF-nontreated A. actinomycetemcomitans-infected mice. From the clinical point of view, the spleen plays a major role in bacterial clearance during bacteremia, as evidenced by its enlarged and morphologically differentiated appearance (32). We also found migration of different cell types in the spleen (33). However, in hLF-treated mice, the inflammation was decreased upon infection and was similar to that in sham-infected animals, as evidenced by the reduction in the spleen weight and decrease in the expression of cytokine mRNA levels (34, 35).
It has been documented that both LPS or bacterial challenge and LF administration lead to chemotaxis of the peripheral neutrophils. The present study has also shown a significant increase in neutrophil migration. At this point, the increased neutrophil migration in hLF administration groups could be due to effects of both A. actinomycetemcomitans as well as hLF. In conclusion, in A. actinomycetemcomitans-induced bacteremia, the present study demonstrated that mice i.v. administered hLF cleared the bacteria more rapidly than those orally administered hLF.
ACKNOWLEDGMENTS
The corresponding author thanks Pauline P. Ward, Orla M. Conneely, and David Briles for providing us the LFKO−/− mice.
This research was supported by the NIH NIDCR grant R21 DE019548 to V.K. The authors also thank the confocal imaging facility, a core facility of Rutgers-NJMS.
Footnotes
Published ahead of print 4 November 2013
REFERENCES
- 1.Kinane DF, Riggio MP, Walker KF, MacKenzie D, Shearer B. 2005. Bacteraemia following periodontal procedures. J. Clin. Periodontol. 32:708–713. 10.1111/j.1600-051X.2005.00741.x [DOI] [PubMed] [Google Scholar]
- 2.Dajani AS, Taubert KA, Wilson W, Bolger AF, Bayer A, Ferrieri P, Gewitz MH, Shulman ST, Nouri S, Newburger JW, Hutto C, Pallasch TJ, Gage TW, Levison ME, Peter G, Zuccaro G., Jr 1997. Prevention of bacterial endocarditis: recommendations by the American Heart Association. Clin. Infect. Dis. 25:1448–1458. 10.1086/516156 [DOI] [PubMed] [Google Scholar]
- 3.Actor JK, Hwang SA, Kruzel ML. 2009. Lactoferrin as a natural immune modulator. Curr. Pharm. Des. 15:1956–1973. 10.2174/138161209788453202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmad G, Sial GZ, Ramadori P, Dudas J, Batusic DS, Ramadori G. 2011. Changes of hepatic lactoferrin gene expression in two mouse models of the acute phase reaction. Int. J. Biochem. Cell Biol. 43:1822–1832. 10.1016/j.biocel.2011.09.002 [DOI] [PubMed] [Google Scholar]
- 5.Teraguchi S, Shin K, Ogata T, Kingaku M, Kaino A, Miyauchi H, Fukuwatari Y, Shimamura S. 1995. Orally administered bovine lactoferrin inhibits bacterial translocation in mice fed bovine milk. Appl. Environ. Microbiol. 61:4131–4134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teraguchi S, Shin K, Ozawa K, Nakamura S, Fukuwatari Y, Tsuyuki S, Namihira H, Shimamura S. 1995. Bacteriostatic effect of orally administered bovine lactoferrin on proliferation of Clostridium species in the gut of mice fed bovine milk. Appl. Environ. Microbiol. 61:501–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teraguchi S, Wakabayashi H, Kuwata H, Yamauchi K, Tamura Y. 2004. Protection against infections by oral lactoferrin: evaluation in animal models. Biometals 17:231–234. 10.1023/B:BIOM.0000027697.83706.32 [DOI] [PubMed] [Google Scholar]
- 8.Zimecki M, Kapp J, Machnicki M, Zagulski T, Wlaszczyk A, Kubler A, Mazurier J, Spik G. 1998. Lactoferrin. Its role in maturation and function of cells of the immune system and protection against shock in mice. Adv. Exp. Med. Biol. 443:331–336 [PubMed] [Google Scholar]
- 9.Yamaguchi N, Kieba IR, Korostoff J, Howard PS, Shenker BJ, Lally ET. 2001. Maintenance of oxidative phosphorylation protects cells from Actinobacillus actinomycetemcomitans leukotoxin-induced apoptosis. Cell. Microbiol. 3:811–823. 10.1046/j.1462-5822.2001.00161.x [DOI] [PubMed] [Google Scholar]
- 10.Bhimani RS, Vendrov Y, Furmanski P. 1999. Influence of lactoferrin feeding and injection against systemic staphylococcal infections in mice. J. Appl. Microbiol. 86:135–144. 10.1046/j.1365-2672.1999.00644.x [DOI] [PubMed] [Google Scholar]
- 11.Velusamy S, Ganeshnarayan K, Markowitz K, Schreiner H, Furgang D, Fine DH, Velliyagounder K. 2013. Lactoferrin knockout mice demonstrate greater susceptibility to Aggregatibacter actinomycetemcomitans-induced periodontal disease. J. Periodontol. 84:1690–1701. 10.1902/jop.2013.120587 [DOI] [PubMed] [Google Scholar]
- 12.Ward PP, Mendoza-Meneses M, Cunningham GA, Conneely OM. 2003. Iron status in mice carrying a targeted disruption of lactoferrin. Mol. Cell. Biol. 23:178–185. 10.1128/MCB.23.1.178-185.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schreiner H, Markowitz K, Miryalkar M, Moore D, Diehl S, Fine DH. 2011. Aggregatibacter actinomycetemcomitans-induced bone loss and antibody response in three rat strains. J. Periodontol. 82:142–150. 10.1902/jop.2010.100250 [DOI] [PubMed] [Google Scholar]
- 14.van den Berg S, Laman JD, Boon L, ten Kate MT, de Knegt GJ, Verdijk RM, Verbrugh HA, Nouwen JL, Bakker-Woudenberg IA. 2013. Distinctive cytokines as biomarkers predicting fatal outcome of severe Staphylococcus aureus bacteremia in mice. PLoS One 8:e59107. 10.1371/journal.pone.0059107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang G, Kitten T, Munro CL, Wellman GC, Mintz KP. 2008. EmaA, a potential virulence determinant of Aggregatibacter actinomycetemcomitans in infective endocarditis. Infect. Immun. 76:2316–2324. 10.1128/IAI.00021-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang G, Mintz KP. 2010. Glycosylation of the collagen adhesin EmaA of Aggregatibacter actinomycetemcomitans is dependent upon the lipopolysaccharide biosynthetic pathway. J. Bacteriol. 192:1395–1404. 10.1128/JB.01453-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang T, Kurita-Ochiai T, Hashizume T, Du Y, Oguchi S, Yamamoto M. 2010. Aggregatibacter actinomycetemcomitans accelerates atherosclerosis with an increase in atherogenic factors in spontaneously hyperlipidemic mice. FEMS Immunol. Med. Microbiol. 59:143–151. 10.1111/j.1574-695X.2010.00674.x [DOI] [PubMed] [Google Scholar]
- 18.Rahamat-Langendoen JC, van Vonderen MG, Engstrom LJ, Manson WL, van Winkelhoff AJ, Mooi-Kokenberg EA. 2011. Brain abscess associated with Aggregatibacter actinomycetemcomitans: case report and review of literature. J. Clin. Periodontol. 38:702–706. 10.1111/j.1600-051X.2011.01737.x [DOI] [PubMed] [Google Scholar]
- 19.Machnicki M, Zimecki M, Zagulski T. 1993. Lactoferrin regulates the release of tumour necrosis factor alpha and interleukin 6 in vivo, Int. J. Exp. Pathol. 74:433–439 [PMC free article] [PubMed] [Google Scholar]
- 20.Miyauchi H, Hashimoto S, Nakajima M, Shinoda I, Fukuwatari Y, Hayasawa H. 1998. Bovine lactoferrin stimulates the phagocytic activity of human neutrophils: identification of its active domain. Cell. Immunol. 187:34–37. 10.1006/cimm.1997.1246 [DOI] [PubMed] [Google Scholar]
- 21.Sanchez L, Calvo M, Brock JH. 1992. Biological role of lactoferrin. Arch. Dis. Child. 67:657–661. 10.1136/adc.67.5.657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bellamy W, Takase M, Yamauchi K, Wakabayashi H, Kawase K, Tomita M. 1992. Identification of the bactericidal domain of lactoferrin. Biochim. Biophys. Acta 1121:130–136. 10.1016/0167-4838(92)90346-F [DOI] [PubMed] [Google Scholar]
- 23.Baveye S, Elass E, Mazurier J, Spik G, Legrand D. 1999. Lactoferrin: a multifunctional glycoprotein involved in the modulation of the inflammatory process. Clin. Chem. Lab. Med. 37:281–286 [DOI] [PubMed] [Google Scholar]
- 24.Spagnuolo PA, Hoffman-Goetz L. 2008. Dietary lactoferrin does not prevent dextran sulfate sodium induced murine intestinal lymphocyte death. Exp. Biol. Med. (Maywood) 233:1099–1108. 10.3181/0802-RM-53 [DOI] [PubMed] [Google Scholar]
- 25.Crouch SP, Slater KJ, Fletcher J. 1992. Regulation of cytokine release from mononuclear cells by the iron-binding protein lactoferrin. Blood 80:235–240 [PubMed] [Google Scholar]
- 26.Zimecki M, Artym J, Chodaczek G, Kocieba M, Kruzel M. 2005. Effects of lactoferrin on the immune response modified by the immobilization stress. Pharmacol. Rep. 57:811–817 http://www.if-pan.krakow.pl/pjp/pdf/2005/6_811.pdf [PubMed] [Google Scholar]
- 27.Haversen LA, Baltzer L, Dolphin G, Hanson LA, Mattsby-Baltzer I. 2003. Anti-inflammatory activities of human lactoferrin in acute dextran sulphate-induced colitis in mice. Scand. J. Immunol. 57:2–10. 10.1046/j.1365-3083.2003.01162.x [DOI] [PubMed] [Google Scholar]
- 28.Kruzel ML, Actor JK, Boldogh I, Zimecki M. 2007. Lactoferrin in health and disease. Postepy Hig. Med. Dosw. 61:261–267 http://www.phmd.pl/pub/phmd/vol_61/10398.pdf [PubMed] [Google Scholar]
- 29.van der Does AM, Hensbergen PJ, Bogaards SJ, Cansoy M, Deelder AM, van Leeuwen HC, Drijfhout JW, van Dissel JT, Nibbering PH. 2012. The human lactoferrin-derived peptide hLF1-11 exerts immunomodulatory effects by specific inhibition of myeloperoxidase activity. J. Immunol. 188:5012–5019. 10.4049/jimmunol.1102777 [DOI] [PubMed] [Google Scholar]
- 30.Maccio A, Madeddu C, Gramignano G, Mulas C, Sanna E, Mantovani G. 2010. Efficacy and safety of oral lactoferrin supplementation in combination with rHuEPO-beta for the treatment of anemia in advanced cancer patients undergoing chemotherapy: open-label, randomized controlled study. Oncologist 15:894–902. 10.1634/theoncologist.2010-0020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sosroseno W, Musa M, Ravichandran M, Ibrahim MF, Bird PS, Seymour GJ. 2008. Effect of inhibition of inducible nitric oxide synthase (iNOS) on the murine splenic immune response induced by Aggregatibacter (Actinobacillus) actinomycetemcomitans lipopolysaccharide. Eur. J. Oral Sci. 116:31–36. 10.1111/j.1600-0722.2007.00501.x [DOI] [PubMed] [Google Scholar]
- 32.Sosroseno W, Bird PS, Seymour GJ. 2009. Effect of exogenous nitric oxide on murine splenic immune response induced by Aggregatibacter actinomycetemcomitans lipopolysaccharide. Anaerobe 15:95–98. 10.1016/j.anaerobe.2009.01.002 [DOI] [PubMed] [Google Scholar]
- 33.Chen LT, Lu L, Broxmeyer HE. 1987. Effects of purified iron-saturated human lactoferrin on spleen morphology in mice infected with Friend virus complex. Am. J. Pathol. 126:285–292 [PMC free article] [PubMed] [Google Scholar]
- 34.Zimecki M, Miedzybrodzki R, Mazurier J, Spik G. 1999. Regulatory effects of lactoferrin and lipopolysaccharide on LFA-1 expression on human peripheral blood mononuclear cells. Arch. Immunol. Ther. Exp. (Warsz.) 47:257–264 [PubMed] [Google Scholar]
- 35.Zimecki M, Artym J, Chodaczek G, Kocieba M, Kruzel ML. 2004. Protective effects of lactoferrin in Escherichia coli-induced bacteremia in mice: relationship to reduced serum TNF alpha level and increased turnover of neutrophils. Inflamm. Res. 53:292–296. 10.1007/s00011-004-1257-1 [DOI] [PubMed] [Google Scholar]
- 36.Leon-Sicairos N, Martinez-Pardo L, Sanchez-Hernandez B, de la Garza M, Carrero JC. 2012. Oral lactoferrin treatment resolves amoebic intracecal infection in C3H/HeJ mice. Biochem. Cell Biol. 90:435–441. 10.1139/o2012-008 [DOI] [PubMed] [Google Scholar]
- 37.Welsh KJ, Hwang SA, Boyd S, Kruzel ML, Hunter RL, Actor JK. 2011. Influence of oral lactoferrin on Mycobacterium tuberculosis induced immunopathology. Tuberculosis (Edinb.) 91(Suppl 1):S105–S113. 10.1016/j.tube.2011.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Drago-Serrano ME, Rivera-Aguilar V, Resendiz-Albor AA, Campos-Rodriguez R. 2010. Lactoferrin increases both resistance to Salmonella typhimurium infection and the production of antibodies in mice. Immunol. Lett. 134:35–46. 10.1016/j.imlet.2010.08.007 [DOI] [PubMed] [Google Scholar]
- 39.Lee HY, Park JH, Seok SH, Baek MW, Kim DJ, Lee BH, Kang PD, Kim YS, Park JH. 2005. Potential antimicrobial effects of human lactoferrin against oral infection with Listeria monocytogenes in mice. J. Med. Microbiol. 54:1049–1054. 10.1099/jmm.0.45918-0 [DOI] [PubMed] [Google Scholar]
- 40.Shin K, Wakabayashi H, Yamauchi K, Teraguchi S, Tamura Y, Kurokawa M, Shiraki K. 2005. Effects of orally administered bovine lactoferrin and lactoperoxidase on influenza virus infection in mice. J. Med. Microbiol. 54:717–723. 10.1099/jmm.0.46018-0 [DOI] [PubMed] [Google Scholar]
- 41.Takakura N, Wakabayashi H, Ishibashi H, Yamauchi K, Teraguchi S, Tamura Y, Yamaguchi H, Abe S. 2004. Effect of orally administered bovine lactoferrin on the immune response in the oral candidiasis murine model. J. Med. Microbiol. 53:495–500. 10.1099/jmm.0.05505-0 [DOI] [PubMed] [Google Scholar]
- 42.Togawa J, Nagase H, Tanaka K, Inamori M, Nakajima A, Ueno N, Saito T, Sekihara H. 2002. Oral administration of lactoferrin reduces colitis in rats via modulation of the immune system and correction of cytokine imbalance. J. Gastroenterol. Hepatol. 17:1291–1298. 10.1046/j.1440-1746.2002.02868.x [DOI] [PubMed] [Google Scholar]
- 43.Zagulski T, Jarzabek Z, Zagulska A, Jaszczak M, Kochanowska IE, Zimecki M. 2001. Lactoferrin stimulates killing and clearance of bacteria but does not prevent mortality of diabetic mice. Arch. Immunol. Ther. Exp. (Warsz.) 49:431–438 [PubMed] [Google Scholar]
- 44.Zimecki M, Artym J, Kocieba M, Weber-Dabrowska B, Lusiak-Szelachowska M, Gorski A. 2008. The concerted action of lactoferrin and bacteriophages in the clearance of bacteria in sublethally infected mice. Postepy Hig. Med. Dosw. 62:42–46 http://www.phmd.pl/pub/phmd/vol_62/11539.pdf [PubMed] [Google Scholar]
- 45.Yamaguchi M, Matsuura M, Kobayashi K, Sasaki H, Yajima T, Kuwata T. 2001. Lactoferrin protects against development of hepatitis caused by sensitization of Kupffer cells by lipopolysaccharide. Clin. Diagn. Lab. Immunol. 8:1234–1239. 10.1128/CDLI.8.6.1234-1239.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Palumbo D, Iannaccone M, Porta A, Capparelli R. 2010. Experimental antibacterial therapy with puroindolines, lactoferrin and lysozyme in Listeria monocytogenes-infected mice. Microbes Infect. 12:538–545. 10.1016/j.micinf.2010.03.010 [DOI] [PubMed] [Google Scholar]
- 47.Zagulski T, Lipinski P, Zagulska A, Jarzabek Z. 1998. Antibacterial system generated by lactoferrin in mice in vivo is primarily a killing system. Int. J. Exp. Pathol. 79:117–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rivera FP, Medina AM, Bezada S, Valencia R, Bernal M, Meza R, Maves RC, Ochoa TJ. 2013. Bovine lactoferrin decreases cholera-toxin-induced intestinal fluid accumulation in mice by ganglioside interaction. PLoS One 8:e59253. 10.1371/journal.pone.0059253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brouwer CP, Welling MM. 2008. Various routes of administration of (99m)Tc-labeled synthetic lactoferrin antimicrobial peptide hLF 1–11 enables monitoring and effective killing of multidrug-resistant Staphylococcus aureus infections in mice. Peptides 29:1109–1117. 10.1016/j.peptides.2008.03.003 [DOI] [PubMed] [Google Scholar]
- 50.Zagulski T, Lipinski P, Zagulska A, Broniek S, Jarzabek Z. 1989. Lactoferrin can protect mice against a lethal dose of Escherichia coli in experimental infection in vivo. Br. J. Exp. Pathol. 70:697–704 [PMC free article] [PubMed] [Google Scholar]


