Abstract
Vancomycin (VAN) is often used to treat methicillin-resistant Staphylococcus aureus (MRSA) bacteremia despite a high incidence of microbiological failure. Recent in vitro analyses of β-lactams in combination with VAN demonstrated synergistic activity against MRSA. The goal of this study was to examine the impact of combination therapy with VAN and a β-lactam (Combo) on the microbiological eradication of MRSA bacteremia compared to VAN alone. This was a retrospective cohort study of patients with MRSA bacteremia who received Combo therapy or VAN alone. Microbiological eradication of MRSA, defined as a negative blood culture obtained after initiation of therapy, was used to evaluate the efficacy of each regimen. A total of 80 patients were included: 50 patients in the Combo group and 30 patients in the VAN-alone group. Microbiological eradication was achieved in 48 patients (96%) in the Combo group compared to 24 patients (80%) in the VAN-alone group (P = 0.021). In a multivariable model, the Combo treatment had a higher likelihood of achieving microbiological eradication (adjusted odds ratio, 11.24; 95% confidence interval, 1.7 to 144.3; P = 0.01). In patients with infective endocarditis (n = 22), 11/11 (100%) who received Combo therapy achieved microbiological eradication compared to 9/11 (81.8%) treated with VAN alone, but the difference was not statistically significant (P = 0.20). Patients with MRSA bacteremia who received Combo therapy were more likely to experience microbiological eradication of MRSA than patients who received VAN alone.
INTRODUCTION
Methicillin-resistant Staphylococcus aureus (MRSA) bacteremia is associated with increased health care costs, morbidity, and mortality as well as worse treatment outcomes than methicillin-susceptible Staphylococcus aureus (MSSA) bacteremia (1, 2). Moreover, a recent study found that 88% of invasive, nosocomial MRSA infections involved a positive blood culture (3). Vancomycin (VAN) has been the mainstay of MRSA treatment for over 40 years, but concerns regarding the efficacy of VAN against MRSA are mounting (4). VAN has been shown to have slow bactericidal activity, poor antistaphylococcal activity, poor tissue penetration, and high rates of infection relapse (1, 5–10).
Given the widespread use of VAN for treating MRSA infections despite its questionable efficacy, several in vitro studies have explored combination therapy using VAN with a β-lactam (BL) against MRSA. An in vitro pharmacokinetic/pharmacodynamic (PK/PD) model simulating in vivo antibiotic exposure demonstrated that VAN in combination with cefazolin improved antibacterial activity against MRSA and heterogeneous vancomycin intermediate-susceptible Staphylococcus aureus (hVISA) isolates compared to VAN alone (11). Another in vitro pharmacokinetic/pharmacodynamic model by Leonard demonstrated increased bactericidal activity against MRSA and hVISA using a combination of VAN and nafcillin compared to VAN alone (12). Piperacillin-tazobactam in combination with VAN has also demonstrated synergistic activity against MRSA and VISA isolates in time-kill studies (13, 14).
BLs are often empirically added to VAN as Gram-negative coverage for many disease states, including pneumonia and septic shock (15, 16). However, despite extensive clinical use of these regimens, little is known about the impact of BLs on VAN activity against MRSA. While in vitro studies have demonstrated synergy between BLs and VAN against MRSA isolates, studies looking at clinical outcomes of these combinations have not been performed. The objective of this study was to examine the impact of combination therapy with VAN and a β-lactam for ≥24 h on the microbiological eradication of MRSA bacteremia compared to VAN alone.
MATERIALS AND METHODS
Study design, setting, and population.
A retrospective cohort study was conducted at the University of New Mexico Hospital (UNMH), a 646-bed tertiary care academic medical center in Albuquerque, NM. This study was approved by the University of New Mexico Human Research Review Committee. This study conforms to the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) recommendations for reporting cohort studies (17). Patients were eligible for study inclusion if they met the following criteria: (i) they were admitted to UNMH between January 2005 and December 2012; (ii) they were ≥18 years of age at the time of admission; (iii) they had had at least one blood culture positive for MRSA with a VAN MIC of ≤2 mg/liter by the BD Phoenix or Vitek automated microbiological system, and the isolate was available for further microbiological and molecular analysis; and (iv) they received either initial treatment with intravenous VAN or a BL ≥24 h concurrently with intravenous VAN. Patients with multiple MRSA-positive blood cultures during the same hospitalization were included for review once, using their first blood culture as the index culture. Patients were excluded from this study if they (i) received <72 h of VAN treatment, (ii) received daptomycin or linezolid before obtaining a negative blood culture, (iii) received more than one dose of clindamycin, doxycycline, or trimethoprim-sulfamethoxazole before obtaining a negative blood culture, (iv) lacked repeat blood culture(s) after the index culture, or (v) had a negative blood culture the same day therapy was started.
Data collection.
Microbiological data from UNMH's reference laboratory (Tricore Reference Laboratories, Inc.) were used in conjunction with information from the electronic medical record to identify patients who met the inclusion criteria. Data from eligible subjects were retrospectively collected using a systematic data collection form. Patient data included the following: age, gender, race, admission date, discharge date or date of death, and admitting diagnosis. Length of hospital stay (LOS) and intensive care unit (ICU) stay were documented as well as the duration of mechanical ventilation if required. The severity of bacteremia using the Pitt bacteremia score was calculated for all patients. Mortality data during hospitalization, including all-cause and attributable to MRSA bacteremia, were also recorded (18). Patient risk factors for MRSA infection were documented using the following categories: (i) immunosuppression, defined as treatment with >10 mg of prednisone or equivalent per day for >14 days prior to infection, neutropenia, human immunodeficiency virus seropositivity or having received chemotherapy within 45 days prior to infection, and/or the use of immunosuppressive medication(s) other than prednisone; (ii) previous health care exposure, defined by home intravenous antibiotics or infusion clinic attendance 30 days prior to infection, hospital admission for ≥2 days within the past 90 days prior to infection, hemodialysis 30 days prior to infection, or being a resident of a nursing home or long-term-care facility; (iii) injection drug use; (iv) hemodialysis; (v) prior exposure to a β-lactam, fluoroquinolone, or VAN for ≥7 days within 30 days prior to infection; (vi) presence of a central venous catheter, skin ulcers, or cellulitis at the time of infection; (vii) homelessness; and (viii) a history of an MRSA infection or being a known MRSA carrier. The presence of the following patient comorbidities was also recorded: cancer, liver disease, congestive heart failure, chronic lung disease, alcoholism, and diabetes mellitus.
The origins of bacteremia, antibiotic susceptibilities, and dates for all MRSA blood cultures were recorded. Origins of MRSA bacteremia were categorized as follows: infective endocarditis (IE), osteomyelitis, skin and soft tissue infection, and other (comprised of catheter-related bloodstream infection, urinary tract infection, pneumonia, prosthesis, or unknown origin). A removable source of infection at the time of the index culture and whether or not the removable source of infection was retained were also documented. For each antibiotic, the dose, frequency, and duration of therapy were documented, and patients were classified into either the VAN-only group or the group receiving combination therapy with VAN and a BL (Combo). The BLs included ampicillin, nafcillin, amoxicillin/clavulanate, piperacillin-tazobactam, cephalexin, cefazolin, cefoxitin, ceftriaxone, ceftazidime, cefotaxime, cefepime, imipenem, and meropenem, alone or in any combination. Appropriateness of antimicrobial therapy was recorded and defined as the initiation of VAN within the first 48 h of index blood culture collection. Nephrotoxicity was also collected, defined as an increase in serum creatinine from baseline by either 0.5 mg/dl or 50%. VAN serum concentrations were also collected. Repeat blood cultures following the index blood culture were recorded to document microbiological clearance of MRSA bacteremia.
Microbiological outcomes.
Microbiological eradication was defined as a negative blood culture obtained while the patient was receiving VAN or Combo therapy with no relapse of infection, which was defined as the isolation of MRSA from blood within 30 days of completing VAN or Combo therapy. Patients whose last documented blood culture was positive before death or whose antimicrobial therapy was changed from VAN or Combo were categorized as microbiological failures, as were patients who experienced relapse of infection. Microbiological eradication was selected as the outcome of interest to most closely correlate with in vitro studies demonstrating an acute impact on bacterial inoculum using VAN in combination with BLs.
Characterization of MRSA isolates.
Subcultures of all clinical MRSA isolates were collected from the reference laboratory at the time blood cultures were identified and stored at −80°C. Vancomycin MICs were determined using Etest (0.016 to 256 mg/liter) (AB Biodisk, Solna, Sweden) methodology, according to the manufacturer's instructions (19). The δ-hemolysin activity on sheep blood agar plates was determined by β-lysin disk assay and was used as a surrogate marker of accessory gene regulator (agr) operon functionality, as previously described (20). S. aureus strains RN6607 and RN9120 were used as agr-positive and agr-negative controls, respectively. Isolates were incubated for 24 h and read independently by two different investigators who were blinded to microbiological outcomes. Absence of δ-hemolysin expression was interpreted as loss of agr function. The agr group was assessed using a previously described quantitative PCR assay (21). Briefly, individual isolates were cultured in tryptic soy broth for 2 h at 37°C with mixing. Zirconia beads and a BeadBeater-type homogenizer (BioSpec, Bartlesville, OK, USA) were used to extract DNA from bacterial pellets resuspended in 10 mM Tris, pH 8.0, 1 mM EDTA. DNA from each isolate was amplified by quantitative PCR using primers and probes corresponding to variable regions of agrC specific for each agr group (21). DNA from isolates of a known agr group were included with each amplification as internal controls. Pulse-field gel electrophoresis (PFGE) was also performed to determine the USA strain type. Individual MRSA isolates were embedded in agarose and lysed in situ, and genomic DNA was digested with the restriction endonuclease SmaI. The restriction fragments were resolved into a pattern of discrete bands in a 1% SeaKem Gold agarose gel by switching the current direction, starting at 2 s and finishing at 40 s, using a linear ramping factor for a total run time of 16.5 h. The DNA fragment patterns were visualized by ethidium bromide staining and analyzed by a computerized gel imaging software program (GelCompar II, version 3.0) (22).
Statistical analysis.
Continuous variables were summarized using the mean and standard deviation (SD) or the median and interquartile range (IQR). Two-sample t tests or Wilcoxon rank-sum tests were used to compare the two groups. Categorical variables were summarized using frequencies and percentages and compared between the two treatment groups using the likelihood ratio χ2 or Fisher's exact test. Univariable analysis was used to identify variables associated with microbiological eradication. Sparse data and zero cells (i.e., quasi-complete separation) were anticipated for some predictors. Therefore, a logistic regression with Firth's penalized maximum-likelihood bias reduction approach was used to ensure convergence to finite parameter estimates in all univariable analyses (23). Predictor variables associated with the outcome variable at α = 0.25 were included in the multivariable model selection procedure. Immunosuppression was added to the selection procedure due to its biological role in the clearance of bacteremia, even though it did not meet the cutoff value. An all-possible-regressions selection approach based on the Bayesian information criterion was used to select the best-fitting, most parsimonious model. The final model was fit using the above-mentioned Firth's procedure to obtain the adjusted odds ratios (AORs) and the corresponding 95% profile likelihood confidence intervals (CIs). The SAS macro FL was used to obtain P values that correspond to the 95% profile likelihood CIs of the AORs (24). All analyses were performed with SAS, version 9.3 (SAS Institute, Cary, NC, USA).
RESULTS
Study population.
Of the 127 patients with MRSA bacteremia during the study period, 80 were included in the final analysis (Fig. 1). Fifty patients were included in the Combo group, and 30 were included in the VAN group. Patient demographic and clinical characteristics were comparable between the two groups (Table 1). However, the patients in the Combo group had a longer LOS and were more likely to be admitted to the ICU. Risk factors for MRSA bacteremia were similar as were origins of MRSA bacteremia. The majority of patients had IE, osteomyelitis, or skin and soft tissue infections. Nineteen patients (23.8%) had a removable source of infection, and the source of infection was removed in all but four patients (Table 1). Seven patients in the Combo group and six patients in the VAN group had catheter-related bacteremia, and catheter removal was performed for all but one patient in each group. All patients with catheter-related bacteremia had evidence of disseminated infection and were treated accordingly. A removable source of infection was retained in two other patients. One patient in the VAN treatment group had spinal hardware, and one patient in the Combo group had a biliary stent. All four of the patients for whom a removable source of infection was retained experienced microbiological cure. The median Pitt bacteremia score was 1 in both treatment groups, and a similar percentage of patients in each group had a Pitt bacteremia score of ≥4, a score indicating critical illness (19). All patients were treated for severe, invasive MRSA bacteremia with lengthy durations of antimicrobial therapy. Seventy-six patients (95%) received appropriate therapy with VAN within 48 h of the index blood culture. Of the four patients who did not receive VAN within 48 h of the index blood culture, three were in the Combo group, and one was in the VAN-alone group; none of these four patients experienced a microbiological failure. Eighty percent of patients received a consultation from an infectious diseases physician (78% in the Combo group and 83.3% in the VAN group, respectively).
FIG 1.
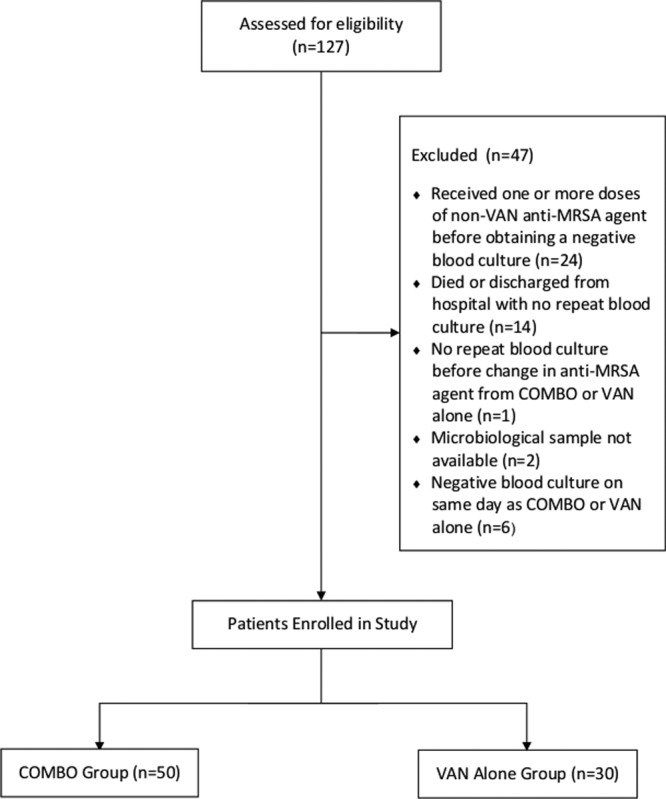
Consolidated standardized reporting of trials (CONSORT) flow diagram of study participants.
TABLE 1.
Demographic and clinical characteristics of the two treatment groupsa
| Variable | Value for the group |
P valueb | |
|---|---|---|---|
| Combo (n = 50) | VAN alone (n = 30) | ||
| Mean age (yr [±SD]) | 51.6 ± 15.0 | 50.5 ± 16.8 | 0.772 |
| Median LOS (days [IQR]) | 20.5 (10.0–41.0) | 12.0 (7.8–28.8) | 0.027c |
| Median VAN therapy duration (days [IQR]) | 23.5 (11.0–34.0) | 30.0 (11.0–44.0) | 0.281c |
| Median VAN serum level (mg/liter [IQR])d | 20.2 (16.2–23.2) | 17.5 (14.1–24.4) | 0.067c |
| No. of male participants (%) | 37 (74.0) | 17 (56.7) | 0.112 |
| Race (no. of patients [%]) | 0.531 | ||
| Caucasian | 18 (36.0) | 14 (46.7) | |
| Hispanic | 19 (38.0) | 8 (26.7) | |
| Othere | 13 (26.0) | 8 (26.7) | |
| Comorbidities (no. of patients [%]) | |||
| Alcoholism | 7 (14.0) | 1 (3.3) | 0.097 |
| Cancer | 11 (22.0) | 4 (13.3) | 0.327 |
| Chronic lung disease | 4 (8.0) | 4 (13.3) | 0.448 |
| Congestive heart failure | 6 (12.0) | 2 (6.7) | 0.429 |
| Diabetes mellitus | 25 (50.0) | 13 (43.3) | 0.563 |
| Liver disease | 6 (12.0) | 6 (20.0) | 0.338 |
| MRSA bacteremia risk factors (no. of patients [%]) | |||
| Cellulitis on admission | 2 (4.0) | 4 (13.3) | 0.190f |
| Central venous catheter | 5 (10.0) | 5 (16.7) | 0.389 |
| Hemodialysis | 6 (12.0) | 6 (20.0) | 0.338 |
| Homelessness | 1 (2.0) | 0 (0.0) | >0.999f |
| Immunosuppression | 9 (18.0) | 3 (10.0) | 0.320 |
| Injection drug use | 9 (18.0) | 7 (23.3) | 0.566 |
| MRSA colonizationg | 10 (20.0) | 8 (26.7) | 0.493 |
| Previous health care exposure | 22 (44.0) | 12 (40.0) | 0.726 |
| Prior antibiotic exposureh | 4 (8.0) | 2 (6.7) | >0.999f |
| Skin ulcers on admission | 9 (18.0) | 1 (3.3) | 0.037 |
| Origin of bacteremia (no. of patients [%]) | |||
| Endocarditis | 11 (22.0) | 11 (36.7) | 0.159 |
| Osteomyelitis | 11 (22.0) | 4 (13.3) | 0.327 |
| Skin and soft tissue infection | 5 (10.0) | 1 (3.3) | 0.402f |
| Otheri | 20 (40.0) | 13 (43.3) | 0.770 |
| More than one source | 3 (6.0) | 1 (3.3) | >0.999f |
| Source control (no. of patients [%]) | |||
| Removable source of infection | 11 (22.0) | 8 (26.7) | 0.637 |
| Removable source of infection retainedj | 2 (4.0) | 2 (6.7) | 0.602 |
| Severity of illness markers | |||
| ICU admission (no. of patients [%]) | 21 (42.0) | 6 (20.0) | 0.039 |
| Ventilator use (no. of patients [%]) | 13 (26.0) | 4 (13.3) | 0.169 |
| Median Pitt bacteremia score (IQR) | 1 (1–3) | 1 (0–2) | 0.238c |
| Pitt bacteremia score of ≥4 (no. of patients [%]) | 9 (18.0) | 4 (13.3) | 0.580 |
LOS, length of stay; IQR, interquartile range; ICU, intensive care unit.
P values were calculated by the likelihood ratio χ2 test, except where otherwise noted.
Wilcoxon rank sum test.
One patient in each treatment group did not have a vancomycin serum level documented in the electronic medical record. Both patients experienced microbiological eradication.
Comprised of African American, Asian American/Pacific Islander and Native American/Alaskan Native, two or more races and unknown/declined to answer.
Fisher's exact test.
History of an MRSA infection or being a known MRSA carrier.
Exposure to a β-lactam, fluoroquinolone, or VAN for ≥7 days within 30 days prior to infection.
Comprised of catheter-related bloodstream infection, urinary tract infection, pneumonia, prosthesis, or bacteremia of unknown origin.
Of the four patients for whom a removable source of infection was retained, none experienced microbiological failure.
At UNMH, initial weight-based VAN dosing is used, followed by individualized, pharmacist-monitored VAN pharmacokinetics based on VAN trough levels to maintain a VAN goal trough of 15 to 20 mg/liter for invasive MRSA infections. No patient received less than 96 h of VAN treatment, and the median duration of VAN therapy was 30 days (interquartile range [IQR], 11 to 44 days) in the VAN-alone group and 24 days (IQR, 11 to 34 days) in the Combo group (P = 0.281). In the Combo group, 34 patients received piperacillin-tazobactam (68%). Additionally, four patients received cefepime (8%), three received ceftriaxone (6%), two patients each (4%) received cefazolin, ampicillin/sulbactam, and meropenem, and one patient each (2%) received cephalexin, ampicillin, and a combination of piperacillin-tazobactam and ceftriaxone. BL doses were consistent with recommended treatment guidelines for Gram-positive and Gram-negative infections. The median duration of BL use was 6 days (IQR, 3 to 9 days). Only one patient in the VAN-alone group and no patients in the Combo group received a dose of clindamycin prior to obtaining a negative blood culture. No patients in either treatment group received doxycycline or trimethoprim-sulfamethoxazole prior to obtaining a negative blood culture. Eight patients in each treatment group received aminoglycoside therapy with gentamicin, and, among these patients, one patient in each treatment group did not achieve microbiological eradication. Additionally, three patients in the VAN group (10%) and six patients in the Combo group (12%) experienced nephrotoxicity.
Characteristics of MRSA isolates.
The microbiological characteristics of the MRSA isolates are shown in Table 2. The median VAN Etest MIC was 2.0 mg/liter (IQR, 1.5 to 2.0 mg/liter) in the Combo group and 1.5 mg/liter (IQR, 1.5 to 2.0 mg/liter) in the VAN-alone group (P = 0.066). The median VAN automated MIC was 1 mg/liter (IQR, 1 to 1 mg/liter) in both groups (P = 0.065). Most of the isolates belonged to agr group I (57.5%; n = 46), followed by agr group II (41.3%; n = 33), and one organism was part of agr group III. The proportion of isolates from agr group I was similar between the Combo and VAN groups (58% and 60%, respectively). Forty percent of isolates in both the Combo and VAN groups were from agr group II. agr functionality was also similar between the two treatment groups, but there was a higher proportion of USA300 strains in the Combo group (64%) than in the VAN-alone group (50%); however, this difference was not statistically significant (P = 0.764).
TABLE 2.
Microbiological characteristics of MRSA isolates
| Parameter | Value for the groupa |
P valueb | |
|---|---|---|---|
| Combo (n = 50) | VAN alone (n = 30) | ||
| Median VAN MIC (mg/liter [IQR])c | 2.0 (1.5–2.0) | 1.5 (1.5–2.0) | 0.066d |
| MRSA straind | 0.213 | ||
| USA100 | 17 (35.4) | 14 (50.0) | |
| USA300 | 31 (64.6) | 14 (50.0) | |
| agr functionalitye | 0.764 | ||
| Functional | 31 (75.6) | 21 (72.4) | |
| Loss of function | 10 (24.4) | 8 (27.6) | |
| agr groupf | 0.943 | ||
| Group I | 29 (59.2) | 18 (60.0) | |
| Group II | 20 (40.8) | 12 (40.0) | |
Except where otherwise noted, data are expressed as number (percentage) of patients.
P values were calculated by the likelihood ratio χ2 test, except where otherwise noted.
Vancomycin Etest MIC. IQR, interquartile range.
Wilcoxon rank sum test.
MRSA strain type and agr were not available for all isolates.
One patient in the Combo group had an isolate from agr group III and was not included in this analysis.
Microbiological outcomes.
Microbiological eradication was achieved in 48 patients (96%) in the Combo group compared to 24 patients (80%) in the VAN group (P = 0.021) (Table 3). Of the two patients who experienced microbiological failure in the Combo group, one received piperacillin-tazobactam, and the other received cephalexin. Only treatment group and hemodialysis were independently associated with microbiological outcome by univariable analysis (Table 4). Adjusted odds ratios for microbiological eradication from the multivariable analysis are shown in Table 5. Patients in the Combo group were 11.24 (95% CI, 1.7 to 144.3 times; P = 0.01) times more likely to achieve microbiological eradication than patients in the VAN-alone group. An inverse relationship was observed between VAN serum level and microbiological eradication (AOR, 0.93; 95% CI, 0.86 to 0.98; P = 0.006). Additionally, hemodialysis patients were almost 24 times less likely to achieve microbiological eradication than nonhemodialysis patients (AOR, 0.042; 95% CI, 0.01 to 0.25; P < 0.001). In the subset of patients who had IE (n = 22), 11/11 patients (100%) who received Combo treatment achieved microbiological eradication, compared to 9/11 patients (81.8%) treated with VAN alone. The odds ratio for microbiological eradication among patients with IE who received the Combo treatment was 6.05 (95% CI, 0.42 to 875.58; P = 0.20). The all-cause mortality was 16.3% (n = 13). The overall MRSA-attributable mortality rate was 10%. Four patients in each group expired due to MRSA bacteremia (8% in the Combo group and 13.3% in the VAN group; P = 0.448).
TABLE 3.
Microbiological eradication in patients with MRSA bacteremia
| Patient group | Eradication frequency by treatment groupa |
P value | |
|---|---|---|---|
| Combo | VAN alone | ||
| All patients | 48/50 (96.0) | 24/30 (80.0) | 0.021 |
| Patients with IE | 11/11 (100.0) | 9/11 (81.8) | 0.200 |
Data are expressed as numbers of patients with eradication of MRSA bacteremia/total number of patients (percentage). One patient in each group experienced a relapse of MRSA to any site. Neither patient who experienced MRSA infection relapse had IE.
TABLE 4.
Univariable analysis of the association between potential predictor variables and microbiological eradication in patients with MRSA bacteremia
| Variableb | Value for the variablea |
||
|---|---|---|---|
| OR | 95% CI | P value | |
| Treatment group (Combo vs VAN alone) | 5.15 | 1.21–29.7 | 0.026 |
| Age (yr) | 0.99 | 0.95–1.04 | 0.719 |
| LOS (days) | 1.04 | 0.99–1.14 | 0.101 |
| Vancomycin serum level (mg/liter)c | 0.97 | 0.93–1.01 | 0.136 |
| Cancer | 1.24 | 0.24–12.41 | 0.816 |
| Hemodialysis | 0.07 | 0.01–0.33 | <0.001 |
| Immunosuppression | 3.51 | 0.39–465.03 | 0.317 |
| Injection drug use | 4.96 | 0.56–654.41 | 0.178 |
| MRSA colonization | 0.76 | 0.17–4.44 | 0.733 |
| Endocarditis | 1.02 | 0.24–5.88 | 0.985 |
| Osteomyelitis | 4.58 | 0.52–604.59 | 0.206 |
| ICU admission | 0.79 | 0.19–3.67 | 0.753 |
| Ventilator use | 1.46 | 0.29–14.56 | 0.676 |
| Pitt bacteremia score of ≥4 | 1.07 | 0.82–1.69 | 0.653 |
| MRSA strain (USA300 vs USA100) | 2.52 | 0.62–11.67 | 0.197 |
| agr functionality | 1.08 | 0.18–4.77 | 0.920 |
The odds ratio (OR) and 95% confidence interval were derived using Firth's penalized maximum likelihood bias reduction approach to logistic regression.
LOS, length of stay; ICU, intensive care unit.
One patient in each treatment group did not have a vancomycin serum level documented in the electronic medical record. Both patients experienced microbiological eradication.
TABLE 5.
Multivariable analysis of the association between potential predictor variables and microbiological eradication in patients with MRSA bacteremia
| Variable | Value for the variablea |
||
|---|---|---|---|
| AOR | 95% CI | P value | |
| Treatment group (Combo vs VAN alone) | 11.24 | 1.72–144.34 | 0.010 |
| Vancomycin serum level (mg/liter)b | 0.93 | 0.86–0.98 | 0.006 |
| Hemodialysis | 0.042 | 0.01–0.25 | <0.001 |
The adjusted odds ratio (AOR) and 95% confidence interval were derived using Firth's penalized maximum-likelihood bias reduction approach to logistic regression.
One patient in each treatment group did not have a vancomycin serum level documented in the electronic medical record. Both patients experienced microbiological eradication.
DISCUSSION
This study describes the microbiological impact of adding a BL to VAN in treating patients with MRSA bacteremia. Patients treated with Combo therapy were more likely to obtain a negative blood culture than patients treated with VAN alone. This relationship persisted after data were adjusted for other predictors associated with microbiological eradication and was independent of the BL dose or duration. Given the low number of microbiological failures in the Combo group, a formal statistical analysis stratified by different BL agents could not be performed. Combo therapy may also lead to faster microbiological eradication of MRSA bacteremia. Nafcillin with VAN demonstrated bactericidal activity against MRSA after only 6.3 h of therapy in an in vitro pharmacokinetic/pharmacodynamic model, whereas VAN alone did not demonstrate bactericidal activity (12). However, in our study the exact time to microbiological eradication could not be determined accurately since obtaining daily blood cultures is not common practice.
The higher rate of microbiological eradication in the Combo group substantiates existing in vitro data that has demonstrated a synergistic effect between VAN and BLs against MRSA (11–14). In the current study, combining BLs with no activity against MRSA with VAN improved the rate of microbiological eradication of MRSA bacteremia compared to treatment with VAN alone. A similar example is the use of ceftriaxone with ampicillin for Enterococcus faecalis IE, despite ceftriaxone's lack of activity against Enterococcus. (25) Our results show an unrecognized, beneficial effect of combination therapy, especially combinations often used together like piperacillin-tazobactam and VAN. This finding also suggests that combination antibiotic therapy against MRSA may be a method to enhance the activity of existing anti-MRSA agents, such as VAN, rather than waiting for novel anti-MRSA agents to be developed. Although combination therapy is effective for certain infections, such as human immunodeficiency virus and Mycobacterium tuberculosis infections, the use of combination therapy has not translated to treatment of MRSA infections (26, 27). This was done to decrease the potential for unwanted antibacterial resistance and to prevent unwanted side effects of broad-spectrum antibiotic therapy. Now, the treatment paradigm may need to change for patients with recurrent or difficult to treat MRSA infections as alternative options are limited.
Patients were not started on Combo therapy for MRSA infections but, rather, for potential polymicrobial infections. Patients in the Combo group were more likely to be in the ICU and had a longer LOS. This is not unexpected as combination antibiotic therapy targeting Gram-positive and Gram-negative organisms is commonly used to treat patients in the ICU (15). The VAN serum concentrations were higher in the Combo group as well, which is likely a reflection of more aggressive VAN dosing used in the ICU. In multivariable analysis, VAN serum level was inversely related with microbiological eradication, likely a result of VAN discontinuation due to supratherapeutic levels. It should be noted that the median VAN serum concentration for each group was therapeutic (>15 mg/liter) based on current MRSA bacteremia treatment guidelines (4). Two recent studies have demonstrated increased rates of acute kidney injury among patients who received piperacillin-tazobactam with VAN compared to VAN alone (28, 29). However, rates of nephrotoxicity were similar between the Combo and VAN groups even though the majority of patients in the Combo group received piperacillin-tazobactam.
Origins of MRSA bacteremia and the proportion of isolates from agr group II, which contribute substantially to the duration of MRSA bacteremia, were also similar between the two treatment groups (30, 31). Multivariable analysis also showed that hemodialysis patients were less likely to experience microbiological eradication, which is consistent with a previous study of persistent MRSA bacteremia (32). MRSA IE is also associated with prolonged bacteremia as well as with high rates of clinical failures (33–35). VAN use is associated with slow treatment response and high failure rates when it is used to treat MRSA IE (31, 36). Yet in the current study, no patients with IE in the Combo group experienced a microbiological failure. While this is a subgroup analysis, these results are promising, given that IE has been shown to be a predictor of VAN failure (36). Our results are consistent with a recent study by Moise et al. in which the addition of a BL to daptomycin against S. aureus bacteremia improved clinical outcomes compared to daptomycin alone (37). The overall inpatient, MRSA-attributable mortality in our study was 10%. This is lower than many previous studies of MRSA bacteremia, including a recent study by Brown and colleagues which found an attributable-mortality rate due to MRSA bacteremia of 16% (38). The lower attributable mortality rate in our study likely resulted from several factors, including the high proportion of patients receiving early and appropriate therapy, the low VAN MICs of the organisms, efforts to eliminate removable sources of infection, and having a high rate of consultation with an infectious diseases physician. Consultation with an infectious diseases physician has been associated with a lower risk of death from MRSA bacteremia (39). Moreover, it should be noted that the MRSA-attributable mortality rate was lower in the Combo group than in the VAN-alone group.
Among the few in vitro studies examining combination therapy against MRSA, none has elucidated a mechanism to explain why these combinations are effective. It is possible that BLs induce an alteration in the MRSA cell wall which allows for improved VAN binding. Against a vancomycin-intermediate, daptomycin-resistant strain of S. aureus, oxacillin was shown to alter the cell wall surface charge to allow for increased daptomycin binding (40). Moreover, despite elevated VAN and daptomycin MICs, the isolate did not demonstrate an increased susceptibility to oxacillin. Increased susceptibility to BLs among S. aureus isolates with elevated VAN and daptomycin MICs is known as the “seesaw effect” and has been demonstrated among daptomycin-resistant strains of S. aureus using in vitro time-kill studies (41). VAN-Bodipy binding was reduced in time-kill studies, demonstrating synergy between BLs and VAN against VISA (42). As such, synergy between VAN and BLs against S. aureus may not be a result of increased VAN binding but, rather, may result from either enhanced VAN interactions with cell wall precursors, as suggested by Werth and colleagues, or an enhancement of BL activity (42). Inhibition of peptidoglycan synthesis by VAN against MRSA has been shown to decrease methicillin resistance (43). We have previously demonstrated that the MIC90 for piperacillin-tazobactam against 20 MRSA isolates decreased from 96 mg/liter to 2 mg/liter in the presence of subinhibitory concentrations of VAN (14). Additionally, using time-kill studies with piperacillin-tazobactam or oxacillin in combination with VAN, we demonstrated increased antibacterial activity of the Combo therapy compared to any of these agents alone (14). We have also demonstrated increased antibacterial activity of piperacillin-tazobactam in combination with VAN against MRSA and VISA compared to either agent alone using a 72-h in vitro PK/PD model (44). In the current study, patients with MRSA bacteremia who received Combo therapy were more likely to experience microbiological eradication of MRSA than patients who received VAN alone even though there is no currently known mechanism to explain this effect. Future studies should examine the impact Combo therapy against MRSA in a prospective manner. Additional in vitro research is also needed to elucidate how BLs and VAN interact with the MRSA cell wall.
Limitations of the current study must be acknowledged. Although this is the first study to report microbiological outcomes associated with VAN in combination with BLs in patients with MRSA bacteremia, this study was a retrospective analysis performed at a single center. Additionally, the majority of MRSA isolates at UNMH have Etest MICs of ≤2 mg/liter with limited clonality. However, many institutions in the United States have isolates with similar MRSA susceptibilities to VAN (45). We did consider examining clinical outcomes. But the treatment durations varied depending on the origin of the MRSA bacteremia, and we hypothesized that the effect of the Combo treatment would be more pronounced in the acute phase of infection based on results from in vitro PK/PD studies evaluating various β-lactams in combination with VAN (11, 12). Additionally, some patients were discharged from the hospital without follow-up being documented in the electronic medical record; thus, assessment of clinical outcomes beyond hospital discharge was not possible for all patients. Because of these factors, we chose to examine microbiological eradication as our primary outcome. In conclusion, our results suggest that combination therapy with VAN and a BL is more likely to achieve microbiological eradication among patients with MRSA bacteremia than treatment with VAN alone. Combination therapy could help preserve the use of VAN as a treatment option for MRSA bacteremia, and its impact on clinical outcomes should be explored further.
ACKNOWLEDGMENTS
We thank Martin Tuan Tran for his preliminary exploration of this data set. We also thank Tricore Reference Laboratories, Inc. (Albuquerque, NM), for the provision of MRSA bloodstream isolates.
C.W. has provided lectures for Cubist Pharmaceuticals. T.J.D., O.I., P.H., J.S., and R.-C.M. have no conflicts of interest to declare. P.H. is supported by NIH grant AI090917.
Footnotes
Published ahead of print 21 October 2013
REFERENCES
- 1.Chu VH, Crosslin DR, Friedman JY, Reed SD, Cabell CH, Griffiths RI, Masselink LE, Kaye KS, Corey GR, Reller LB, Stryjewski ME, Schulman KA, Fowler VG., Jr 2005. Staphylococcus aureus bacteremia in patients with prosthetic devices: costs and outcomes. Am. J. Med. 118:1416. 10.1016/j.amjmed.2005.06.011 [DOI] [PubMed] [Google Scholar]
- 2.Cosgrove E, Sakoulas G, Perencevich EN, Schwaber MJ, Karchmer AW, Carmeli Y. 2003. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clin. Infect. Dis. 36:53–59. 10.1086/345476 [DOI] [PubMed] [Google Scholar]
- 3.Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, Harrison LH, Lynfield R, Dumyati R, Townes JM. 2007. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 298:1763–1771. 10.1001/jama.298.15.1763 [DOI] [PubMed] [Google Scholar]
- 4.Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE. 2011. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin. Infect. Dis. 52:e18–e55. 10.1093/cid/ciq146 [DOI] [PubMed] [Google Scholar]
- 5.Kim SH, Kim KH, Kim HB, Kim NJ, Kim EC, Oh M, Choe KW. 2008. Outcome of vancomycin treatment in patients with methicillin-susceptible Staphylococcus aureus bacteremia. Antimicrob. Agents Chemother. 52:192–197. 10.1128/AAC.00700-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stryjewski ME, Szczech LA, Benjamin DK, Inrig JK, Kanafani ZA, Engemann JJ, Chu VH, Joyce MJ, Reller LB, Corey GR. 2007. Use of vancomycin or first-generation cephalosporins for the treatment of hemodialysis-dependent patients with methicillin-susceptible Staphylococcus aureus bacteremia. Clin. Infect. Dis. 44:190–196. 10.1086/510386 [DOI] [PubMed] [Google Scholar]
- 7.Boucher HW, Sakoulas G. 2007. Perspectives on daptomycin resistance, with emphasis on resistance in Staphylococcus aureus. Clin. Infect. Dis. 45:601–608. 10.1086/520655 [DOI] [PubMed] [Google Scholar]
- 8.Welsh KJ, Skrobarcek KA, Abbott AN, Lewis CT, Kruzel MC, Lewis EM, Gardiner JM, Mohr JF, Armitige LY, Wanger A. 2011. Predictors of relapse of methicillin-resistant Staphylococcus aureus bacteremia after treatment with vancomycin. J. Clin. Microbiol. 49:3669–3672. 10.1128/JCM.05287-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kollef MH. 2007. Limitations of vancomycin in the management of resistant staphylococcal infections. Clin. Infect. Dis. 45:S191–S195. 10.1086/519470 [DOI] [PubMed] [Google Scholar]
- 10.Sakoulas G, Moise-Broder PA, Schentag J, Forrest A, Moellering RC, Jr, Eliopoulos GM. 2004. Relationship of MIC and bactericidal activity to efficacy of vancomycin for treatment of methicillin-resistant Staphylococcus aureus bacteremia. J. Clin. Microbiol. 42:2398–2402. 10.1128/JCM.42.6.2398-2402.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hagihara M, Wiskirchen DE, Kuti JL, Nicolau DP. 2012. In vitro pharmacodynamics of vancomycin and cefazolin alone and in combination against methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 56:202–207. 10.1128/AAC.05473-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leonard SN. 2012. Synergy between vancomycin and nafcillin against Staphylococcus aureus in an in vitro pharmacokinetic/pharmacodynamic Model. PLoS One 7:e42103. 10.1371/journal.pone.0042103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palmer SM, Rybak MJ. 1997. An evaluation of the bactericidal activity of ampicillin/sulbactam, piperacillin/tazobactam, imipenem or nafcillin alone and in combination with vancomycin against methicillin-resistant Staphylococcus aureus (MRSA) in time-kill curves with infected fibrin clots. J. Antimicrob. Chemother. 39:515–518. 10.1093/jac/39.4.515 [DOI] [PubMed] [Google Scholar]
- 14.Dilworth TJ, Sliwinski-Heath J, Ryan K, Dodd M, Mercier RC.Abstr. 52nd Intersci. Conf. Antimicrob. Agents Chemother., abstr E-1472.2012. [Google Scholar]
- 15.Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, Reinhart K, Angus DC, Brun-Buisson C, Beale R. 2008. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Intensive Care Med. 34:17–60. 10.1007/s00134-007-0934-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niederman M, Craven D, Bonten M, Chastre J, Craig W, Fagon J, Hall J, Jacoby G, Kollef M, Luna C. 2005. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am. J. Respir. Crit. Care Med. 171:388–416. 10.1164/rccm.200405-644ST [DOI] [PubMed] [Google Scholar]
- 17.Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. 2007. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Prev. Med. 45:247–251. 10.1016/j.ypmed.2007.08.012 [DOI] [PubMed] [Google Scholar]
- 18.Chow JW, Yu VL. 1999. Combination antibiotic therapy versus monotherapy for gram-negative bacteraemia: a commentary. Int. J. Antimicrob. Agents. 11:7–12. 10.1016/S0924-8579(98)00060-0 [DOI] [PubMed] [Google Scholar]
- 19.BioMerieux 2009. Etest for MIC determination (package insert). BioMerieux, Inc., Solna, Sweden [Google Scholar]
- 20.Sakoulas G, Eliopoulos GM, Moellering RC, Wennersten C, Venkataraman L, Novick RP, Gold HS. 2002. Accessory gene regulator (agr) locus in geographically diverse Staphylococcus aureus isolates with reduced susceptibility to vancomycin. Antimicrob. Agents Chemother. 46:1492–1502. 10.1128/AAC.46.5.1492-1502.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Francois P, Koessler T, Huyghe A, Harbarth S, Bento S, Lew D, Etienne J, Pittet D, Schrenzel J. 2006. Rapid Staphylococcus aureus agr type determination by a novel multiplex real-time quantitative PCR assay J. Clin. Microbiol. 44:1892–1895. 10.1128/JCM.44.5.1892-1895.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McDougal LK, Steward CD, Killgore GE, Chaitram JM, McAllister SK, Tenover FC. 2003. Pulsed-field gel electrophoresis typing of oxacillin-resistant Staphylococcus aureus isolates from the United States: establishing a national database. J. Clin. Microbiol. 41:5113–5120. 10.1128/JCM.41.11.5113-5120.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Firth D. 1993. Bias reduction of maximum likelihood estimates. Biometrika 80:27–38. 10.1093/biomet/80.1.27 [DOI] [Google Scholar]
- 24.Heinze G, Schemper M. 2002. A solution to the problem of separation in logistic regression. Stat. Med. 21:2409–2419. 10.1002/sim.1047 [DOI] [PubMed] [Google Scholar]
- 25.Fernández-Hidalgo N, Almirante B, Gavaldà J, Gurgui M, Peña C, de Alarcón A, Ruiz J, Vilacosta I, Montejo M, Vallejo N. 2013. Ampicillin plus ceftriaxone is as effective as ampicillin plus gentamicin for treating Enterococcus faecalis infective endocarditis. Clin. Infect. Dis. 56:1261–1268. 10.1093/cid/cit052 [DOI] [PubMed] [Google Scholar]
- 26.Stanley SK, Kaplan JE, Fauci A, Gordin F, Bartlett J, Goosby E, Harrington DM, Smith M, Chang S, Anderson J. 1998. Guidelines for the use of antiretroviral agents in HIV-infected adults and adolescents. MMWR Recommend. Rep. 47(RR5):43–82 [Google Scholar]
- 27.Blumberg HM, Leonard MK, Jr, Jasmer RM. 2005. Update on the treatment of tuberculosis and latent tuberculosis infection. JAMA 293:2776–2784. 10.1001/jama.293.22.2776 [DOI] [PubMed] [Google Scholar]
- 28.Hellwig T, Hammerquist R, Loecker B, Shields J. 2011. 301: Retrospective evaluation of the incidence of vancomycin and/or piperacillin-tazobactam induced acute renal failure. Crit. Care Med. 39(Suppl 12):79. [Google Scholar]
- 29.Min E, Box K, Lane J, Sanchez J, Coimbra R, Doucet J, Potenza B, Wargel L. 2011. 714: Acute kidney injury in patients receiving concomitant vancomycin and piperacillin/tazobactam. Crit. Care Med. 39(Suppl 12):20021178536 [Google Scholar]
- 30.Lowy FD. 1998. Staphylococcus aureus infections. N. Engl. J. Med. 339:520–532. 10.1056/NEJM199808203390806 [DOI] [PubMed] [Google Scholar]
- 31.Moise PA, Forrest A, Bayer AS, Yan Q, Xiong YQ, Yeaman MR, George Sakoulas G. 2010. Factors influencing time to vancomycin-induced clearance of nonendocarditis methicillin-resistant Staphylococcus aureus bacteremia: role of platelet microbicidal protein killing and agr genotypes J. Infect. Dis. 201:233–240. 10.1086/649429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hawkins C, Huang J, Jin N, Noskin GA, Zembower TR, Bolon M. 2007. Persistent Staphylococcus aureus bacteremia: an analysis of risk factors and outcomes. Arch. Intern. Med. 167:1861. 10.1001/archinte.167.17.1861 [DOI] [PubMed] [Google Scholar]
- 33.Levine DP, Fromm BS, Reddy BR. 1991. Slow response to vancomycin or vancomycin plus rifampin in methicillin-resistant Staphylococcus aureus endocarditis. Ann. Intern. Med. 115:674. 10.7326/0003-4819-115-9-674 [DOI] [PubMed] [Google Scholar]
- 34.Markowitz N, Quinn EL, Saravolatz LD. 1992. Trimethoprim-sulfamethoxazole compared with vancomycin for the treatment of Staphylococcus aureus infection. Ann. Intern. Med. 117:390. 10.7326/0003-4819-117-5-390 [DOI] [PubMed] [Google Scholar]
- 35.Roder BL, Wandall DA, Frimodt-Moller N, Espersen F, Skinhoj P, Rosdahl VT. 1999. Clinical features of Staphylococcus aureus endocarditis: a 10-year experience in Denmark. Arch. Intern. Med. 159:462. 10.1001/archinte.159.5.462 [DOI] [PubMed] [Google Scholar]
- 36.Walraven CJ, North MS, Marr-Lyon L, Deming P, Sakoulas G, Mercier RC. 2011. Site of infection rather than vancomycin MIC predicts vancomycin treatment failure in methicillin-resistant Staphylococcus aureus bacteraemia. J. Antimicrob. Chemother. 66:2386–2392. 10.1093/jac/dkr301 [DOI] [PubMed] [Google Scholar]
- 37.Moise PA, Amodio-Groton M, Rashid M, Lamp KC, Hoffman-Roberts HL, Sakoulas G, Yoon MJ, Schweitzer S, Rastogi A. 2013. Multicenter evaluation of the clinical outcomes of daptomycin with and without concomitant β-lactams in patients with Staphylococcus aureus bacteremia and mild to moderate renal impairment: a multicenter evaluation. Antimicrob. Agents Chemother. 57:1192–1200. 10.1128/AAC.02192-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brown J, Brown K, Forrest A. 2012. Vancomycin AUC24/MIC ratio in patients with complicated bacteremia and infective endocarditis due to methicillin-resistant Staphylococcus aureus and its association with attributable mortality during hospitalization. Antimicrob. Agents Chemother. 56:634–638. 10.1128/AAC.05609-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pastagia M, Kleinman LC, Lacerda de la Cruz EC, Jenkins SG. 2012. Predicting risk for death from MRSA bacteremia. Emerg. Infect. Dis. 18:1072–1080. 10.3201/eid1807.101371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dhand A, Bayer AS, Pogliano J, Yang SJ, Bolaris M, Nizet V, Wang G, Sakoulas G. 2011. Use of antistaphylococcal β-Lactams to increase daptomycin activity in eradicating persistent bacteremia due to methicillin-resistant Staphylococcus aureus: role of enhanced daptomycin binding. Clin. Infect. Dis. 53:158–163. 10.1093/cid/cir340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang SJ, Xiong YQ, Boyle-Vavra S, Daum R, Jones T, Bayer AS. 2010. Daptomycin-oxacillin combinations in treatment of experimental endocarditis caused by daptomycin-nonsusceptible strains of methicillin-resistant Staphylococcus aureus with evolving oxacillin susceptibility (the “seesaw effect”). Antimicrob. Agents Chemother. 54:3161–3169. 10.1128/AAC.00487-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Werth BJ, Vidaillac C, Murray KP, Newton KL, Sakoulas G, Nonejuie P, Pogliano J, Rybak MJ. 2013. Novel combinations of vancomycin plus ceftaroline or oxacillin against methicillin-resistant vancomycin-intermediate Staphylococcus aureus (VISA) and heterogeneous VISA. Antimicrob. Agents Chemother. 57:2376–2379. 10.1128/AAC.02354-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sieradzki K, Tomasz A. 1997. Suppression of beta-lactam antibiotic resistance in a methicillin-resistant Staphylococcus aureus through synergic action of early cell wall inhibitors and some other antibiotics. J. Antimicrob. Chemother. 39:47–51. 10.1093/jac/39.suppl_1.47 [DOI] [PubMed] [Google Scholar]
- 44.Dilworth TJ, Leonard S, Mercier RC.Abstr. 53rd Intersci. Conf. Antimicrob. Agents Chemother., abstr A-023.2013. [Google Scholar]
- 45.Richter SS, Satola SW, Crispell EK, Heilmann KP, Dohrn CL, Riahi F, Costello AJ, Diekema DJ, Doern GV. 2011. Detection of Staphylococcus aureus isolates with heterogeneous intermediate-level resistance to vancomycin in the United States. J. Clin. Microbiol. 49:4203–4207. 10.1128/JCM.01152-11 [DOI] [PMC free article] [PubMed] [Google Scholar]


