Abstract
GSK1322322 is a potent peptide deformylase inhibitor with in vitro and in vivo activity against multidrug-resistant skin and respiratory pathogens. This report provides plasma and intrapulmonary pharmacokinetics, safety, and tolerability of GSK1322322 after repeat (twice daily intravenous dosing for 4 days) dosing at 1,500 mg. Plasma samples were collected over the last 12-hour dosing interval of repeat dosing following the day 4 morning dose (the last dose). Bronchoalveolar lavage samples were collected once in each subject, either before or at 2 or 6 h after the last intravenous dose. Plasma area under the concentration-time curve (AUC0–τ) was 66.7 μg · h/ml, and maximum concentration of drug in serum (Cmax) was 25.4 μg/ml following repeat doses of intravenous GSK1322322. The time course of epithelial lining fluid (ELF) and alveolar macrophages (AM) mirrored the plasma concentration-time profile. The AUC0–τ for ELF and AM were 78.9 μg · h/ml and 169 μg · h/ml, respectively. The AUC0–τ ratios of ELF and AM to total plasma were 1.2 and 2.5, respectively. These ratios increased to 3.5 and 7.4, respectively, when unbound plasma was considered. These results are supportive of GSK1322322 as a potential antimicrobial agent for the treatment of lower respiratory tract bacterial infections caused by susceptible pathogens. (This study has been registered at ClinicalTrials.gov under registration number NCT01610388.)
INTRODUCTION
There is a crucial need for the development of new antibiotics with novel mechanisms of action against multidrug-resistant pathogens (1, 2). Peptide deformylase (PDF) has become a promising and attractive bacterial target to explore for the discovery of new antibacterial agents (3, 4). GSK1322322 is a potent PDF inhibitor from the hydrazinopyrimidine class and has demonstrated in vitro and in vivo antibacterial activity against skin and respiratory tract pathogens, including methicillin-resistant Staphylococcus aureus (5–7). Phase 1 safety and pharmacokinetic studies for single and multiple doses of an oral dosage formulation of GSK1322322 have recently been reported (8–10). A clinical trial evaluating the first time in human (FTIH) use of intravenous administration of GSK1322322 has been completed (ClinicalTrials.gov identifier, NCT01610388).
Knowledge of intrapulmonary drug concentrations has been advocated to assist in the selection and design of anti-infective dosing regimens to effectively treat lower respiratory tract infections (11, 12). Among the compartments of the lung, epithelial lining fluid (ELF) has been suggested as an important site of infection for lower respiratory tract pathogens such as Streptococcus pneumoniae, Moraxella catarrhalis, and Haemophilus influenzae. In addition, pulmonary alveolar macrophages (AM) have been proposed as the site for intracellular or atypical pathogens such as Mycoplasma pneumoniae, Chlamydophila pneumoniae, and Legionella pneumophila.
The primary objectives of this report are to describe the plasma and intrapulmonary pharmacokinetics, safety, and tolerability of GSK1322322 after repeat dosing (1,500 mg twice daily intravenous dosing for 4 days) in healthy subjects.
(This work was presented in part at the Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, CA, September 2012.)
MATERIALS AND METHODS
Study design and subjects.
This was a phase I, randomized, double-blind, placebo-controlled, single and repeat dose escalation study to evaluate the safety, tolerability, and pharmacokinetics of oral and intravenous GSK1322322 in healthy subjects. This study was the first time in human (FTIH) administration of GSK1322322 as an intravenous formulation. The study was approved by the Western Institutional Review Board and conducted at DaVita Clinical Research (Minneapolis, MN). Written informed consent was obtained from each subject before study entry.
This report is limited to the subjects enrolled into cohort C (third dose-escalation cohort) of the larger intravenous FTIH study. This portion of the study evaluated both plasma and intrapulmonary pharmacokinetics of GSK1322322 at a dosage level of 1,500 mg in healthy adult subjects. The dosage formulation included lyophilized vials containing the free base form of GSK1322322.
Inclusion criteria included healthy male or female subjects between 18 and 65 years of age, with body weight of ≥50 kg and body mass index (BMI) between 18.5 and 29.9 kg/m2, and no clinically significant abnormalities based on medical history, vital signs, physical examination, laboratory tests (chemistry, hematology, urinalysis), and electrocardiographs (ECGs). Females were of nonchildbearing potential or postmenopausal. Male subjects with female partners of childbearing potential were required to use protocol-defined birth control methods.
Exclusion criteria included pregnant or lactating females, prestudy test results positive for hepatitis B surface antigen, hepatitis C antibody, HIV antibody or drug and alcohol screen, current or chronic history of liver disease or known hepatic or biliary abnormalities, and contraindications for bronchoalveolar lavage (BAL) including hypercapnia of >50 mm Hg, refractory hypoxemia, reactive airway disease or asthma, unstable angina or acute myocardial infarction within the past 6 months, heart failure, and severe hemostatic alterations. Subjects could not have a hypersensitivity history to GSK1322322, atropine, midazolam, fentanyl, lidocaine, codeine, or heparin that contraindicated their participation. Prescription or nonprescription drugs (including vitamins and herbal and dietary supplements) were not allowed within 7 days (or 14 days if the drug is a potent enzyme inducer) or 5 half-lives (whichever is longer) before the first dose of GSK1322322. Red wine, Seville oranges, grapefruit, or grapefruit juice could not be consumed within 7 days before the first dose of GSK1322322. Subjects must not have had urinary cotinine levels indicative of smoking or a history of regular use of tobacco- or nicotine-containing products within 6 months before being screened. Cardiovascular exclusion criteria included a history of previous myocardial infarction, protocol-defined ECG changes, conduction abnormality, and any significant arrhythmia. Subjects could not have donated blood or blood products in excess of 500 ml within a 56-day period prior to study participation. Subjects could not have a history of regular alcohol consumption within 6 months of the study, have received an investigational drug or participated in a clinical trial within the past 30 days, or been exposed to more than four new chemical entities within 12 months of the first dose of GSK1322322.
Subjects were randomized to receive either placebo (n = 3) or GSK1322322 (n = 18). Subjects assigned to GSK1322322 received 1,500-mg doses of GSK1322322 intravenously every 12 h for a total of seven doses. All intravenous doses were infused over 60 min via a controlled infusion pump, and exact infusion times were recorded. Subjects randomized to placebo followed the same intravenous administration schedules and collection of pharmacokinetic samples as subjects receiving GSK1322322.
Pharmacokinetic samples.
One pharmacokinetic sampling period was assessed in this study. Blood samples for the measurement of GSK1322322 concentrations in plasma were collected during the final 12-hour dosing interval. Sampling times included predose (within 15 min before) and at 0.25, 0.5, 1 (end of infusion), 1.5, 2, 4, 6, 8, and 12 h after the start of the final (seventh) intravenous infusion of GSK1322322. All blood samples (approximately 2 ml) were taken from an indwelling cannula, collected into EDTA tubes, and immediately placed on ice and centrifuged at 3,000 × g for 15 min. Supernatant plasma was transferred to matrix screw-cap tubes and stored at −20°C until shipped to the analytical laboratory.
Each subject underwent one standardized bronchoscopy and BAL procedure before or after the last intravenous dose of GSK1322322. The scheduled collection times included predose (12 h after the previous [sixth] dose) or at 2 or 6 h after the start of the final (seventh) intravenous infusion of GSK1322322. The 2-, 6-, and 12-hour sampling times were selected to provide concentration-time data over the entire 12-hour dosing interval and represent the maximum (peak), midpoint, and minimum (trough) intrapulmonary concentrations, respectively.
Bronchoscopy and BAL.
Standardized bronchoscopy and BAL procedures have been previously described (13). In brief, subjects received the following medications prior to the bronchoscopy procedure: nebulized 0.5 mg atropine with 4% lidocaine, 1 to 2 mg of midazolam, and 25 to 75 μg of fentanyl. A 2% concentration of viscous lidocaine was applied to the distal end of the bronchoscope prior to insertion. A fiber-optic bronchoscope (models P-20 and P-20D; Olympus America Inc., Melville, NY) was inserted into a subsegment of the right middle lobe. A 4% concentration of lidocaine was administered to the vocal chords. If needed, a 1% concentration of lidocaine was administered to the lower airway.
Four 50-ml aliquots of sterile 0.9% normal saline solution were instilled into the middle lobe, and each specimen was immediately aspirated and placed in ice. The aspirate from the first 50-ml instillation (BAL-1) was collected separately. The aspirates recovered from the second, third, and fourth instillations were pooled (BAL-2). The volumes of both BAL fluid samples were measured and recorded. A 4-ml aliquot was removed from the BAL samples and immediately sent to the laboratory for cell count and differential count. The remaining volume of BAL fluid was immediately centrifuged at 400 × g for 5 min to separate ELF (supernatant) and AM (pellet). The remaining supernatant and pellet were removed separately and placed into amber tubes. The supernatant was frozen immediately at −20°C. The pellet was resuspended in deionized water at 20% of the supernatant volume that was removed and then frozen at −20°C. A 2-ml aliquot of supernatant was also separated, stored in amber tubes, and frozen for quantification of urea.
Blood pressure, heart rate, respiratory rate, pulse, and oxygen saturation were evaluated and recorded before, at the end of, and between 30 and 60 min after the end of the bronchoscopy procedure. A 2-ml blood sample to determine urea concentrations was obtained just before the scheduled bronchoscopy procedure and was kept on ice until centrifuged. Blood samples were centrifuged at 3,000 × g for 15 min, serum was separated, and samples were stored at −20°C until the assays were performed. A physical examination and an assessment of clinical laboratory parameters were repeated in all subjects after the completion of study procedures.
Laboratory and safety assessment.
Safety was assessed by evaluating reported and observed adverse events, reviewing ECGs, measuring vital signs, baseline 24-hour Holter monitoring, and conducting physical examinations and clinical laboratory tests (i.e., chemistry, hematology, and urinalysis). Twelve-lead ECGs were obtained at screening, before and after the single-dose phase for oral and intravenous administration, before and after the first and last doses of the multiple-dose phase, and 7 to 10 days after the last intravenous dose.
Bioanalytical procedures for determination of GSK1322322 concentrations.
Concentrations of GSK1322322 in plasma and pulmonary samples were measured by ultrahigh-performance liquid chromatography-tandem mass spectrometry (UHPLC-MS/MS) at Bioanalytical Science and Toxicokinetics (GlaxoSmithKline, King of Prussia, PA). Briefly, the analytical system consisted of an Acquity UPLC system (Waters, Milford, MA), an Acquity UPLC HSS T3 analytical column (1.8-μm particle size, 2.1 by 50 mm; Waters, Milford, MA), and an API-5500 mass spectrometer (Applied Biosystems/MDS Sciex, Canada). GSK1322322 was extracted from samples after the addition of an isotopically labeled internal standard ([13C2, 15N2]GSK1322322). Extracts were analyzed by UHPLC-MS/MS using a TurboIonSpray interface and multiple reaction monitoring. Computer systems used to acquire and quantify data included Analyst software (version 1.5; Applied Biosystems/MDS Sciex, Canada) and Study Management System 2000 (version 2.3; GlaxoSmithKline).
GSK1322322 was extracted from plasma samples using a validated protein precipitation procedure. Using a 25-μl aliquot, the lower and upper limits of quantification for the plasma assay were 5.0 ng/ml and 5,000 ng/ml, respectively. The standard curve for GSK1322322 in plasma was linear (r2 ≥ 0.999) over the concentration range of 5 to 5,000 ng/ml. The accuracy (percent bias) ranged from −4.0% to 9.5%. The within- and between-run precision values (percent coefficient of variation [%CV]) were ≤6.0% and ≤4.4%, respectively.
GSK1322322 was extracted from 300 μl of ELF (supernatant from BAL fluid) and 50 μl of AM (reconstituted centrifuged pellet from BAL fluid) by a validated solid-phase extraction procedure. The standard curve for GSK1322322 in ELF was linear (r2 ≥ 0.9984) over the concentration range of 0.5 to 1,000 ng/ml. The accuracy ranged from −10.8% to 10.8%. The within- and between-run precision values (%CV) were ≤3.3% and ≤7.5%, respectively. The standard curve for GSK1322322 in AM was linear (r2 ≥ 0.9978) over the concentration range of 5 to 5,000 ng/ml. The accuracy ranged from −14.7% to 13.0%. The within- and between-run precision (%CV) values were ≤6.7% and ≤12.5%, respectively.
For all matrixes, quality control (QC) samples were prepared at three different analyte concentrations and analyzed with each batch of sample against separately prepared calibration standards. For the analysis to be acceptable, no more than one-third of the total QC results were to deviate from the nominal concentration by more than 15% and at least 50% of the results from each QC concentration should be within 15% of nominal. Validation experiments also tested the assay selectivity in the presence of atropine, lidocaine, midazolam, and fentanyl. None of these coadministered drugs had any effect on the quantification of GSK1322322 in the various matrices. The applicable analytical runs met all predefined run acceptance criteria.
Urea concentration determination.
Concentrations of urea in serum and BAL fluid supernatant were determined using the Beckman Coulter urea nitrogen reagent (kit number 0SR6134; Brea, CA) on an AU480 Chemistry Analyzer (Beckman Coulter, Inc., Brea, CA) at MedTox Laboratories (St. Paul, MN). For the BAL fluid samples, a modification in the manufacturer's procedure was made, and standard curves were prepared in normal saline. The standard curves for serum and BAL fluid ranged from 1.00 to 100 mg/dl and 0.05 to 8.0 mg/dl, respectively. Both standard curves were linear, and interday coefficients of variations were <5%.
Calculation of the volume of ELF and GSK1322322 concentrations in ELF and AM.
The urea dilution method described by Rennard and colleagues was used to determine the apparent volume of ELF (VELF) (14). The VELF was estimated from the ratio of urea concentrations in BAL fluid (ureaBAL) and plasma (ureaplasma) by using the equation VELF = VBAL × ureaBAL/ureaplasma, where VBAL is the volume of aspired BAL2 fluid.
The concentration of GSK1322322 in ELF (GSK322ELF) was determined from the equation GSK322ELF = GSK322BAL × VBAL/VELF, where GSK322BAL is the measured concentration of GSK1322322 in the BAL aspirate supernatant.
The concentration of GSK1322322 in AM (GSK322AM) was determined from the equation GSK322AM = GSK322pellet/VAC, where GSK322pellet is the measured amount of GSK1322322 in the BAL pellet from the cell suspension and VAC is the volume of alveolar cells. The concentration of white blood cells in BAL fluid prior to centrifugation was determined. A differential cell count determined the percentage of macrophages and monocytes in BAL fluid. A mean cell volume of 2.42 μl/106 cells was used in the calculations for VAC (15).
Pharmacokinetic analysis.
Pharmacokinetic parameters were determined for each subject by noncompartmental methods using the computer software WinNonlin (version 5.2; Pharsight Corporation, Cary, NC). Pharmacokinetics parameters following multiple intravenous (i.v.) doses included maximum concentration of drug in serum (Cmax), AUC during the 12-hour dosing interval (AUC0–12 or AUC0–τ), and systemic clearance (CL). Plasma pharmacokinetic parameters were summarized as geometric mean values and between-subject percent coefficient of variation (%CVb).
The AUC0–τ for ELF and AM were calculated with the linear trapezoidal method using the mean ELF and AM concentrations of GSK1322322 at the bronchopulmonary sampling times of 2, 6, and 12 h. The 12-hour concentration values (from the sixth dose) were used both as a time zero and as a 12-hour value for calculating AUC0–τ. Ratios of AUC0–12 and concentration values at each sampling time were determined by dividing values in ELF or AM by those in the plasma. Total and unbound plasma GSK1323322 values were used to determine these penetration ratios. Fraction unbound in plasma (34%) was calculated using the protein binding value of 66% for GSK1322322 (9).
RESULTS
Subjects.
A total of 22 subjects met inclusion criteria, signed an informed consent, and participated in the study. The characteristics of the 19 study subjects receiving GSK1322322 as a single oral dose are reported in Table 1. One of the enrolled subjects withdrew informed consent after completing the single oral dose pharmacokinetic study. Eighteen subjects received intravenous dosing of GSK1322322. Subsequently, a second subject was withdrawn from the study due to moderate adverse event (pruritic rash) during the third day of the multiple-dose intravenous pharmacokinetic study. The remaining 17 subjects completed 4 days of intravenous dosing of GSK1322322 and underwent bronchoscopy with BAL at their randomized sampling time (Table 1). Three male subjects (age range, 24 to 36 years) served as a control group and did not receive GSK1322322.
TABLE 1.
Characteristics of study subjects receiving 1,500 mg of GSK1322322a
| Cohortb | No. of subjects and sexc | Age (yr) | Height (cm) | Wt (kg) | Body mass index (kg/m2) | Total cell count/mm3 in BAL fluid | Macrophages/monocytes (%) |
|---|---|---|---|---|---|---|---|
| 2-hour | 5 M, 1 F | 36.8 ± 13.5 | 181.7 ± 11.5 | 85.4 ± 12.9 | 25.8 ± 2.1 | 102 ± 51 | 86 ± 13 |
| 6-hour | 4 M, 1 F | 37.2 ± 15.0 | 170.4 ± 14.3 | 70.5 ± 14.2 | 24.2 ± 3.7 | 111 ± 26 | 83 ± 8 |
| 12-hour | 3 M, 3 F | 51.3 ± 9.0 | 175.5 ± 2.4 | 78.9 ± 14.2 | 25.5 ± 4.0 | 102 ± 39 | 89 ± 13 |
Data are expressed as means ± SD except for sex.
Seventeen subjects received multiple i.v. doses of GSK1322322 and had bronchoalveolar lavage fluid collected.
M, male; F, female.
The most frequently reported drug-related adverse events in subjects receiving intravenous GSK1322322 were headache (n = 8), infusion site pain (n = 5) and irritation (n = 4), and diarrhea (n = 4). All adverse events were mild or moderate in intensity. One subject was withdrawn after the multiple intravenous doses of GSK1322322 due to moderate pruritic rash on the neck and chest, behind the ear, and at the infusion site location. The rash appeared gradually, and the subject was treated with methylprednisolone and diphenhydramine. The rash resolved within 3 days after discontinuation of GSK1322322.
No significant trends or changes from baseline in vital signs, chemistry, and hematology data were observed. No subjects had Bazett's corrected QT (QTcB) and/or Friedericia's corrected QT (QTcF) values of >480 ms. One subject had changes from baseline in QTcB and QTc values that were >60 ms.
Pharmacokinetics.
The geometric mean (%CVb) pharmacokinetic parameters of GSK1322322 in plasma following multiple intravenous doses of 1,500 mg included Cmax of 25.4 (27.1%) μg/ml, AUC0–τ of 66.7 (25.0%) μg · h/ml, and CL of 22.5 (25.0%) liters/h. Similarities between this CL value and CL values from other dose levels and between single and repeated intravenous doses were consistent with linear pharmacokinetics (16).
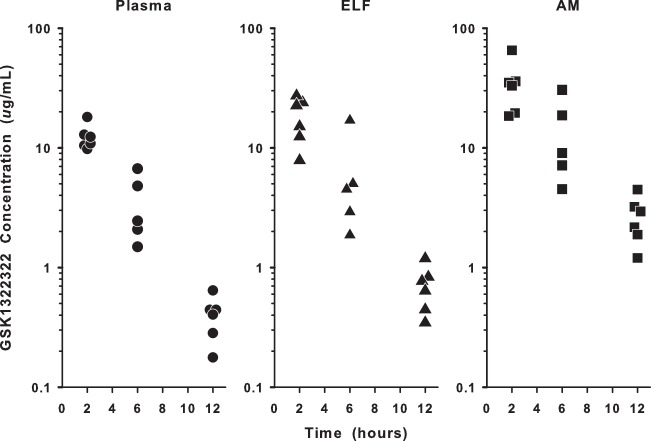
The individual concentrations of GSK1322322 in total plasma, ELF, and AM during the 12-hour interval following the last intravenous dose are shown in Fig. 1. The mean (± standard deviation [SD]) steady-state concentrations of GSK1322322 measured in plasma (total), ELF, and AM are reported in Table 2. The highest observed concentrations for all three matrices occurred at the 2-hour sampling time, and GSK1322322 concentrations in ELF and AM showed a time pattern similar to plasma concentrations (Fig. 1 and 2). The ratios of concentrations in ELF and AM to concentrations in total plasma ranged from 1.4- to 2.0-fold and 2.6- to 6.5-fold, respectively. The ratio of concentrations in ELF and AM to concentrations in unbound plasma ranged from 4.1- to 5.8-fold and 7.7- to 19.2-fold, respectively.
FIG 1.
Individual measured concentrations of GSK1322322 in plasma (total), epithelial lining fluid (ELF), and alveolar macrophages (AM) at 2, 6, and 12 h. The y axis is in log scale.
TABLE 2.
GSK1322322 concentrations in plasma (total), epithelial lining fluid (ELF), and alveolar macrophages (AM) at sampling time of bronchoscopy and bronchoalveoar lavage fluid
| Sampling time (h) | Mean concn ± SD (μg/ml) of GSK1322322 in: |
||
|---|---|---|---|
| Total plasma | ELF | AM | |
| 2 | 13.2 ± 3.80a | 18.2 ± 7.55b | 34.6 ± 16.9b |
| 6 | 3.19 ± 1.40a | 6.26 ± 6.12c | 14.0 ± 10.7c |
| 12 | 0.406 ± 0.178a | 0.703 ± 0.301b | 2.65 ± 1.15b |
There were 17 concentrations in plasma at each sampling time.
There were 6 concentrations in ELF and AM at the 2- and 12-hour sampling times.
There were 5 concentrations in ELF and AM at the 6-hour sampling time.
FIG 2.
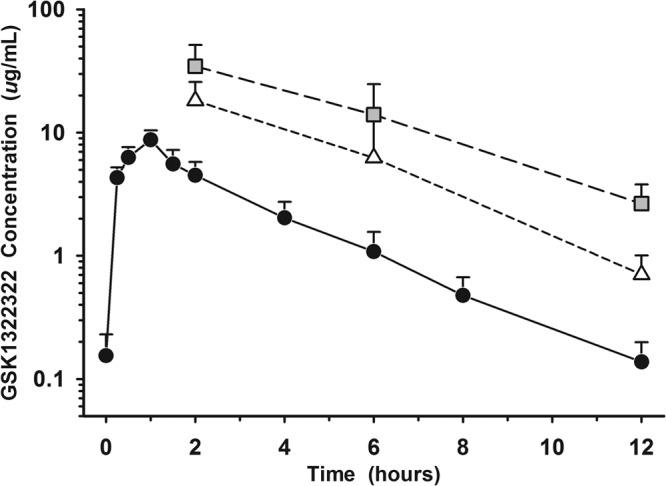
Mean (± SD) concentration-versus-time profiles of GSK1322322 in plasma (unbound), epithelial lining fluid (ELF), and alveolar macrophages (AM) after repeat intravenous dosing of 1,500 mg twice daily. Closed circles represent unbound plasma, open triangles represent ELF, and gray squares represent AM. The y axis is in log scale.
Following repeated intravenous doses of GSK1322322 at 1,500 mg, the total and unbound plasma AUC0–τ averaged 66.7 μg · h/ml and 22.7 μg · h/ml, respectively. Based on the mean concentrations at each sampling time (Table 2), the calculated AUC0–τ values for ELF and AM were 78.9 μg · h/ml and 169 μg · h/ml, respectively. The ratios of ELF and AM to total plasma based on the AUC0–τ values were 1.2 and 2.5, respectively, whereas the ratios of ELF and AM to unbound plasma were 3.5 and 7.4, respectively.
DISCUSSION
This clinical trial was the first time in human (FTIH) administration of GSK1322322 as an intravenous formulation. Repeat intravenous administration of 1,500 mg twice daily of GSK1322322 for 4 days was well tolerated. The most frequently observed drug-related adverse events included headache, infusion-related reactions (site pain and irritation), and diarrhea. All adverse events were mild to moderate in severity, and no serious adverse events were observed in this study. One subject withdrew from the study because of a pruritic skin rash on the third day of intravenous administration. Other than infusion-related reactions, the overall adverse events profile appeared to be similar to that observed with repeat dosing of an oral powder-in-bottle formulation of GSK1322322 (500 mg to 1,500 mg twice daily) for 10 days (10).
Following repeated intravenous administration of 1,500 mg, GSK1322322 readily appeared in ELF and AM and had intrapulmonary concentration-time profiles similar to plasma with minimal hysteresis (Fig. 2). The ELF and AM concentrations were greater than the MIC90 values for Staphylococcus aureus and Haemophilus influenzae (4 μg/ml), Streptococcus pneumoniae (2 μg/ml), and Moraxella catarrhalis (1 μg/ml) for approximately 50% of the 12-hour dosing interval (7). The mean ELF and AM concentrations were 1.4- to 2.0-fold and 2.6- to 6.5-fold of the concurrent total concentrations in plasma, respectively, and 4.1- to 5.8-fold and 7.7- to 19.2-fold of the concentrations in unbound plasma, respectively. The AUC0–τ ratios of ELF and AM to total plasma were 1.2 and 2.5, respectively, and 3.5 and 7.4 for the unbound plasma, respectively. These data demonstrated that GSK1322322 penetrated into extracellular (i.e., ELF) and intracellular (i.e., AM) compartments in the lung following intravenous administration. Once the appropriate pharmacokinetic-pharmacodynamic target (i.e., drug exposure indexed to MIC) is determined, the observed plasma and intrapulmonary concentrations of GSK1322322 will assist in dosage regimen evaluations for the treatment of lower respiratory infections caused by susceptible bacterial organisms.
While the clinical significance of concentrations in ELF and AM remains unknown for GSK1322322, the knowledge of drug penetration into the extracellular and intracellular space of the lungs should be of assistance in evaluating and designing specific dosing regimens for use against potential pathogens (11, 12). Further studies in patients with pulmonary infections are warranted to confirm and explore the importance of intrapulmonary concentrations, pharmacodynamic parameters, and clinical outcomes.
In summary, this report provides safety and tolerability data after the intravenous administration of GSK1322322 at multiple doses of 1,500 mg. Intrapulmonary penetration of GSK1322322 after multiple intravenous doses was extensive, with the AUC0–τ ratios of ELF and AM to unbound plasma being 3.5 and 7.4, respectively. The time course of concentrations in ELF and AM generally followed the plasma concentration-time profile. Results from this study lend support to exploring GSK1322322 as a potential antimicrobial agent for the treatment of lower respiratory tract bacterial infections caused by susceptible pathogens.
ACKNOWLEDGMENTS
O.J.N., L.S.J., J.Z.Z., C.L.B., L.C., and E.D. are employees of GlaxoSmithKline. K.A.R. is a consultant to GlaxoSmithKline.
We thank Bonnie Orr for her technical assistance in performing the ELF procedures.
Footnotes
Published ahead of print 4 November 2013
REFERENCES
- 1.Spellberg B, Bartlett JG, Gilbert DN. 2013. The future of antibiotics and resistance. N. Engl. J. Med. 368:299–302. 10.1056/NEJMp1215093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Livermore DM. 2011. Discovery research: the scientific challenge of finding new antibiotics. J. Antimicrob. Chemother. 66:1941–1944. 10.1093/jac/dkr262 [DOI] [PubMed] [Google Scholar]
- 3.Apfel CM, Locher H, Evers S, Takacs B, Hubschwerlen C, Pirson W, Page MGP, Keck W. 2001. Peptide deformylase as an antibacterial drug target: target validation and resistance development. Antimicrob. Agents Chemother. 45:1058–1064. 10.1128/AAC.45.4.1058-1064.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jain R, Chan D, White RJ, Patel DV, Yuan Z. 2005. Bacterial peptide deformylase inhibitors: a new class of antibacterial agents. Curr. Med. Chem. 12:1607–1621. 10.2174/0929867054367194 [DOI] [PubMed] [Google Scholar]
- 5.Sutcliffe JA. 2011. Antibiotics in development targeting protein synthesis. Ann. N. Y. Acad. Sci. 1241:122–152. 10.1111/j.1749-6632.2011.06323.x [DOI] [PubMed] [Google Scholar]
- 6.Ross JE, Scangarella-Oman NE, Miller LA, Sader HS, Jones RN. 2011. Determination of disk diffusion and MIC quality control ranges for GSK1322322, a novel peptide deformylase inhibitor. J. Clin. Microbiol. 49:3928–3930. 10.1128/JCM.01213-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Dwyer K, Hackel M, Hightower S, Hoban D, Bouchillon S, Qin D, Aubart K, Zalacain M, Butler D. 2013. Comparative analysis of the antibacterial activity of a novel peptide deformylase inhibitor, GSK1322322. Antimicrob. Agents Chemother. 57:2333–2342. 10.1128/AAC.02566-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naderer OJ, Dumont E, Zhu J, Kurtinecz M, Jones LS. 2013. Effect of H2 blockade and food on single-dose pharmacokinetics of GSK1322322, a peptide deformylase inhibitor antibacterial. Antimicrob. Agents Chemother. 57:2556–2561. 10.1128/AAC.02505-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naderer OJ, Dumont E, Zhu J, Kurtinecz M, Jones LS. 2013. Single-dose safety, tolerability, and pharmacokinetics of the antibiotic GSK1322322, a novel peptide deformylase inhibitor. Antimicrob. Agents Chemother. 57:2005–2009. 10.1128/AAC.01779-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naderer OJ, Dumont E, Zhu J, Kurtinecz M, Jones LS. 2013. Safety, tolerability, and pharmacokinetics of repeat dosing of the antibiotic GSK1322322, a peptide deformylase inhibitor: a randomized placebo-controlled study. J. Antimicrob. Chemother. 68:1901–1909. 10.1093/JAC/dkt097 [DOI] [PubMed] [Google Scholar]
- 11.Rodvold KA, George JM, Yoo L. 2011. Penetration of anti-infective agents into pulmonary epithelial lining fluid: focus on antibacterial agents. Clin. Pharmacokinet. 50:637–664. 10.2165/11594090-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 12.Ambrose PG, Bhavnani SM, Ellis-Grosse EJ, Drusano GL. 2010. Pharmacokinetic-pharmacodynamic considerations in the design of hospital-acquired or ventilator-acquired bacterial pneumonia studies: look before you leap! Clin. Infect. Dis. 51(Suppl 1):S103–S110. 10.1086/653057 [DOI] [PubMed] [Google Scholar]
- 13.Shelton MJ, Lovern M, Ng-Cashin J, Jones L, Gould E, Gauvin J, Rodvold KA. 2011. Zanamivir pharmacokinetics and pulmonary penetration into epithelial lining fluid following intravenous or oral inhaled administration to healthy adult subjects. Antimicrob. Agents Chemother. 55:5178–5184. 10.1128/AAC.00703-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rennard SI, Basset G, Lecossier D, O'Donnell KM, Pinkston P, Martin PG, Crystal RG. 1986. Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker of dilution. J. Appl. Physiol. 60:532–538 [DOI] [PubMed] [Google Scholar]
- 15.Baldwin DR, Wise R, Andrews JM, Ashby JP, Honeybourne D. 1990. Azithromycin concentrations at the sites of pulmonary infections. Eur. Respir. J. 3:886–890 [PubMed] [Google Scholar]
- 16.Naderer OJ, Jones LS, Zhu J, Kurtinecz M, Dumont E. 2013. Safety, tolerability, and pharmacokinetics of oral and intravenous administration of GSK1322322, a peptide deformylase inhibitor. J. Clin. Pharmacol. 53:1168–1176. 10.1002/jcph.150 [DOI] [PubMed] [Google Scholar]