Abstract
The distribution of metronidazole in the central nervous system has only been described based on cerebrospinal fluid data. However, extracellular fluid (ECF) concentrations may better predict its antimicrobial effect and/or side effects. We sought to explore by microdialysis brain ECF metronidazole distribution in patients with acute brain injury. Four brain-injured patients monitored by cerebral microdialysis received 500 mg of metronidazole over 0.5 h every 8 h. Brain dialysates and blood samples were collected at steady state over 8 h. Probe recoveries were evaluated by in vivo retrodialysis in each patient for metronidazole. Metronidazole and OH-metronidazole were assayed by high-pressure liquid chromatography, and a noncompartmental pharmacokinetic analysis was performed. Probe recovery was equal to 78.8% ± 1.3% for metronidazole in patients. Unbound brain metronidazole concentration-time curves were delayed compared to unbound plasma concentration-time curves but with a mean metronidazole unbound brain/plasma AUC0–τ ratio equal to 102% ± 19% (ranging from 87 to 124%). The unbound plasma concentration-time profiles for OH-metronidazole were flat, with mean average steady-state concentrations equal to 4.0 ± 0.7 μg ml−1. This microdialysis study describes the steady-state brain distribution of metronidazole in patients and confirms its extensive distribution.
INTRODUCTION
Metronidazole, a nitroimidazole antibiotic, is useful for treating infections by Bacteroides spp. and many anaerobic bacteria. Since metronidazole is supposed to penetrate extensively into central nervous system (CNS), it has been described in literature as being responsible for both peripheral (1) and central (2–6) neurotoxicity, especially after a prolonged use of metronidazole (7). In both cases, symptoms and lesions on magnetic resonance imaging may spontaneously regress after discontinuation of treatment. While the pathogenesis as yet remains unclear, the most likely hypothesis is an axonal swelling due to metronidazole-induced vasogenic edema, which could be linked to an impairment of vitamin B1 action, because of metronidazole conversion to a thiamine analog (8). Thus, characterizing the distribution of metronidazole in cerebral tissue may contribute to managing the dosing regimen in order to prevent side effects while preserving maximal antibacterial efficiency.
Differences in anatomy, enzymatic activity or bulk-flow exist between blood-brain-barrier (BBB) and blood-cerebrospinal fluid (CSF) barrier (9), which could result in differences in drug distribution between the CSF and brain extracellular fluid (ECF). Current metronidazole doses rely on a few studies that show an extensive distribution of metronidazole into the CSF (10–12). However, most of the previous CSF pharmacokinetic studies of metronidazole used nonspecific microbiological assays that cannot distinguish parent drug from metabolites (13–16) and at present no study has explored the distribution of metronidazole in the brain ECF.
Intracerebral microdialysis is the state-of-the-art in vivo technique allowing ECF sampling to study the distribution of exogenous compounds, such as antibiotics, in the brain (17). The main interest of this technique is to continuously measure brain unbound concentrations as a function of time, thus providing information on drug transport across the BBB (17, 18), information that may be in assessing desirable activity and/or neurotoxicity in the CNS. However, one of the crucial issues in the use of quantitative microdialysis for estimating accurate unbound drug concentrations is the determination of probe in vivo recovery, which may not be easy in a clinical setting (18). The main goal of the present study was to explore the cerebral ECF distribution of metronidazole in patients with acute brain injury by comparing unbound concentrations in brain and plasma.
MATERIALS AND METHODS
Patients.
This study was performed in the neurointensive care unit at the University Hospital of Poitiers (France) and was approved by the local ethics committee (CPP OUEST III, protocol 2008-003311-12). Written informed consent was obtained from a legal representative of the four patients enrolled. The patients (four men), aged 52 to 65 years, were brain injured, sedated with midazolam and fentanyl, and mechanically ventilated. The demographic characteristics are detailed in Table 1. All received metronidazole (B Braun, Boulogne-Billancourt, France) and cefotaxime (Panpharma, Fougères, France) for the clinical management of a lung infection at respective dosing regimens of 500 mg and 2 g three times per day. Routine monitoring for acute brain injury included brain-specific monitoring of the intracranial pressure (Micro-Sensor ICP monitoring system; Codman & Shurtleff, Inc., Raynham, MA), measurement of the partial pressure of oxygen in brain tissue (PbO2) measurement (Licox; Integra Neurosciences, Lyon, France), and cerebral microdialysis (CMA-70, polyamide membrane, 20 kDa, μdialysis; Advanced Medical Products, France) for lactate, pyruvate, and glucose concentration determinations.
TABLE 1.
Patient demographic characteristics (n = 4)
| Parameter | Patient |
|||
|---|---|---|---|---|
| P1 | P2 | P3 | P4 | |
| Age (yrs) | 55 | 64 | 52 | 65 |
| Ht (cm) | 180 | 172 | 175 | 172 |
| Sex | M | M | M | M |
| Wt (kg) | 90 | 90 | 77 | 79 |
| Creatinine clearance (ml min−1)a | 171 | 118 | 86 | 144 |
| Serum albumin (g liter−1) | 22 | NAc | 31 | 42 |
| Serum total proteins (g liter−1) | 66 | 61 | 64 | 67 |
| Admission typeb | TBI | TBI | SAH | TBI |
| No. of previous administrations | 17 | 6 | 6 | 8 |
Calculated by using the MDRD (modification in diet of renal disease) equation.
TBI, trauma brain injury; SAH, subarachnoid hemorrhage.
NA, not available.
Microdialysis probe implantation.
Microdialysis probe implantation and equilibration at a flow rate of 0.3 μl min−1 were performed as previously described (17, 19).
Drug administration and sampling.
The metronidazole brain pharmacokinetic study was conducted at steady state between days 3 and 6, corresponding to 6 to 17 metronidazole administrations. After collection of baseline dialysates and blood samples, 500 mg of metronidazole was infused over 0.5 h. Brain dialysates were collected over the 8-h period at 0.5-h intervals during the first 3 h and at 1-h intervals for the remainder of the experiment. Eight to 13 blood samples were collected over the dosing interval. Blood samples were centrifuged at 2,000 × g for 15 min at 4°C, and plasma was collected to determine the total metronidazole and OH-metronidazole concentrations. Two extra plasma samples were collected, one early and one later postdosing, to determine unbound concentrations of both molecules by ultrafiltration at 2,500 × g for 30 min at 4°C (Centrifree; Millipore, Billerica, MA). Immediately after collection, dialysates, ultrafiltrates (UF), and plasma samples were kept at −80°C until analysis.
In vivo recovery calculation of metronidazole concentrations.
For each patient, in vivo probe recovery was determined using a “retrodialysis-by-drug” method over 2.5 h at the end of the experiment, as previously described (19). The next metronidazole injection was delayed in order to perform recovery estimation. Briefly, microdialysis probe was perfused with a 50-μg ml−1solution of metronidazole in CNS perfusion fluid and, after a 1-h equilibration period, three 0.5-h interval dialysates were collected. The in vivo relative recovery by loss was calculated for each dialysate collected, and the mean value was used to correct the dialysate concentrations (19). The metronidazole residual unbound concentration in brain was taken into consideration to estimate recovery, as previously described (20). Briefly, in vivo recovery was calculated for each interval as presented in the following equation: in vivo recovery = [(Cin + Cext) − Cout]/Cin, where Cin and Cout are the perfusate and dialysate metronidazole concentrations and Cext is the extrapolated concentration of metronidazole in the dialysate at the midpoint of the retrodialysis interval of collection. We observed that Cext was always relatively low (3 to 15%) compared to Cin.
Metronidazole and OH-metronidazole assay.
Metronidazole and OH-metronidazole concentrations were determined by high-pressure liquid chromatography with UV detection. The chromatographic system consisted of an Xterra C18 column (150 by 3.8 mm [inner diameter]; Waters, France), a Hitachi L-2130 pump (VWR, Fontenay sous Bois, France), and a Hitachi L-2200 autosampler (VWR) connected to a UV detector (SPD 10A; Schimadzu, Marne la Vallée, France) at 310 nm. The data were recorded and analyzed on EZ Chrom-Integrator (VWR). The mobile phase consisted of a solution of 0.01 M KH2PO4 mixed with acetonitrile (86/14 [vol/vol]) at a flow rate of 0.4 ml/min. Brain dialysates and UF samples were injected directly after dilution with an internal standard solution of dimetridazole (0.5 ng ml−1). For both metronidazole and OH-metronidazole, eight calibration standards using concentrations between 0.25 and 20 μg ml−1 were performed. The dialysate and UF metronidazole and OH-metronidazole intra- and interday variabilities were respectively characterized at four (0.25, 0.5, 2, and 20 μg ml−1) and three (0.5, 2, and 15 μg ml−1) concentrations, respectively, and were always <15%. Plasma samples (100 μl) were treated by the addition of 200 μl of acetonitrile containing internal standard (2.5 μg ml−1) for deproteinization. In plasma, seven calibration standards were prepared using concentrations between 0.5 and 40 μg ml−1. The plasma intra- and interday variabilities were characterized at four (1, 2, 10, and 40 μg ml−1) and three (1, 5, and 30 μg ml−1) concentrations, respectively, and were always <15%.
Pharmacokinetic analysis.
In each patient, two individual unbound fraction values (fu) of metronidazole and OH-metronidazole were calculated as the ratio of metronidazole or OH-metronidazole concentrations in UF to corresponding total plasma concentrations. The mean value was used to convert total concentrations into unbound concentrations. Pharmacokinetic parameters were estimated from unbound plasma and ECF brain unbound concentrations by individual noncompartmental analysis (Phoenix WinNonlin 6.2; Pharsight, St. Louis, MO) as previously described (17, 19). Briefly, areas under the plasma and brain ECF unbound concentration-time curves between two consecutive dosing at steady-state (AUC0–τ) were calculated by using the linear trapezoidal rule. The elimination rate constant ke and corresponding half-life (t1/2) were determined in the terminal phase of the decline, and the metronidazole clearance (CLss,u) and volume of distribution (Vss,u) were calculated. The average steady-state unbound concentration (Caverage) was calculated as the ratio of AUC0–τ to τ, where τ was equal to 8 h.
Statistical analysis.
Results are expressed as means ± the standard deviations. The metronidazole half-life and mean maximum unbound concentration values in plasma and brain were compared by using a nonparametric Wilcoxon test at a significance level of P < 0.05.
RESULTS
Estimated in vivo metronidazole probe recoveries in patients were relatively high with limited intra and between patients variability (73.9 to 82.9%) (Table 2). Metronidazole and OH-metronidazole plasma protein binding was very limited, with estimated mean unbound fractions in plasma (fu) of 86.5% ± 8.9% and 79.0% ± 13.0%, respectively.
TABLE 2.
In vivo probe recovery of metronidazole and pharmacokinetic parameters determined at steady state by noncompartmental analysis in critical care patients receiving 30-min intravenous infusions of 500 mg of metronidazole every 8 h
| Patienta | Metronidazole pharmacokinetic parameterb |
|||||
|---|---|---|---|---|---|---|
| Vss,u (liters) | CLss,u (liters h−1) | t1/2 plasma (h) | t1/2 brain (h) | Brain ECF/unbound plasma AUC ratio | Mean % in vivo recovery ± SD (n = 3) | |
| Individual patients | ||||||
| P1 | 66.7 | 4.0 | 6.7 | 4.2 | 0.87 | 82.9 ± 0.5 |
| P2 | 56.4 | 3.4 | 9.2 | 4.5 | 0.83 | 79.5 ± 3.4 |
| P3 | 53.6 | 3.2 | 4.9 | 4.8 | 1.24 | 79.1 ± 1.4 |
| P4 | 65.6 | 3.9 | 4.3 | 4.4 | 1.16 | 73.9 ± 4.2 |
| Mean data | ||||||
| Mean | 60.6 | 7.9 | 6.3 | 4.5 | 1.02 | |
| SD | 6.6 | 2.8 | 2.2 | 0.2 | 0.19 | |
Both individual and mean patient data are presented.
The values for t1/2 plasma and t1/2 brain were not significantly different.
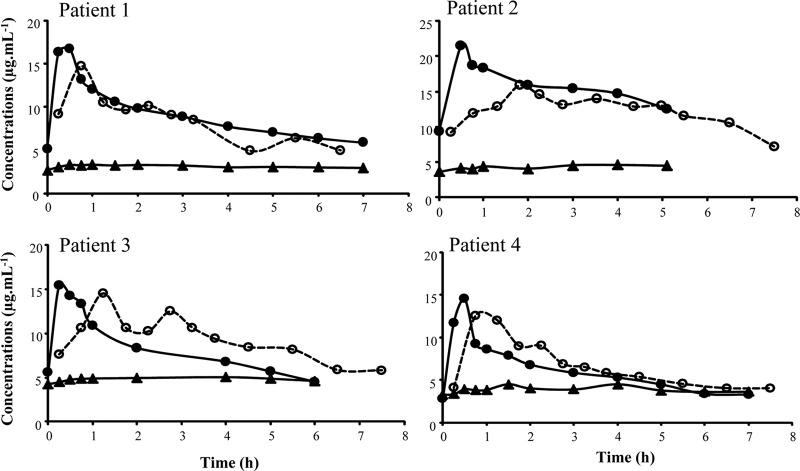
Unbound metronidazole concentration-time curves in the brain were delayed compared to unbound plasma concentration-time curves, with a maximal time peak observed at 69 ± 30 min, but the brain maximal concentration was only slightly and not significantly lower than the corresponding value in plasma (Cmax,ub = 14.5 ± 1.2 μg ml−1 versus Cmax,up = 17.1 ± 3.1 μg ml−1) (Fig. 1). Mean metronidazole brain to unbound plasma AUC0–τ ratio was equal to 102% ± 19%. The pharmacokinetic parameters are presented in Table 2.
FIG 1.
Individual steady-state unbound plasma (●, full line) and brain ECF (○, dashed line) concentrations of metronidazole and unbound plasma concentration of OH-metronidazole (▲, full line) after 500-mg metronidazole infusion over 0.5 h every 8 h in critical care patients.
Unbound OH-metronidazole plasma concentration-versus-time profiles were flat, with corresponding mean average steady-state concentrations of 4.0 ± 0.7 μg ml−1 (Fig. 1). Similar profiles were obtained in brain dialysates (not shown); however, because it was impossible to estimate in vivo OH-metronidazole probe recovery, ECF brain concentrations of this metabolite could not be determined.
DISCUSSION
We used intracerebral microdialysis to investigate the CNS distribution of metronidazole in patients. In vivo probe recoveries estimated by the retrodialysis-by-drug approach were always >70% in all patients (Table 2), which is 1.5- to 3-fold higher than the recoveries we previously reported for cefotaxime and meropenem in patients (17, 19, 21) and which may be considered optimal. The experimental conditions here, including the flow rate and the selected probes, were identical (0.3 μl min−1, CMA-70, 20 kDa) to those used in previous cefotaxime studies (17, 19), whereas the same flow rate but larger-cutoff probes (CMA-71, 100 kDa) were used for our meropenem study (21). However, the metronidazole probe recoveries in patient brains were not only particularly high but also virtually identical between individuals (Table 2).
Numerous pharmacokinetic studies of metronidazole have been performed in healthy volunteers (22–24) and in various specific populations (10, 12), but only two studies have been conducted in critically ill patients (25, 26). In the present study, the mean metronidazole fu (86.5% ± 8.9%) was in accordance with previous results (10). Since the unbound fractions of metronidazole were close to unity, our pharmacokinetic parameters estimated from unbound concentrations can be directly compared to parameters previously reported for total concentrations. Thus, the Vss,u in the present study (60.6 ± 6.6 liters, Table 2) was close to the value estimated in septic shock patients after a single administration (53.5 ± 4 liters) (26). However, our CLss,u (7.9 ± 2.8 liters h−1) was 2-fold higher than in septic shock patients (56.2 ml min−1 or 3.4 liters h−1) (26). Consequently, our metronidazole t1/2 (6.3 ± 2.2 h, Table 2) is much lower than previously reported in critical care patients (13.2 ± 5.3 h) and in better agreement with the t1/2 estimated in healthy volunteers (between 7.3 to 7.9 h) (22–24). The most likely explanation for this discrepancy is the impaired renal function that is usual in patients with a septic shock (26).
This is the first microdialysis study to explore brain ECF distribution of metronidazole in patients with acute brain injury. We have confirmed the extensive distribution of metronidazole in brain, with a mean AUC ratio close to unity (Table 2). These new data suggest that metronidazole brain ECF distribution is governed by passive diffusion, whereas for cefotaxime the mean brain/plasma AUC ratio was much lower (26.1% ± 12.1%) (19), a finding in accordance with the fact that some β-lactam antibiotics are known to be transported by efflux transporters, including organic anion transporter 3, peptide transporter 2, or multidrug-resistant associated protein 4 (27–29).
These findings demonstrate that the extensive distribution of metronidazole within brain ECF contributes to the CNS toxicity (2–6) observed occasionally during treatments with this antibiotic. Although brain ECF concentrations of metronidazole observed here were always >4 μg/ml, which should be sufficiently high for ensuring proper antimicrobial efficacy against most targeted bacteria, no firm conclusion about antimicrobial efficacy of metronidazole for the treatment of cerebromeningeal infections can be drawn for at least two reasons. First, the bacterial location—i.e., the ECF, the CSF, or both—remains unclear, meaning that CSF concentrations and ECF levels could be complementary for predicting antimicrobial efficacy. Second, our patients did not present with CNS infection, which may alter BBB permeability by opening thigh junction or interfering with efflux pump activity (30–32), although such a permeation effect should have greater consequences for antibiotics with limited CNS distribution than for those, such as metronidazole, with extensive and mostly passive diffusion. However, this important issue should be clarified in the future.
This study is the first to describe an extensive distribution of metronidazole in patient brain ECF by using microdialysis. An accompanying paper examines the measurement of metronidazole and hydroxymetronidazole concentrations in the CSF of patients with external ventricular drain (33).
ACKNOWLEDGMENT
This study was supported by a PHRC grant from the French Ministère de l'Education et de la Recherche.
Footnotes
Published ahead of print 25 November 2013
REFERENCES
- 1.Bernstein LH, Frank MS, Brandt LJ, Boley SJ. 1980. Healing of perineal Crohn's disease with metronidazole. Gastroenterology 79:599. [PubMed] [Google Scholar]
- 2.Kim E, Na DG, Kim EY, Kim JH, Son KR, Chang KH. 2007. MR imaging of metronidazole-induced encephalopathy: lesion distribution and diffusion-weighted imaging findings. Am. J. Neuroradiol. 28:1652–1658. 10.3174/ajnr.A0655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel K, Green-Hopkins I, Lu S, Tunkel AR. 2008. Cerebellar ataxia following prolonged use of metronidazole: case report and literature review. Int. J. Infect. Dis. 12:e111–114. 10.1016/j.ijid.2008.03.006 [DOI] [PubMed] [Google Scholar]
- 4.Seok JI, Yi H, Song YM, Lee WY. 2003. Metronidazole-induced encephalopathy and inferior olivary hypertrophy: lesion analysis with diffusion-weighted imaging and apparent diffusion coefficient maps. Arch. Neurol. 60:1796–1800. 10.1001/archneur.60.12.1796 [DOI] [PubMed] [Google Scholar]
- 5.Kalia V, Vibhuti, Saggar K. 2010. Case report: MRI of the brain in metronidazole toxicity. Indian J. Radiol. Imaging 20:195–197. 10.4103/0971-3026.69355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradley WG, Karlsson IJ, Rassol CG. 1977. Metronidazole neuropathy. BMJ ii:610–611. 10.1136/bmj.2.6087.610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ingham HR, Eaton S, Venables CW, Adams PC. 1978. Bacteroides fragilis resistant to metronidazole after long-term therapy. Lancet i:214. [DOI] [PubMed] [Google Scholar]
- 8.Moosa ANV, Perkins D. 2010. Neurological picture: MRI of metronidazole induced cerebellar ataxia. J. Neurol. Neurosurg. Psychiatr. 81:754–755. 10.1136/jnnp.2008.165308 [DOI] [PubMed] [Google Scholar]
- 9.Westerhout J, Danhof M, De Lange ECM. 2011. Preclinical prediction of human brain target site concentrations: considerations in extrapolating to the clinical setting. J. Pharm. Sci. 100:3577–3593. 10.1002/jps.22604 [DOI] [PubMed] [Google Scholar]
- 10.Lamp KC, Freeman CD, Klutman NE, Lacy MK. 1999. Pharmacokinetics and pharmacodynamics of the nitroimidazole antimicrobials. Clin. Pharmacokinet. 36:353–373. 10.2165/00003088-199936050-00004 [DOI] [PubMed] [Google Scholar]
- 11.Easmon CS, Ison CA, Kaye CM, Timewell RM, Dawson SG. 1982. Pharmacokinetics of metronidazole and its principal metabolites and their activity against Gardnerella vaginalis. Br. J. Vener. Dis. 58:246–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lau AH, Lam NP, Piscitelli SC, Wilkes L, Danziger LH. 1992. Clinical pharmacokinetics of metronidazole and other nitroimidazole anti-infectives. Clin. Pharmacokinet. 23:328–364. 10.2165/00003088-199223050-00002 [DOI] [PubMed] [Google Scholar]
- 13.Davies AH. 1967. Metronidazole in human infections with syphilis. Br. J. Vener. Dis. 43:197–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feldman WE. 1976. Bacteroides fragilis ventriculitis and meningitis: report of two cases. AMA Am. J. Dis. Child. 130:880–883. 10.1001/archpedi.1976.02120090090017 [DOI] [PubMed] [Google Scholar]
- 15.Jokipii AM, Myllyla VV, Hokkanen E, Jokipii L. 1977. Penetration of the blood brain barrier by metronidazole and tinidazole. J. Antimicrob. Chemother. 3:239–245. 10.1093/jac/3.3.239 [DOI] [PubMed] [Google Scholar]
- 16.O'Grady LR, Ralph ED. 1976. Anaerobic meningitis and bacteremia caused by Fusobacterium species. AMA Am. J. Dis. Child. 130:871–873 [DOI] [PubMed] [Google Scholar]
- 17.Frasca D, Dahyot-Fizelier C, Couet W, Debaene B, Mimoz O, Marchand S. 2012. Brain microdialysis distribution study of cefotaxime in a patient with traumatic brain injury. Br. J. Anaesthesia 109:830–831. 10.1093/bja/aes369 [DOI] [PubMed] [Google Scholar]
- 18.Dahyot C, Marchand S, Bodin M, Debeane B, Mimoz O, Couet W. 2008. Application of basic pharmacokinetic concepts to analysis of microdialysis data: illustration with imipenem muscle distribution. Clin. Pharmacokinet. 47:181–189. 10.2165/00003088-200847030-00004 [DOI] [PubMed] [Google Scholar]
- 19.Dahyot-Fizelier C, Frasca D, Gregoire N, Adier C, Mimoz O, Debaene B, Couet W, Marchand S. 2013. Microdialysis study of cefotaxime cerebral distribution in patients with acute brain injury. Antimicrob. Agents Chemother. 57:2738–2742. 10.1128/AAC.02570-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karjagin J, Lefeuvre S, Oselin K, Kipper K, Marchand S, Tikkerberi A, Starkopf J, Couet W, Sawchuk RJ. 2008. Pharmacokinetics of meropenem determined by microdialysis in the peritoneal fluid of patients with severe peritonitis associated with septic shock. Clin. Pharmacol. Ther. 83:452–459. 10.1038/sj.clpt.6100312 [DOI] [PubMed] [Google Scholar]
- 21.Dahyot-Fizelier C, Timofeev I, Marchand S, Hutchinson P, Debaene B, Menon D, Mimoz O, Gupta A, Couet W. 2010. Brain microdialysis study of meropenem in two patients with acute brain injury. Antimicrob. Agents Chemother. 54:3502–3504. 10.1128/AAC.01725-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Houghton GW, Thorne PS, Smith J, Templeton R, Collier J. 1979. Comparison of the pharmacokinetics of metronidazole in healthy female volunteers following either a single oral or intravenous dose. Br. J. Clin. Pharmacol. 8:337–341. 10.1111/j.1365-2125.1979.tb04715.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mattila J, Mannisto PT, Mantyla R, Nykanen S, Lamminsivu U. 1983. Comparative pharmacokinetics of metronidazole and tinidazole as influenced by administration route. Antimicrob. Agents Chemother. 23:721–725. 10.1128/AAC.23.5.721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muscara MN, Pedrazzoli J, Jr, Miranda EL, Ferraz JG, Hofstatter E, Leite G, Magalhaes AF, Leonardi S, De Nucci G. 1995. Plasma hydroxy-metronidazole/metronidazole ratio in patients with liver disease and in healthy volunteers. Br. J. Clin. Pharmacol. 40:477–480. 10.1111/j.1365-2125.1995.tb05792.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plaisance KI, Quintiliani R, Nightingale CH. 1988. The pharmacokinetics of metronidazole and its metabolites in critically ill patients. J. Antimicrob. Chemother. 21:195–200. 10.1093/jac/21.2.195 [DOI] [PubMed] [Google Scholar]
- 26.Karjagin J, Pahkla R, Karki T, Starkopf J. 2005. Distribution of metronidazole in muscle tissue of patients with septic shock and its efficacy against Bacteroides fragilis in vitro. J. Antimicrob. Chemother. 55:341–346. 10.1093/jac/dkh544 [DOI] [PubMed] [Google Scholar]
- 27.Akanuma S, Uchida Y, Ohtsuki S, Kamiie J, Tachikawa M, Terasaki T, Hosoya K. 2011. Molecular-weight-dependent, anionic-substrate-preferential transport of Î2-lactam antibiotics via multidrug resistance-associated protein 4. Drug Metab. Pharmacokinet. 26:602–611. 10.2133/dmpk.DMPK-11-RG-063 [DOI] [PubMed] [Google Scholar]
- 28.Shen H, Ocheltree SM, Hu Y, Keep RF, Smith DE. 2007. Impact of genetic knockout of PEPT2 on cefadroxil pharmacokinetics, renal tubular reabsorption, and brain penetration in mice. Drug Metab. Dispos. 35:1209–1216. 10.1124/dmd.107.015263 [DOI] [PubMed] [Google Scholar]
- 29.Spector R. 2010. Nature and consequences of mammalian brain and CSF efflux transporters: four decades of progress. J. Neurochem. 112:13–23. 10.1111/j.1471-4159.2009.06451.x [DOI] [PubMed] [Google Scholar]
- 30.Quagliarello V, Scheld WM. 1992. Bacterial meningitis: pathogenesis, pathophysiology, and progress. N. Engl. J. Med. 327:864–872. 10.1056/NEJM199209173271208 [DOI] [PubMed] [Google Scholar]
- 31.Gerber J, Nau R. 2010. Mechanisms of injury in bacterial meningitis. Curr. Opin. Neurol. 23:312–318. 10.1097/WCO.0b013e32833950dd [DOI] [PubMed] [Google Scholar]
- 32.Miller DS. 2010. Regulation of P-glycoprotein and other ABC drug transporters at the blood-brain barrier. Trends Pharmacol. Sci. 31:246–254. 10.1016/j.tips.2010.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frasca D, Dahyot-Fizelier C, Adier C, Mimoz O, Debaene B, Couet W, Marchand S. 2014. Metronidazole and hydroxymetronidazole central nervous system distribution: 2. cerebrospinal fluid concentration measurements in patients with external ventricular drain. Antimicrob. Agents Chemother. 58:1024–1027. 10.1128/AAC.01762-13 [DOI] [PMC free article] [PubMed] [Google Scholar]