Abstract
Antibiotic overconsumption is the main force driving the emergence of multidrug-resistant bacterial strains. To promote better antibiotic use in France, a nationwide campaign has been run every year from October to March since 2002. In 2007, it was shown that winter outpatient antibiotic consumption had decreased by 26.5% compared to the 2000-2002 baseline period. Here, we quantified outpatient antibiotic use between 2000 and 2010 as a follow-up analysis of the nationwide campaign. Reimbursed outpatient antibiotic prescriptions were extracted from computerized French National Health Insurance databases. Entire series and age group and antibiotic class analyses were computed. Time series analyses used autoregressive moving-average models with exogenous variables and intervention functions. Two periods were considered: October to March “campaign” periods and April to September “warm” periods. Compared to the precampaign (2000-2002) baseline period, the numbers of weekly antibiotic prescriptions per 1,000 inhabitants during campaign periods decreased until winter 2006 to 2007 (−30% [95% confidence interval {CI}, −36.3 to −23.8%]; P < 0.001) and then stabilized except for individuals >60 years of age, for whom prescriptions reached the precampaign level. During the warm periods from April to September, no significant differences were estimated compared to the baseline level for the entire series, but seniors had an increasing trend that became significant as of 2005, reaching +21% (95% CI, +12.9 to +29.6%) in 2009 (P < 0.0001). These results highlight the need for a better understanding of antibiotic use by the elderly, requiring research with targeted and tailored public health actions for this population.
INTRODUCTION
Over the last 4 decades, the emergence and dissemination of multidrug-resistant bacterial strains have become major global health issues (1). Because of antibiotic resistance, management of bacterial infections is becoming more complex, and numbers of treatment failures are increasing. In the European Union, an estimated 25,000 deaths are caused by drug-resistant bacteria annually (2). In the community, β-lactam-resistant Streptococcus pneumoniae is one of the best-known bacteria that has disseminated during the past 20 years. Antibiotic overuse is the main force driving the spread of multidrug-resistant bacteria (3, 4). In developed countries, antibiotics are prescribed mostly in outpatient settings for upper respiratory tract infections. However, because most of these infections are viral, antibiotics are usually unnecessary (5, 6, 7).
In the early 2000s, France had the highest rate of antibiotic-resistant S. pneumoniae in Europe and was identified as one of the highest antimicrobial users worldwide (3). In this context, a nationwide public health campaign was launched (“Antibiotics Are Not Automatic!” and “Antibiotics, Used Unnecessarily, Lose Their Potency!”) and has been repeated every winter from October to March (period of highest antibiotic consumption) since 2002, with the main goal of decreasing prescriptions in the community, particularly for children. In addition, the 7-valent pneumococcal conjugate vaccine (PCV7) was introduced in 2003 to prevent invasive S. pneumoniae disease in children <2 years old at risk because of medical or living conditions. It was further expanded to every child <2 years old.
Between 2002 and 2007, winter outpatient antibiotic consumption decreased by 26.5% compared to the baseline 2000-2002 period (8). Because no detailed and prolonged longitudinal antibiotic consumption evaluation has been performed since then, it is not known whether the decreasing trend of outpatient antibiotic consumption has been sustained. Moreover, such information, according to age group and antibiotic class, is crucial to refining the communication strategies directed toward physicians. Therefore, by using time series analyses, we quantified the evolutions of outpatient antibiotic use between 2002 and 2010 for the entire series and by age group and therapeutic class.
MATERIALS AND METHODS
Data sources.
French National Health Insurance (NHI) covers all medical care provided by outpatient and private-practice physicians and pharmacies. We used aggregated 2000-2010 data on all outpatient antibiotics prescribed and reimbursed, computed from the databases of the 2 main NHI agencies that cover salaried workers and self-employed individuals (>90% of the French population). Each file contains drug-related information, the prescription date, and the patient's sex, year of birth, and region of residence. The study concerned only systemic antibiotics (anatomical therapeutic chemical class J01) used in the community. To take into account French population growth during the study period, we used demographic data obtained from the French National Institute for Statistics and Economic Studies (INSEE) (http://www.insee.fr/). The results are presented as weekly rates of antibiotic prescriptions per 1,000 inhabitants.
Weekly flu-like syndrome (FLS) incidence was provided by the French Sentinel Network (http://websenti.u707.jussieu.fr/sentiweb/) (9), which defines FLS as the combination of the following clinical symptoms: sudden onset of fever of ≥39°C, myalgias, and respiratory symptoms (e.g., dyspnea and/or cough). The data are presented as FLS incidence per 100,000 inhabitants.
Statistical analyses.
We considered 2 periods: the first, called the “campaign” period (October to March), coincides with the targeted public service campaign, and the second, the “warm” period, corresponds to April to September. More specifically, we examined 2 truncated series, both lasting 26 weeks: campaign periods started at week 40 of year n and finished at week 13 of year n + 1, whereas warm periods started at week 14 of year n and finished at week 39 of the same year. Time series analyses examined the evolution of outpatient antibiotic consumption per 1,000 inhabitants during the successive periods. Use of autoregressive moving-average models with exogenous variables (ARMAX) allowed us to adjust for FLS incidence and include intervention functions (10–13).
The intervention ARMAX models were built in 2 steps. The first concerned the 2000-2002 period, before the first public health campaign, considering antibiotic consumption during this time as the baseline. Because of seasonal fluctuations, a trigonometric function had to be estimated for the 2000-2002 period. This function was then removed from the entire series so that the residual series was in a stationary mode. We then fitted an autoregressive moving-average (ARMA) model to the observed 2000-2002 data. Because it was assumed that those campaigns did not modify seasonal fluctuations or change the ARMA model structure but affected only the means, in the second step, we added 15 dummy variables to the model (8 for each campaign period [c1 to c8] and 7 for each warm period [w1 to w7]). For each series, an ARMAX model was estimated and properly fitted to the observed antibiotic consumption, leaving a Gaussian white noise residual series. The FLS incidence was added to this model, using a simple transfer function when possible. The construction and the writing of the model were partly described previously (see Supporting Text S1 in reference 14). Because FLS incidence by age group is not available in France, no adjustment could be made for the age group analyses.
We quantified the estimated difference in absolute number and percent change of weekly antibiotic prescriptions per 1,000 inhabitants for each campaign and warm period versus the corresponding baseline period, meaning that we calculated the difference between the estimated number predicted by the model and the one expected under the assumption of no change since the baseline period.
Analyses were computed for the entire series and by age group (0 to 5, 6 to 15, 16 to 60, or >60 years) and by antibiotic class (penicillins, cephalosporins, macrolides, or quinolones). Computations were done with SAS 9.1 software, with a P value of <0.05 defining significance.
RESULTS
Nine series were considered between July 2000 and March 2010 (see Fig. 1 for the entire series). Strong seasonality of FLS and antibiotic prescriptions was observed, with the highest rates being found during the winter campaign periods. Between 2000 and 2010, the weekly numbers of reimbursed antibiotic prescriptions ranged between 9 and 33 per 1,000 inhabitants, with a mean of 19 (95% confidence interval [CI], 9.6 to 28.3) (Table 1). Considering antibiotic classes, mean numbers of prescriptions ranged between 1.6 for quinolones and 7.7 for penicillins. Penicillins were the most prescribed antibiotic class, representing >40% of the total antibiotic consumption; cephalosporins and macrolides were similarly consumed, each representing around 20%, while quinolones, much less used, represented only 8%. Considering age groups, very different consumptions were observed, with the highest for 0- to 5-year-old age group (mean = 44.4) and the lowest for 16- to 60- and >60-year-old age groups (mean = 17.0). The 0- to 5- and >60-year-old age groups each consumed 18% of all antibiotics.
FIG 1.
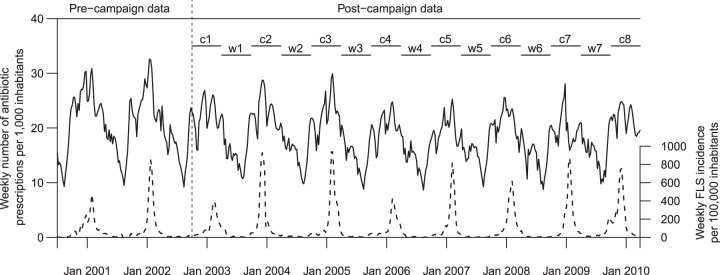
Overall weekly antibiotic consumption per 1,000 inhabitants (plain) and flu-like syndrome incidence (dotted) between 2000 and 2010, periods and intervention functions used for the construction of the ARMAX model. Dummy variables are c1 to c8 (campaign period) and w1 to w7 (warm period).
TABLE 1.
Consumption of antibiotics overall, per antibiotic class, and per age group
| Series | No. of antibiotic prescriptions/1,000 inhabitants |
% of total consumption | ||
|---|---|---|---|---|
| Mean (95% CI) | Min | Max | ||
| Entire series | 18.94 (9.60–28.28) | 8.65 | 32.61 | 100 |
| Antibiotic class | ||||
| Penicillins | 7.67 (3.80–11.55) | 3.51 | 14.00 | 40.50 |
| Cephalosporins | 4.33 (1.30–7.36) | 1.42 | 8.67 | 22.86 |
| Macrolides | 4.29 (1.63–6.96) | 1.50 | 8.50 | 22.65 |
| Quinolones | 1.62 (1.15–2.09) | 0.91 | 2.30 | 8.55 |
| Age group (yr) | ||||
| 0–5 | 44.38 (8.12–80.63) | 10.75 | 101.15 | 17.42 |
| 6–15 | 17.45 (4.50–30.39) | 6.14 | 46.56 | 11.46 |
| 16–60 | 16.66 (9.56–23.75) | 8.28 | 27.53 | 52.92 |
| >60 | 16.98 (9.76–24.20) | 8.25 | 30.09 | 18.20 |
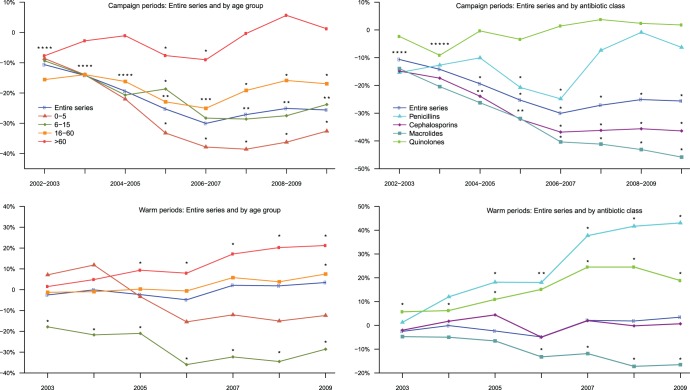
An ARMAX model fulfilling goodness-of-fit criteria was obtained for each series (see Fig. 2 for the entire series). The estimated differences in antibiotic consumption per 1,000 inhabitants during the campaign and warm periods between 2002 and 2010 are reported in Fig. 2 for the entire series (absolute numbers and percentages) as well as in Tables 2 and 3 (in absolute numbers) and Fig. 3 (in percentages) for all the considered series. Additional results in percentages are available in Tables S1 and S2 in the supplemental material. Different trends were observed. During the campaign periods, for the entire series, a maximum significant decrease of 30% (95% CI, −36.3 to −23.8%) was estimated in 2006 to 2007, and thereafter, reductions were smaller but stable (between −25% and −27%) and still significant compared to the baseline value (P < 0.0001). The same trend was found for the 0- to 5-, 6- to 15-, and 16- to 60-year-old age groups, with a higher reduction for the youngest children (−39% [95% CI, −50.8 to −26.3%] in 2007 to 2008) (Table 2). During the last campaign period, estimated decreases were −33% (95% CI, −45.4 to −19.8%; P < 0.0001), −24% (95% CI, −33.1 to −14.5%; P < 0.0001), and −17% (95% CI, −22.7 to −11.2%; P < 0.0001) for the 0- to 5-, 6- to 15-, and 16- to 60-year-old age groups, respectively. For the oldest group (>60 years), the trend was very different, with fluctuations around baseline values and only 2 significant decreases, with the largest being observed in 2006 to 2007 (−9.0% [95% CI, −14.9 to −3.2%]; P = 0.004). During the warm periods, no significant differences were estimated versus the baseline for the entire series and the youngest age group (Table 3). For 6- to 15-year-olds, the trend was comparable to that observed during campaign periods, with a maximum diminution in 2006 (−36% [95% CI, −49.5 to −22.5%]; P < 0.0001), which subsequently stabilized (between −32% and −29%) and remained significant (P < 0.0001). For subjects >15 years of age, an increasing trend became marginally significant in 2009 for those 16 to 60 years old (+7.5% [95% CI, −0.5 to +15.5%]; P = 0.074) and as of 2005 for those >60 years old, reaching +21% in 2009 (95% CI, +12.9 to +29.6%; P < 0.0001) (Table 3).Trends by therapeutic class showed disparities. During the campaign periods, as for the entire series, prescription rates for cephalosporins declined significantly until 2006 to 2007 (−37% [95% CI, −45.3 to −28.3%]; P < 0.0001) and then stabilized at −36% (P < 0.0001) (Table 2). The same trend was observed for macrolides (−40% [95% CI, −46.7 to −34.0%] in 2006 to 2007; P < 0.0001), but the decrease continued more weakly (−46% [95% CI, −52.6 to −39.0%] in 2009 to 2010; P < 0.0001). Quinolone use remained at the baseline level. The trend for penicillins showed a maximum significant decrease of −25% (95% CI, −36.2 to −13.4%) in 2006 to 2007 (P < 0.0001), followed by nonsignificant differences from baseline value. During warm periods, as for the entire series, no significant differences versus the baseline were estimated for cephalosporins or macrolides between 2003 and 2006, but the latter began to significantly decline as of 2006, at −13% (95% CI, −23.1 to −3.4%; P = 0.009), ending in 2009, at −17% (95% CI, −27.4 to −5.7%; P = 0.003) (Table 3). More surprising were the trends of quinolones and penicillins: prescriptions for the former increased significantly during all warm periods, with 2 maxima in 2007 and 2008 of +25% (P < 0.0001), and prescriptions for the latter rose significantly as of 2005, reaching its maximum of +43% (95% CI, +25.3 to + 60.8%; P < 0.0001) in 2009.
FIG 2.

Overall weekly antibiotic consumption per 1,000 inhabitants between 2000 and 2010, ARMAX model predictions, and estimated differences in percentages for campaign and warm periods. Black lines are expected antibiotic consumption levels during campaign and warm periods under the assumption of no change since the baseline period (2000 to 2002). Red lines are antibiotic consumption levels during campaign and warm periods predicted by the model. The indicated percentages are relative changes compared to the baseline period. ∗∗∗, P < 0.001 compared to baseline values (t test).
TABLE 2.
Estimated changes of the weekly absolute numbers of antibiotic prescriptions per 1,000 inhabitants compared to the baseline 2000-2002 period during campaign periods (week 40 of year n to week 13 of year n + 1) for the entire series and by antibiotic class and age groupa
| Series | Baseline no. of antibiotic prescriptions/1,000 inhabitants | Estimated change of wkly absolute no. of antibiotic prescriptions/1,000 inhabitants (95% CI) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 2002–2003 | 2003–2004 | 2004–2005 | 2005–2006 | 2006–2007 | 2007–2008 | 2008–2009 | 2009–2010 | ||
| Entire series (FLS adjusted) | 25.24 | −2.69*** (−3.70–−1.68) | −3.58*** (−4.89–−2.27) | −4.90*** (−6.35–−3.45) | −6.39*** (−7.91–−4.87) | −7.58*** (−9.15–−6.01) | −6.85*** (−8.45–−5.25) | −6.33*** (−7.96–−4.70) | −6.46*** (−8.15–−4.77) |
| Entire series (FLS unadjusted) | 25.24 | −2.94*** (−4.33–−1.55) | −3.52*** (−5.22–−1.81) | −4.90*** (−6.76–−3.04) | −6.55*** (−8.48–−4.63) | −7.49*** (−9.46–−5.52) | −6.88*** (−8.88–−4.88) | −6.07*** (−8.10–−4.04) | −5.97*** (−8.10–−3.85) |
| Antibiotic class (FLS adjusted) | |||||||||
| Penicillins | 10.72 | −1.64*** (−2.49–−0.79) | −1.36* (−2.41–−0.31) | −1.08 (−2.23–0.07) | −2.22*** (−3.41–−1.03) | −2.66*** (−3.88–−1.44) | −0.79 (−2.03–0.45) | −0.09 (−1.33–1.15) | −0.67 (−1.96–0.62) |
| Cephalosporins | 6.65 | −0.98*** (−1.35–−0.62) | −1.15*** (−1.62–−0.69) | −1.59*** (−2.11–−1.07) | −2.14*** (−2.69–−1.59) | −2.45*** (−3.01–−1.88) | −2.41*** (−2.98–−1.83) | −2.37*** (−2.95–−1.78) | −2.42*** (−3.03–−1.81) |
| Macrolides | 6.51 | −0.91*** (−1.17–−0.64) | −1.33*** (−1.67–−0.99) | −1.71*** (−2.09–−1.33) | −2.08*** (−2.48–−1.68) | −2.63*** (−3.04–−2.21) | −2.68*** (−3.10–−2.26) | −2.81*** (−3.24–−2.38) | −2.99*** (−3.43–−2.54) |
| Quinolones | 1.66 | −0.04 (−0.12–0.04) | −0.15*** (−0.24–−0.06) | −0.01 (−0.09–0.08) | −0.06 (−0.14–0.03) | 0.02 (−0.06–0.11) | 0.06 (−0.03–0.15) | 0.04 (−0.05–0.13) | 0.03 (−0.06–0.12) |
| Age group (yr) (FLS unadjusted) | |||||||||
| 0–5 | 65.78 | −5.66 (−11.97–0.65) | −9.20* (−17.28–−1.12) | −14.47*** (−22.54–−6.40) | −21.85*** (−29.85–−13.86) | −24.89*** (−32.89–−16.89) | −25.37*** (−33.42–−17.32) | −23.84*** (−31.88–−15.81) | −21.44*** (−29.85–−13.03) |
| 6–15 | 26.68 | −2.50* (−4.49–−0.51) | −3.77*** (−5.88–−1.65) | −5.52*** (−7.68–−3.36) | −4.98*** (−7.19–−2.77) | −7.55*** (−9.79–−5.31) | −7.63*** (−9.90–−5.36) | −7.34*** (−9.65–−5.02) | −6.35*** (−8.83–−3.87) |
| 16–60 | 21.31 | −3.32*** (−4.12–−2.51) | −2.97*** (−3.98–−1.96) | −3.45*** (−4.54–−2.36) | −4.88*** (−6.00–−3.76) | −5.34*** (−6.48–−4.20) | −4.08*** (−5.25–−2.91) | −3.38*** (−4.55–−2.20) | −3.61*** (−4.83–−2.38) |
| >60 | 18.43 | −1.42** (−2.31–−0.52) | −0.51 (−1.53–0.51) | −0.20 (−1.26–0.87) | −1.41* (−2.48–−0.33) | −1.66** (−2.74–−0.58) | −0.06 (−1.18–1.05) | 1.04 (−0.05–2.14) | 0.23 (−0.88–1.33) |
*, P < 0.05; **, P < 0.01; ***, P < 0.001 (compared to baseline [t test]). FLS, flu-like syndrome.
TABLE 3.
Estimated changes of the weekly absolute numbers of antibiotic prescriptions per 1,000 inhabitants compared to the baseline 2000-2002 period during warm periods (week 14 of year n to week 39 of the same year) for the entire series and by antibiotic class and age groupa
| Series | Baseline no. of antibiotic prescriptions/1,000 inhabitants | Estimated change of wkly absolute no. of antibiotic prescriptions/1,000 inhabitants (95% CI) |
||||||
|---|---|---|---|---|---|---|---|---|
| 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | ||
| Entire series (FLS adjusted) | 16.54 | −0.42 (−1.43–0.59) | −0.01 (−1.2–1.18) | −0.39 (−1.8–1.03) | −0.80 (−2.30–0.70) | 0.35 (−1.21–1.91) | 0.30 (−1.31–1.92) | 0.57 (−1.08–2.22) |
| Entire series (FLS unadjusted) | 16.54 | −0.05 (−1.43–1.32) | 0.54 (−1.15–2.24) | 0.26 (−1.59–2.10) | −0.28 (−2.20–1.64) | 0.89 (−1.08–2.86) | 1.14 (−0.88–3.15) | 1.46 (−0.58–3.51) |
| Antibiotic class (FLS adjusted) | ||||||||
| Penicillins | 7.14 | 0.09 (−0.76–0.94) | 0.86 (−0.19–1.91) | 1.30* (0.15–2.44) | 1.29* (0.09–2.48) | 2.69* (1.47–3.91) | 2.98* (1.73–4.22) | 3.07* (1.81–4.34) |
| Cephalosporins | 3.63 | −0.07 (−0.43–0.28) | 0.06 (−0.39–0.52) | 0.16 (−0.35–0.67) | −0.18 (−0.72–0.36) | 0.07 (−0.49–0.64) | −0.01 (−0.59–0.57) | 0.02 (−0.57–0.62) |
| Macrolides | 3.99 | −0.19 (−0.45–0.07) | −0.20 (−0.53–0.13) | −0.26 (−0.63–0.11) | −0.53* (−0.92–−0.13) | −0.47* (−0.88–−0.06) | −0.69* (−1.11–−0.27) | −0.66* (−1.09–−0.23) |
| Quinolones | 1.39 | 0.08* (0.00–0.16) | 0.09* (0.00–0.17) | 0.15* (0.06–0.24) | 0.21* (0.12–0.30) | 0.34* (0.25–0.43) | 0.34* (0.25–0.43) | 0.26* (0.17–0.35) |
| Age group (yr) (FLS unadjusted) | ||||||||
| 0–5 | 38.40 | 2.74 (−3.77–9.25) | 4.55 (−3.45–12.56) | −1.28 (−9.32–6.76) | −5.94 (−13.94–2.07) | −4.64 (−12.66–3.38) | −5.78 (−13.82–2.26) | −4.76 (−12.83–3.32) |
| 6–15 | 16.04 | −2.87* (−4.85–−0.88) | −3.48* (−5.56–−1.41) | −3.37* (−5.48–−1.25) | −5.77* (−7.94–−3.60) | −5.18* (−7.38–−2.98) | −5.54* (−7.78–−3.30) | −4.58* (−6.88–−2.29) |
| 16–60 | 14.76 | −0.19 (−1.01–0.64) | −0.14 (−1.16–0.87) | 0.04 (−1.04–1.13) | −0.09 (−1.21–1.03) | 0.86 (−0.29–2.00) | 0.56 (−0.61–1.73) | 1.11 (−0.07–2.29) |
| >60 | 13.08 | 0.20 (−0.70–1.10) | 0.64 (−0.38–1.62) | 1.22* (0.17–2.28) | 1.04 (−0.03–2.11) | 2.24*** (1.16–3.32) | 2.65*** (1.54–3.75) | 2.78*** (1.68–3.87) |
*, P < 0.05; ***, P < 0.001 (compared to baseline [t test]).
FIG 3.
Estimated percent differences compared to the baseline period (2000 to 2002) during campaign (top) and warm (bottom) periods for the entire series and by age group and therapeutic class. ∗, P < 0.05 compared to baseline values (t test). Asterisks were lined up when the space between dots was too small.
DISCUSSION
Between 2002 and 2010, outpatient antibiotic use during October to March campaign periods showed a decline of −26% (adjusted for FLS fluctuations), with no significant change during the warm periods of April to September. This reduction reached a maximum of −30% in winter 2006 to 2007 and then stabilized at around −26%. The maximum decrease was observed for 0- to 5-year-old children and was followed by stabilization since 2006 to 2007. For seniors >60 years old, antibiotic use fluctuated around the baseline level during campaign periods but rose substantially during warm periods and significantly since 2005. Also, trends differed according to therapeutic class, with less use of all but quinolones and, more surprisingly, significantly increased penicillin prescription starting in 2007.
Our results are consistent with analyses of Sabuncu et al. (8), with an overall decrease of ∼30% until winter 2006 to 2007 and the steepest decline for children <5 years old. The ARMAX models used by Sabuncu et al. differed from ours, supporting the changes estimated with these models.
Considering the entire series, decreases during campaign periods and no changes during warm periods were observed. Between 2002 to 2003 and 2006 to 2007, the reduction was linear, going from −10% to −30%, starting during the first national campaign in 2002 to 2003. In children <5 years old, the introduction of PCV7 in 2002 to 2003 might have contributed to the observed decrease in antibiotic use. However, a substantial coverage of vaccination was obtained only as of 2005 (15). Therefore, until the 2004-2005 campaign, the decrease was most likely attributable to national campaigns.
During 2006 to 2007, all antibiotic consumption stabilized, raising the question of the sustainability of the behavioral change promoted by the campaign. However, the yearly seasonal exposure of the general public and physicians to the campaign over a prolonged period should exert a sustained effect. Considering age groups, the greatest impact was on children ≤15 years old, particularly 0- to 5-year-olds, with almost 40% fewer prescriptions in winter 2007 to 2008. Similar findings were obtained with antibiotic data from a survey of medical prescriptions by a panel of French private practitioners: for children, the greatest reductions were observed for diagnoses of rhinopharyngitis (16). Also, repeated cross-sectional studies on antibiotic treatments for children attending day care centers in southeastern France showed that the proportion of children who had received antibiotics during the previous 3 months fell from 48.0% in 2004 to 29.7% in 2008 (17). While those results are encouraging, they are more uncertain in adults, especially for seniors (>60 years), whose decreased antibiotic consumption was weakly significant during the 2006-2007 campaign period and increased regularly thereafter. Notably, the campaigns did not specifically target this age group. Of greater concern is their significantly increased antibiotic use during warm periods since 2005. To take into account the demographic changes of seniors during the study period, separate analyses have been done for individuals 61 to 70, 71 to 80, and >80 years old (see Tables S3 and S4 in the supplemental material). An age effect can be observed, with increasing antibiotic consumption with an increase in age. However, the trend was identical for all the age groups during the study period, indicating the absence of a differential effect among this age group (see Fig. S1 in the supplemental material).
Prescriptions for all therapeutic classes except quinolones declined until 2007. Similarly, a previous study found that all antibiotic classes except fluoroquinolones decreased for 7- to 18-year-olds (16). Here again, the campaign seems to have been effective during the first 5 years for almost all therapeutic classes. Since 2007, cephalosporin consumption has remained stable, and macrolide use has continued to decline. Above all, penicillin consumption has been increasing significantly since 2007. Notably, penicillin consumption has increased significantly for subjects >60 years old since 2007, reaching +16% and +27% during the 2008-2009 campaign and 2009 warm periods, respectively (data not shown). Moreover, quinolone consumption by this group has also significantly increased since 2005, but this class represents a smaller proportion of all antibiotics (data not shown).
Many countries have initiated campaigns to promote better-targeted antibiotic use. Although there seems to be a downward trend of antibiotic consumption in most countries for which data are available, several European countries without major public health programs (Denmark, Italy, and Ireland) have observed increasing trends of antibiotic use over the last decade (18). Our results are consistent with those reported in other countries with large-scale national campaigns to promote better antibiotic prescription. For example, in Belgium, a country comparable to France in terms of antibiotic overuse, a 36% reduction was seen between 1999 to 2000 and 2006 to 2007 (19). In Sweden, where antibiotic use is the lowest in Europe, the detection of multiresistant pneumococcal clones together with an increasing trend of antibiotic consumption led to the implementation of a comprehensive program to preserve antibiotic efficacies in the mid-1990s. In this context, macrolide consumption decreased by 65% between 1995 and 2004, representing the sharpest decline among all therapeutic classes (20). The campaign had no impact on quinolones.
This long-term monitoring of outpatient antibiotic prescriptions was made possible by the existing large NHI databases that provide individual data on antibiotic reimbursements for >90% of the French population. These weekly data allowed analysis of time trends in outpatient antibiotic use because they are not affected by an information bias. We used ARMAX models that are well suited for time series analyses. These models were selected independently, and very similar underlying structures (ARMA model) were found for all the series, supporting the validity of those models. Seasonal FLS variations that could affect antibiotic prescriptions and hamper the interpretation of the trends were accounted for in the entire series and therapeutic class analyses. Unfortunately, FLS incidence could not be adjusted for age group analyses. However, percent changes might have differed if we had adjusted for the age group FLS incidence, but the trend would probably not have been markedly modified. Analyses with and without adjustment for FLS incidence have been performed for the entire series, and the results are similar (Tables 2 and 3). There is no reason to believe that it would be markedly different for age group analyses.
This analysis has several limitations. First, preintervention data are limited (2 years), as we did not have data for before July 2000. We cannot exclude a change in antibiotic consumption before the start of the nationwide campaigns. However, between 2000 and 2002, the outpatient antibiotic consumption rate seemed to be stable, so the potential change would probably be negligible compared to the changes observed after the start of the campaigns. Second, considering the context, we could not have a control group. Therefore, as underscored by Sabuncu et al. (8), because of the absence of a control group and the limited preintervention data, a cause-effect relationship between the campaigns and reduced antibiotic use cannot be established. Also, the absence of data on the diagnoses that led to antibiotic prescriptions limits the understanding of the impact of the campaigns. Unfortunately, studies on antibiotic prescriptions including indications for treatment are scarce and focused only on pediatric prescriptions (16, 17), as children are the predominant consumers of antibiotics and were thus the main target of the national campaign.
In conclusion, overall outpatient antibiotic use in France declined significantly between 2002 and 2010, but an upward trend has been observed since 2007. Moreover, trends markedly differed between age groups and therapeutic classes. Particularly, antibiotic consumption by the elderly (>60 years old) tended to increase. This observation highlights that a better understanding of antibiotic use by senior outpatients is urgently needed to target and tailor public health actions for this population. With respect to therapeutic classes, further research is warranted to understand the determinants of prescription that could explain the contrasting trends.
Supplementary Material
ACKNOWLEDGMENTS
This study has received funding from the French Government's Investissement d'Avenir program, Laboratoire d'Excellence Integrative Biology of Emerging Infectious Diseases (grant no. ANR-10-LABX-62-IBEID).
We are grateful to the two main French National Health Insurances agencies (General Scheme and Social Scheme), for access to all outpatient antibiotics reimbursed. Finally, this article has benefited from constructive comments made by the anonymous referees, whose help is gratefully acknowledged.
Footnotes
Published ahead of print 14 October 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.01813-13.
REFERENCES
- 1.French GL. 2010. The continuing crisis in antibiotic resistance. Int. J. Antimicrob. Agents. 36(Suppl 3):S3–S7. 10.1016/S0924-8579(10)70003-0 [DOI] [PubMed] [Google Scholar]
- 2.European Centre for Disease Prevention and Control, European Medicines Agency 2009. The bacterial challenge: time to react. European Centre for Disease Prevention and Control, Stockholm, Sweden: http://www.emea.europa.eu/docs/en_GB/document_library/Report/2009/11/WC500008770.pdf [Google Scholar]
- 3.Goossens H, Ferech M, Vander Stichele R, Elseviers M. 2005. Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet 365:579–587. 10.1016/S0140-6736(05)17907-0 [DOI] [PubMed] [Google Scholar]
- 4.Van de Sande-Bruinsma N, Grundmann H, Verloo D, Tiemersma E, Monen J, Goosens H, Ferech M, European Antimicrobial Resistance Surveillance System Group, European Surveillance of Antimicrobial Consumption Project Group 2008. Antimicrobial drug use and resistance in Europe. Emerging Infect. Dis. 14:1722–1730. 10.3201/eid1411.070467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gonzales R, Steiner JF, Sande MA. 1997. Antibiotic prescribing for adults with colds, upper respiratory tract infections, and bronchitis by ambulatory care physicians. JAMA 278:901–904. 10.1001/jama.1997.03550110039033 [DOI] [PubMed] [Google Scholar]
- 6.Nyquist AC, Gonzales R, Steiner JF, Sande MA. 1998. Antibiotic prescribing for children with colds, upper respiratory tract infections, and bronchitis. JAMA 279:875–877. 10.1001/jama.279.11.875 [DOI] [PubMed] [Google Scholar]
- 7.Gonzales R, Malone DC, Maselli JH, Sande MA. 2001. Excessive antibiotic use for acute respiratory infections in the United States. Clin. Infect. Dis. 33:757–762. 10.1086/322627 [DOI] [PubMed] [Google Scholar]
- 8.Sabuncu E, David J, Bernède-Bauduin C, Pépin S, Leroy M, Boëlle PY, Watier L, Guillemot D. 2009. Significant reduction of antibiotic use in the community after a nationwide campaign in France, 2002-2007. PLoS Med. 6:e1000084. 10.1371/journal.pmed.1000084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valleron AJ, Bouvet E, Garnerin P, Ménarès J, Heard I, Letrait S, Lefaucheux J. 1986. A computer network for the surveillance of communicable diseases: the French experiment. Am. J. Public Health 76:1289–1292. 10.2105/AJPH.76.11.1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Box GE, Jenkins GM. 1976. Time series analysis: forecasting and control, revised. Holden-Day, San Francisco, CA [Google Scholar]
- 11.Shumway R, Stoffer D. 2000. Time series analysis and its applications. Springer, New York, NY [Google Scholar]
- 12.Helfenstein U. 1996. Box-Jenkins modelling in medical research. Stat. Methods Med. Res. 5:3–22. 10.1177/096228029600500102 [DOI] [PubMed] [Google Scholar]
- 13.Box GE, Tiao GC. 1975. Intervention analysis with applications to economic and environmental problems. J. Am. Stat. Assoc. 70:70–79. 10.1080/01621459.1975.10480264 [DOI] [Google Scholar]
- 14.Bernier A, Ligier C, Guillemot D, Watier L. 2013. Did media attention of the 2009 A(H1N1) influenza epidemic increase outpatient antibiotic use in France? A time-series analysis. PLoS One 8:e69075. 10.1371/journal.pone.0069075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Institut National de Veille Sanitaire 2013. Couverture par le vaccin antipneumococcique conjugé. Institut National de Veille Sanitaire, Saint-Maurice, France: http://www.invs.sante.fr/Dossiers-thematiques/Maladies-infectieuses/Maladies-a-prevention-vaccinale/Couverture-vaccinale/Donnees/Pneumocoque Accessed 15 March 2013 [Google Scholar]
- 16.Dommergues MA, Hentgen V. 2012. Decreased paediatric antibiotic consumption in France between 2000 and 2010. Scand. J. Infect. Dis. 44:495–501. 10.3109/00365548.2012.669840 [DOI] [PubMed] [Google Scholar]
- 17.Dunais B, Van Dijken C, Bruno P, Touboul P, Carsenti-Dellamonica H, Pradier C. 2011. Antibiotic prescriptions in French day-care centres: 1999-2008. Arch. Dis. Child. 96:1033–1037. 10.1136/adc.2010.207969 [DOI] [PubMed] [Google Scholar]
- 18.Huttner B, Goossens H, Verheij T, Harbarth S. 2010. Characteristics and outcomes of public campaigns aimed at improving the use of antibiotics in outpatients in high-income countries. Lancet Infect. Dis. 10:17–31. 10.1016/S1473-3099(09)70305-6 [DOI] [PubMed] [Google Scholar]
- 19.Goossens H, Coenen S, Costers M, De Corte S, De Sutter A, Gordts B, Laurier L, Struelens M. 2008. Achievements of the Belgian Antibiotic Policy Coordination Committee (BAPCOC). Euro Surveill. 13(46):pii=19036 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19036 [PubMed] [Google Scholar]
- 20.Mölstad S, Erntell M, Hanberger H, Melander E, Norman C, Skoog G, Lundborg CS, Söderström A, Torell E, Cars O. 2008. Sustained reduction of antibiotic use and low bacterial resistance: 10-year follow-up of the Swedish Strama programme. Lancet Infect. Dis. 8:125–132. 10.1016/S1473-3099(08)70017-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.