Abstract
Pharmacokinetic exposure and the MIC of fluoroquinolones are important determinants of their efficacy against Mycobacterium tuberculosis. Population modeling was used to describe the steady-state plasma pharmacokinetics of moxifloxacin in 241 tuberculosis (TB) patients in southern Africa. Monte Carlo simulations were applied to obtain the area under the unbound concentration-time curve from 0 to 24 h (fAUC0–24) after daily doses of 400 mg or 800 mg moxifloxacin and 800 mg ofloxacin. The MIC distributions of ofloxacin and moxifloxacin were determined for 197 drug-resistant clinical isolates of Mycobacterium tuberculosis. For a specific MIC, the probability of target attainment (PTA) was determined for target fAUC0–24/MIC ratios of ≥53 and ≥100. The PTAs were combined with the MIC distributions to calculate the cumulative fraction of response (CFR) for multidrug-resistant (MDR) Mycobacterium tuberculosis strains. Even with the less stringent target ratio of ≥53, moxifloxacin at 400 mg and ofloxacin at 800 mg achieved CFRs of only 84% and 58% for multidrug-resistant isolates with resistance to an injectable drug, while the 800-mg moxifloxacin dose achieved a CFR of 98%. Using a target ratio of ≥100 for multidrug-resistant strains (without resistance to injectable agents or fluoroquinolones), the CFR was 88% for moxifloxacin and only 43% for ofloxacin, and the higher dose of 800 mg moxifloxacin was needed to achieve a CFR target of >90%. Our results indicate that moxifloxacin is more efficacious than ofloxacin in the treatment of MDR-TB. Further studies should determine the optimal pharmacodynamic target for moxifloxacin in a multidrug regimen and clarify safety issues when it is administered at higher doses.
INTRODUCTION
Fluoroquinolones play an important role in the treatment of multidrug-resistant tuberculosis (MDR-TB) (1), which is defined as resistance to both rifampin and isoniazid (2). Fluoroquinolones differ from each other in their efficacy against Mycobacterium tuberculosis as measured by the ratio of the area under the unbound concentration-time curve from 0 to 24 h (fAUC0–24/MIC) and also display differences in their clinical pharmacokinetics. The in vitro bactericidal activity of moxifloxacin against M. tuberculosis is superior to that of ofloxacin (3); its improved potency has also been confirmed in mice (4). The substitution of ethambutol by moxifloxacin, but not ofloxacin, in combination with isoniazid, rifampin, and pyrazinamide in the treatment of susceptible TB resulted in faster culture conversion (5, 6). New fluoroquinolones are usually preferred to the earlier-generation ones (7), but ofloxacin is still widely used to treat MDR-TB because of its affordability and availability.
Moxifloxacin is rapidly absorbed, and the major fraction of the dose reaches the systemic circulation within 2 h (8, 9). It has a long half-life in humans (8, 9), with moderate renal excretion of 6% to 20% of total elimination after intravenous administration (9). Moxifloxacin is a substrate of inducible p-glycoprotein (10), sulfotransferases (11), and glucuronosyltransferases (12). Coadministration of moxifloxacin with rifapentine (enzyme and transporter inducer) gave 17.2% (8) and 8% (13) decreases in moxifloxacin exposure in healthy volunteers (dosed three times a week) and tuberculosis patients (dosed once or twice weekly), respectively. Ofloxacin is rapidly absorbed, with peak concentrations reached within 2 h and with a half-life of 6 h, and those characteristics are comparable between healthy volunteers (14) and infected patients (15). Ofloxacin is primarily renally eliminated (16); its concentrations were reported to increase linearly with dose, but elimination of ofloxacin decreases with declining renal function and increasing age (17).
The critical concentration for drug susceptibility is defined as the lowest concentration of a drug that inhibits ≥95% of wild-type strains lacking mechanisms of acquired or mutational resistance to the specific drug (18). Accordingly, the World Health Organization (WHO) recommends susceptibility testing breakpoint concentrations for moxifloxacin and ofloxacin of 0.25 and 2.0 mg/liter, respectively (19). The efficacy of fluoroquinolones has been related to the fAUC0–24/MIC ratio (20). Based on in vitro, murine, and clinical studies, a fAUC0–24/MIC ratio of at least 100 to 125 has been proposed as a reliable predictor of bactericidal activity against Gram-positive and Gram-negative bacteria (21, 22). The hollow-fiber bioreactor system (HFS) has suggested a minimum target fAUC0–24/MIC ratio of 53 for M. tuberculosis as the identified target for suppressing the outgrowth of moxifloxacin-resistant mutants but not necessarily optimal bactericidal activity (23).
In this study, we aimed to describe the population pharmacokinetics of moxifloxacin using data from 241 South African and Zimbabwean patients with pulmonary tuberculosis who participated in the RIFAQUIN study ISRCTN 44153044 (24, 25). Monte Carlo simulations were then employed to assess the probability of reaching the fAUC0–24/MIC ratio target using moxifloxacin and ofloxacin at the recommended doses for MDR-TB (2). For ofloxacin pharamcokinetics assays, we used a population model that we reported previously (26), while the MIC distributions of moxifloxacin and ofloxacin for drug-resistant M. tuberculosis isolates were previously determined (27).
MATERIALS AND METHODS
Study population.
Patients (n = 241) with pulmonary TB received an initial intensive phase of therapy that included daily rifampin and moxifloxacin for 2 months. For the continuation phase, they were treated with either 400 mg moxifloxacin once weekly together with 1,200 mg rifapentine or with 400 mg moxifloxacin twice weekly with 900 mg of rifapentine. Pharmacokinetic sampling was carried out during the fourth month of therapy. Previously published pharmacokinetic data obtained during rifapentine and moxifloxacin cotreatment of 28 patients (13) were combined with concentration-time data obtained from 213 additional patients who participated in the RIFAQUIN study (24, 25). The doses of rifapentine and moxifloxacin were taken with 240 ml of water 15 min after the patients received 2 hard-boiled eggs with bread. Four hours after dosing, a light meal, snacks, and fluids were provided. Pharmacokinetic samples were obtained immediately before dosing and at 1, 2, 3, 5, 7, 10, 12, 26, and 50 h after the dose in 28 patients. For the remaining 213 patients, samples were obtained at 2 (± 0.5) h, 5 (± 0.5) h, and 24 (± 3) h or 48 (± 3) h after dosing. HIV-positive patients who required antiretroviral treatment at randomization were excluded. Separate written informed consent for the pharmacokinetic study was obtained from the RIFAQUIN study participants in Harare (Zimbabwe) and in Johannesburg (Gauteng) and Worcester (Western Cape, South Africa). The study protocol was reviewed and approved by the London-Surrey Borders Research Ethics Committee (reference no. 07/Q0806/58), the Research Ethics Committee of the University of Cape Town, the Medicines Control Council of South Africa, the Medicines Research Council of Zimbabwe, and the Medicines Control Authority of Zimbabwe.
Drug determination.
After blood collection, plasma was separated and immediately stored at −80°C. Moxifloxacin concentrations were determined using liquid chromatography-tandem mass spectrometry (LC-MS/MS) as previously described (13). The lower limit of quantification was 0.063 mg/liter.
MICs of clinical isolates.
MICs of moxifloxacin and ofloxacin were determined for 197 drug-resistant M. tuberculosis isolates from patients in the Western Cape, South Africa, by Bactec MIGIT 960 as previously described (27). The 0.25 mg/liter and 2.0 mg/liter concentrations of moxifloxacin and ofloxacin were used as susceptibility breakpoints to differentiate between susceptible and resistant strains as suggested by WHO (19).
Pharmacokinetic analysis.
Moxifloxacin plasma concentration-time data were analyzed using a nonlinear mixed-effects model as implemented in NONMEM 7.2 (28). The execution of runs was through Perl-speaks-NONMEM (29), and graphical diagnostics were created using Xpose 4 (30). The use of allometric scaling testing total body weight (WT), fat-free mass (FFM) (31), and fat mass (FAT) as predictors was applied on clearance (CL), intercompartmental clearance (Q), and volume of distribution of the central (Vc) and peripheral (Vp) compartments, as previously described (13). Various structural models were tested, including one- or two-compartment distribution with first-order absorption and elimination rate constants, absorption lag time, and transit compartment absorption (32). Estimation of typical population pharmacokinetic parameters, along with their random interindividual variability (IIV), was performed using a first-order conditional estimation method with ε-η interaction (FOCE INTER). A lognormal distribution for IIV was assumed, and additive and/or proportional models for the residual unexplained variability (RUV) were evaluated. Data below the lower limit of quantification (LLOQ) were described using the M3 method (33). The covariate relationships were screened by using a stepwise approach and forward inclusion using a delta objective function (ΔOFV) of ≥3.84 (P ≤ 0.05) as the cutoff for inclusion, followed by a backward elimination using a ΔOFV of ≥6.83 (P ≤ 0.01) for covariate retention. The tested covariates included age, HIV status, sex, site, and regimen/arm (drug administration once weekly versus twice weekly). The detected covariate effects were included in the final model if clinically significant (a cutoff of 20% was used). Estimates of the precision of parameters were obtained from a nonparametric bootstrap (n = 200).
Model evaluation.
Model selection was based on graphical assessment of conditional weighted residuals (CWRES) versus time, basic goodness-of-fit plots (GOF), changes in the NONMEM objective function (OFV), estimates of the precision of parameters as provided by the NONMEM covariance step (if successfully completed), and, most importantly, visual predictive checks (VPC) (34).
Pharmacokinetic simulations and probability of target attainment.
The final pharmacokinetic model was used to perform Monte Carlo simulations for 10,000 individuals after administration of multiple daily doses of 400 mg moxifloxacin to obtain steady-state fAUC0–24 values. Daily doses of 800 mg of moxifloxacin were also explored. The simulated fAUC0–24 values were obtained by using covariate distributions similar to those used for the population on which the model was developed and assuming 50% plasma protein binding for moxifloxacin (9, 35, 36). Similar simulations were performed to obtain the fAUC0–24 values for ofloxacin using a previously published model, developed from South African patients with MDR-TB (26), and an unbound fraction value of 0.75 in humans (16).The estimated fAUC0–24/MIC ratios were obtained by dividing fAUC0–24 values by MICs ranging from 0.125 to 8 mg/liter. The MIC distributions of moxifloxacin and ofloxacin of drug-resistant M. tuberculosis isolates were from a separate study in patients from the Western Cape, South Africa (27). For the comparison, we used targets of fAUC0–24/MIC ≥ 100 and fAUC0–24/MIC ≥ 53. The probability of target attainment (PTA) was calculated as the proportion of individuals achieving fAUC0–24/MIC ≥ 100 (or ≥ 53) for a specific MIC. The cumulative fraction of response (CFR) (37) was calculated as the weighted average of the PTA across the MIC strata, as shown below:
The PTA at each MICi level was multiplied by the relative frequency of that MIC in the study population, p(MICi). Our target was CFR ≥ 90%.
RESULTS
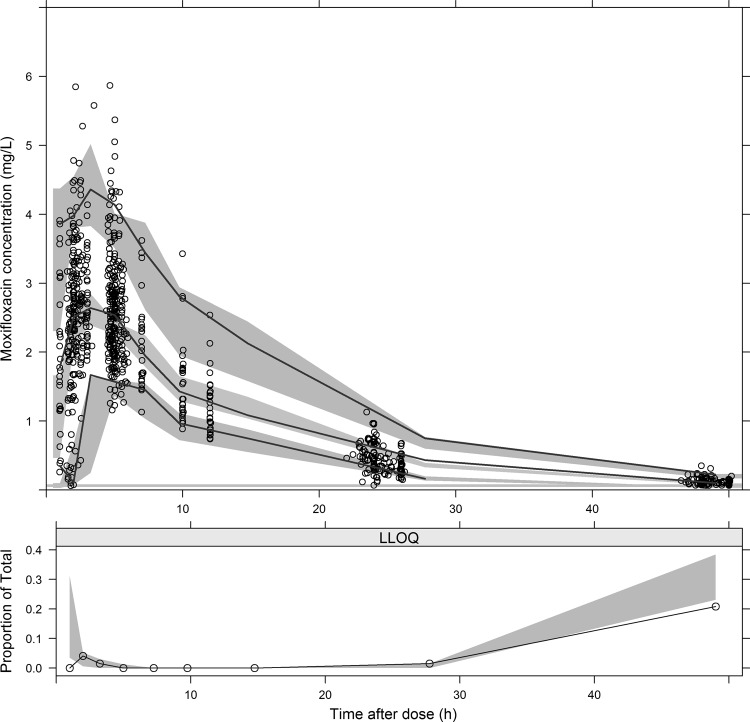
Although the RIFAQUIN study patients had drug-susceptible pulmonary tuberculosis, while the patients in the ofloxacin pharmacokinetic study had MDR-TB, their demographic and patient characteristics were similar and differed only by HIV status and sex (Table 1). The 241 patients on moxifloxacin provided 856 concentration-time points, and only 4% were below LLOQ. As in our previous analysis (13), the population pharmacokinetics of moxifloxacin was well described by a two-compartment model with first-order elimination and transit absorption compartments. FFM was used for allometric scaling of CL, Q and Vc, while Vp was better scaled with FAT. The final parameter estimates are shown in Table 2, and a VPC of the final model is shown in Fig. 1. No significant differences in the pharmacokinetic parameters were found between the once-weekly and twice-weekly dosing approaches, and no additional covariates were included except for body size, which was incorporated via allometric scaling. The Monte Carlo simulations predicted a median AUC0–24 value of 38.7 after 400-mg daily moxifloxacin administration, while the 2.5th and 97.5th percentiles were 21.9 and 69.6 mg·h/liter, respectively.
TABLE 1.
Characteristics of patients who received moxifloxacin in the RIFAQUIN trial or ofloxacin in a previous studya
| Parameter | Value(s) |
||
|---|---|---|---|
| Patients on moxifloxacin (13, 24, 25) | Patients on moxifloxacin (24, 25) | Patients on ofloxacin (26) | |
| Total no. of patients | 28 | 213 | 65 |
| No. (%) of males | 19 (68) | 134 (63) | 52 (80) |
| No. (%) HIV+ (%) | 3 (11) | 43 (20) | 35 (54) |
| Median age, range (yrs) | 39.7 (19.8–53.4) | 31.6 (22.8–56.6) | 34 (19–70) |
| Median wt, range (kg) | 52.0 (41.0–71.0) | 56.0 (37.7–74.0) | 55 (35–91.8) |
| Median ht, range (cm) | 163 (151–176) | 167 (151–184) | 167 (127–189) |
| BMI, range (kg/m2) | 19.6 (13.2–31.1) | 20.1 (11.1–32.5) | 19.3 (12.4–39.3) |
| No. (%) of patients on twice-weekly doses | 15 (54) | 101 (47) | N/A |
BMI, body mass index; N/A, not applicable.
TABLE 2.
Parameter estimates of the final moxifloxacin pharmacokinetic modela
| Parameter | Value(s) (RSE[%]) |
|
|---|---|---|
| Typical | IIVb | |
| CL (liters/h) | 10.6 (2.68) | 18.7 (4.05) |
| Vc (liters) | 114 (1.36) | |
| ka (h−1) | 1.50 (2.15) | 69.9 (3.62) |
| MTT (h) | 0.723 (7.02) | 73.4 (2.58) |
| No. of transit compartments | 11.6 (2.39) | |
| Q (liters/h) | 2.14 (2.92) | 32.9 (3.17) |
| Vp (liters) | 89.8 (3.66) | |
| F | 1 FIX | 17.7 (3.28) |
| Proportional error (%) | 7.85 (1.44) | |
RSE, relative standard error reported on the approximate standard-deviation scale obtained from a bootstrap sample size of 200; CL, oral clearance; Vc, volume of distribution in the central compartment; ka, first-order absorption rate constant; MTT, absorption mean transit time; Q, intercompartmental clearance; Vp, volume of distribution in the peripheral compartment; F, oral bioavailability fixed to 1 (since we did not have intravenous injection data). In this table, we report the values of parameters directly estimated by the model. To obtain CL/F, the values of CL must be combined with those of F. Since the typical value of F was fixed to 1, the typical value of CL/F has the same value as CL, while the between-subject variability (BSV) of CL/F needs to take into account both the BSV in CL and that in F. A similar consideration is valid for Vc/F, Q/F, and Vp/F.
IIV, interindividual variability expressed as percent coefficient of variation (% CV).
FIG 1.
Visual predictive check (VPC) for the final moxifloxacin population pharmacokinetic model. In the upper panel, the lower, middle, and upper solid lines are the 5th, median, and 95th percentiles of the observed plasma concentration, respectively, while the shaded areas are the 95% confidence intervals for the same percentiles of the simulated data. The lower panel shows the fraction of observed data below the lower limit of quantification (LOQ), which is represented by the solid line. The shaded area shows the simulation-based 95% confidence interval around the median of the LOQ data.
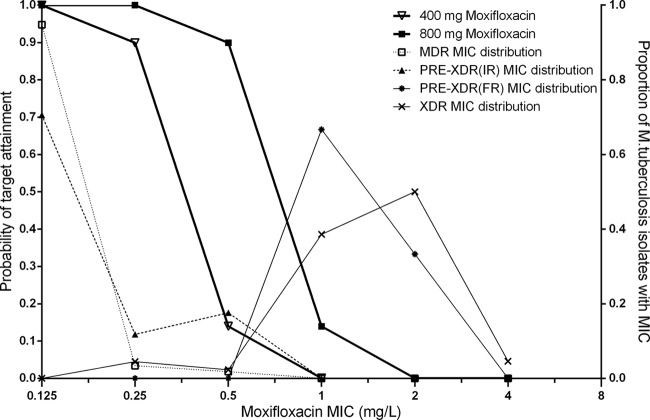
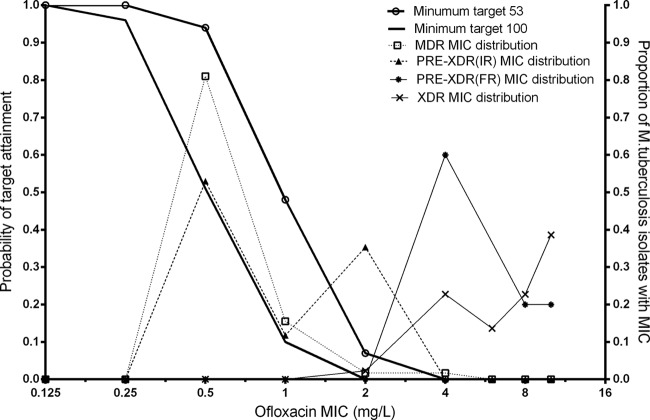
The MIC distributions of moxifloxacin and ofloxacin are listed in Table 3. The PTA with a target fAUC0–24/MIC ratio of ≥53 across the range of MIC values for daily 400-mg and 800-mg moxifloxacin doses is shown in Fig. 2, while the PTA for daily 800-mg ofloxacin doses is shown in Fig. 3. Table 4 shows the CFR for daily 400 mg and 800 mg moxifloxacin and for daily 800 mg ofloxacin with a target fAUC0–24/MIC value of either ≥53 or ≥100. Moxifloxacin at 400 mg had a higher CFR than ofloxacin at 800 mg in both scenarios (target ratios of 53 and 100).
TABLE 3.
The MIC distributions of moxifloxacin and ofloxacin in 197 Mycobacterium tuberculosis isolates
| Resistance profilea | No. of isolates with indicated drug MIC (mg/liter) |
Total no. of isolates | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ≤0.125 | >0.125 ≤ 0.25 | >0.25 ≤ 0.5 | >0.5 ≤ 1.0 | >1.0 ≤ 2.0 | >2.0 ≤ 4.0 | >4.0 ≤ 6.0 | >6.0 ≤ 8.0 | ≥10.0 | ||
| Moxifloxacin | ||||||||||
| INH | 68 | 68 | ||||||||
| RIF | 5 | 5 | ||||||||
| MDR | 55 | 2 | 1 | 58 | ||||||
| MDR+INJ | 12 | 2 | 3 | 17 | ||||||
| MDR+FLQ | 3 | 2 | 5 | |||||||
| XDR | 2 | 1 | 17 | 22 | 2 | 44 | ||||
| Ofloxacin | ||||||||||
| INH | 59 | 9 | 68 | |||||||
| RIF | 5 | 5 | ||||||||
| MDR | 47 | 9 | 1 | 1 | 58 | |||||
| MDR+INJ | 9 | 2 | 6 | 17 | ||||||
| MDR+FLQ | 3 | 1 | 1 | 5 | ||||||
| XDR | 1 | 10 | 6 | 10 | 17 | 44 | ||||
Resistance to either isoniazid (INH) or rifampin (RIF) represented monoresistance. MDR, resistance to both INH and RIF; MDR+INJ, MDR plus resistant to an injectable; MDR+FLQ, MDR plus resistant to either fluoroquinolone; XDR, MDR plus resistance to both a FLQ and an injectable.
FIG 2.
Probability of target attainment (target fAUC0–24/MIC ratio ≥ 53) versus Mycobacterium tuberculosis isolate MICs for 400-mg and 800-mg moxifloxacin doses. MDR and XDR data represent MIC distributions from multidrug-resistant and extensively drug-resistant isolates, respectively. PRE-XDR(IR) and PRE-XDR(FR) data represent MIC distributions from isolates resistant to injectables and fluroroquinolones, respectively.
FIG 3.
Probability of target attainment (target fAUC0–24/MIC ratio ≥ 53 or 100) versus Mycobacterium tuberculosis isolate MICs for 800-mg ofloxacin dose. MDR and XDR data represent MIC distributions from multidrug-resistant and extensively drug-resistant isolates, respectively. PRE-XDR(IR) and PRE-XDR(FR) data represent MIC distributions from isolates resistant to injectables and fluroroquinolones, respectively.
TABLE 4.
The cumulative fractions of response for daily doses of 400 mg and 800 mg of moxifloxacin and 800 mg of ofloxacin for target fAUC0–24/MIC ratios of 53 (23) and 100 (21, 22, and 38)a
| fAUC0–24/MIC ratio and M. tuberculosis strain phenotype | CFR expectation |
||
|---|---|---|---|
| 400 mg moxifloxacin | 800 mg moxifloxacin | 800 mg ofloxacin | |
| ≥53 | |||
| MDR | 0.98 | 1.00 | 0.84 |
| MDR+INJ | 0.84 | 0.98 | 0.58 |
| MDR+FLQ | 0.00 | 0.09 | 0.00 |
| XDR | 0.04 | 0.12 | 0.00 |
| ≥100 | |||
| MDR | 0.88 | 0.98 | 0.43 |
| MDR+INJ | 0.68 | 0.85 | 0.28 |
| MDR+FLQ | 0.00 | 0.00 | 0.00 |
| XDR | 0.01 | 0.04 | 0.00 |
CFR, cumulative fraction of response; MDR, resistance to both isoniazid (INH) and rifampin (RIF); MDR+INJ, MDR plus resistant to an injectable; MDR+FLQ, MDR plus resistant to either fluoroquinolone; XDR, MDR plus resistance to both a FLQ and an injectable.
DISCUSSION
Our results revealed that the CFR for 400 mg moxifloxacin was 98% versus 84% for 800 mg ofloxacin by using a target fAUC0–24/MIC ratio of ≥53. With the more stringent target ratio of ≥100, the differences in the performances of the drugs were even more marked, and both regimens fell short of the 90% CFR threshold (the CFR for moxifloxacin was 88% versus 43% for ofloxacin). On the other hand, with 800-mg doses of moxifloxacin in the same patients with MDR-TB and the target ratio of ≥100, a CFR of 98% would be achieved (Table 4 and Fig. 3). The higher moxifloxacin dose (800 mg) also achieved the pharmacodynamic target ratio of ≥53 in 98% of MDR-TB patients with resistance to an injectable agent (Table 4 and Fig. 3), whereas the standard 400-mg dose had a marginal CFR of 84% (Table 4).
Moxifloxacin has structural differences from ofloxacin at the C-7 position that reduce the ability of the bacterium to efflux moxifloxacin across the cell wall, thus lowering the MIC. Moxifloxacin also has intracellular killing kinetics superior to those of ofloxacin. Experimental data show that moxifloxacin MICs in macrophages increased by only 2-fold compared to the MIC in extracellular broth, while 4-fold increases were demonstrated for ofloxacin (20).
Using a target fAUC0–24/MIC ratio of ≥53, the currently recommended 400-mg daily dose of moxifloxacin obtained a PTA greater than 90% when the isolates had MICs ≤ 0.25 mg/liter. On the other hand, ofloxacin failed to achieve a PTA of more than 90% when the MIC was >0.5 mg/liter, as found in about 20% of the isolates, classified by standard procedures as resistant to rifampin and isoniazid but not to injectable second-line drugs (such as capreomycin, kanamycin, and amikacin) or to fluoroquinolones. Hence, our findings suggest that a 4-fold reduction in the susceptibility breakpoint for ofloxacin, which is currently set at 2.0 mg/liter, may be warranted. However, clinical correlates for the fAUC0–24/MIC targets are lacking for patients with tuberculosis, and using the target of 100 would suggest revision of the ofloxacin susceptibility breakpoint down to 0.25 mg/liter. It should be noted that the target ratio of 53 which we used for comparisons of fluoroquinolones was derived only for moxifloxacin and that this value is not necessarily applicable to ofloxacin. The current doses for moxifloxacin (400 mg) and ofloxacin (800 mg) may thus be suboptimal for the treatment of drug-resistant tuberculosis if a pharmacodynamic target of fAUC0–24/MIC ≥ 100 correlates better with successful clinical outcomes. Our simulations suggest susceptibility breakpoints of 0.125 mg/liter for 400-mg doses of moxifloxacin and 0.25 mg/liter for 800 mg ofloxacin. Doubling the dose of ofloxacin is unlikely to achieve acceptable PTA in many patients as previously reported (26). On the other hand, our simulations show that doubling the moxifloxacin dose to 800 mg daily could lead to acceptable PTA (Fig. 2), and this is consistent with previous reports (23). Higher doses of moxifloxacin may increase moxifloxacin side effects, including prolongation of the time between the start of the Q wave and the end of the T wave in the heart's electrical cycle (QT interval) (39), and this concern is particularly serious, given the long duration of MDR-TB treatment. However, limited studies seem to suggest the safety of higher doses. A recent study by Ruslami et al. (40) which evaluated daily 800-mg doses of moxifloxacin did not show increased toxicity, while a study by Alffenaar et al. showed tolerability at 600 mg and 800 mg moxifloxacin (41). An ongoing clinical trial by Alffenaar et al. is evaluating the safety of moxifloxacin at escalated doses of 600 and 800 mg (NCT01329250; http://clinicaltrials.gov/show/NCT01329250).
The continued use of fluoroquinolones in suboptimal doses may hinder their use in the future due to the development of fluoroquinolone resistance (42). The target fAUC0–24/MIC ratio of ≥53 is based on studies showing suppression of resistance emergence with moxifloxacin monotherapy in a HFS (23). In our study, 400 mg moxifloxacin was shown to attain a CFR ≥ 90% for M. tuberculosis strains resistant to isoniazid and rifampin but not injectable agents, while ofloxacin at 800 mg daily did not. However, for MDR-TB strains resistant to injectable agents, only the 800-mg daily doses of moxifloxacin achieved a CFR ≥ 90%. The target ratio of ≥100 is based on review of studies in Gram-positive and Gram-negative bacteria conducted in animals (22) and humans (21). In patients, values of 125 to 250 were associated with clinical cure and speed of bacterial eradication for Gram-negative infections of the respiratory tract (43), and the target value of >100 was linked to decreased emergence of bacterial resistance (44). For Gram-negative organisms, a target of 100 to 125 achieved acceptable activity, although more-rapid eradication was achieved with a target fAUC0–24/MIC ratio of ≥250 when ciprofloxacin, grepafloxacin, levofloxacin, and gatifloxacin were evaluated (43). Considering sterilizing activity, including killing of M. tuberculosis within macrophages, the target of 100 may be more appropriate, as penetration to the site of action should be considered (20). Fluoroquinolones generally achieve higher concentrations in epithelial lining fluid (ELF) than in plasma (45), which means that our PTA and CFR would be higher at the site of action than when plasma concentrations are used. Compared with other fluoroquinolones, moxifloxacin has been found to have greater efficacy than levofloxacin in mice despite a lower plasma AUC/MIC ratio (46), presumably due to higher intracellular concentrations of moxifloxacin. Levofloxacin, however, penetrates into cerebrospinal fluid of patients with tuberculosis meningitis better than ciprofloxacin and gatifloxacin (47). In comparison to another moxifloxacin population pharmacokinetic model (48), we found a reduced IIV for V but a significant IIV for CL and F. Our estimate of CL was 25% higher than that reported by Peloquin et al.; this may have been due to the differences in the study populations, but it may also have been a consequence of the differences in dosing schedules, sampling times, and structural models used to interpret the data.
Due to limited sample size, our MIC data may not represent the true distribution for some drug resistance categories. The M. tuberculosis isolates used to determine the MICs originated from patients in the same region as those contributing data to the pharmacokinetic model (Table 1). Given the limited geographical distributions of our study population and M. tuberculosis isolates contributing to our analysis, we cannot assume that the PTA and especially the CFR analyses will be applicable to other populations outside the region. In addition, our results compare the activities of moxifloxacin with those of ofloxacin using pharmacodynamic targets derived in experiments using the drugs administered alone (as monotherapy). Previous studies have shown that a combination of rifampin (a rifamycin) and moxifloxacin suppresses resistance emergence but at the price of slightly slowing bacterial killing (49, 50). Our comparisons did not take into account within-regimen synergy or antagonism (50), although these effects are unlikely to differ considerably within the fluoroquinolone class. Our pharmacodynamic targets are based on experimental models which differ from the organism-drug interface in patients. Importantly, the diversity of the M. tuberculosis growth states encountered in patients is not accounted for. Moreover, our analysis assumes unbound plasma concentrations as a marker of exposure, while tissue-free drug concentrations would be more appropriate.
Conclusions.
Our analyses based on the pharmacokinetic and drug susceptibility distributions in African patients indicate that, in the currently used doses, moxifloxacin is more efficacious than ofloxacin for the treatment of MDR-TB. Doubling the dose of moxifloxacin to 800 mg daily improves the CFR. However, further clinical studies are required to evaluate the safety and tolerability of moxifloxacin at higher doses.
ACKNOWLEDGMENTS
This study was supported by European and Developing Countries Clinical Trials Partnership and the Wellcome Trust (WT081199/Z/06/Z). S.P.Z. is supported by the Wellcome Trust, United Kingdom (grant number WT081199/Z/06/Z), and P.D. is supported by the Wellcome Trust, United Kingdom (program grant 5374). E.C. was supported by Clinical Infectious Diseases Research Initiative (CIDRI) Wellcome Trust Fund grant 41216.
We thank the South African Tuberculosis Vaccine Initiative (SATVI), South Africa Aurum Institute for Health Research, Johannesburg, South Africa; Biomedical Research and Training Institute, Harare, Zimbabwe; and Harare City Health Department, Harare, Zimbabwe, for hosting the clinical study. We also thank Manshil Misra (Cape Town site), Ronnie Matambo (Harare site), and Marietha Luttig (Johannesburg site), who were responsible for data collection at the sites. We also gratefully acknowledge the financial and intellectual support from Novartis Pharma toward building modeling and simulation skills at the Division of Clinical Pharmacology, University of Cape Town.
Footnotes
Published ahead of print 4 November 2013
REFERENCES
- 1.Falzon D, Jaramillo E, Schunemann HJ, Arentz M, Bauer M, Bayona J, Blanc L, Caminero JA, Daley CL, Duncombe C, Fitzpatrick C, Gebhard A, Getahun H, Henkens M, Holtz TH, Keravec J, Keshavjee S, Khan AJ, Kulier R, Leimane V, Lienhardt C, Lu C, Mariandyshev A, Migliori GB, Mirzayev F, Mitnick CD, Nunn P, Nwagboniwe G, Oxlade O, Palmero D, Pavlinac P, Quelapio MI, Raviglione MC, Rich ML, Royce S, Rusch-Gerdes S, Salakaia A, Sarin R, Sculier D, Varaine F, Vitoria M, Walson JL, Wares F, Weyer K, White RA, Zignol M. 2011. WHO guidelines for the programmatic management of drug-resistant tuberculosis: 2011 update. Eur. Respir. J. 38:516–528. 10.1183/09031936.00073611 [DOI] [PubMed] [Google Scholar]
- 2.WHO 2008. Guidelines for the programmatic management of drug-resistant tuberculosis. World Health Organization, Geneva, Switzerland: http://whqlibdoc.who.int/publications/2008/9789241547581_eng.pdf Accessed 23 March 2013 [Google Scholar]
- 3.Hu Y, Coates AR, Mitchison DA. 2003. Sterilizing activities of fluoroquinolones against rifampin-tolerant populations of Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 47:653–657. 10.1128/AAC.47.2.653-657.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoshimatsu T, Nuermberger E, Tyagi S, Chaisson R, Bishai W, Grosset J. 2002. Bactericidal activity of increasing daily and weekly doses of moxifloxacin in murine tuberculosis. Antimicrob. Agents Chemother. 46:1875–1879. 10.1128/AAC.46.6.1875-1879.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conde MB, Efron A, Loredo C, De Souza GR, Graca NP, Cezar MC, Ram M, Chaudhary MA, Bishai WR, Kritski AL, Chaisson RE. 2009. Moxifloxacin versus ethambutol in the initial treatment of tuberculosis: a double-blind, randomised, controlled phase II trial. Lancet 373:1183–1189. 10.1016/S0140-6736(09)60333-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rustomjee R, Lienhardt C, Kanyok T, Davies GR, Levin J, Mthiyane T, Reddy C, Sturm AW, Sirgel FA, Allen J, Coleman DJ, Fourie B, Mitchison DA. 2008. A phase II study of the sterilising activities of ofloxacin, gatifloxacin and moxifloxacin in pulmonary tuberculosis. Int. J. Tuberc. Lung Dis. 12:128–138 [PubMed] [Google Scholar]
- 7.WHO 2011. Guidelines for the programmatic management of drug-resistant tuberculosis update. World Health Organization, Geneva, Switzerland: http://whqlibdoc.who.int/publications/2011/9789241501583_eng.pdf Accessed 14 May 2013 [Google Scholar]
- 8.Dooley K, Flexner C, Hackman J, Peloquin CA, Nuermberger E, Chaisson RE, Dorman SE. 2008. Repeated administration of high-dose intermittent rifapentine reduces rifapentine and moxifloxacin plasma concentrations. Antimicrob. Agents Chemother. 52:4037–4042. 10.1128/AAC.00554-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siefert HM, Domdey-Bette A, Henninger K, Hucke F, Kohlsdorfer C, Stass HH. 1999. Pharmacokinetics of the 8-methoxyquinolone, moxifloxacin: a comparison in humans and other mammalian species. J. Antimicrob. Chemother. 43(Suppl B):69–76. 10.1093/jac/43.suppl_2.69 [DOI] [PubMed] [Google Scholar]
- 10.Brillault J, De Castro WV, Harnois T, Kitzis A, Olivier JC, Couet W. 2009. P-glycoprotein-mediated transport of moxifloxacin in a Calu-3 lung epithelial cell model. Antimicrob. Agents Chemother. 53:1457–1462. 10.1128/AAC.01253-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Senggunprai L, Yoshinari K, Yamazoe Y. 2009. Selective role of sulfotransferase 2A1 (SULT2A1) in the N-sulfoconjugation of quinolone drugs in humans. Drug Metab. Dispos. 37:1711–1717. 10.1124/dmd.109.027441 [DOI] [PubMed] [Google Scholar]
- 12.Tachibana M, Tanaka M, Masubuchi Y, Horie T. 2005. Acyl glucuronidation of fluoroquinolone antibiotics by the UDP-glucuronosyltransferase 1A subfamily in human liver microsomes. Drug Metab. Dispos. 33:803–811. 10.1124/dmd.104.003178 [DOI] [PubMed] [Google Scholar]
- 13.Zvada SP, Denti P, Geldenhuys H, Meredith S, van As D, Hatherill M, Hanekom W, Wiesner L, Simonsson US, Jindani A, Harrison T, McIlleron HM. Moxifloxacin population pharmacokinetics in patients with pulmonary tuberculosis and the effect of intermittent high-dose rifapentine. Antimicrob. Agents Chemother. 56:4471–4473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yuk JH, Nightingale CH, Quintiliani R, Sweeney KR. 1991. Bioavailability and pharmacokinetics of ofloxacin in healthy volunteers. Antimicrob. Agents Chemother. 35:384–386. 10.1128/AAC.35.2.384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belousov OB, Gutkin AB, Sokolov AV, Tishchenkova IF, Efremenkova OV. 1996. Clinical and pharmacokinetic evaluation of ofloxacin under various regimens of administration in patients with bronchopulmonary infections. Antibiot. Khimioter. 41:47–49 (In Russian.) [PubMed] [Google Scholar]
- 16.Lode H, Hoffken G, Olschewski P, Sievers B, Kirch A, Borner K, Koeppe P. 1987. Pharmacokinetics of ofloxacin after parenteral and oral administration. Antimicrob. Agents Chemother. 31:1338–1342. 10.1128/AAC.31.9.1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stambaugh JJ, Berning SE, Bulpitt AE, Hollender ES, Narita M, Ashkin D, Peloquin CA. 2002. Ofloxacin population pharmacokinetics in patients with tuberculosis. Int. J. Tuberc. Lung Dis. 6:503–509 [DOI] [PubMed] [Google Scholar]
- 18.Canetti G, Fox W, Khomenko A, Mahler HT, Menon NK, Mitchison DA, Rist N, Smelev NA. 1969. Advances in techniques of testing mycobacterial drug sensitivity, and the use of sensitivity tests in tuberculosis control programmes. Bull. World Health Organ. 41:21–43 [PMC free article] [PubMed] [Google Scholar]
- 19.WHO 2008. Policy guidance on drug-susceptibility testing (DST) of second-line antituberculosis drugs. WHO/HTM/TB/2008.392. World Health Organization, Geneva, Switzerland: http://www.who.int/tb/publications/2008/whohtmtb_2008_392/en/index.html Accessed 27 February 2013 [PubMed] [Google Scholar]
- 20.Shandil RK, Jayaram R, Kaur P, Gaonkar S, Suresh BL, Mahesh BN, Jayashree R, Nandi V, Bharath S, Balasubramanian V. 2007. Moxifloxacin, ofloxacin, sparfloxacin, and ciprofloxacin against Mycobacterium tuberculosis: evaluation of in vitro and pharmacodynamic indices that best predict in vivo efficacy. Antimicrob. Agents Chemother. 51:576–582. 10.1128/AAC.00414-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schentag JJ, Meagher AK, Forrest A. 2003. Fluoroquinolone AUIC break points and the link to bacterial killing rates. Part 2: human trials. Ann. Pharmacother. 37:1478–1488 [DOI] [PubMed] [Google Scholar]
- 22.Schentag JJ, Meagher AK, Forrest A. 2003. Fluoroquinolone AUIC break points and the link to bacterial killing rates. Part 1: In vitro and animal models. Ann. Pharmacother. 37:1287–1298 [DOI] [PubMed] [Google Scholar]
- 23.Gumbo T, Louie A, Deziel MR, Parsons LM, Salfinger M, Drusano GL. 2004. Selection of a moxifloxacin dose that suppresses drug resistance in Mycobacterium tuberculosis, by use of an in vitro pharmacodynamic infection model and mathematical modeling. J. Infect. Dis. 190:1642–1651. 10.1086/424849 [DOI] [PubMed] [Google Scholar]
- 24.Jindani A, Hatherill M, Charalambous S, Mungofa S, Zizhou S, van Dijk J, Shepherd J, Phillips P, Nunn A, Mitchison D, Team RT. 2013. A multicentre randomized clinical trial to evaluate high-dose rifapentine with a quinolone for treatment of pulmonary TB: the RIFAQUIN Trial. Paper 147LB 20th Conference on Retrovirus and Opportunistic Infections. Georgia World Congress Center, Atlanta, GA [Google Scholar]
- 25.RIFAQUIN 2008. An international multicentre controlled clinical trial to evaluate high dose rifapentine and a quinolone in the treatment of pulmonary tuberculosis. ISRCTN 44153044. http://ipc.nxgenomics.org/intertb/download/RIFAQUIN_Protocol_v_1.8_15_April_2011_FINAL.pdf Accessed 27 February 2013
- 26.Chigutsa E, Meredith S, Wiesner L, Padayatchi N, Harding J, Moodley P, Mac Kenzie WR, Weiner M, McIlleron H, Kirkpatrick CM. 2012. Population pharmacokinetics and pharmacodynamics of ofloxacin in South African patients with multidrug-resistant tuberculosis. Antimicrob. Agents Chemother. 56:3857–3863. 10.1128/AAC.00048-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sirgel FA, Warren RM, Streicher EM, Victor TC, van Helden PD, Bottger EC. 2012. gyrA mutations and phenotypic susceptibility levels to ofloxacin and moxifloxacin in clinical isolates of Mycobacterium tuberculosis. J. Antimicrob. Chemother. 67:1088–1093. 10.1093/jac/dks033 [DOI] [PubMed] [Google Scholar]
- 28.Beal S, Sheiner LB, Boeckmann A, Bauer RJ. 2009. NONMEM user's guides (1989-2009). Icon Development Solutions, Ellicott City, MD [Google Scholar]
- 29.Lindbom L, Pihlgren P, Jonsson EN. 2005. PsN-Toolkit—a collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM. Comput. Methods Programs Biomed. 79:241–257. 10.1016/j.cmpb.2005.04.005 [DOI] [PubMed] [Google Scholar]
- 30.Jonsson EN, Karlsson MO. 1999. Xpose—an S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput. Methods Programs Biomed. 58:51–64 [DOI] [PubMed] [Google Scholar]
- 31.Anderson BJ, Holford NH. 2008. Mechanism-based concepts of size and maturity in pharmacokinetics. Annu. Rev. Pharmacol. Toxicol. 48:303–332. 10.1146/annurev.pharmtox.48.113006.094708 [DOI] [PubMed] [Google Scholar]
- 32.Savic RM, Jonker DM, Kerbusch T, Karlsson MO. 2007. Implementation of a transit compartment model for describing drug absorption in pharmacokinetic studies. J. Pharmacokinet. Pharmacodyn. 34:711–726. 10.1007/s10928-007-9066-0 [DOI] [PubMed] [Google Scholar]
- 33.Bergstrand M, Karlsson MO. 2009. Handling data below the limit of quantification in mixed effect models. AAPS J. 11:371–380. 10.1208/s12248-009-9112-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holford N. 2005. The visual predictive check—superiority to standard diagnostic (Rorschach) plots, abstr. 738Abstr. 14th Meet. Popul. Approach Group Eur. (PAGE) [Google Scholar]
- 35.Andersson MI, MacGowan AP. 2003. Development of the quinolones. J. Antimicrob. Chemother. 51(Suppl 1):1–11. 10.1093/jac/dkg212 [DOI] [PubMed] [Google Scholar]
- 36.Zhanel GG, Ennis K, Vercaigne L, Walkty A, Gin AS, Embil J, Smith H, Hoban DJ. 2002. A critical review of the fluoroquinolones: focus on respiratory infections. Drugs 62:13–59. 10.2165/00003495-200262010-00002 [DOI] [PubMed] [Google Scholar]
- 37.Mouton JW, Dudley MN, Cars O, Derendorf H, Drusano GL. 2005. Standardization of pharmacokinetic/pharmacodynamic (PK/PD) terminology for anti-infective drugs: an update. J. Antimicrob. Chemother. 55:601–607. 10.1093/jac/dki079 [DOI] [PubMed] [Google Scholar]
- 38.Schaaf HS, Marais BJ, Hesseling AC, Brittle W, Donald PR. 2009. Surveillance of antituberculosis resistance amonnst children from the Western Cape Province of South Africa—an upward trend. Am. J. Public Health 99:1486–1490. 10.2105/AJPH.2008.143271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Falagas ME, Rafailidis PI, Rosmarakis ES. 2007. Arrhythmias associated with fluoroquinolone therapy. Int. J. Antimicrob. Agents 29:374–379. 10.1016/j.ijantimicag.2006.11.011 [DOI] [PubMed] [Google Scholar]
- 40.Ruslami R, Ganiem AR, Aarnoutse RE, van Crevel R; study team 2013. Rifampicin and moxifloxacin for tuberculous meningitis—authors' reply. Lancet Infect. Dis. 13:570. 10.1016/S1473-3099(13)70156-7 [DOI] [PubMed] [Google Scholar]
- 41.Alffenaar JW, van Altena R, Bokkerink HJ, Luijckx GJ, van Soolingen D, Aarnoutse RE, van der Werf TS. 2009. Pharmacokinetics of moxifloxacin in cerebrospinal fluid and plasma in patients with tuberculous meningitis. Clin. Infect. Dis. 49:1080–1082. 10.1086/605576 [DOI] [PubMed] [Google Scholar]
- 42.Ginsburg AS, Grosset JH, Bishai WR. 2003. Fluoroquinolones, tuberculosis, and resistance. Lancet Infect. Dis. 3:432–442. 10.1016/S1473-3099(03)00671-6 [DOI] [PubMed] [Google Scholar]
- 43.Forrest A, Nix DE, Ballow CH, Goss TF, Birmingham MC, Schentag JJ. 1993. Pharmacodynamics of intravenous ciprofloxacin in seriously ill patients. Antimicrob. Agents Chemother. 37:1073–1081. 10.1128/AAC.37.5.1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thomas JK, Forrest A, Bhavnani SM, Hyatt JM, Cheng A, Ballow CH, Schentag JJ. 1998. Pharmacodynamic evaluation of factors associated with the development of bacterial resistance in acutely ill patients during therapy. Antimicrob. Agents Chemother. 42:521–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kiem S, Schentag JJ. 2008. Interpretation of antibiotic concentration ratios measured in epithelial lining fluid. Antimicrob. Agents Chemother. 52:24–36. 10.1128/AAC.00133-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ahmad Z, Tyagi S, Minkowski A, Peloquin CA, Grosset JH, Nuermberger EL. 2013. Contribution of moxifloxacin or levofloxacin in second-line regimens with or without continuation of pyrazinamide in murine tuberculosis. Am. J. Respir. Crit. Care Med. 188:97–102. 10.1164/rccm.201212-2328OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thwaites GE, Bhavnani SM, Chau TT, Hammel JP, Torok ME, Van Wart SA, Mai PP, Reynolds DK, Caws M, Dung NT, Hien TT, Kulawy R, Farrar J, Ambrose PG. 2011. Randomized pharmacokinetic and pharmacodynamic comparison of fluoroquinolones for tuberculous meningitis. Antimicrob. Agents Chemother. 55:3244–3253. 10.1128/AAC.00064-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peloquin CA, Hadad DJ, Molino LP, Palaci M, Boom WH, Dietze R, Johnson JL. 2008. Population pharmacokinetics of levofloxacin, gatifloxacin, and moxifloxacin in adults with pulmonary tuberculosis. Antimicrob. Agents Chemother. 52:852–857. 10.1128/AAC.01036-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Drusano GL, Sgambati N, Eichas A, Brown DL, Kulawy R, Louie A. 2010. The combination of rifampin plus moxifloxacin is synergistic for suppression of resistance but antagonistic for cell kill of Mycobacterium tuberculosis as determined in a hollow-fiber infection model. mBio 1:e00139–10. 10.1128/mBio.00139-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Balasubramanian V, Solapure S, Gaonkar S, Mahesh Kumar KN, Shandil RK, Deshpande A, Kumar N, Vishwas KG, Panduga V, Reddy J, Ganguly S, Louie A, Drusano GL. 2012. Effect of coadministration of moxifloxacin and rifampin on Mycobacterium tuberculosis in a murine aerosol infection model. Antimicrob. Agents Chemother. 56:3054–3057. 10.1128/AAC.06383-11 [DOI] [PMC free article] [PubMed] [Google Scholar]