Abstract
Treatment of tuberculosis (TB) is impaired by the long duration and complexity of therapy and the rising incidence of drug resistance. There is an urgent need for new agents with improved efficacy, safety, and compatibility with combination chemotherapies. Oxazolidinones offer a potential new class of TB drugs, and linezolid—the only currently approved oxazolidinone—has proven highly effective against extensively drug-resistant (XDR) TB in experimental trials. However, widespread use of linezolid is prohibited by its significant toxicities. AZD5847, a novel oxazolidinone, demonstrates improved in vitro bactericidal activity against both extracellular and intracellular M. tuberculosis compared to that of linezolid. Killing kinetics in broth media and in macrophages indicate that the rate and extent of kill obtained with AZD5847 are superior to those obtained with linezolid. Moreover, the efficacy of AZD5847 was additive when tested along with a variety of conventional TB agents, indicating that AZD5847 may function well in combination therapies. AZD5847 appears to function similarly to linezolid through impairment of the mycobacterial 50S ribosomal subunit. Future studies should be undertaken to further characterize the pharmacodynamics and pharmacokinetics of AZD5847 in both in vitro and animal models as well is in human clinical trials.
INTRODUCTION
Tuberculosis (TB), caused by Mycobacterium tuberculosis, continues to pose an enormous threat to global public health (1). In 2010, nearly 9 million people were infected with M. tuberculosis and 1.4 million died from the disease (1, 2). While the vast majority of cases of drug-sensitive TB are curable with appropriate drug therapy, rates of treatment success have stagnated due to the long duration and complexity of therapy and the rising incidence of drug resistance (1, 3, 4). Standard TB therapy alone involves four drugs taken for at least 6 months, and treatment for multidrug-resistant (MDR) and extensively drug-resistant (XDR) TB requires administration of more toxic antibiotics—including at least one injectable—for durations of 1 to 2 years (5). Often, patients are coinfected with HIV, which further complicates treatment due to deleterious potential drug-drug interactions between TB medications and antiretrovirals (6). Despite the limitations of current treatment options, only one new class of TB drugs has been approved in the past 40 years (7). There is an urgent need for new TB drugs with greater efficacy, reduced toxicities, and improved compatibility with antiretrovirals.
Recent evidence demonstrates that linezolid, the only member of the oxazolidinone class of antimicrobials currently on the market, has efficacy against MDR TB (8–11). The potential of linezolid for the treatment of TB is limited due to the incidence of serious adverse effects, including cytopenias, neuropathies, lactic acidosis, and rhabdomyolysis (12, 13). Risk increases with increasing dose and duration, which is particularly problematic, given the typical extended course of chemotherapy for TB. A number of research initiatives, therefore, have begun to pursue development of new oxazolidinones with greater efficacy and reduced toxicity which would have the potential to become an important new component of the drug arsenal against TB, including MDR and XDR TB. One such new oxazolidinone, sutezolid (PNU-100480), for example, has completed a phase 2a study (14). We report here the findings of preclinical studies of another novel oxazolidinone, AZD5847, which may exhibit an improved safety profile and superior efficacy against M. tuberculosis compared to those of linezolid.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Pandrug-susceptible M. tuberculosis ATCC 27294 (strain H37Rv) was derived from a single seed lot previously isolated from infected mouse lung tissue and stored at −70°C. The methods for seed lot preparation and storage have been reported earlier (15). H37Rv cultures for experiments were performed either in Middlebrook 7H9 broth (7H9) supplemented with 10% (vol/vol) albumin-dextrose-catalase (Difco Laboratories, Detroit, MI), 0.05% (vol/vol) Tween 80 (Sigma, St. Louis, MO), and 0.2% (vol/vol) glycerol or on Middlebrook 7H11 agar (7H11) supplemented with 10% (vol/vol) oleic acid-albumin-dextrose-catalase (Difco). Incubations were done at 37°C in roller bottles or culture plates sealed in CO2-permeable polyethylene bags.
For the macromolecular incorporation assay, the surrogate organism M. bovis BCG was used. These studies were performed in AstraZeneca, Bangalore, India, as per the institutional biosafety committee guidelines approved by the government of India. Derived from a frozen seed lot, the M. bovis isolate was initially grown for 4 to 5 days in 7H9 under the conditions described for M. tuberculosis liquid culture until an optical density at 600 nm (OD600) of 0.3 was attained, at which point it was used directly for the assays. As described further in subsequent methods, the assay also involved the use of a mixture of radiotracers: 10 μCi/ml of [1-14C]acetate (45 to 60 mCi/mmol; PerkinElmer, Waltham, Mass.) and 10 μCi/ml of [5,6-3H]uracil (49 Ci/mmol), 25 μCi/ml of [8-3H]adenine (20 to 25 Ci/mmol), and 25 μCi/ml of l-[4,5-3H] leucine (120 to 190 Ci/mmol) (GE Healthcare, Little Chalfont, United Kingdom).
The agents AZD5847 (Fig. 1; AstraZeneca, London, United Kingdom), isoniazid, rifampin, ethambutol, streptomycin, and sparfloxacin (Sigma), and moxifloxacin and linezolid (Sai Quest, Hyderabad, India) were dissolved and serially diluted in dimethyl sulfoxide before being added to media where appropriate and at the concentrations stated in subsequent methods.
FIG 1.
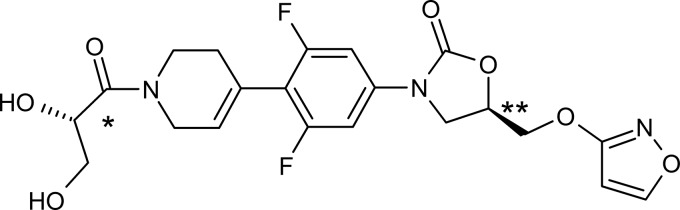
Structure of AZD5847. *, (S) configuration; **, (R) configuration). No evidence of isomerization was found.
MIC determination.
We determined the MIC of AZD5847 by a standard microdilution method as described earlier (16). The assay was performed in duplicate 96-well microtiter plates (Tarsons, Kolkata, India) in which all peripheral wells were filled with sterile distilled water. In the remaining wells, we dispensed serial 2-fold dilutions of AZD5847 at concentrations ranging from 0.06 to 32 μg/ml. The wells in column 11 contained no drug and served as culture controls. 7H9 containing H37Rv was added to each well to provide a final start inoculum of approximately 5 × 105 CFU/ml. After 7 days of incubation, 40 μl of a freshly prepared 1:1 mixture of 10× alamarBlue (Accumed International, Westlake, OH) and 10% Tween 80 was added to each of the wells. The plates were incubated for an additional 24 h, and the colors of all wells were recorded. A blue color was interpreted as no growth, whereas a pink color was scored as growth. We defined the MIC as the lowest drug concentration at which a color change from blue to pink was prevented.
The MIC against clinical isolates of M. tuberculosis (both drug-susceptible and single-drug-resistant strains) was determined using the same protocol described above. However, the incubation period was extended up to 3 weeks until the no-drug control wells showed visible turbidity. The bacterial growth was monitored turbidometrically, and the lowest concentration of drug which showed no visible growth was scored as the MIC. With the single-drug-resistant strains, the respective resistance marker drug was included as a positive control to confirm their phenotype.
In vitro combination studies in broth.
We determined the effects of combining AZD5847 with several TB drugs through the use of a checkerboard titration, which has been previously described (17). Briefly, using 96-well microtiter plates, we diluted one drug vertically (rows A to H) and a second drug horizontally (columns 1 to 10) to obtain various combinations of the two drugs in a final volume of 100 μl for each well. We then added to each well 100 μl of H37Rv-infected 7H9 to provide an inoculum of approximately 5 × 105 CFU/ml. The plates were incubated for 7 days, and the MIC was interpreted as described earlier (17). To evaluate whether the paired combinations of agents had additive efficacy in inhibiting M. tuberculosis (i.e., no interaction) or inhibitory efficacy more or less than the sum of their effects alone (synergy versus antagonism), we calculated the fractional inhibitory concentration index (ΣFIC) using the following formula: (MICdrug A in combination/MICdrug A alone) + (MICdrug B in combination/MICdrug B alone). For the purposes of interpretation, we defined synergy as an ΣFIC of ≤0.5, antagonism as an ΣFIC of ≥4.0, and additivity (no interaction) as ΣFIC values between 0.5 and 4.0.
Killing kinetics of AZD5847 in broth and macrophages.
We evaluated the killing kinetics of AZD587 and linezolid in both 7H9 and bone marrow-derived macrophages. We used 24-well microtiter plates to generate serial 2-fold dilutions of the given agent with concentrations ranging from 0.5 to 256 μg/ml.
For the assay in broth, we added to each well 20 μl of H37Rv-infected 7H9 to provide an inoculum of approximately 3 × 108 CFU/ml. The plates were then incubated at 37°C in humidified 5% CO2. On days 0, 1, 4, 7, and 14, we removed aliquots of the culture, centrifuged them to wash off the drug, resuspended them in 500 μl of fresh 7H9, and then plated them on 7H11. After incubating the plates at 37°C in humidified 5% CO2 for 21 to 28 days, we performed bacterial enumeration. Data were expressed as the log10 number of CFU recovered for each drug concentration.
For evaluation of the killing kinetics within macrophages, bone marrow-derived macrophages were obtained from BALB/c mice. The mice were euthanized by exposure to CO2 as per ethical guidelines. The studies were approved by the institutional animal ethics committee approved by the government of India. The femur and tibia were excised, the bones were trimmed at each end, and the marrow was flushed out with cold RPMI 1640 medium (Sigma) using a 26-gauge needle. Cell suspensions were then washed twice with fresh medium. To each well of a fresh 24-well microtiter plate, approximately 2 × 106 cells/ml suspended in supplemented RPMI 1640 (RPMI 1640 with 10% fetal bovine serum [Sigma] and 20% culture supernatant of cells of the L929 [ATCC CCL1] mouse fibroblast cell line) were added. The cells were then incubated at 37°C in humidified 5% CO2 for 7 days, during which the media were changed twice. On day 8 of culture, we infected the macrophages for 2 h with H37Rv at a multiplicity of infection (MOI) of 10 under routine culture conditions. The monolayers were then twice washed with prewarmed phosphate-buffered saline (PBS) to remove extracellular bacteria and resuspended in fresh supplemented RPMI 1640. Cell suspensions were then added to 24-well microtiter plates with variable concentrations of the given agent (AZD5847 or linezolid), as described above. The 24-well plates were incubated but periodically examined to note any observable adverse changes in cell morphology potentially due to drug toxicity. At 0, 4, and 10 days into the incubation, portions of the monolayers were removed, gently washed with PBS, lysed with 0.04% sodium dodecyl sulfate, and plated on 7H11. After 18 to 20 days at 37°C with humidified 5% CO2, the 7H11 plates were removed from incubation and the bacterial colonies were enumerated. Data were again expressed as the log10 number of CFU recovered for each drug concentration.
For modeling of the kill kinetics in broth, we first estimated the kill rate constants (equation 1) and then determined the estimated maximum effect (Emax) using a sigmoidal Emax model (equation 2). We modeled the kill kinetics in macrophages similarly, except that a different approach was used to calculate the kill constants (equation 3).
| (1) |
| (2) |
| (3) |
where Kg is the growth rate constant, Kk is the kill rate constant, X is the cell density (number of CFU/ml) Xmax is the maximum cell density (number of CFU/ml) reached, C is the drug concentration, A is the exponent used to describe the lag in growth/kill, t is time, EC50 is the concentration required for a half-maximal kill rate (equation 1) or the concentration required for half of the maximum effect (equation 2), Emax is the number of CFU/ml without drug treatment, E0 is the minimum number of CFU/ml with drug treatment, and H is the Hill coefficient or sigmoidicity factor.
Macromolecular incorporation assay.
Fourfold serial dilutions of AZD5847 were made to attain concentrations ranging from 0.015 to 64 μg/ml. Twenty-five microliters of each drug dilution was added to 225 μl of 7H9 (1:10 dilution). Then, a 96-well microtiter plate was prepared by adding to each well 20 μl of a given drug concentration, 40 μl of the radiotracer mixture, and 40 μl of M. bovis BCG culture. A duplicate 96-well plate without drug dilutions was also generated to serve as no-drug controls. Then, 100 μl of 20% trichloroacetic acid (TCA) was added to the wells in the first 3 columns of the no-drug control plate to stop these reactions and provide the 0-min incorporation values. Following a 2-h incubation, the reactions in the other wells were similarly stopped. The plates were incubated for 12 h at 4°C to allow precipitation of TCA. We then filtered the contents of each plate with a filtration manifold to remove excess radioactivity and washed the pellets three times with 5% TCA and once with ethanol. We air dried the filter plates for 2 h inside a laminar flow hood and then added 50 μl of scintillation fluid to each well. A 1450 Micro Beta Trilux instrument was used to determine scintillation counts, and the 50% inhibitory concentrations (IC50s) were calculated. The IC50 was defined as the minimum concentration of drug at which 50% of the radiolabel incorporation was inhibited. Using the same start cell number used in the incorporation assay, we determined the MIC values against M. bovis BCG. Specific inhibition of a particular pathway was inferred when the ratio of the radiolabel IC50 to the M. bovis BCG MIC was equal to or less than 2.
Determination of resistance frequency.
The single-step selection method was used to generate mutants spontaneously resistant to AZD5847 and linezolid. Briefly, we centrifuged a mid-logarithmic-phase liquid culture and concentrated it 100 times to achieve an approximate bacterial count of 1010 CFU/ml. The bacterial cells were spread on 7H11 plates containing 4, 8, and 16 μg/ml of either compound, which corresponded to 4×, 8×, and 16× the MIC. Triplicate drug-free plates were also made as controls. Control plates were incubated for 4 weeks, followed by bacterial enumeration. The drug-containing plates were incubated for up to 8 weeks to confirm the final number of spontaneously resistant colonies.
For each dilution of a given drug, the spontaneous rate of resistance was calculated by dividing the number of colonies on a drug-containing plate by the number of colonies on the drug-free plate. In addition, 12 colonies were chosen at random from the drug-containing plates, grown in 7H9, and subjected to drug susceptibility testing against AZD5847, linezolid, and several conventional TB agents.
Genetic mapping of resistance-conferring mutations.
We isolated chromosomal DNA from well-characterized clones resistant to AZD5847 and/or linezolid by boiling the cultures for 20 min. The supernatants were then subjected to PCR analysis to amplify various candidate ribosomal genes that are known to confer resistance to oxazolidinones. The rrl gene coding for 23S rRNA was amplified using primers Mtu_rrl_For (5′-GGCTAGCGGTGGCGTGTTCT-3′) and Mtu_rrl_Rev (5′-CGGATGTGGTTGCGAGTTTG-3′). The rplC gene coding for ribosomal protein L3 was amplified using primers Mtb_rplC_For (5′-GCCAGCGTCGACGTCAACAT-3′) and Mtb_rplC_Rev (5′-GCGTCTTGACGTCGATTTTG-3′). For both genes, PCR was performed with the following cycling parameters: 94°C for 30 s, 70°C for 45 s, and 72°C for 2 min for 30 cycles. PCR products were cleaned (PCR purification kit; Qiagen, Venlo, Netherlands), quantitated, and sequenced (Microsynth, Balgach, Switzerland). The sequences from the resistant clones were then aligned against wild-type H37Rv genome sequences using Vector NTI software (Life Technologies, Carlsbad, CA) to detect point mutations in the target genes.
Statistical analysis.
The colony counts obtained from plating experiments were transformed to log10(x + 1), where x equals the total number of viable tubercle bacilli calculated to be present in a given sample. The log10 number of CFU/ml was plotted against the log of the concentration of AZD5847, and a simple Emax model was fitted to the data for estimating EC50 and Emax. Initial kill rates were estimated after plotting the number of CFU/ml against the time of incubation. We fit the killing kinetics models to the log10 number of CFU/ml versus time (days) or versus concentration (μg/ml) data using WinNonlin Phoenix software (v6.2; Pharsight, St. Louis, MO). All statistical calculations were done with Prism software (v5; GraphPad, San Diego, CA). Statistical differences among means were derived using Student's t test.
RESULTS
AZD5847 [(5R)-3-(4-{1-[(2S)-2,3-dihydroxypropanoyl]-1,2,3,6-tetrahydropyridin-4-yl}-3,5-difluorophenyl-5-[(isoxazol-3-yloxy)methyl]-1,3-oxazolan-2-one] belongs to the oxazolidinone drug class and has a molecular formula of C21H21F2N3O7 and a relative molecular mass of 465.4 Da (18). The agent has 2 chiral centers [Fig. 1; *, (S) configuration; **, (R) configuration) with no evidence of isomerization. AZD5847 has no measurable pKa over the physiological pH range, and its melting point is 153°C.
MIC and killing kinetics in broth and macrophages.
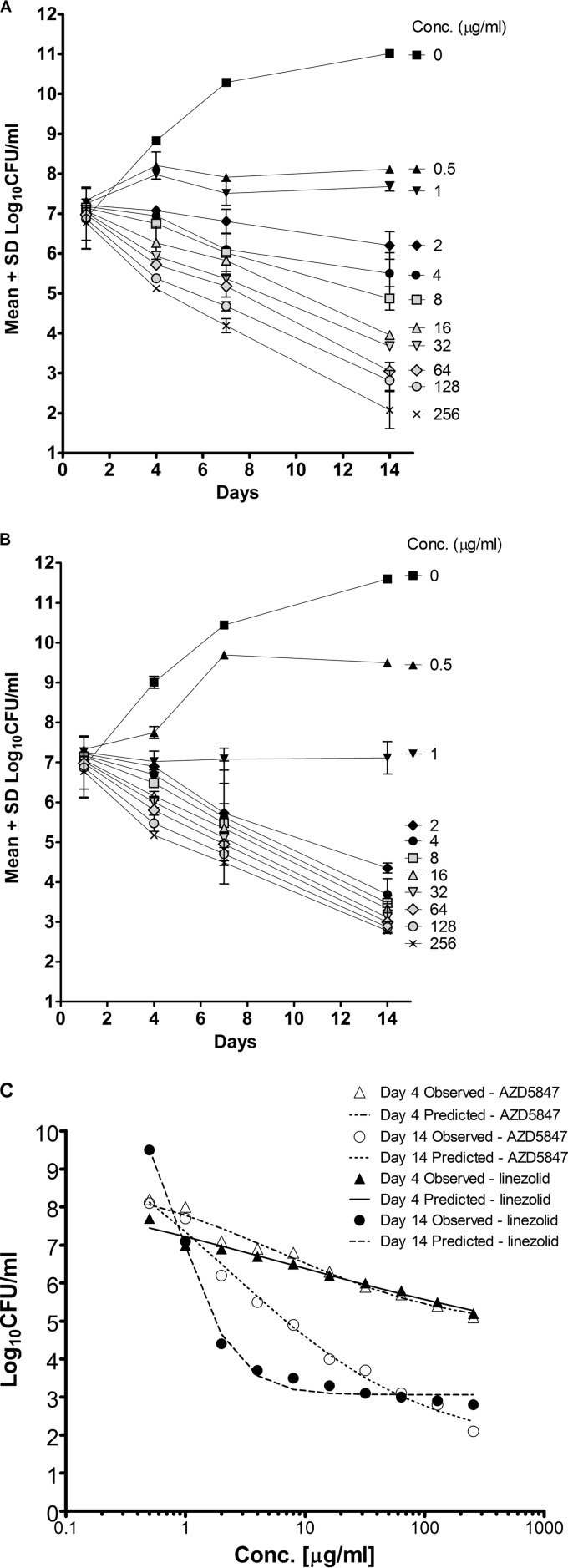
The MIC of AZD5847 against M. tuberculosis H37Rv in Middlebrook 7H9 was 1 μg/ml. AZD5847 retained its MIC (0.25 to 1 μg/ml) against a panel of clinical isolates of M. tuberculosis, including single-drug-resistant strains (Table 1). AZD5847 exhibited concentration- and time-dependent killing kinetics against extracellular M. tuberculosis in 7H9 under aerobic growth conditions. The net Emax (Emax − E0) for AZD5847 progressively increased from 4.1 log10 CFU/ml on day 4 to 9.4 log10 CFU/ml on day 14. The limit of quantitation (LOQ) in terms of bacterial survivors (LOQ, 50 CFU/ml) was reached by the end of the experiment (Fig. 2A). There was a dose-dependent increase in killing over the concentration range tested. The EC50 (equation 2) decreased from 6.1 μg/ml on day 4 to 2.3 μg/ml on day 14. In contrast, linezolid exhibited time-dependent killing kinetics against extracellular M. tuberculosis in 7H9, resulting in a 9-fold decrease in the EC50 (equation 2) from day 4 to day 14 (8.1 to 0.9 μg/ml) under aerobic growth conditions, and the LOQ was not reached even at 14 days of exposure at 256× MIC (Fig. 2B). The Hill slope was significantly higher for linezolid, given that the bacterial killing was more time dependent for linezolid than it was for AZD5847 (19). As shown in Table 2 and Fig. 2C, the kill rate constant obtained for AZD5847 using the pharmacodynamic model was higher than that obtained for linezolid, which resulted in a higher net Emax for AZD5847.
TABLE 1.
Distribution of MICs across various reference and clinical isolates of M. tuberculosis, including single-drug-resistant strainsa
| M. tuberculosis strain | Origin | MIC (μg/ml) |
|||||
|---|---|---|---|---|---|---|---|
| STR | INH | RIF | EMB | OFL | AZD5847 | ||
| ATCC 27294 | Reference strain | 0.25–0.5 | 0.03 | 0.015 | 2–4 | 0.5–1 | 1 |
| ATCC 35811 | Reference strain | 0.25–0.5 | 0.03 | 0.06 | 8 | 0.5–1 | 0.5 |
| ATCC 25618 | Reference strain | 0.25–0.5 | 0.06 | 0.03 | 4 | 1 | 0.5 |
| Erdman | Reference strain | 0.125–0.25 | 0.06 | 0.03 | 4 | 0.25 | 0.5 |
| Beijing | Reference strain | 0.125–0.25 | 0.06 | 0.03 | 8 | 0.5 | 0.25 |
| Harlingen | Reference strain | ≤0.5 | 0.03 | 0.03 | 8 | 0.5 | 0.25 |
| CDC1551 | Reference strain | 0.25 | 0.03 | 0.03 | 8 | 0.5 | 0.5 |
| ATCC 35820 STRr | Reference strain | >8 | 0.03 | 0.03 | 4 | 0.5 | 1 |
| ATCC 35822 INHr | Reference strain | 0.25–0.5 | >128 | 0.015 | 4 | 0.5–1 | 1 |
| DKU220 | Sputum isolate | 0.125 | 0.125 | 0.015 | 4 | 0.5 | 0.5 |
| DK97A | Sputum isolate | 0.125 | 0.06 | 0.03 | 4 | 0.5 | 0.13 |
| DP299S | Sputum isolate | 0.25 | 0.03 | 0.06 | 4 | 0.5 | 1 |
| 136570 STRr | Sputum isolate | >4 | 0.03 | 0.03 | 2 | 0.5 | 1 |
| 912253 INHr | Sputum isolate | 0.25 | >1 | 0.03 | 4 | 0.5 | 0.5 |
| JALMA RIFr | Sputum isolate | 0.25 | 0.06 | >0.5 | 4 | 0.5 | 0.5 |
| 19000 RIFr | Sputum isolate | 0.06–0.125 | 0.06 | >8 | 4 | 0.25–0.5 | 0.5 |
| 17003 EMBr | Sputum isolate | ≤0.5 | 0.03 | 0.03 | >32 | 0.5 | 0.13 |
| 12119 OFLr | Sputum isolate | 0.125–0.5 | 0.06 | 0.008 | 2 | >4 | 1 |
The MIC was determined following drug exposure, and growth was monitored by turbidometry. STR, streptomycin; INH, isoniazid; RIF, rifampin; EMB, ethambutol; OFL, ofloxacin.
FIG 2.
Killing kinetics of AZD5847 (A) and linezolid (B) in 7H9. Data for the mean log10 number of CFU/ml along with the standard deviation are plotted against time (in days). (C) The concentration-response curve against M. tuberculosis in broth on days 4 and 14 for AZD5847 and linezolid is shown. Predicted lines were obtained by fitting these data to the Emax model (equation 2, as described in Materials and Methods).
TABLE 2.
Kill rates, EC50s, and Emaxs derived from 7H9 and murine bone marrow-derived macrophage killing kinetics with AZD5847 and linezolid
| Growth condition and drug | Kg (1/day) | Kk (1/day) | EC50a (μg/ml) | Net Emaxb (log10 CFU/ml) | Hill coefficienta |
|---|---|---|---|---|---|
| 7H9 | |||||
| Linezolid | 1.27 (13)c | 1.97 (10) | 0.69 (11) | 8.5 | 1.5 (11) |
| AZD5847 | 1.15 (12) | 2.20 (10) | 1.24 (36) | 9.4 | 0.4 (15) |
| Murine BMDMsd | |||||
| Linezolid | 0.15 (19) | 0.18 (24) | 1.4 (39) | 0.88 | 2.58 (83) |
| AZD5847 | 0.13 (19) | 0.52 (75) | 8.5 (161) | >1.6 | 0.97 (58) |
EC50 data and Hill coefficients were derived from equation 1.
Net Emax is Emax − E0, and data were derived from equation 2. Data for the assay in 7H9 are from day 14, and data for the assay in murine bone marrow-derived macrophages are from day 10.
Data in parentheses are coefficients of variation (in percent) for the parameter estimates.
BMDM, bone marrow-derived macrophages.
AZD5847 exhibited exposure-dependent killing kinetics against intracellular M. tuberculosis in murine bone marrow-derived macrophages. Efficacy in this model was studied up to 16 μg/ml, since there were signs of macrophage cytotoxicity beyond 32 μg/ml. The compound exhibited a dose-dependent increase in bacterial killing over the concentration range tested. AZD5847, at 16 μg/ml, resulted in a decrease of 1.5 log10 CFU/ml after 10 days in culture (Fig. 3A). As shown in Table 2, the kill rate constant for AZD5847 was 3-fold greater than that for linezolid, resulting in a net Emax for AZD5847 that was approximately 0.7 log unit higher (Fig. 3B). Linezolid had a significantly higher Hill slope, since the bactericidal effect of this compound was more time dependent that of AZD5847. For AZD5847, the net Emax continued to increase as drug concentrations increased, whereas for linezolid the extent of kill was saturated at 8 μg/ml (Fig. 3C).
FIG 3.
Killing kinetics of AZD5847 (A) and linezolid (B) in murine bone marrow-derived macrophages infected with M. tuberculosis. (C) The concentration-response curve against M. tuberculosis in bone marrow-derived macrophages on day 10 for AZD5847 and linezolid is shown. Lines were obtained by fitting these data to the Emax model (equation 2, as described in Materials and Methods).
We tested AZD5847 in combination with a number of more conventional TB drugs. As illustrated in Table 3, the effects of AZD5847 in combination with isoniazid, rifampin, ethambutol, streptomycin, and moxifloxacin were additive in every case, with the FIC index ranging from 0.8 to 1.2.
TABLE 3.
In pairwise combination studies, AZD5847 exerts additivity with known TB drugs
| Drug combination | MIC (μg/ml) |
ΣFICa | |
|---|---|---|---|
| Alone | Combinationb | ||
| AZD5847 | 0.5 | 0.5 | 1.13 |
| Isoniazid | 0.03 | 0.004 | |
| AZD5847 | 0.5 | 0.125 | 0.8 |
| Rifampin | 0.015 | 0.008 | |
| AZD5847 | 0.5 | 0.5 | 1.12 |
| Ethambutol | 1 | 0.125 | |
| AZD5847 | 0.5 | 0.5 | 1.24 |
| Streptomycin | 0.25 | 0.06 | |
| AZD5847 | 0.5 | 0.5 | 1.06 |
| Moxifloxacin | 0.125 | 0.008 | |
A ΣFIC (fractional inhibitory concentration [FIC] index) value of ≤0.5 denotes synergy, one of ≥4.0 denotes antagonism, and values in between denote additivity.
The MIC in the combination was determined using a checkerboard method.
Mechanism-of-action studies.
On the basis of previous studies of other oxazolidinone drugs (20), the expected mechanism of action for AZD5847 was inhibition of protein synthesis, although this was confirmed using a macromolecular incorporation assay. This assay is useful for those targets that have a direct role in the synthesis of macromolecules. Inhibition of the incorporation of radiolabeled precursors into different macromolecules (fatty acids, DNA, RNA, and proteins) is monitored, and differential IC50s for the 4 different biosynthetic processes are determined. The IC50/MIC values are then calculated to help establish how specifically a given agent inhibits each of the processes. The MIC of AZD5847 against M. bovis BCG under the experimental conditions was 4 μg/ml, while the IC50 for the inhibition of leucine incorporation was 0.2 μg/ml. Therefore, the IC50/MIC ratio for leucine was 0.05 (Table 4). In contrast, the IC50/MIC ratios for acetate, adenine, and uracil were >16, indicating specific inhibition of protein synthesis.
TABLE 4.
Effect on macromolecular synthesis inferred from inhibition of incorporation of radiolabeled precursors in M. bovis BCG
| Drug | Radiolabel | IC50 (μg/ml) | MIC (μg/ml) | IC50/MIC | Pathway inhibited |
|---|---|---|---|---|---|
| Cerulenin | [14C]acetate | 0.15 | 8 | 0.02 | Fatty acid |
| Sparfloxacin | [3H]adenine | 1.3 | 1 | 1.3 | DNA synthesis |
| Chloramphenicol | [3H]leucine | 3.67 | 16 | 0.23 | Protein Synthesis |
| Rifampin | [3H]uracil | 0.03 | 0.125 | 0.24 | RNA synthesis |
| AZD5847 | [3H]leucine | 0.2 | 4 | 0.05 | Protein Synthesis |
| AZD5847 | [14C]acetate | >64 | 4 | >16 | Not fatty acid |
| AZD5847 | [3H]adenine | >64 | 4 | >16 | Not DNA |
| AZD5847 | [3H]uracil | >64 | 4 | >16 | Not RNA |
In vitro resistance profile of AZD5847.
In vitro, spontaneous mutants resistant to AZD5847 arose at frequencies of 5.3 × 10−8 at 4× MIC and 5.5 × 10−8 at 8× MIC. In comparison, spontaneous mutants resistant to linezolid arose at frequencies of 1.1 × 10−8 at 4× MIC and 1.5 × 10−8 at 8 × MIC. We recovered no mutants resistant to either AZD5847 or linezolid on plates containing 16× MIC of either agent, suggesting that this may be the mutant prevention concentration (MPC) for both compounds. Neither AZD5847- nor linezolid-resistant mutants showed cross-resistance to isoniazid, rifampin, or moxifloxacin. AZD5847- and linezolid-resistant mutants were cross-resistant to each other, with a 16- to 32-fold increase in the MIC (Table 5).
TABLE 5.
Spontaneous resistance to AZD5847 and linezolid maps to the rrl and rplC genes in M. tuberculosisa
| Strain | MIC (μg/ml) |
Mutation |
||||||
|---|---|---|---|---|---|---|---|---|
| AZD5847 | Linezolid | Moxifloxacin | Isoniazid | Rifampin | rrl gene | rplC gene | L3 protein | |
| H37Rv (ATCC 27294) | 1 | 1 | 0.13 | 0.06 | 0.02 | WT | WT | WT |
| 5847r-4.1 | 16 | 16 | 0.13 | 0.06 | 0.02 | WT | T460C | Cys154Arg |
| 5847r-4.6 | 16 | 16 | 0.13 | 0.06 | 0.02 | WT | T460C | Cys154Arg |
| 5847r-8.8 | 16 | 16 | 0.13 | 0.06 | 0.02 | WT | T460C | Cys154Arg |
| 5847r-8.9 | 16 | 16 | 0.13 | 0.06 | 0.02 | WT | T460C | Cys154Arg |
| 5847r-8.10 | 16 | 8 | 0.13 | 0.06 | 0.02 | G2270T | WT | WT |
| LNZr-8.1 | 16 | 16 | 0.13 | 0.06 | 0.02 | WT | T460C | Cys154Arg |
| LNZr-8.3 | 160 | 16 | 0.13 | 0.06 | 0.02 | WT | T460C | Cys154Arg |
| LNZr-8.7 | 16 | 32 | 0.13 | 0.125 | 0.02 | WT | T460C | Cys154Arg |
WT, wild type; LNZr, linezolid-resistant M. tuberculosis; 5847r, AZD5847-resistant M. tuberculosis.
Sequence analysis was performed on a random subset of spontaneous mutants that showed an increase in the MIC for AZD5847 and/or linezolid. Known target genes rrl (encoding 23S rRNA) and rplC, rplD, and rplW (encoding ribosomal proteins L3, L4, and L23, respectively), which have been implicated in the acquisition of resistance to oxazolidinones, were amplified using PCR (21). One out of five AZD5847-resistant mutants showed a G2270T mutation in the rrl gene, whereas the remaining 4 clones showed a T460C mutation in the rplC gene (Table 5). Interestingly, all 3 linezolid-resistant mutants had changes that mapped to the rplC gene, as described earlier (21), and none mapped to the rrl gene.
DISCUSSION
We describe here the in vitro activity profile of the novel oxazolidinone AZD5847. Although prior research has suggested that it has potent activity against a variety of Gram-positive bacteria (18), little had been done to assess the compound's specific bactericidal properties or mechanism of action against mycobacteria. Importantly, we found that in murine bone marrow-derived macrophages, AZD5847 was highly effective against intracellular M. tuberculosis, producing a greater than 1-log-unit reduction in intracellular bacilli when they were exposed to a concentration of 16 μg/ml for 10 days. In comparison, at the same concentration, the oxazolidinone agent linezolid was associated with a less than 0.5-log-unit reduction in intracellular M. tuberculosis. The capacity for M. tuberculosis to persist within hosts for long periods, sometimes many decades, is believed to be due in part to its ability to take refuge within host macrophages (22). Therefore, efficacy at killing intracellular bacilli is considered a key metric by which any new potential TB agent should be evaluated. The exposure-dependent killing and higher kill rates exhibited by AZD5847 against both extracellular and intracellular M. tuberculosis or a surrogate mycobacterium may translate to efficacy better than that of linezolid in vivo and provide the basis for development of an important new TB drug.
TB treatment requires multidrug chemotherapy regimens, and therefore, success relies not simply on the profiles of its individual component agents but also on their compatibility. The additive efficacy of AZD5847 in combination with several first-line TB drugs supports the potential clinical utility of this agent in conventional as well as some emerging combination TB therapies (23). Further testing in animal models and with a wider panel of potential combination agents will better characterize these opportunities. The cumulative experience of developing combinations of drugs for the treatment of TB has well illustrated that one drug can have profound effects on the exposure, efficacy, and toxicity of a companion agent through alterations in host absorption, distribution, metabolism, or excretion. In the case of treatment of patients coinfected with HIV, these challenges can be compounded by the addition of antiretroviral combinations, possibly with strong inducers of cytochrome P450 enzymes. Future consideration of drugs to be combined with AZD5847 will have to take into account these potential drug-drug interactions.
Through the use of a mycobacterial surrogate organism, M. bovis BCG, and radiolabeled precursor molecules, we were able to demonstrate more than a 20-fold reduction in the protein concentration compared to the concentrations of other macromolecules after incubation, demonstrating that protein synthesis is a major target of AZD5847 inside the mycobacterial cell. This aligns well with what is known about the mechanism of action of linezolid. Numerous studies have shown that linezolid targets the 50S ribosomal subunit by blocking the binding of tRNA and thereby inhibiting bacterial cell growth. High-resolution structural modeling of linezolid bound to the 50S ribosomal subunit has revealed that the binding occurs in a deep cleft of the 23S rRNA (24).
The majority of the AZD5847-resistant strains as well as all linezolid-resistant strains isolated in this study harbored mutations in the rplC gene coding for the L3 ribosomal protein. This is consistent with prior sequencing of spontaneous linezolid-resistant mutants that showed mutations primarily at nucleotide position 2061 of the 23S rRNA gene (25), and recent studies have shown specific mutations in ribosomal proteins L3 and L4 that are associated with linezolid resistance in M. tuberculosis (21). Since the mutated nucleotide residue T460C was identical across all AZD5847- and linezolid-resistant clones, they exhibited complete cross-resistance. The reported T460C nucleotide mutation that results in a Cys154Arg amino acid change has been reported in both laboratory-generated and clinical strains of linezolid-resistant M. tuberculosis (21). Surprisingly, in this study we found a single AZD5847-resistant clone with a G2270T mutation in the rrl gene encoding the 23S rRNA. This is a novel mutation that has not previously been reported to confer resistance to linezolid, let alone AZD5847.
As exemplified by the success of linezolid, the oxazolidinone class of TB agents has shown promise in the treatment of MDR and XDR TB (12, 26). In a retrospective, nonrandomized, unblinded observational study, 90% culture conversion was observed in linezolid-treated patients with TB resistant to >7 drugs, which compared favorably to the 25% culture conversion observed among patients on regimens without linezolid. In the first controlled phase 2 study of linezolid, patients with XDR TB given linezolid as an add-on to their current failing regimen experienced a subsequent sputum culture conversion rate of 89% within 6 months (11). While highly encouraging with regard to potential efficacy, the safety profile of linezolid was not ideal. Adverse effects like peripheral and optic neuropathies and myelosuppression led to the withdrawal of linezolid in many patients prior to the completion of treatment. New oxazolidinones such as AZD5847 offer structural differentiation from linezolid that may lead to better safety profiles while maintaining high clinical efficacy against TB.
AZD5847, a novel oxazolidinone, demonstrates improved in vitro bactericidal activity against both extracellular and intracellular M. tuberculosis compared to that of linezolid. Killing kinetics in broth media and in macrophages indicate that the rate and extent of kill obtained with AZD5847 are superior to those obtained with linezolid. Moreover, the efficacy of AZD5847 was additive when tested along with a variety of more conventional TB agents, indicating that AZD5847 may function well in combination therapies, a fundamental characteristic of TB regimens. Future studies should be undertaken to further characterize the pharmacodynamics and pharmacokinetics of AZD5847 in both in vitro and animal models as well as in human clinical trials.
ACKNOWLEDGMENTS
We thank Tanjore Balganesh for his constant support and encouragement. We also thank Bill Bishai for critical reading of the manuscript prior to submission.
This work was not supported by any external funding.
All of us were employees of AstraZeneca when this research work was performed. We state that we have no conflict of interest and have received no payment in the preparation of the manuscript.
Footnotes
Published ahead of print 4 November 2013
REFERENCES
- 1.World Health Organization 2012. Global tuberculosis report 2012. World Health Organization, Geneva, Switzerland: http://www.who.int/tb/publications/global_report/en/ [Google Scholar]
- 2.Dye C, Williams BG. 2010. The population dynamics and control of TB. Science 328:856–861. 10.1126/science.1185449 [DOI] [PubMed] [Google Scholar]
- 3.Sacks LV, Behrman RE. 2008. Developing new drugs for the treatment of drug resistant tuberculosis: a regulatory perspective. Tuberculosis (Edinb.) 88(Suppl 1):S93–S100. 10.1016/S1472-9792(08)70040-4 [DOI] [PubMed] [Google Scholar]
- 4.Orenstein EW, Basu S, Shah NS, Andrews JR, Friedland GH, Moll AP, Gandhi NR, Galvani AP. 2009. Treatment outcomes among patients with multidrug-resistant tuberculosis: systematic review and meta-analysis. Lancet Infect. Dis. 9:153–161. 10.1016/S1473-3099(09)70041-6 [DOI] [PubMed] [Google Scholar]
- 5.Johnston JC, Shahidi NC, Sadatsafavi M, Fitzgerald JM. 2009. Treatment outcomes of multidrug-resistant tuberculosis: a systematic review and meta-analysis. PLoS One 4:e6914. 10.1371/journal.pone.0006914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pepper DJ, Meintjes GA, McIlleron H, Wilkinson RJ. 2007. Combined therapy for tuberculosis and HIV-1: the challenge for drug discovery. Drug Discov. Today 12:980–989. 10.1016/j.drudis.2007.08.001 [DOI] [PubMed] [Google Scholar]
- 7.Jones D. 2013. Tuberculosis success. Nat. Rev. Drug Discov. 12:175–176. 10.1038/nrd3957 [DOI] [PubMed] [Google Scholar]
- 8.Fortun J, Martin-Davila P, Navas E, Perez-Elias MJ, Cobo J, Tato M, De la Pedrosa EG, Gomez-Mampaso E, Moreno S. 2005. Linezolid for the treatment of multidrug-resistant tuberculosis. J. Antimicrob. Chemother. 56:180–185. 10.1093/jac/dki148 [DOI] [PubMed] [Google Scholar]
- 9.Condos R, Hadgiangelis N, Leibert E, Jacquette G, Harkin T, Rom WN. 2008. Case series report of a linezolid-containing regimen for extensively drug-resistant tuberculosis. Chest 134:187–192. 10.1378/chest.07-1988 [DOI] [PubMed] [Google Scholar]
- 10.Sotgiu G, Centis R, D'Ambrosio L, Alffenaar JW, Anger HA, Caminero JA, Castiglia P, De Lorenzo S, Ferrara G, Koh WJ, Schecter GF, Shim TS, Singla R, Skrahina A, Spanevello A, Udwadia ZF, Villar M, Zampogna E, Zellweger JP, Zumla A, Migliori GB. 2012. Efficacy, safety and tolerability of linezolid containing regimens in treating MDR-TB and XDR-TB: systematic review and meta-analysis. Eur. Respir. J. 40:1430–1442. 10.1183/09031936.00022912 [DOI] [PubMed] [Google Scholar]
- 11.Lee M, Lee J, Carroll MW, Choi H, Min S, Song T, Via LE, Goldfeder LC, Kang E, Jin B, Park H, Kwak H, Kim H, Jeon H-S, Jeong I, Joh JS, Chen RY, Olivier KN, Shaw PA, Follmann D, Song SD, Lee J-K, Lee D, Kim CT, Dartois V, Park S-K, Cho S-N, Barry CE. 2012. Linezolid for treatment of chronic extensively drug-resistant tuberculosis. N. Engl. J. Med. 367:1508–1518. 10.1056/NEJMoa1201964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Migliori GB, Eker B, Richardson MD, Sotgiu G, Zellweger JP, Skrahina A, Ortmann J, Girardi E, Hoffmann H, Besozzi G, Bevilacqua N, Kirsten D, Centis R, Lange C, TBNET Study Group 2009. A retrospective TBNET assessment of linezolid safety, tolerability and efficacy in multidrug-resistant tuberculosis. Eur. Respir. J. 34:387–393. 10.1183/09031936.00009509 [DOI] [PubMed] [Google Scholar]
- 13.Carroll MW, Choi H, Min S, Hwang S, Park H, Song T, Park Y, Jeon HS, Goldfeder LC, Via LE, Lebron J, Jin B, Cai Y, Barry CE, III, Lee M. 2012. Rhabdomyolysis in a patient treated with linezolid for extensively drug resistant (XDR) tuberculosis. Clin. Infect. Dis. 54:1624–1627. 10.1093/cid/cis293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wallis RS, Jakubiec WM, Kumar V, Silvia AM, Paige D, Dimitrova D, Li X, Ladutko L, Campbell S, Friedland G, Mitton-Fry M, Miller PF. 2010. Pharmacokinetics and whole-blood bactericidal activity against Mycobacterium tuberculosis of single doses of PNU-100480 in healthy volunteers. J. Infect. Dis. 202:745–751. 10.1086/655471 [DOI] [PubMed] [Google Scholar]
- 15.Jayaram R, Gaonkar S, Kaur P, Suresh BL, Mahesh BN, Jayashree R, Nandi V, Bharat S, Shandil RK, Kantharaj E, Balasubramanian V. 2003. Pharmacokinetics-pharmacodynamics of rifampin in an aerosol infection model of tuberculosis. Antimicrob. Agents Chemother. 47:2118–2124. 10.1128/AAC.47.7.2118-2124.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shirude PS, Madhavapeddi P, Tucker JA, Murugan K, Patil V, Basavarajappa H, Raichurkar AV, Humnabadkar V, Hussein S, Sharma S, Ramya VK, Narayan CB, Balganesh TS, Sambandamurthy VK. 2013. Aminopyrazinamides: novel and specific GyrB inhibitors that kill replicating and nonreplicating Mycobacterium tuberculosis. ACS Chem. Biol. 8:519–523. 10.1021/cb300510w [DOI] [PubMed] [Google Scholar]
- 17.Solapure S, Dinesh N, Shandil R, Ramachandran V, Sharma S, Bhattacharjee D, Ganguly S, Reddy J, Ahuja V, Panduga V, Parab M, Vishwas KG, Kumar N, Balganesh M, Balasubramanian V. 2013. In vitro and in vivo efficacy of β-lactams against replicating and slowly growing/nonreplicating Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 57:2506–2510. 10.1128/AAC.00023-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Czock D, Keller F. 2007. Mechanism-based pharmacokinetic-pharmacodynamic modeling of antimicrobial drug effects. J. Pharmacokinet. Pharmacodyn. 34:727–751. 10.1007/s10928-007-9069-x [DOI] [PubMed] [Google Scholar]
- 19.Shaw KJ, Barbachyn MR. 2011. The oxazolidinones: past, present, and future. Ann. N. Y. Acad. Sci. 1241:48–70. 10.1111/j.1749-6632.2011.06330.x [DOI] [PubMed] [Google Scholar]
- 20.Beckert P, Hillemann D, Kohl TA, Kalinowski J, Richter E, Niemann S, Feuerriegel S. 2012. rplC T460C identified as a dominant mutation in linezolid resistant Mycobacterium tuberculosis strains. Antimicrob. Agents Chemother. 56:2743–2745. 10.1128/AAC.06227-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wookey A, Turner PJ, Greenhalgh JM, Eastwood M, Clarke J, Seftonet V. 2004. AZD2563, a novel oxazolidinone: definition of antibacterial spectrum, assessment of bactericidal potential and the impact of miscellaneous factors on activity in vitro. Clin. Microbiol. Infect. 10:247–254. 10.1111/j.1198-743X.2004.00770.x [DOI] [PubMed] [Google Scholar]
- 22.Rohde KH, Veiga DF, Caldwell S, Balázsi G, Russell DG. 2012. Linking the transcriptional profiles and the physiological states of Mycobacterium tuberculosis during an extended intracellular infection. PLoS Pathog. 8:e1002769. 10.1371/journal.ppat.1002769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zumla A, Hafner R, Lienhardt C, Hoelscher M, Nunn A. 2012. Advancing the development of tuberculosis therapy. Nat. Rev. Drug Discov. 11:171–172. 10.1038/nrd3694 [DOI] [PubMed] [Google Scholar]
- 24.Leach KL, Swaney SM, Colca JR, McDonald WG, Blinn JR, Thomasco LM, Gadwood RC, Shinabarger D, Xiong L, Mankin AS. 2007. The site of action of oxazolidinone antibiotics in living bacteria and in human mitochondria. Mol. Cell 26:393–402. 10.1016/j.molcel.2007.04.005 [DOI] [PubMed] [Google Scholar]
- 25.Hillemann D, Rusch-Gerdes S, Richter E. 2008. In vitro-selected linezolid-resistant Mycobacterium tuberculosis mutants. Antimicrob. Agents Chemother. 52:800–801. 10.1128/AAC.01189-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cox H, Ford N. 2012. Linezolid for the treatment of complicated drug-resistant tuberculosis: a systematic review and meta-analysis. Int. J. Tuberc. Lung Dis. 16:447–454. 10.5588/ijtld.11.0451 [DOI] [PubMed] [Google Scholar]