Abstract
Clostridium difficile is a leading cause of health care-associated diarrhea with significant morbidity and mortality, and new options for the treatment of C. difficile-associated diarrhea (CDAD) are needed. Cadazolid is a new oxazolidinone-type antibiotic that is currently in clinical development for treatment of CDAD. Here, we report the in vitro and in vivo antibacterial evaluation of cadazolid against C. difficile. Cadazolid showed potent in vitro activity against C. difficile with a MIC range of 0.125 to 0.5 μg/ml, including strains resistant to linezolid and fluoroquinolones. In time-kill kinetics experiments, cadazolid showed a bactericidal effect against C. difficile isolates, with >99.9% killing in 24 h, and was more bactericidal than vancomycin. In contrast to metronidazole and vancomycin, cadazolid strongly inhibited de novo toxin A and B formation in stationary-phase cultures of toxigenic C. difficile. Cadazolid also inhibited C. difficile spore formation substantially at growth-inhibitory concentrations. In the hamster and mouse models for CDAD, cadazolid was active, conferring full protection from diarrhea and death with a potency similar to that of vancomycin. These findings support further investigations of cadazolid for the treatment of CDAD.
INTRODUCTION
Clostridium difficile infection (CDI), or CDAD for C. difficile-associated diarrhea, is a major health care problem and a leading cause of morbidity and mortality in elderly hospitalized patients (1, 2). During the past decade, there has been a renewed interest in CDAD triggered by an increase in both frequency and severity of the disease in the Western world and the discovery of new hypervirulent strains (3–6), as well as an increased incidence of CDAD in the community (7). CDAD results from overgrowth of toxin-producing strains in the colon, typically following disturbances of the normal protective enteric flora. Clinical symptoms range from asymptomatic colonization to diarrhea, severe pseudomembranous colitis, sepsis, and death. The main virulence factors of C. difficile are two high-molecular-weight toxins, the enterotoxin toxin A (TcdA) and the cytotoxin toxin B (TcdB), while the contribution of the binary toxin remains unclear (8). Toxin A and toxin B cause damage to the intestinal epithelial barrier and promote mucosal inflammation. In fact, the main clinical symptoms of CDAD (secretory diarrhea and inflammation of the colonic mucosa) can be explained by the action of toxins A and B (8). Moreover, C. difficile produces endospores that are resistant to antibiotic treatment and routine disinfection (9). Spores surviving in the gut of patients and in the hospital environment may play a major role in reinfection and relapse of CDAD. Current antibiotic therapy for CDAD includes vancomycin and metronidazole, which have limited treatment success in severe disease, and high recurrence rates of up to 30% have been observed with these treatments (10). Only one new antibiotic, fidaxomicin (11, 12), has been approved in the last 30 years for this indication. In clinical studies, this antibiotic was not inferior to vancomycin in treating acute infections, with less recurrence (12, 13). However, recurrence rates for fidaxomicin were still high for infections involving the hypervirulent strain NAP1/BI/027 (24% recurrence rate) (13) and for patients treated for an episode of recurrent CDAD (20% recurrence rate) (14); thus, there remains a need for new drugs with improved efficacy.
Cadazolid (formerly ACT-179811) is a new oxazolidinone-type antibiotic (Fig. 1) currently in clinical development for CDAD. Cadazolid showed potent in vitro activity against C. difficile clinical isolates (15, 16) and in a human gut model of CDAD, while having only a very limited impact on bacteria of the normal gut microflora (17). In phase 1 studies, this compound was well tolerated, with a very low systemic exposure resulting in a high concentration in the colon (18). Recently, phase 2 trials in CDAD showed clinical cure rates similar to those of vancomycin while having lower recurrence rates, resulting in higher sustained-cure rates (19).
FIG 1.
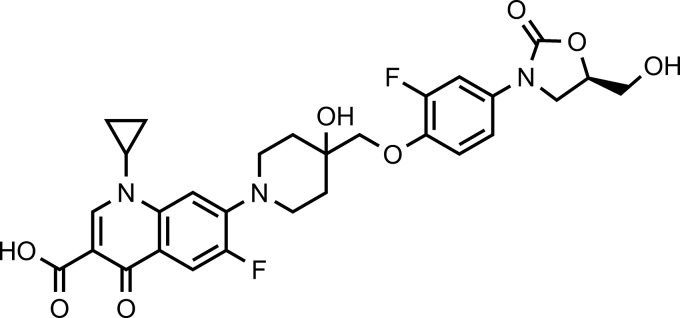
Chemical structure of cadazolid (1-cyclopropyl-6-fluoro-7-{4-[2-fluoro-4-((R)-5-hydroxymethyl-2-oxo-oxazolidin-3-yl)-phenoxymethyl]-4-hydroxy-piperidin-1-yl}-4-oxo-1,4-dihydro-quinoline-3-carboxylic acid).
In this study, we report the in vitro activity of cadazolid against C. difficile, the bactericidal effect, and the effect on toxin and spore formation as well as the efficacy in the mouse and hamster models of CDAD. Vancomycin was chosen as the main comparator, while other antibiotics were included when appropriate for differentiation. Linezolid and moxifloxacin were included as comparators for in vitro assays because of cadazolid's structural similarity to oxazolidinone and quinolone antibiotics (Fig. 1).
(Part of this work was presented at the 23rd European Congress of Clinical Microbiology and Infectious Diseases [ECCMID], Berlin, Germany, 2013, and the 52nd Interscience Conference on Antimicrobial Agents and Chemotherapy [ICAAC], San Francisco, CA, 2012.)
MATERIALS AND METHODS
Bacterial strains and antimicrobial agents.
Strains used in this study were from the Actelion in-house strain collection. Clinical isolates were originally obtained between 2000 and 2011 from various, mostly European hospitals. Reference strains were obtained from the ATCC and NCTC. Most clinical isolates of C. difficile were kindly provided by M. Wilcox (Leeds, United Kingdom) and D. Gerding (Hines, IL). Toxigenic C. difficile strain ATCC 9689 and hypervirulent ribotype 027 strain NCTC 13366 were used for kill-curve, toxin, and spore formation experiments. C. difficile VPI 10463 (ATCC 43255) was used in animal experiments.
Cadazolid (ACT-179811; purity, 98.8%) was synthesized at Actelion Pharmaceuticals Ltd. Other antibiotics were obtained from Sigma-Aldrich.
In vitro antibacterial activity and time-kill assays.
MICs were determined following the guidelines of the Clinical and Laboratory Standards Institute (CLSI) using the agar dilution method for testing anaerobes (20). For time-kill assays, compounds were added to exponentially growing 10-ml cultures (inoculum concentration of 106 to 107 CFU/ml) in brain heart infusion broth supplemented with yeast extract and l-cysteine (BHIS) (9). At different time points, samples were retrieved for CFU determination on Brazier's cefoxitin-cycloserine-egg yolk (CCEY) agar plates (LabM) supplemented with 4% egg yolk emulsion (Oxoid), 1% laked horse blood (Oxoid), and 5 μg/ml lysozyme (Fluka 62971) after 48 h of incubation at 37°C. The detection limit was 50 CFU/ml. Effects of drug carryover were monitored with undiluted and diluted culture samples spiked with the test drug. No evidence of growth inhibition due to drug carryover effects was observed at the drug concentrations tested.
Effects of cadazolid on toxin production.
Experiments to assess the effects of cadazolid and comparator antibiotics on C. difficile toxin formation were done by determination of toxin A and B concentrations in stationary-phase cultures of toxigenic C. difficile (21–23). Briefly, cultures were grown in brain heart infusion broth (BHI) until early stationary phase, harvested by centrifugation (10 min, 3,500 rpm), and resuspended with preanaerobized BHI at 50% of the original volume, which corresponded to a final cell density of 5 × 107 to 1 × 108 CFU/ml. Then antibiotics were added at sub- and supra-MICs (t = 0 h) and further incubated for 24 h at 37°C. Controls with dimethyl sulfoxide (DMSO) (for cadazolid) or H2O (for vancomycin) were also included. For determination of toxin concentrations, 1-ml culture samples were centrifuged for 2 to 3 min at 10,000 × g, and then 450 to 500 μl supernatant was filter sterilized using Vecta Spin Micro filters (Anopore, Whatman 6830-0201). Appropriate dilutions of culture supernatants were analyzed by enzyme-linked immunosorbent assay (ELISA) (Ridascreen Clostridium difficile Toxin A/B; R-Biopharm AG, Darmstadt, Germany) following the manufacturer's instructions. The test detects C. difficile toxins A and B simultaneously, and total toxin concentrations were initially expressed as the optical density at 415 nm (OD415). The total toxin concentration in the supernatant of the untreated control after 24 h of incubation was set as 100%, and the effect of antibiotic treatment was expressed as percent inhibition compared to this control. Purified Clostridium difficile toxin A and toxin B (C3977 and C4102, respectively; Sigma) were used as controls.
Effect on spore formation.
In order to investigate the effects of cadazolid and vancomycin on spore formation, antibiotics were added to growing cultures of C. difficile at late exponential growth phase, and ethanol-resistant spore and total viable cell counts were determined for up to 5 days (21, 24). The antibiotic concentrations chosen were the highest concentrations (MIC fold change) that had no significant impact on C. difficile growth under the test conditions (sub-growth-inhibitory concentrations) and represented 0.5× and 1× the agar dilution MICs. Fourteen-milliliter tubes containing 7 ml BHIS were incubated at 37°C in the anaerobic chamber until an OD600 of 0.8 was reached (late exponential growth phase). Antibiotics were then added at different concentrations as indicated. Cadazolid was added in DMSO (final DMSO concentration, 0.3% [vol/vol]), vancomycin was added in water, and the control tube received 0.3% (vol/vol) DMSO. Tubes were incubated for up to 5 days at 37°C under anaerobic conditions. Assessment of total viable cells was performed as detailed above. Spore counts were done after selective killing of vegetative cells by treatment with 50% ethanol for 1 h and subsequent CFU count on Brazier's agar (21).
All in vitro experiments with C. difficile were performed in an anaerobic glove box (Coy Laboratory) in an atmosphere of 85% N2–10% CO2–5% H2 using preanaerobized media.
Hamster and mouse model of CDAD.
All experiments were performed in agreement with the Swiss Federal Ordinance for animal protection and the animal welfare committee of the Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, USA.
For the hamster model of CDAD, male Golden Syrian hamsters (aged 45 to 70 days; approximately 100 g [range, 98 g to 124 g]) were housed in cages (2 per cage) with free access to chow (Purina 5000) and tap water. Control animals received no clindamycin and were not challenged with C. difficile. All other animals were given a single injection of clindamycin phosphate (10 mg/kg of body weight subcutaneously [s.c.]) and 1 day later were challenged by gavage with 105 CFU of toxigenic C difficile strain VPI 10463 (ATCC 43255; challenge was given on experimental day 0). Groups of 10 animals each received either inert vehicle (0.5% [wt/wt] methylcellulose containing 0.05% [wt/vol] Tween 80; 1 ml), vancomycin (in sterile water), or cadazolid (in 0.5% [wt/wt] methylcellulose containing 0.05% [wt/vol] Tween 80) by gavage daily for 5 days beginning 2 h after C. difficile administration. Hamsters were weighed daily and checked three times daily for morbidity and presence or absence of diarrhea (wet tail). Hamsters judged to be in a moribund state (i.e., exhibiting at least one of the following signs: extended period of weight loss progressing to an emaciated state, anorexia for 24 to 48 h, prolonged lethargy [more than 3 days], signs of paralysis, skin erosions or trauma, hunched posture, distended abdomen) were euthanized by a single injection of sodium pentobarbital.
For the C. difficile mouse model, the protocol published by Chen et al. was followed (25). In brief, 8- to 12-week-old female C57BL/6 mice were kept in individually ventilated cage systems (SlimLine; Tecniplast, Buguggiate, Italy) and given access to water and food ad libitum. To sensitize the mice for C. difficile infections, animals received an antibiotic cocktail containing kanamycin (0.4 mg/ml), gentamicin (0.035 mg/ml), colistin (850 U/ml), metronidazole (0.215 mg/ml), and vancomycin (0.045 mg/ml) in the drinking water for 3 days. Two days after withdrawal of the antibiotic cocktail, mice were treated with clindamycin (10 mg/kg in 10 ml, intraperitoneally [i.p.]). One day after clindamycin application, mice were infected per os (p.o.) with 105 CFU of a spore suspension of C. difficile VPI 10463. Cadazolid or vancomycin was administered p.o. once daily for 4 days, starting either 2 or 24 h postinfection (no impact of treatment start on efficacy was observed [data not shown]). Animals (n = 5 to 10 per group) were monitored daily for moribund state and diarrhea during the treatment phase and for up to 18 days postinfection. Mice judged to be in a moribund state (i.e., exhibiting at least two of the following signs: extended period of weight loss progressing to an emaciated state, anorexia for 24 to 48 h, prolonged lethargy [more than 3 days], signs of paralysis, skin erosions or trauma, hunched posture, distended abdomen) were euthanized by CO2 inhalation, and survival time was recorded. Kaplan-Meier curves of pooled survival data from multiple studies were compared using the Cox regression (26) and log rank tests. A P value of <0.05 was considered statistically significant.
RESULTS
In vitro antibacterial activity against C. difficile.
The in vitro activity of cadazolid was tested against 23 strains of C. difficile, including 15 clinical isolates of different ribotype as well as reference strains from the ATCC (Table 1). To investigate potential cross-resistance, strains resistant to linezolid and/or fluoroquinolones (moxifloxacin) were included. Cadazolid was active against all C. difficile strains tested, with a MIC range of 0.125 to 0.5 μg/ml (Table 1). MIC90s of cadazolid, vancomycin, metronidazole, moxifloxacin, and linezolid were 0.25, 1, 1, 16, and 16 μg/ml, respectively. Interestingly, cadazolid MICs were not, or only marginally, increased in the four linezolid-resistant strains and were not affected by resistance to fluoroquinolones such as in the hypervirulent NAP1/BI/027 strain NCTC 13366 (Table 1). The highest MICs for cadazolid (0.5 μg/ml) were obtained for the two strains resistant to both linezolid and fluoroquinolones, but the shift was only 2- to 4-fold compared to the most susceptible strains. Overall, cadazolid was 8- to 64-fold more potent than linezolid, >64-fold more potent than ciprofloxacin, and 8- to >64-fold more potent than moxifloxacin.
TABLE 1.
In vitro activity of cadazolid and comparator antibiotics against Clostridium difficile, including strains with defined resistance
| C. difficile strain | Resistance profile | MIC (μg/ml) |
||||
|---|---|---|---|---|---|---|
| Cadazolid | Linezolid | Moxifloxacin | Vancomycin | Metronidazole | ||
| All strains (n = 23)a | 0.125–0.5 | 1–32 | 1–32 | 0.5–4 | 0.06–2 | |
| ATCC 9689 | 0.25 | 2 | 2 | 1 | 0.5 | |
| ATCC 700057 | 0.125–0.25 | 2 | 2 | 1 | 0.25 | |
| VPI 10463 | 0.25 | 2 | 2 | 0.5 | 0.5 | |
| NCTC 13366 | 027/BI/NAP1, FQr (GyrA: Thr82-Ile) | 0.25 | 1 | 32 | 1 | 1 |
| A-1290 | LZDr | 0.125–0.25 | 16–32 | 2 | 1 | 0.125 |
| A-1291 | LZDr | 0.25–0.5 | 16–32 | 1 | 1–2 | 0.125 |
| A-1410 | LZDr, FQr (GyrA: Thr82-Ile) | 0.5 | 32–64 | 16 | 1 | 1 |
| A-1412 | LZDr, FQr (GyrA: Thr82-Ile) | 0.5 | 32–64 | 16 | 1 | 0.125 |
Includes 15 random European clinical isolates with ribotypes 027, 078,001, 002, 005, 012, 014, and 106 and the 8 selected strains shown. Abbreviations: r, resistant; FQ, fluoroquinolone; LZD, linezolid.
Time-kill kinetics.
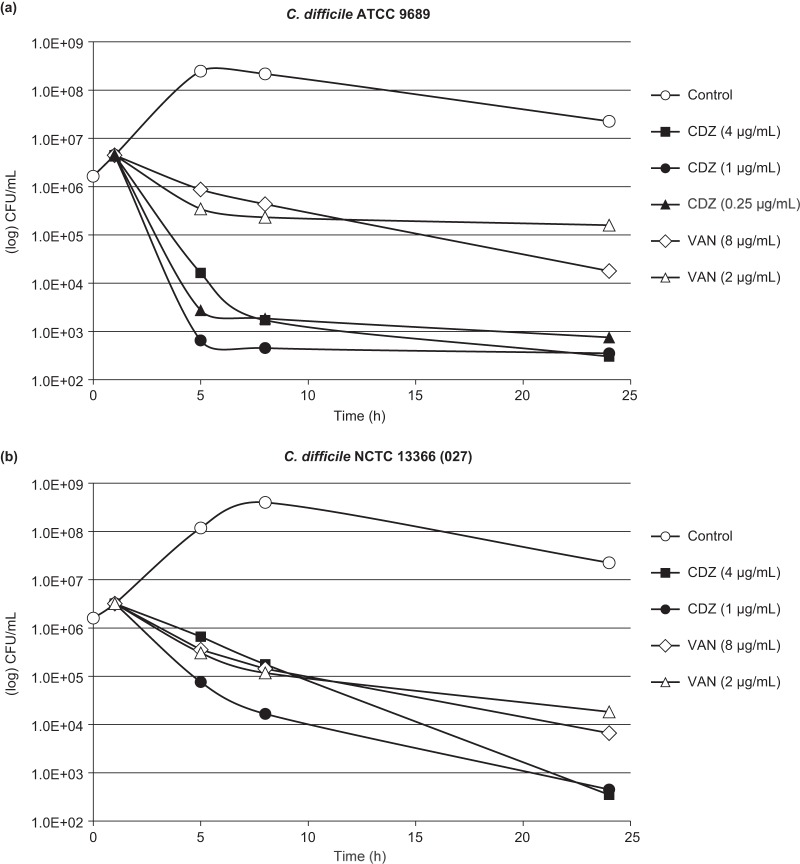
Cadazolid showed a time-dependent bactericidal effect against C. difficile in time-kill experiments (Fig. 2). A ≥3-log CFU reduction was achieved within 24 h at all concentrations tested (representing 1× to 16× the MIC) for both the ATCC 9689 strain and the hypervirulent and fluoroquinolone-resistant ribotype 027 strain NCTC 13366. While a comparably slower initial killing rate was observed for the 027 strain, the final magnitude of killing after 24 h was similar to that of the ATCC 9689 strain. In contrast, vancomycin tested in parallel at concentrations of 2× to 8× the MIC showed a slower initial rate of killing and did not reach the 3-log CFU reduction endpoint (Fig. 2).
FIG 2.
Time-kill kinetics of cadazolid (CDZ) and vancomycin (VAN) with C. difficile ATCC 9689 (a) and NCTC 13366 (ribotype 027) (b). Antibiotics were added in the exponential growth phase at t of 1 h.
Effects on C. difficile toxin production.
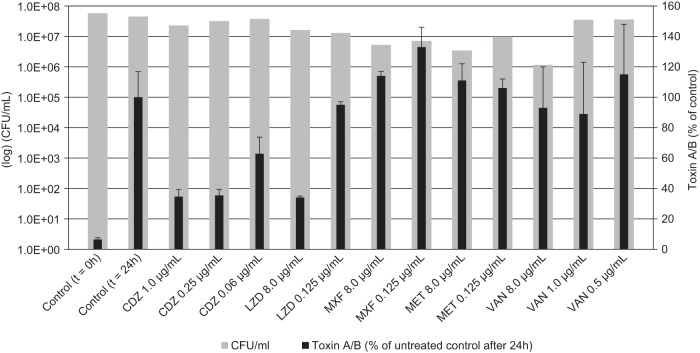
Only toxigenic strains of C. difficile, i.e., strains that produce either both toxins (toxin A and B) or toxin B alone, are able to cause C. difficile-associated disease (8). It has been hypothesized that reduction of the formation of toxins and other virulence factors would contribute to effective treatment of CDAD (21). As C. difficile toxins are produced mainly during the late exponential and stationary growth phases, they can be formed in the absence of bacterial growth and replication (22). Nonreplicating bacteria, on the other hand, are not or only very slowly killed by most antibiotics, especially those targeting replication and cell division. Therefore, in this study, we used high-density stationary-phase cultures to investigate the effects of cadazolid and other antibiotics on C. difficile toxin formation. Cultures of toxigenic C. difficile ATCC 9689 were treated with sub-MIC and supra-MIC antibiotic concentrations for 24 h, and the concentrations of toxins A and B in the culture supernatants were determined by ELISA (Fig. 3). Cadazolid at concentrations of 1× and 4× the MIC showed a strong inhibition of toxin formation compared to the untreated control, while the viable bacterial count (CFU) was not affected. A substantial inhibition of toxin formation was also observed at 0.06 μg/ml, representing 0.25× MIC. In contrast, vancomycin, metronidazole, and moxifloxacin did not show a major effect on toxin formation under these conditions, even when tested at supra-MIC. A trend for an increased toxin formation compared to the control was observed for cultures with sub-MICs of vancoymcin or moxifloxacin. Linezolid, on the other hand, showed an inhibitory effect on toxin formation similar to that of cadazolid when tested at 4× MIC. Since the culture was in a stationary (nonreplicating) phase, none of the tested antibiotics showed significant killing effects as judged by CFU counts (Fig. 3).
FIG 3.
Effects of cadazolid and other antibacterial agents on toxin formation in stationary-phase cultures of toxigenic C. difficile ATCC 9689. Cultures were either left untreated (control) or treated with sub-MIC and supra-MICs of antibiotics for 24 h. Cell viability (colony count) and total toxin (toxins A and B) concentrations were determined at t of 0 h and 24 h. The total toxin concentration after 24 h in the untreated culture was set as 100%, and the relative toxin concentrations in the treated samples are shown. Abbreviations: CDZ, cadazolid; LZD, linezolid; MXF, moxifloxacin; MET, metronidazole; VAN, vancomycin.
Similar results were obtained in an experiment using stationary-phase cultures of hypervirulent C. difficile strain NCTC 13366 and a cell-based cytotoxicity test to measure toxin concentrations. While cadazolid (and linezolid) inhibited de novo cytotoxin formation nearly completely, vancomycin and metronidazole exhibited only marginal effects (see Fig. S1 in the supplemental material).
Effects on C. difficile spore formation.
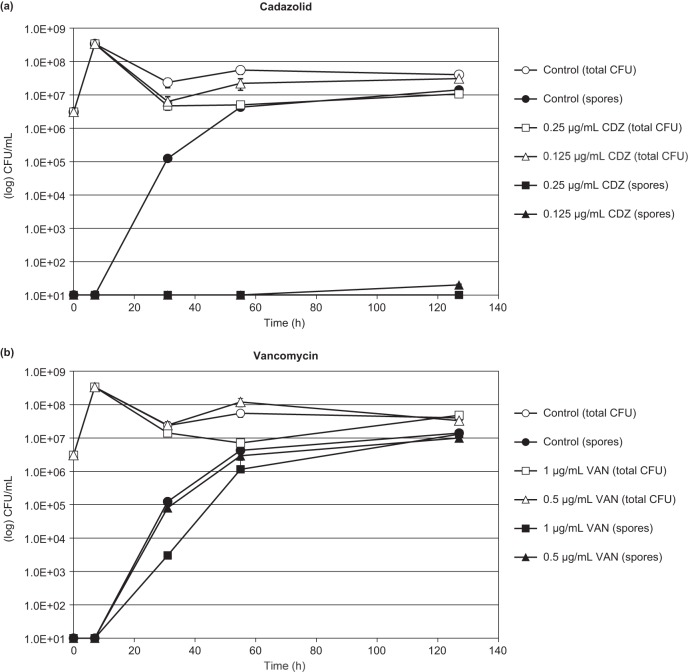
In order to investigate the effects on spore formation, cadazolid and vancomycin were added at sub-growth-inhibitory concentrations to cultures of C. difficile at late exponential growth phase, and ethanol-resistant spores and total viable cell counts were scored for up to 5 days. In untreated cultures, spore concentrations increased over time, and after 5 days spores represented the majority of the total viable counts (Fig. 4). Cadazolid inhibited spore formation markedly at sub-growth-inhibitory concentrations. No new spore formation was observed in the presence of cadazolid at 1× MIC for up to 5 days, while at 0.5× MIC spore formation was strongly inhibited (Fig. 4). In contrast, vancomycin (tested at 0.5× and 1× MIC) did not inhibit or delay spore formation. Similar results were obtained in a second strain of C. difficile (see Fig. S2 in the supplemental material), where cadazolid strongly inhibited (1× MIC) or delayed (0.5× MIC) spore formation while vancomycin had no effect.
FIG 4.
Effect of sub-growth-inhibitory concentrations of cadazolid (a) and vancomycin (b) on spore formation in C. difficile ATCC 9689. Antibiotics were added to late exponential cultures (t = 6 h) at concentrations representing 0.5× and 1× the agar dilution MIC. Controls received no antibiotics. Total viable cells and spores (after ethanol treatment) were enumerated by colony count. Abbreviations: CDZ, cadazolid; VAN, vancomycin.
Efficacy in animal models of CDAD.
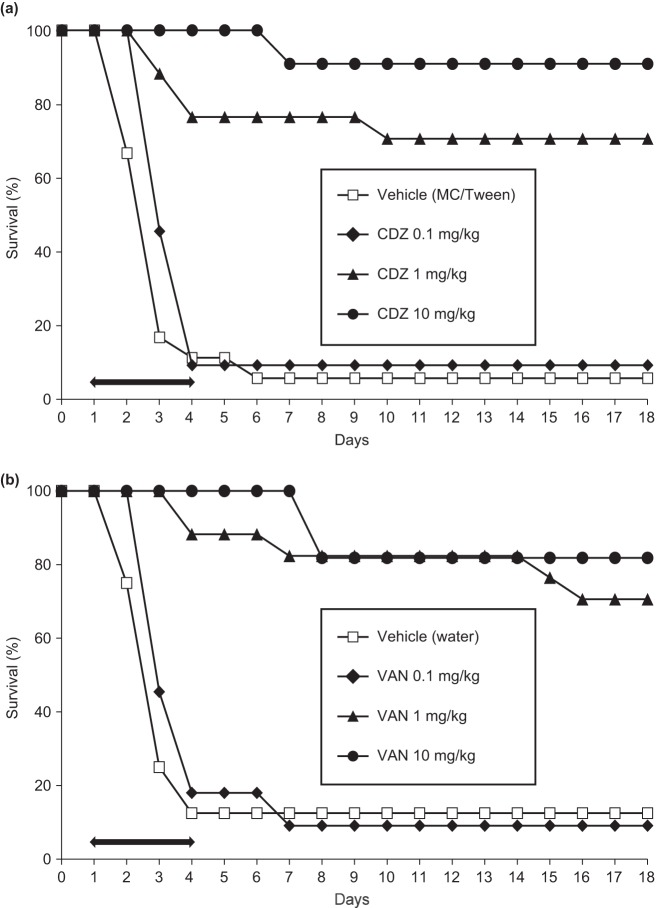
Cadazolid dose dependently prevented mortality and diarrhea when given to presensitized and C. difficile-infected C57BL/6 mice (Fig. 5). Dose-dependent efficacy was observed during and after treatment time and was reproducible between experiments. In 17 pooled independent experiments, the overall survival rates of mice treated with vehicle or cadazolid at doses of 0.1, 1, and 10 mg/kg were 18, 47, 97, and 100%, respectively, after the treatment period (day 5), while the corresponding values were 12, 40, 92, and 88%, respectively, at day 18 postinfection (Table 2). According to Cox proportional hazards regression analysis, cadazolid decreased the risk of death of infected mice during the 18-day monitoring period by 56, 96, and 95%, respectively, indicating a significant sustained treatment effect compared to vehicle-treated mice (P < 0.001 for all three dose groups). Overall, 0.1 mg/kg cadazolid was significantly less protective than 1 mg/kg and 10 mg/kg (P < 0.001), while 1 mg/kg was not significantly different from 10 mg/kg (P = 0.7). In three studies, cadazolid was compared to vancomycin side by side, and the pooled overall survival rates were similar at the same doses (Table 2).
FIG 5.
Efficacy of cadazolid (a) and vancomycin (b) in the mouse model of C. difficile infection. Mice were pretreated with clindamycin and an antibiotic cocktail and infected with C. difficile VPI 10463 on day 0. Cadazolid, vancomycin, or vehicles were administered per os once daily for 4 days starting on day 1. One representative of three similar experiments is shown. The treatment period is marked with a black arrow. MC/Tween, 0.5% (wt/wt) methylcellulose containing 0.05% (wt/vol) Tween 80. Abbreviations: CDZ, cadazolid; VAN, vancomycin.
TABLE 2.
Efficacy of cadazolid and vancomycin in the mouse model of CDAD
| Compound (no. of pooled studies) | % survival (total no. of mice tested)a |
|||||||
|---|---|---|---|---|---|---|---|---|
| During treatment phase, day 5 postinfection |
Posttreatment phase, day 18 postinfection |
|||||||
| Vehicle control | Drug dose (mg/kg) |
Vehicle control | Drug dose (mg/kg) |
|||||
| 0.1 | 1 | 10 | 0.1 | 1 | 10 | |||
| Cadazolid (all)b | 17.5 (137) | 46.7 (107) | 96.5 (114) | 100 (107) | 12.4 (137) | 40.2 (107) | 92.1 (114) | 87.9 (107) |
| Cadazolid (3)c | 11 (18) | 9.1 (11) | 76 (17) | 100 (11) | 5.6 (18) | 9.1 (11) | 70.5 (17) | 91 (11) |
| Vancomycin (3)c | 12.5 (16) | 9.1 (11) | 88 (17) | 100 (11) | 12.5 (16) | 9.1 (11) | 70.5 (17) | 82 (11) |
Mice (n = 5 to 7 per group) were treated for 4 days once daily.
Pooled data of all studies with cadazolid compared to vehicle (n = 17).
Pooled data of 3 studies with side-to-side comparison of cadazolid and vancomycin.
Cadazolid also dose dependently prevented mortality when given to presensitized and C. difficile-infected Golden Syrian hamsters (Table 3). In three independent studies, both cadazolid and vancomycin prevented diarrhea-related death during the treatment phase (days 1 to 5) at doses of 3 to 300 mg/kg (100% survival), while doses of 0.3 mg/kg and vehicle were not protective (<20% survival). Survival during posttreatment phase (days 6 to 28) was variable (0 to 100%) for both cadazolid and vancomycin, with poor reproducibility between experiments, and therefore the results were difficult to interpret (Table 3; see also Fig. S3 in the supplemental material).
TABLE 3.
Efficacy of cadazolid and vancomycin in the hamster model of CDADa
| Phase and study no. | Drug dose (mg/kg) and % survival |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cadazolid |
Vancomycin |
|||||||||
| During treatment, day 5 postinfection | ||||||||||
| 1 | VC, 0 | 10, 100 | 30, 100 | 100, 100 | VC, 0 | 50, 100 | ||||
| 2 | VC, 0 | 12.5, 100 | 25, 100 | 50, 100 | VC, ND | 12.5, 100 | 25, 100 | 50, 100 | ||
| 3 | VC, 20 | 0.3, 30 | 3, 100 | 30, 100 | 300, 100 | VC, ND | 0.3, 10 | 3, 100 | 30, 100 | 300, 100 |
| Posttreatment, day 28 postinfection | ||||||||||
| 1 | VC, 0 | 10, 40 | 30, 100 | 100, 80 | VC, 0 | 50, 100 | ||||
| 2 | VC, 0 | 12.5, 40 | 25, 60 | 50, 40 | VC, ND | 12.5, 30 | 25, 40 | 50, 100 | ||
| 3 | VC, 20 | 0.3, 20 | 3, 20 | 30, 0 | 300, 60 | VC, ND | 0.3, 0 | 3, 60 | 30, 80 | 300, 80 |
Hamsters (n = 10 or 11 per group) were treated for 5 days once daily. Survival rates (%) are in boldface. ND, not done; VC, vehicle control.
DISCUSSION
The data presented here indicate that cadazolid has potent in vitro activity against C. difficile, including strains resistant or nonsusceptible to linezolid and/or fluoroquinolone, in agreement with other studies involving a higher number of clinical isolates (15, 16). While cadazolid is also active against other Gram-positive pathogens, including Staphylococcus aureus and enterococci, its activity is comparably weak or not detectable against most Gram-negative bacteria (27). It is believed that a narrow-spectrum antibiotic with no or limited effect on members of the normal gut flora, notably the Bacteroides group, is an important factor for sustained treatment success against CDAD with less recurrence, as exemplified clinically for fidaxomicin (28). In an in vitro human gut model using therapeutic doses of cadazolid, the drug had very limited impact on the normal gut microflora, notably sparing the Bacteroides group (17). However, in the end clinical data will be needed to demonstrate the impact of cadazolid on the gut microbiota.
The in vitro bactericidal effect of cadazolid against C. difficile was more pronounced than that of vancomycin. Generally, oxazolidinone antibiotics such as linezolid show only a static effect on most aerobic bacteria; however, bactericidal effects against C. difficile have been observed for linezolid and oxazolidinone derivatives (23), in agreement with our findings for cadazolid.
Only toxigenic strains of C. difficile, i.e., strains that produce either both toxins (toxin A and B) or toxin B alone are able to cause disease (8). Therefore, it can be anticipated that inhibition of the formation of toxins and other virulence factors may contribute to effective treatment of CDAD (21). The data presented in this study indicate that cadazolid and linezolid inhibit toxin formation in nongrowing C. difficile in the absence of bacterial killing, while vancomycin, metronidazole, and moxifloxacin do not. Inhibition of toxin formation in vitro has been reported also for fidaxomicin (29), for REP3123, a methionyl-tRNA synthetase inhibitor (21), and for a new investigational biaryl oxazolidinone antibiotic (23). All of these compounds inhibit bacterial protein synthesis, either indirectly by blocking gene transcription (fidaxomicin) or directly by interfering with translation. In contrast, vancomycin (acting on cell wall synthesis) and metronidazole (interacting with DNA metabolism) were weak inhibitors of C. difficile toxin formation in in vitro assays (21, 23). Studies addressing the mode of action of cadazolid revealed that it primarily acts as an inhibitor of protein synthesis, while inhibition of DNA synthesis was observed as a weaker additional mode of action as reported in a companion article by Locher et al. (30). These findings are in agreement with the inhibitory effect of cadazolid on de novo C. difficile toxin formation as well as the potent activity against linezolid- and fluoroquinolone-resistant strains reported here.
Spores surviving in the gut of patients or in the environment may play a major role in reinfection and relapse of CDAD (2, 9) after antibiotic treatment, and it has been speculated that inhibition of spore formation may be beneficial for the treatment of CDAD by potentially reducing the persistence and the spread of the organism (21, 24). In vitro promotion of spore formation at sub-growth-inhibitory concentrations has been reported for the antibiotics metronidazole and vancomycin (21). Our data indicate that cadazolid does not promote the formation of spores; on the contrary, it strongly inhibited or delayed spore formation at sub-growth-inhibitory concentrations, while this was not observed for vancomycin. Inhibition of spore formation in vitro has been shown also for fidaxomicin (24) as well as for REP3123 (21) and may be linked to a mode of action leading to inhibition of de novo protein synthesis.
In vitro activity of cadazolid translated well to in vivo efficacy in two independent animal models of CDAD. Remarkably, the potency of cadazolid for prevention of diarrhea and mortality was comparable in the mouse and hamster models. CDAD develops comparably in both models, independently from the somewhat more fatal progression of disease in hamsters than in mice. This is in line with the notion that in both models disease progresses from diarrhea to death and that in both models histopathological changes in cecum and large intestine are comparable (25, 31–34). In the hamster model in this study, efficacy during the posttreatment phase was variable between experiments and remained difficult to interpret. This may be due to the low number of experiments and animals assessed. When looking at the posttreatment phase in mice, using a larger number of animals, statistical comparison between cadazolid and vancomycin became possible. There, the sustained treatment success of cadazolid and vancomycin appeared to be comparable. However, the predictive value of animal models for sustained cure in human CDAD remains open. During the development of two of the most recent treatment options against human CDAD, fidaxomicin and surotomycin, the recurrence rates in the hamster model were comparable to those of vancomycin (35, 36) and the mouse model was not employed. However, in human trials both compounds presented to be superior in the sustained treatment success compared to vancomycin (12, 13, 36, 37).
The data presented here indicate that cadazolid has promising in vitro and in vivo activity against C. difficile and support further clinical studies.
Supplementary Material
ACKNOWLEDGMENTS
We thank M. Wilcox, Leeds (United Kingdom), and D. Gerding, Chicago, IL (USA), for providing C. difficile strains and for helpful advice and discussions.
Potential conflicts of interest: H. H. Locher, P. Seiler, S. Schroeder, P. Pfaff, M. Enderlin, A. Klenk, E. Fournier, C. Hubschwerlen, D. Ritz, and W. Keck are or have been employees and stockholders of Actelion Pharmaceuticals Ltd.
X. Chen is supported in part by National Institutes of Health grant AI 095256 and has acted as Principal Investigator for a research grant to BIDMC from Merck. C. P. Kelly is supported in part by National Institutes of Health grants AI 095256, DK 07760, AI 103612, and AI 099458, he has acted as a consultant and scientific advisor to Astellas, CSL Behring, Cubist, Glaxo Smith Kline, Ironwood, Merck, Novartis, Optimer, PaxVax, Pfizer, Regeneron, Sanofi-Pasteur, and VHSquared, and he has acted as Principal Investigator for research grants to BIDMC from Claremont BioSolutions, CSL Behring, Merck, and Optimer.
Footnotes
Published ahead of print 25 November 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.01830-13.
REFERENCES
- 1.Ananthakrishnan AN. 2011. Clostridium difficile infection: epidemiology, risk factors and management. Nat. Rev. Gastroenterol. Hepatol. 8:17–26. 10.1038/nrgastro.2010.190 [DOI] [PubMed] [Google Scholar]
- 2.Rupnik M, Wilcox MH, Gerding DN. 2009. Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nat. Rev. Microbiol. 7:526–536. 10.1038/nrmicro2164 [DOI] [PubMed] [Google Scholar]
- 3.O'Connor JR, Johnson S, Gerding DN. 2009. Clostridium difficile infection caused by the epidemic BI/NAP1/027 strain. Gastroenterology 136:1913–1924. 10.1053/j.gastro.2009.02.073 [DOI] [PubMed] [Google Scholar]
- 4.McDonald LC, Killgore GE, Thompson A, Owens RC, Jr, Kazakova SV, Sambol SP, Johnson S, Gerding DN. 2005. An epidemic, toxin gene-variant strain of Clostridium difficile. N. Engl. J. Med. 353:2433–2441. 10.1056/NEJMoa051590 [DOI] [PubMed] [Google Scholar]
- 5.Gerding DN. 2010. Global epidemiology of Clostridium difficile in 2010. Infect. Control Hosp. Epidemiol. 31(S1):S32–S34. 10.1086/655998 [DOI] [PubMed] [Google Scholar]
- 6.Kelly CP, LaMont JT. 2008. Clostridium difficile—more difficult than ever. N. Engl. J. Med. 359:1932–1940. 10.1056/NEJMra0707500 [DOI] [PubMed] [Google Scholar]
- 7.Kuntz JL, Johnson ES, Raebel MA, Petrik AF, Yang X, Thorp ML, Spindel SJ, Neil N, Smith DH. 2012. Epidemiology and healthcare costs of incident Clostridium difficile infections identified in the outpatient healthcare setting. Infect. Control Hosp. Epidemiol. 33:1031–1038. 10.1086/667733 [DOI] [PubMed] [Google Scholar]
- 8.Kuehne SA, Cartman ST, Minton NP. 2011. Both, toxin A and toxin B, are important in Clostridium difficile infection. Gut Microbes 2:252–255. 10.4161/gmic.2.4.16109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burns DA, Minton NP. 2011. Sporulation studies in Clostridium difficile. J. Microbiol. Methods 87:133–138. 10.1016/j.mimet.2011.07.017 [DOI] [PubMed] [Google Scholar]
- 10.Kamboj M, Khosa P, Kaltsas A, Babady NE, Son C, Sepkowitch KA. 2011. Relapse versus reinfection: surveillance of Clostridium difficile infection. Clin. Infect. Dis. 53:1003–1006. 10.1093/cid/cir643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hardesty JS, Juang P. 2011. Fidaxomicin: a macrocyclic antibiotic for the treatment of Clostridium difficile infection. Pharmacotherapy 31:877–886. 10.1592/phco.31.9.877 [DOI] [PubMed] [Google Scholar]
- 12.Cornely OA, Crook DW, Esposito R, Poirier A, Somero MS, Weiss K, Sears P, Gorbach S, OPT-80-004 Clinical Study Group 2012. Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: a double-blind, non-inferiority, randomised controlled trial. Lancet Infect. Dis. 12:281–289. 10.1016/S1473-3099(11)70374-7 [DOI] [PubMed] [Google Scholar]
- 13.Louie TJ, Miller MA, Mullane KM, Weiss K, Lentnek A, Golan Y, Gorbach S, Sears P, Shue YK, OPT-80-003 Clinical Study Group 2011. Fidaxomicin versus vancomycin for Clostridium difficile infection. N. Engl. J. Med. 364:422–431. 10.1056/NEJMoa0910812 [DOI] [PubMed] [Google Scholar]
- 14.Cornely OA, Miller MA, Louie TJ, Crook TW, Gorbach SL. 2012. Treatment of first recurrence of Clostridium difficile infection: fidaxomicin versus vancomycin. Clin. Infect. Dis. 55(Suppl 2):S154–S161. 10.1093/cid/cis462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hecht DW, Osmolski JR, Sambol S, Cheknis A, Gerding DN. 2012. In vitro activity of cadazolid against 209 toxigenic isolates of Clostridium difficile, abstr E-808 Abstr. 52nd Intersci. Conf. Antimicrob. Agents Chemother., San Francisco, CA. American Society for Microbiology, Washington, DC [Google Scholar]
- 16.Rashid MU, Martinez Lozano H, Weintraub A, Nord CE. 2013. In vitro activity of cadazolid against Clostridium difficile strains isolated from primary and recurrent infections in Stockholm, Sweden. Anaerobe 20:32–35. 10.1016/j.anaerobe.2013.02.003 [DOI] [PubMed] [Google Scholar]
- 17.Chilton CH, Crowther G, Baines S, Todhunter S, Freeman J, Locher HH, Athanasious A, Wilcox M. 2013. 14 October 2013. In vitro activity of cadazolid against clinically relevant Clostridium difficile isolates and in an in vitro gut model of C. difficile infection. J. Antimicrob. Chemother. 10.1093/jac/dkt411 [DOI] [PubMed] [Google Scholar]
- 18.Baldoni D, Gutierrez M, Timmer W, Dingemanse J. 2013. 8 October 2013. Cadazolid, a novel antibiotic with potent activity against Clostridium difficile: safety, tolerability and pharmacokinetics in healthy subjects following single and multiple oral doses. J. Antimicrob. Chemother. 2013. 10.1093/jac/dkt401 [DOI] [PubMed] [Google Scholar]
- 19.Louie TJ, Buitrago M, Cornely OA, Kracker H, Rangaraju M, Charef P. 2013. Multicentre, double-blind, randomised, phase 2 study evaluating the novel antibiotic, cadazolid, in subjects with Clostridium difficile-associated diarrhea, abstract/poster LB-2956 Abstr. 53rd Eur. Cong. Clin. Microbiol. Infect. Dis., Berlin, Germany [Google Scholar]
- 20.CLSI 2007. Methods for antimicrobial susceptibility testing of anaerobic bacteria: approved standard—7th ed, M11-A7. Clinical and Laboratory Standards Institute, Wayne, PA: [PubMed] [Google Scholar]
- 21.Ochsner UA, Bell SJ, O'Leary AL, Hoang T, Stone KC, Young CL, Critchley IA, Janjic N. 2009. Inhibitory effect of REP3123 on toxin and spore formation in Clostridium difficile, and in vivo efficacy in a hamster gastrointestinal infection model. J. Antimicrob. Chemother. 63:964–971. 10.1093/jac/dkp042 [DOI] [PubMed] [Google Scholar]
- 22.Gerber M, Walch C, Loffler B, Tischendorf K, Reischl U, Ackermann G. 2008. Effect of sub-MIC concentrations of metronidazole, vancomycin, clindamycin and linezolid on toxin gene transcription and production in Clostridium difficile. J. Med. Microbiol. 57:776–783. 10.1099/jmm.0.47739-0 [DOI] [PubMed] [Google Scholar]
- 23.Mathur T, Kumar M, Barman TK, Kumar GR, Kalia V, Raj VS, Das B, Bhatnagar PK. 2011. Activity of RBx 11760, a novel biaryl oxazolidinone, against Clostridium difficile. J. Antimicrob. Chemother. 66:1087–1095. 10.1093/jac/dkr033 [DOI] [PubMed] [Google Scholar]
- 24.Babakhani F, Bouillaut L, Gomez A, Sears P, Nguyen N, Sonenshein AL. 2012. Fidaxomicin inhibits spore production in Clostridium difficile. Clin. Infect. Dis. 55(Suppl 2):S162–S169. 10.1093/cid/cis453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen X, Katchar K, Goldsmith JD, Nathakumar N, Cheknis A, Gerding DN, Kelly CP. 2008. A mouse model of Clostridium difficile-associated disease. Gastroenterology 135:1984–1992. 10.1053/j.gastro.2008.09.002 [DOI] [PubMed] [Google Scholar]
- 26.Cox DR. 1972. Regression models and life tables (with discussion). J. R. Statist. Soc. B 34:187–220 [Google Scholar]
- 27.Locher HH, Pfaff P, Schroeder S, Specklin JL, Hubschwerlen C, Keck W. 2012. Cadazolid, a novel quinolonyl-oxazolidinone antibiotic with potent activity against Clostridium difficile: in vitro antibacterial activity and propensity for resistance development, abstr C1-1346 Abstr. 52nd Intersci. Conf. Antimicrob. Agents Chemother., San Francisco, CA. American Society for Microbiology, Washington, DC [Google Scholar]
- 28.Louie TJ, Emery J, Krulicki W, Byrne B, Mah M. 2009. OPT-80 eliminates Clostridium difficile and is sparing of bacteroides species during treatment of C. difficile infection. Antimicrob. Agents Chemother. 53:261–263. 10.1128/AAC.01443-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bouillaut L, Sims C, Gomez A, Sears P, Seddon J, Sonenshein A. 2012. Fidaxomicin inhibits production of toxin A and toxin B in Clostridium difficile. J. Hosp. Med. 7(Suppl 2):77 http://www.shmabstracts.com/abstract.asp?MeetingID=783&id=97620 [Google Scholar]
- 30.Locher HH, Caspers P, Bruyère T, Schroeder S, Pfaff P, Knezevic A, Keck W, Ritz D. 2014. Investigations of the mode of action and resistance development of cadazolid, a new antibiotic for treatment of Clostridium difficile infections. Antimicrob. Agents Chemother. 58:901–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kokkotou E, Moss AC, Michos A, Espinoza D, Cloud JW, Mustafa N, O'Brien N, Pothoulakis C, Kelly CP. 2008. Comparative efficacies of rifaximin and vancomycin for treatment of Clostridium difficile-associated diarrhea and prevention of disease recurrence in hamsters. Antimicrob. Agents Chemother. 52:1121–1126. 10.1128/AAC.01143-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bartlett JG, Onderdonk AB, Cisneros RL, Kasper DL. 1977. Clindamycin-associated colitis due to a toxin-producing species of Clostridium in hamsters. J. Infect. Dis. 136:701–705. 10.1093/infdis/136.5.701 [DOI] [PubMed] [Google Scholar]
- 33.Sambol SP, Merrigan MM, Tang JK, Johnson S, Gerding DN. 2002. Colonization for the prevention of Clostridium difficile disease in hamsters. J. Infect. Dis. 186:1781–1789. 10.1086/345676 [DOI] [PubMed] [Google Scholar]
- 34.Warren CA, Van Opstal EJ, Riggins M, Li Y, Moore JH, Kolling GL, Guerrant RL, Hoffman PS. 2013. Vancomycin treatment's association with delayed intestinal tissue injury, clostridial overgrowth, and recurrence of Clostridium difficile infection in mice. Antimicrob. Agents Chemother. 57:689–696. 10.1128/AAC.00877-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swanson RN, Hardy DJ, Shipkowitz NL, Hanson CW, Ramer NC, Fernandes PB, Clement JJ. 1991. In vitro and in vivo evaluation of tiacumicins B and C against Clostridium difficile. Antimicrob. Agents Chemother. 35:1108–1111. 10.1128/AAC.35.6.1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mascio CT, Mortin LI, Howland KT, Van Praagh AD, Zhang S, Arya A, Chuong CL, Chang C, Li T, Silverman JA. 2012. In vitro and in vivo characterization of CB-183,315, a novel lipopeptide antibiotic for treatment of Clostridium difficile. Antimicrob. Agents Chemother. 56:5023–5030. 10.1128/AAC.00057-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patino H, Stevens C, Louie TJ, Bernardo P, Friedland I. 2011. Abstr. 51st Intersci. Conf. Antimicrob. Agents Chemother., Chicago, IL, abstr K-205a. American Society for Microbiology, Washington, DC [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.