Abstract
Danoprevir (DNV) is a hepatitis C virus (HCV) protease inhibitor that achieves high sustained virologic response (SVR) rates in combination with peginterferon alfa-2a–ribavirin in treatment-naive HCV genotype 1 (G1)-infected patients. This study explored the efficacy and safety of ritonavir-boosted DNV (DNVr) plus peginterferon alfa-2a–ribavirin in G1-infected prior peginterferon-ribavirin null responders. Null responders (<2-log10 reduction in HCV RNA level at week 12) were given an open-label combination of 100 mg of ritonavir and 100 mg of DNV (100/100 mg DNVr) every 12 h (q12h) plus peginterferon alfa-2a–ribavirin for 12 weeks. All patients achieving an early virologic response (EVR; ≥2-log10 decrease in HCV RNA by week 12) continued treatment with peginterferon alfa-2a–ribavirin; those without an EVR discontinued all study drugs. Twenty-four prior null responders were enrolled; 16 patients (67%) were infected with HCV G1b, and 8 (33%) were infected with G1a. Ninety-six percent of patients had an IL28B non-CC genotype. A sustained virologic response at 24 weeks posttreatment (SVR24) was achieved in 67% of patients, with a higher rate in G1b-infected (88%) than G1a-infected (25%) patients. Resistance-related breakthrough occurred in 4/8 G1a and 1/16 G1b patients through the DNV resistance-associated variant (RAV) NS3 R155K. NS3 R155K was also detected in 2/2 G1a patients who relapsed. Treatment was well tolerated. Two patients withdrew prematurely from study medications due to adverse events. Two serious adverse events were reported; both occurred after completion of DNVr therapy and were considered unrelated to treatment. No grade 3 or 4 alanine aminotransferase (ALT) elevations were observed. DNVr plus peginterferon alfa-2a–ribavirin demonstrated high SVR24 rates in HCV G1b-infected prior null responders and was well tolerated. (This study has been registered at ClinicalTrials.gov under registration no. NCT01185860.)
INTRODUCTION
Approximately 150 million people are chronically infected with the hepatitis C virus (HCV) worldwide, with over 350,000 deaths each year attributable to HCV infection (1, 2). Among the six major HCV genotypes, genotype 1 (G1) is the most prevalent worldwide (3). Until recently, the standard of care for treatment-naive patients was peginterferon alfa plus ribavirin (PegIFN-RBV); however 50% to 60% of HCV G1-infected patients treated with this regimen do not achieve a sustained virologic response (SVR) (4–7). Patients who have failed previous treatment for chronic hepatitis C are classified as nonresponders. Null responders are the least responsive population of this class and are defined as persons whose HCV RNA levels did not decline by at least 2 log10 IU/ml at week 12 of PegIFN-RBV treatment (8). Furthermore, null responders respond poorly to retreatment with PegIFN-RBV, achieving SVR rates of only 5% (9).
Significant advances have recently been made in the treatment of HCV G1-infected patients with the approval of the first protease inhibitors (PIs), which have led to substantial improvements over PegIFN-RBV in SVR rates and the option of abbreviated therapy for some patients (8). Among treatment-naive G1-infected patients, SVR rates of 63% to 75% can be achieved by adding boceprevir or telaprevir to PegIFN-RBV (10–12). SVR rates in null responders are also increased by treatment with telaprevir or boceprevir in combination with PegIFN-RBV; however, rates remain relatively low (29% to 38%) (9, 13).
Treatment with boceprevir and telaprevir also demonstrates a number of limitations; adverse events (AEs) occur more frequently in PI-treated patients than in patients treated with PegIFN-RBV alone. These PIs are associated with specific toxicities (anemia, skin rash, and perianal discomfort with telaprevir; anemia and dysgeusia with boceprevir), which reduce tolerability compared with PegIFN-RBV treatment alone (9–11, 14). In addition, all patients who receive treatment with boceprevir and telaprevir are subject to an increased pill burden, dosing frequency, and need for administration with a fatty meal (telaprevir) that may reduce adherence (15). Indeed, clinical trial data of boceprevir indicate that adherence is most important for treatment-experienced patients, among whom lower SVR rates have been observed for those patients with less than 60% adherence to a regimen that requires dosing with boceprevir every 8 hours (q8h) and less than 80% adherence to treatment duration (16). Finally, regimens containing telaprevir and boceprevir in combination with PegIFN-RBV are associated with increased complexity of lead-in strategies and response-guided therapies. Thus, there is an unmet need for alternative treatment options for the HCV G1-infected prior null responder population to improve efficacy, safety, and tolerability and reduce complexity of administration compared to currently available regimens.
A number of newer PIs, as well as direct-acting antivirals (DAAs) with different modes of action, are currently being evaluated in clinical trials for efficacy and safety in null responders, including simeprevir (TMC-435), faldaprevir (BI 201335), ABT-450/r, vaniprevir (MK-7009), and asunaprevir (BMS-650032) (17). Current strategies include a single DAA combined with PegIFN-RBV (triple therapy), combination DAAs with PegIFN-RBV (quadruple therapy), and combinations of multiple DAAs with and without RBV (interferon-free therapy). Danoprevir (RG7227; DNV) is a highly selective, macrocyclic inhibitor of the HCV protease and is currently in clinical development in combination with low-dose ritonavir (DNVr) (18–21). Biochemical characterization of DNV displayed subnanomolar in vitro 50% inhibitory concentration (IC50) values for DNV against genotypes G1a (0.20 nM) and G1b (0.23 nM) (18). Ritonavir boosting (coadministration of 100 mg of ritonavir) of DNV has been shown to optimize the pharmacokinetic (PK) profile of DNV, allowing for lower dosing and enabling higher plasma trough concentrations with lower patient exposure to DNV, while still maintaining antiviral activity (19–21). In addition, coadministering ritonavir with DNV has been shown to significantly inhibit DNV metabolism, including the formation of reactive metabolites, which may reduce the risk of alanine aminotransferase (ALT) elevations that have been previously observed with high-dose unboosted DNV (22).
We have previously shown that treatment with DNVr plus PegIFN alfa-2a–RBV for 15 days was well tolerated in treatment-naive HCV G1-infected patients and led to robust reductions in serum HCV RNA levels (19). The present study assessed the efficacy, safety, and pharmacokinetics of DNVr in combination with PegIFN alfa-2a–RBV in HCV G1-infected patients who were null responders to prior PegIFN-RBV therapy.
MATERIALS AND METHODS
This study comprised the final cohort of patients enrolled in a larger study (19) and investigated the efficacy and safety of DNVr plus PegIFN alfa-2a–RBV in G1-infected patients. This was a multicenter study conducted between August 2009 (first patient screened) and January 2012 (last patient, last visit) (ClinicalTrials.gov registration no. NCT01185860), where DNVr plus PegIFN alfa-2a–RBV was administered as open-label treatment.
Study population.
Eligible patients were adults aged between 18 and 65 years with chronic HCV G1 infection who were prior null responders to PegIFN-RBV treatment, with an HCV RNA level of ≥1 × 105 IU/ml. Null responders were defined as patients (i) having completed at least 12 weeks of therapy with good compliance, and (ii) having a <1-log10 reduction in HCV RNA level from baseline at week 4 or a <2-log10 reduction at week 12 of treatment. Other inclusion criteria included a body mass index between 18 and 35 kg/m2 inclusive and a minimum weight of 45 kg; a liver biopsy or noninvasive procedure (e.g., FibroScan) was required in the 24 months prior to treatment start showing absence of cirrhosis. If transient elastography (FibroScan) was used, a liver stiffness cutoff value of 12.5 kPa was generally adopted as the diagnostic threshold for cirrhosis.
Exclusion criteria included decompensated liver disease, impaired liver function (defined by a history of ascites, hepatic encephalopathy, hepatocellular carcinoma, or bleeding esophageal varices), chronic liver disease attributed to a cause other than HCV, pregnancy, or coinfection with hepatitis B virus (HBV) or HIV. Patients were also ineligible if they had an international normalized ratio of ≥1.5, serum ALT level of >5 times the upper limit of normal (ULN), an absolute neutrophil count of ≤1.5 × 109/liter, a platelet count of ≤100 × 109/liter, hemoglobin concentration of <12.0 g/dl (if female) or <13.0 g/dl (if male), or an increased risk of anemia.
Patients were also excluded from the study if they had participated in a clinical trial and received treatment with an experimental HCV protease inhibitor or if they had recent use of drugs or nutrients known to induce or inhibit cytochrome P450 (CYP) enzymes. Use of antacids, H-2 blockers, proton pump inhibitors, systemic immunosuppressive drugs, chemotherapeutic agents, radiation therapy, oral or inhaled corticosteroids, and topical class 1 and 2 steroids was prohibited during the study. Detailed patient eligibility criteria and prohibited medications are as previously described (19).
Study design.
Eligible patients received treatment with oral DNV at 100 mg twice daily (q12h) plus oral ritonavir (Norvir; Abbott Laboratories, Chicago, IL, USA) at 100 mg (100/100 mg DNVr) q12h for 12 weeks. In addition, all patients received concurrent treatment with 180 μg of subcutaneous peginterferon alfa-2a (40 kDa) (Pegasys; Roche, Basel, Switzerland) once weekly plus ribavirin at 1,000 mg/day (bodyweight of <75 kg) or 1,200 mg/day (bodyweight of ≥75 kg) q12h orally. All patients who achieved an early virologic response (EVR), defined as a ≥2-log10 decrease in HCV RNA level by week 12, continued treatment with PegIFN alfa-2a–RBV for up to 36 additional weeks. For those patients who did not demonstrate an EVR, all study drugs were discontinued. All patients were followed for an additional 24 weeks after the last dose of study medication.
Patients discontinued treatment with DNVr under the following circumstances: virologic breakthrough (defined as a sustained [at least two consecutive measurements] ≥1-log10 IU/ml increase in viral load above nadir, where the nadir is a ≥0.5-log10 IU/ml decrease in HCV RNA from baseline, or an HCV RNA level of >43 IU/ml at two consecutive time points following an HCV RNA level of <15 IU/ml); any confirmed clinically significant AIDS Clinical Trial Group (ACTG) grade 4 laboratory abnormality; any confirmed ACTG grade 3 ALT elevation associated with grade 2 total bilirubin laboratory abnormalities; any confirmed positive cardiac troponin result. Continuation, discontinuation, or dose adjustments of PegIFN alfa-2a–RBV were at the discretion of the investigator.
During the study, the protocol was amended because of an unacceptable rate of virologic breakthrough in the first eight HCV G1a-infected patients enrolled, such that all patients enrolled after 6 July 2010 were required to be infected with G1b HCV.
The protocol was approved by the Multi Regional Ethics Committee (New Zealand), the Bioethics Committee at Medical Academy in Warsaw (Poland), and CPP Sud-Méditerranée III (France) and was conducted in accordance with the Declaration of Helsinki and tenets of Good Clinical Practice. Written informed consent was obtained from all patients before they underwent any study-related activities.
Efficacy outcomes.
Viral load was measured predosing of any study medication (baseline) and 60 min before administration of the morning dose on days 3, 6, 9, 12, and 15. On-treatment HCV RNA levels were also measured at weeks 4, 8, 12, 16, 20, 24, 36, and 48. Serum HCV RNA levels were determined by real-time PCR assay (COBAS AmpliPrep/COBAS TaqMan HCV test; lower limit of detection [LLOD], 15 IU/ml) (Roche Diagnostics North America, Indianapolis, IN).
The antiviral efficacy endpoints for this study were the proportion of patients with undetectable HCV RNA at week 4 (rapid virologic response, RVR), week 12 (complete early virologic response, cEVR), week 48 (end of treatment, EOT), and week 72 (sustained virologic response at 24 weeks posttreatment, SVR24).
PK assessments.
To determine individual and mean plasma DNV pharmacokinetic (PK) parameters, blood samples were collected prior to drug intake and at 0.5, 1, 2, 3, 4, 6, 8, and 12 h postdosing on day 14 (multiple oral doses of DNVr). Morning predose blood samples were collected on days 2, 3, 6, 9, 12, 15, 28, 42, 56, 70, and 84 (week 12). Further details on PK methodology are as previously described (19). Plasma PK parameters for DNV, including the area under the concentration-time curve from 0 h to τ (length of the dosing interval) at steady state (AUC0-τ,SS), observed maximum plasma concentration at steady state (Cmax,SS), the time to reach Cmax,SS, and the observed trough concentration at steady state (Ctrough,SS), were estimated using noncompartmental analysis, actual dosing, and PK collection times.
Resistance monitoring.
Resistance monitoring was performed by population sequence analysis of the complete HCV NS3/NS4A (NS3/4A) coding region. Baseline NS3/4A sequencing was performed for all patients. Samples from patients who experienced viral breakthrough, partial response (viral load of ≥1,000 IU/ml at the end of 4 weeks of DNVr treatment or initial viral load decline followed by stabilization while on DNVr treatment), nonresponse (<0.5-IU/ml decrease in viral load from nadir), or relapse (detection of HCV RNA during follow-up in a patient with undetectable HCV RNA at EOT) were selected for NS3/4A sequence analysis. In addition, sequencing was performed for the first sample after viral breakthrough or relapse and the EOT sample in nonresponders and partial responders. For patients who discontinued treatment early, NS3/4A sequencing was performed at the last follow-up time point. Further details on resistance monitoring methodology are as previously described (19).
Safety assessments.
Safety and tolerability were assessed by monitoring electrocardiograms, vital signs, laboratory tests, and clinical AEs throughout the study.
Statistical analysis.
The sample size was chosen based on practical considerations of the study design and not on statistical power calculation. Thus, no formal hypothesis testing was performed or formal statistical inference was used in this study. Summary statistics for steady-state DNV PK parameters are reported. All patients who received at least one dose of study medication and had at least one postbaseline assessment were included in the efficacy and safety analyses. In the efficacy analysis, patients were considered nonresponders on visits when HCV RNA data were missing.
RESULTS
A total of 24 prior null responders to PegIFN-RBV therapy were enrolled in the study and received at least one dose of the study drug. The majority of patients were male (79%), White (83%), with a mean age (SD) of 47.7 (9.71) years, and infected with HCV G1b (67%) (Table 1). There was a predominance of G1b- versus G1a-infected patients due to a protocol amendment, whereby enrollment of G1a patients was stopped because of an unacceptable rate of virologic breakthrough, and the study was then restricted to G1b patients. Among the 23 individuals who consented to DNA analysis, 22 (96%) had an IL28B non-CC genotype (Table 1).
TABLE 1.
Baseline characteristics of patients
| Characteristic | Value for the characteristic (n = 24)a |
|---|---|
| No. of male patients (%) | 19 (79) |
| Age (yr [SD]) | 47.7 (9.71) |
| Race (no. of patients [%]) | |
| White | 20 (83) |
| Other | 4 (17) |
| Weight (kg [SD]) | 78.0 (12.33) |
| Body mass index (kg/m2 [SD]) | 25.3 (3.00) |
| Median HCV RNA level (IU/ml [range]) | 6.56 (5.78–7.27) |
| HCV subtype (no. of patients [%])b | |
| 1a | 8 (33) |
| 1b | 16 (67) |
| IL28B genotype (no. of patients [%])c | |
| CC | 1/23 (4) |
| CT | 14/23 (61) |
| TT | 8/23 (35) |
| Median ALT (IU/ml [range])d | 79.5 (24–275) |
Participants received ritonavir-boosted danoprevir (DNVr) 100/100 mg q12h plus PegIFN alfa-2a–RBV. Data presented as mean (standard deviation) unless stated otherwise.
The predominance of HCV G1b-infected versus G1a-infected patients was due to a protocol amendment, whereby enrollment of G1a patients was stopped and restricted to G1b patients because of high rates of virologic breakthrough.
Twenty-three of 24 patients consented to IL28B genotyping.
ALT, alanine aminotransferase.
Efficacy.
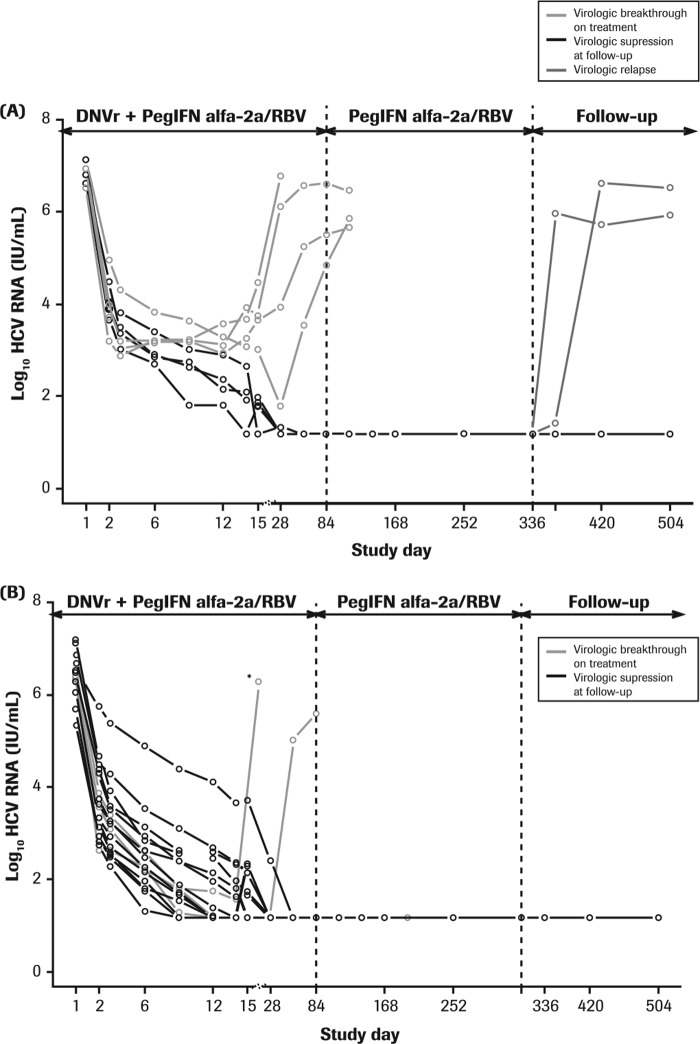
All individual patient HCV RNA levels declined after the initiation of treatment with DNVr plus PegIFN alfa-2a–RBV (Fig. 1). The earliest time point for undetectable HCV RNA (<15 IU/ml) was observed at day 9 of treatment, by which time 13% (3/24) of patients had achieved undetectable HCV RNA levels, and all these patients had been infected with HCV G1b. By day 15 of treatment, 13% (1/8) and 56% (9/16) of G1a- and G1b-infected patients, respectively, had undetectable HCV RNA levels (<15 IU/ml) (Fig. 1).
FIG 1.
Individual HCV RNA levels in G1a-infected (A) and G1b-infected (B) prior null responders during 12 weeks of treatment with DNVr plus PegIFN alfa-2a–RBV, followed by 36 weeks of PegIFN alfa-2a–RBV and the study follow-up period. DNVr, ritonavir-boosted danoprevir; G, genotype; PegIFN alfa-2a–RBV; peginterferon alfa-2a (40KD) at 180 μg/week plus 1,000 mg or 1,200 mg/day ribavirin. *, patient discontinued treatment at day 14 due to an adverse event.
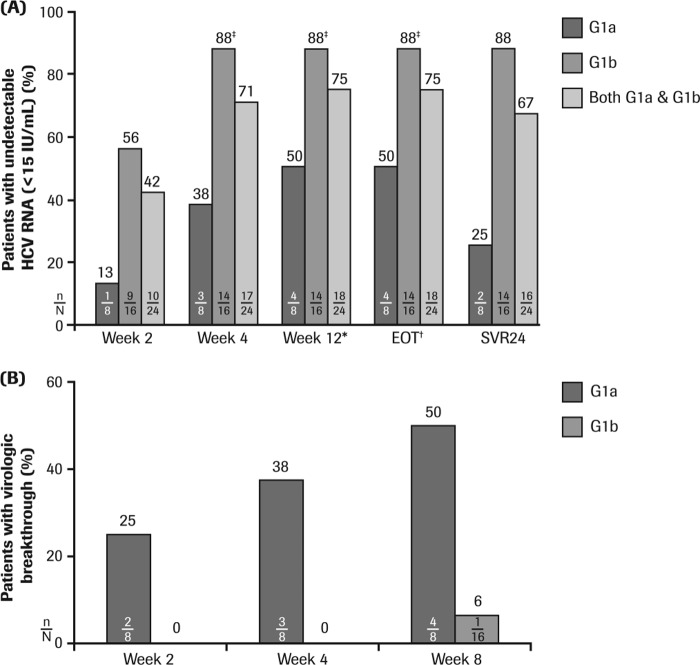
Early on-treatment virologic response rates are shown in Fig. 2A. Virologic response rates increased with duration of treatment with DNVr plus PegIFN alfa-2a–RBV, with 42% of the total patient population achieving undetectable HCV RNA levels by week 2, 71% by week 4, and 75% by week 12 of therapy with DNVr plus PegIFN alfa-2a–RBV. On-treatment virologic response rates observed in HCV G1b-infected patients were higher than rates in HCV G1a-infected patients at all time points analyzed (Fig. 2A).
FIG 2.
(A) Mean virologic response (HCV RNA of <15 IU/ml) and SVR24 rates with DNVr plus PegIFN alfa-2a–RBV in prior null responders. (B) Cumulative virologic breakthrough according to HCV G1 subtype with DNVr plus PegIFN alfa-2a–RBV in prior null responders. *, end of treatment with DNVr plus PegIFN alfa-2a–RBV; †, end of all study medication (12 weeks of DNVr plus PegIFN alfa-2a–RBV followed by 36 weeks of PegIFN alfa-2a–RBV); ‡, one patient with HCV G1b infection stopped treatment at day 14 due to an adverse event. DNVr, ritonavir-boosted danoprevir; EOT, end of treatment; G, genotype; PegIFN alfa-2a–RBV; 180 μg/week peginterferon alfa-2a (40KD) plus 1,000 mg or 1,200 mg/day ribavirin; SVR24, sustained virologic response (undetectable HCV RNA [<15 IU/ml]) at 24 weeks posttreatment. n/N, number of patients with undetectable HCV RNA (A) or virologic breakthrough (B)/total number of patients.
Following treatment with DNVr plus PegIFN alfa-2a–RBV, 75% (18/24) of patients achieved an EVR and continued with an additional 36 weeks of PegIFN alfa-2a–RBV. Overall, 67% (16/24) of patients achieved an SVR24, with a higher rate in HCV G1b-infected (88%; 14/16) than G1a-infected (25%; 2/8) patients (Fig. 2A). Further, relapse occurred in 50% of G1a and 0% of G1b patients who achieved an undetectable HCV RNA level by EOT.
Viral resistance.
Resistance monitoring was carried out for all patients who experienced either viral breakthrough or relapse or for those patients who discontinued treatment early. No patient had a partial response or nonresponse while being treated with DNVr plus PegIFN alfa-2a–RBV. Of the 24 prior null responder patients treated with DNVr plus PegIFN alfa-2a–RBV for up to 12 weeks, 4/8 (50%) G1a patients and 1/16 (6%) G1b patients experienced virologic breakthrough (Fig. 2B). No patient experienced virologic breakthrough while on the PegIFN alfa-2a–RBV phase of treatment (up to 36 weeks). Among these five patients, virologic breakthrough occurred by week 2 in two G1a patients, by week 4 in one G1a patient, and by week 8 in one G1a and one G1b patient. In addition, one G1b-infected patient discontinued treatment at week 2 (with an HCV RNA load of 37 IU/ml). In the 18 patients who maintained complete viral suppression at EOT, relapse occurred between weeks 2 and 4 posttreatment in 2/4 (50%) G1a patients, whereas no relapse was observed in the 14 G1b patients.
Sequence analysis of the HCV NS3/4A coding region revealed that none of the 24 enrolled patients had preexisting DNV-associated resistance variants at baseline. All five cases of virologic breakthrough and two cases of relapse were associated with selection of the R155K variant in the NS3 protease region that confers resistance to DNV (Table 2). The R155K variant persisted after DNVr had been discontinued and through week 24 of the follow-up period for 3/4 G1a patients with virologic breakthrough. For the fourth G1a patient, the 155K substitution did not persist and was replaced by 155R (wild-type residue) by the last follow-up time point, which corresponded to 240 days after the end of DNVr treatment. The R155K resistance-associated variant (RAV) persisted through follow-up week 24 for the one G1b patient who experienced virologic breakthrough. In addition, NS3/4A sequence analysis of samples obtained at the last follow-up visit of the study (follow-up week 24) for the G1b-infected patient who discontinued at week 2 showed no evidence of known DNV resistance substitutions, compared with the baseline sample.
TABLE 2.
Changes in NS3/4A amino acid residues from baseline in patients who experienced either viral breakthrough or relapse or discontinued treatment early
| Event (HCV genotype) and patient no. | Time point | Changes in NS3/4A amino acid residues from baselinea |
|---|---|---|
| Viral breakthrough (G1a) | ||
| 1 | Day 15 | R155K |
| Follow-up week 4 | R155K | |
| Follow-up week 12 | R155K | |
| Follow-up week 24 | R155K | |
| 2 | Week 4b | R155K |
| Follow-up week 4 | Q110H, R155K | |
| Follow-up week 12 | Q110H, R155K | |
| Follow-up week 24 | Q110H, R155K | |
| 3 | Week 8b | V36M, I132V/I, R155K, C159C/R |
| Follow-up week 4 | V36M, I132I/V, R155K, T/A477T | |
| Follow-up week 12 | V36M, R155K, T/A477T | |
| Follow-up week 24 | V36M/V, I132I/V, R155K, T/A477T | |
| 4 | Day 16 | R155K, P482P/S |
| Follow-up week 4 | I132I/V, R155K | |
| Follow-up week 12 | I132I/V, R155K | |
| Follow-up week 24 | V347I/V | |
| Viral breakthrough (G1b) | ||
| 5 | Week 8 | R155K, V654A/V |
| Week 10 (unscheduled) | R155K, V654A/V | |
| Follow-up week 4 | R155K, V654A/V | |
| Follow-up week 12 | R155K | |
| Follow-up week 24b | R155K | |
| Viral relapse (G1a) | ||
| 6 | Follow-up week 4 | P89P/S, R155K |
| Follow-up week 12 | R155K | |
| Follow-up week 24 | No change | |
| 7 | Follow-up week 12 | R155K, N/S174S, A/V358V, I/T586I |
| Follow-up week 24b | N/S174S | |
| Premature discontinuation (G1b)c | ||
| 8 | Follow-up week 24 | V18I, A72T, I669V |
The danoprevir resistance-associated variant is highlighted in bold.
Only the sequence of the NS3 protease was obtained.
No DNV resistance.
Pharmacokinetics.
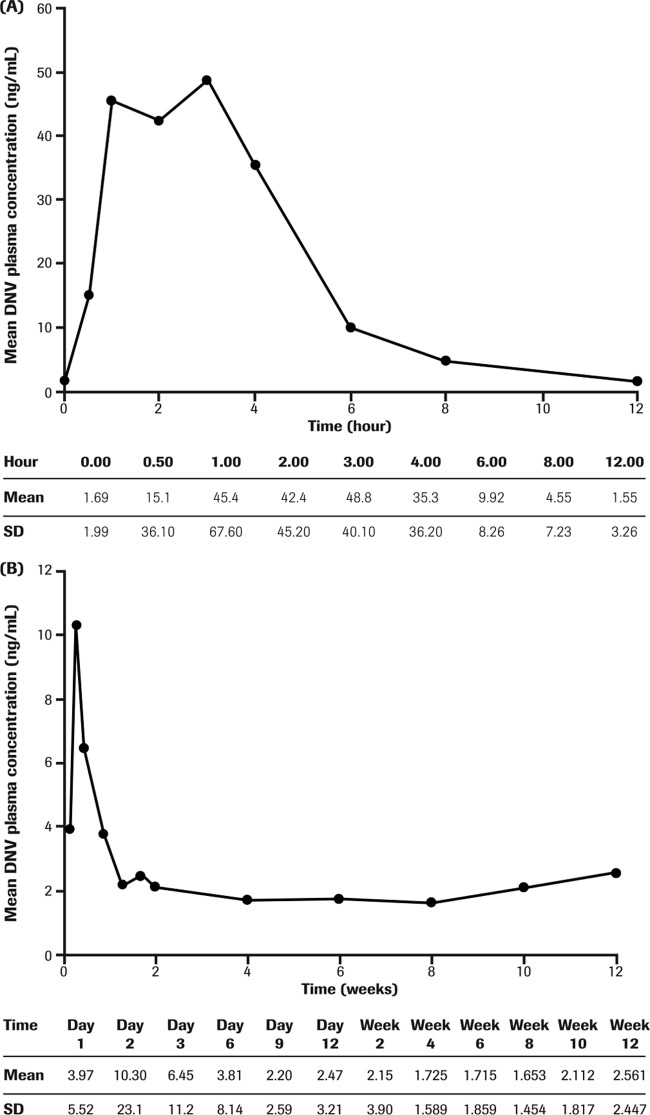
Steady-state pharmacokinetic parameters for 100/100 mg of DNVr were available for 23/24 patients and are summarized in Table 3. The mean DNV concentration-time profile in prior null responders on day 14 is presented in Fig. 3A. In the presence of low-dose ritonavir, DNV concentrations increased during the first 2 days and then gradually declined toward steady state after 6 to 10 days of dosing (Fig. 3B). Following achievement of steady state, median DNV trough concentrations were generally similar across treatment weeks for null responders receiving 100/100 mg of DNVr in combination with PegIFN alfa-2a–RBV for 12 weeks (Table 3). Interpatient variability was moderate to high for the AUC0-τ (coefficient of variation [%] [CV%], 63.7), Cmax (CV%, 68.5), clearance (CL)/F (CV%, 76.0), volume of distribution (V)/F (CV%, 90.8), and Cmin (CV%, 210) DNV pharmacokinetic parameters in this study population.
TABLE 3.
Danoprevir steady-state pharmacokinetic parameters for the DNVr regimen in HCV genotype 1-infected prior null responder patients
| DNV steady-state pharmacokinetic parametera | Value for the parameter (n = 23)c |
|---|---|
| AUC0-τ,SS (ng · h/ml)b | 224 (143) |
| Cmax,SS (ng/ml) | 90.9 (62.2) |
| Cmin (ng/ml)b | 1.55 (3.26) |
| Median Tmax (h [range]) | 2.05 (0.50–8.00) |
| t1/2 (h)b | 2.19 (1.58) |
| Median Ctrough,SS range (weeks 2 to 12 [ng/ml])b | 0.74–1.53 |
| CLSS/F (liters/h)b | 715 (543) |
| VSS/F (liters)b | 2,230 (2,030) |
AUC0-τ,ss, the steady-state area under the concentration-time curve over the dosing interval from 0 to τ; CLSS/F, apparent oral clearance at steady state; Cmax,SS, the observed maximum plasma concentration at steady state; Cmin, minimum plasma concentration within a dosing interval; Ctrough,SS, the observed trough concentration at steady state; DNVr, ritonavir-boosted danoprevir; PK, pharmacokinetic; Tmax, time to maximum plasma concentration; t1/2, drug elimination half-life; VSS/F, apparent oral volume of distribution at steady state.
PK data are not available for the one patient who discontinued treatment at week 2.
All data are means (standard deviations) unless otherwise stated. DNVr was administered at 100/100 mg q12h.
FIG 3.
Mean DNV steady-state plasma concentration-time profile (A) and predose trough concentrations versus treatment day to week 12 in null responder G1-infected patients (B) (n = 23). DNV, danoprevir; G, genotype. PK data are not available for the one patient who discontinued treatment at week 2.
Safety.
Treatment with 100/100 mg DNVr q12h plus PegIFN alfa-2a–RBV for up to 12 weeks was safe and well tolerated (Table 4). The most common AEs occurring in ≥6 (25%) patients were headache, fatigue, pyrexia, nausea, arthralgia, myalgia, and neutropenia. Most AEs were mild to moderate in severity.
TABLE 4.
Summary of treatment-emergent adverse events and grade 3 or 4 laboratory parametersa
| Parameter or conditionb | Value (n = 24)e |
|---|---|
| No. of patients with ≥1 AE (%) | 24 (100) |
| No. of AEs | 145 |
| No. of withdrawals due to AE(s) (%) | 2 (8) |
| No. of treatment-emergent AEs (%)c | |
| Headache | 15 (63) |
| Fatigue | 6 (25) |
| Pyrexia | 6 (25) |
| Nausea | 6 (25) |
| Arthralgia | 6 (25) |
| Myalgia | 6 (25) |
| Neutropenia | 6 (25) |
| Anemia | 4 (17) |
| Influenza-like illness | 4 (17) |
| Abdominal pain | 3 (13) |
| Hemorrhoids | 3 (13) |
| Asthenia | 3 (13) |
| Injection site erythema | 2 (8) |
| Formication | 2 (8) |
| Diarrhea | 2 (8) |
| Mouth ulceration | 2 (8) |
| Bronchitis | 2 (8) |
| Upper respiratory tract infection | 2 (8) |
| Depressed mood | 2 (8) |
| Insomnia | 2 (8) |
| Rash | 2 (8) |
| Cough | 2 (8) |
| Weight decreased | 2 (8) |
| Hypothyroidism | 2 (8) |
| No. of SAEs (%)d | 2 (8) |
| No. of deaths (%) | 0 (0) |
| No. of grade 3 or 4 laboratory abnormalities (%) | |
| ALT | 0 (0) |
| AST | 1 (4) |
| Bilirubin | 1 (4) |
| Hemoglobin | 1 (4) |
| Neutrophil count | 8 (33) |
| Platelet count | 0 (0) |
Patients received ritonavir-boosted danoprevir (DNVr; 100 mg of ritonavir and 100 mg of danoprevir) every 12 hours plus PegIFN alfa-2a–RBV.
AE, adverse event; SAE, serious adverse event.
AEs reported by at least two patients.
SAEs were cryoglobulinemia (n = 1) on day 178; cellulitis (n = 1) on day 94. Both SAEs were considered unrelated to treatment by the investigator.
n, number of patients.
Two patients withdrew prematurely from study medications due to AEs. One patient, who had a medical history of depression and low mood symptoms, discontinued treatment on study day 14 due to a severe AE of interferon-related mood swings, and the other patient discontinued treatment at week 25 due to a serious AE (SAE) of type II cryoglobulinemia, described below.
A total of two SAEs were reported in two patients after completion of treatment with DNVr plus PegIFN alfa-2a–RBV in the experimental phase. One patient, with a history of type II cryoglobulinemia, experienced an SAE of cryoglobulinemia on day 178, 94 days after the last dose of DNVr, which led to discontinuation of PegIFN alfa-2a–RBV. The cryoglobulinemia was not resolved as of the last report. The second patient experienced an SAE of cellulitis on day 94, which resolved by study day 154 without an interruption in treatment. Both SAEs were considered by the investigator to be unrelated to treatment.
Grade 3 or 4 neutropenia occurred in eight patients (two G1a-infected and six G1b-infected patients; 33%), with six patients experiencing neutropenia during the experimental phase of treatment with DNVr plus PegIFN alfa-2a–RBV (occurring in one G1a patient in week 12 and in five G1b patients between days 8 and 28) and two patients during treatment with PegIFN alfa-2a–RBV alone, following 12 weeks treatment with DNVr (week 20 [G1a] and week 24 [G1b]). All patients responded to dose adjustment of PegIFN alfa-2a. Grade 3 anemia occurred in one G1b patient at week 24 of treatment (this patient also experienced grade 3 neutropenia), and one patient had an isolated grade 3 total bilirubin level at day 8 that resolved by the next assessment at day 13. No treatment-emergent grade 3 or 4 ALT elevation was observed during the study.
DISCUSSION
In this study, we assessed the efficacy and safety of DNVr with PegIFN alfa-2a–RBV in a cohort of HCV G1-infected patients who were null responders to prior PegIFN-RBV therapy. This treatment regimen was efficacious, safe, and well tolerated in this null responder study population. Overall, SVR24 rates of 67% were achieved with DNVr plus PegIFN alfa-2a–RBV for 12 weeks followed by 36 weeks of PegIFN alfa-2a–RBV. Furthermore, high SVR24 rates were observed in G1b-infected (88%) compared with G1a-infected (25%) patients. Thus, the longer-term efficacy and achievement of SVR and the safety of the treatment regimen of 100/100 mg of DNVr plus PegIFN alfa-2a–RBV were confirmed in null responders, compared with a previous study demonstrating antiviral activity over 15 days in treatment-naive patients (19).
The DAUPHINE study investigated the efficacy and safety of DNVr in combination with PegIFN alfa-2a–RBV in treatment-naive G1- or G4-infected patients. This study reports high SVR24 rates for patients treated with 100/100 or 200/100 mg of DNVr plus PegIFN alfa-2a–RBV for 24 weeks (21). The current study extends the efficacy findings of the DAUPHINE study by demonstrating that a regimen of 100/100 mg of DNVr plus PegIFN alfa-2a–RBV is highly efficacious in null responders with G1b infection as well as in treatment-naive patients.
In addition to the current study, several other protease inhibitors have been evaluated in clinical trials in null responders. For example, simeprevir in combination with PegIFN-RBV has been shown to achieve SVR24 rates of 51% versus 19% with PegIFN-RBV (23). In the phase II SILEN-C2 trial, SVR24 rates of 35% were observed with the addition of 240 mg of BI201335 once a day (QD) to the PegIFN-RBV regimen (24). However, in the absence of head-to-head clinical trials, it should be noted that direct comparison of the efficacy of these PI-based regimens to the current study regimen of DNVr plus PegIFN alfa-2a–RBV is limited by differences in study designs, patient populations, and lower limits of detection for the HCV RNA assay.
Virologic response rates differed significantly between HCV G1 subtypes. The majority of patients infected with G1b HCV (88%) achieved an SVR24 compared with only 25% of G1a-infected patients. Low SVR24 rates in G1a-infected patients were due to high rates of DNV resistance-associated virologic breakthrough and relapse. Similar findings showing lower SVR24 rates in G1a-infected than G1b-infected prior null responders were observed in the ASPIRE study, which showed that 150 mg of simeprevir plus PegIFN-RBV yielded SVR24 rates of 42% versus 58% for G1a- and G1b-infected patients, respectively (23).
During PI-based therapy, the emergence of antiviral-resistant variants is frequently associated with virologic breakthrough and relapse (8). Indeed, in this present study, all seven patients (six G1a and one G1b) who experienced either virologic breakthrough or relapse were shown to have DNV-associated resistance through the NS3 R155K substitution. The higher frequency of RAVs in DNVr-treated patients infected with HCV subtype G1a than G1b is consistent with published data of other PIs and other studies of DNVr. G1a has a lower genetic barrier to resistance than G1b, with only a single nucleotide change in the NS3 protein (AGG to AAG) required to confer resistance in HCV G1a, while two sequential nucleotide changes are required in HCV G1b (CGG to AAG) (21, 25).
The low SVR24 rates observed in G1a-infected patients in this study suggest a possible requirement for the addition of a second DAA, with a higher barrier to resistance, to DNVr-containing regimens. The feasibility of combining unboosted DNV and the nucleoside HCV polymerase inhibitor mericitabine in an interferon-free regimen was previously investigated in a short-term pilot study (26). Furthermore, in a more recent ongoing trial, both interferon-free and interferon-containing 24-week regimens of DNVr and mericitabine are being explored in null responders (27). Recently, Lok and colleagues combined two DAAs with different mechanisms of action and barriers to resistance, the NS5A replication complex inhibitor daclatasvir and the NS3 PI asunaprevir, with and without PegIFN alfa-2a–RBV(28, 29). The interferon-free combination of daclatasvir and 200 mg of asunaprevir once daily yielded an SVR12 rate of 63% in G1b patients, and the interferon-containing regimen (200 mg of asunaprevir once daily) led to a 95% (20/21) SVR12 rate in null responders consisting of mainly G1a-infected patients (G1a-infected patients, 90%; G1b-infected patients, 10%) (28, 29). Furthermore, the interferon-free regimen consisting of the combination of three DAAs—the NS3 PI ABT-450 boosted with ritonavir, the NS5A inhibitor ABT-267, and the nonnucleoside polymerase inhibitor ABT-333 plus RBV for 12 weeks—has shown high efficacy rates for both G1a-infected (89%; 25/28) and G1b-infected (100%; 17/17) null responders (30).
DNV exposure increased over the first 2 days and then declined toward steady state by approximately day 6 of dosing, reflecting the mixed inhibition/induction effects of ritonavir (31). Following achievement of steady-state DNV levels, trough concentrations remained stable across treatment weeks. DNV, even when coadministered with ritonavir, has a relatively short elimination half-life (2.19 h) that results in a high DNV peak/trough ratio (∼60). For anti-HCV agents, maintaining Ctrough concentrations above in vitro IC50 or in vivo 50% effective concentration (EC50) values is a critical target for antiviral activity (32). However, despite the fairly high peak/trough ratio, the regimen of DNVr plus PegIFN alfa-2a–RBV has been shown to be efficacious, safe, and well tolerated in larger phase 2b studies with DNVr doses up to 200/100 mg of DNVr q12h (21). Pharmacokinetic parameters from this study suggest that following multiple dosing of 100/100 mg of DNVr q12h, DNV plasma concentrations at steady state appeared higher in null responders than those previously reported for treatment-naive patients receiving 100/100 mg of DNVr q12h (19). However, the number of treatment-naive patients in the cohort receiving 100/100 mg of DNVr q12h was relatively small (n = 9), with high intersubject variability observed for DNV pharmacokinetic parameters. Subsequently, DNV pharmacokinetic data from phase II DNVr studies in both treatment-naive and null responder patients have become available, substantially increasing the size of the DNV pharmacokinetic database (data on file). Using this larger pharmacokinetic data set, DNV exposure appears similar between treatment-naive and null responder patients. Therefore, the difference observed in this phase I study is likely due to the small sample size and high pharmacokinetic variability.
Treatment with DNVr plus PegIFN-RBV was safe and well tolerated. The two SAEs that were reported were deemed unrelated to study treatment. In contrast, boceprevir and telaprevir have significant toxicity issues. In the registered studies in treatment-naive and treatment-experienced patients, treatment with either boceprevir or telaprevir was associated with a higher incidence of anemia, dysgeusia, rash, and dry skin than in PegIFN-RBV control groups (9–12, 14). Additionally, grade 3 anemia, neutropenia, leukopenia, rash, and pruritus were all more frequent in the telaprevir treatment groups (9). In the current study, no cases of severe rash and only one case of grade 3 anemia were reported. Grade 3 or 4 neutropenia occurred in eight patients (33%), and all patients responded to dose adjustment of PegIFN alfa-2a. AEs in the current study were predominantly mild or moderate in severity and similar in nature to those associated with PegIFN alfa-2a–RBV therapy (3).
Rare and reversible grade 4 ALT elevations were previously observed in four patients treated with high-dose unboosted DNV in combination with PegIFN alfa-2a–RBV (33). Reactive metabolites play a significant role in drug-induced liver injury (34). Ritonavir has been shown to inhibit CYP-mediated metabolism and substantially reduce the formation of oxidative DNV metabolites (22). Further, cumulative evidence indicates that coadministration of ritonavir with DNV can reduce or eliminate the risk of ALT elevations associated with the formation of reactive metabolites from DNV (22). Consistent with the benefits of ritonavir-boosting of DNV, no grade 3 or 4 ALT elevations were observed in this study. Similarly, no treatment-emergent grade 4 ALT elevations have been observed in a larger study of treatment-naive patients treated with DNVr plus PegIFN alfa-2a–RBV for 24 weeks in the ongoing phase IIb DAUPHINE study (21).
The current study is limited by the small patient population, and findings are therefore descriptive in nature and lack statistical power. The demographic scope and inclusion criteria also reflect limitations of the study, whereby patients with cirrhosis were excluded, and the population was predominantly male and White. In addition, small numbers preclude the possibility of examining predictors of response. Moreover, since a control arm was not included in this study, no comparison of the safety profile and efficacy could be made between DNVr plus PegIFN alfa-2a–RBV and treatment with PegIFN alfa-2a–RBV alone in null responders. Thus, further studies in larger numbers of prior null responders would be needed to confirm the efficacy and safety findings of this study and also the generalizability of these findings to patients with other baseline characteristics.
In conclusion, treatment with DNVr in combination with PegIFN-RBV for 12 weeks followed by 36 weeks of PegIFN alfa-2a–RBV was safe and well tolerated and provided high SVR24 rates in patients with HCV G1b infection who were null responders to previous PegIFN-RBV therapy. In contrast, response rates were low, and viral breakthrough rates were high in patients with G1a infection. DNVr plus PegIFN alfa-2a–RBV treatment was generally safe and well tolerated. Overall, these results show great promise for the treatment of G1b-infected prior null responders with DNVr. However, the findings of this study also suggest that further research is required using combinations of DAAs with nonoverlapping resistance profiles to specifically target the treatment of prior null responder patients infected with the more difficult to treat G1a HCV. Indeed, an ongoing clinical study, MATTERHORN, is investigating the efficacy and safety of DNVr in combination with a second DAA, the nucleoside polymerase inhibitor mericitabine, and PegIFN alfa-2a–RBV. MATTERHORN includes a large cohort of patients, and preliminary data from this study show promise for the treatment of HCV G1a- or G1b-infected null responders, with SVR4 rates of 73% and 100%, respectively, among patients with available follow-up data (27).
ACKNOWLEDGMENTS
This research was funded by F. Hoffmann-La Roche, Ltd. Support for third-party writing assistance for the manuscript was provided by F. Hoffmann-La Roche, Ltd.
All authors had access to the study data and reviewed and approved the final manuscript.
Footnotes
Published ahead of print 2 December 2013
REFERENCES
- 1.World Health Organizations 2012. Hepatitis C fact sheet no. 164. World Health Organization, Geneva, Switzerland: http://www.who.int/mediacentre/factsheets/fs164/en/index.html Accessed 11 September 2012 [Google Scholar]
- 2.Hatzakis A, Wait S, Bruix J, Buti M, Carballo M, Cavaleri M, Colombo M, Delarocque-Astagneau E, Dusheiko G, Esmat G, Esteban R, Goldberg D, Gore C, Lok AS, Manns M, Marcellin P, Papatheodoridis G, Peterle A, Prati D, Piorkowsky N, Rizzetto M, Roudot-Thoraval F, Soriano V, Thomas HC, Thursz M, Valla D, van Damme P, Veldhuijzen IK, Wedemeyer H, Wiessing L, Zanetti AR, Janssen HL. 2011. The state of hepatitis B and C in Europe: report from the hepatitis B and C summit conference. J. Viral Hepat. 18(Suppl 1):1–16. 10.1111/j.1365-2893.2011.01499.x [DOI] [PubMed] [Google Scholar]
- 3.Ghany MG, Strader DB, Thomas DL, Seeff LB. 2009. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology 49:1335–1374. 10.1002/hep.22759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Goncales FL, Jr, Haussinger D, Diago M, Carosi G, Dhumeaux D, Craxi A, Lin A, Hoffman J, Yu J. 2002. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N. Engl. J. Med. 347:975–982. 10.1056/NEJMoa020047 [DOI] [PubMed] [Google Scholar]
- 5.McHutchison JG, Lawitz EJ, Shiffman ML, Muir AJ, Galler GW, McCone J, Nyberg LM, Lee WM, Ghalib RH, Schiff ER, Galati JS, Bacon BR, Davis MN, Mukhopadhyay P, Koury K, Noviello S, Pedicone LD, Brass CA, Albrecht JK, Sulkowski MS, Study Team IDEAL 2009. Peginterferon alfa-2b or alfa-2a with ribavirin for treatment of hepatitis C infection. N. Engl. J. Med. 361:580–593. 10.1056/NEJMoa0808010 [DOI] [PubMed] [Google Scholar]
- 6.Hadziyannis SJ, Sette H, Jr, Morgan TR, Balan V, Diago M, Marcellin P, Ramadori G, Bodenheimer H, Jr, Bernstein D, Rizzetto M, Zeuzem S, Pockros PJ, Lin A, Ackrill AM. 2004. Peginterferon-α2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann. Intern. Med. 140:346–355. 10.7326/0003-4819-140-5-200403020-00010 [DOI] [PubMed] [Google Scholar]
- 7.Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M, Albrecht JK. 2001. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 358:958–965. 10.1016/S0140-6736(01)06102-5 [DOI] [PubMed] [Google Scholar]
- 8.Ghany MG, Nelson DR, Strader DB, Thomas DL, Seeff LB. 2011. An update on treatment of genotype 1 chronic hepatitis C virus infection: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology 54:1433–1444. 10.1002/hep.24641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeuzem S, Andreone P, Pol S, Lawitz E, Diago M, Roberts S, Focaccia R, Younossi Z, Foster GR, Horban A, Ferenci P, Nevens F, Mullhaupt B, Pockros P, Terg R, Shouval D, van Hoek B, Weiland O, van Heeswijk R, De Meyer S, Luo D, Boogaerts G, Polo R, Picchio G, Beumont M. 2011. Telaprevir for retreatment of HCV infection. N. Engl. J. Med. 364:2417–2428. 10.1056/NEJMoa1013086 [DOI] [PubMed] [Google Scholar]
- 10.Poordad F, McCone, Bacon JBR, Bruno S, Manns MP, Sulkowski MS, Jacobson IM, Reddy KR, Goodman ZD, Boparai N, DiNubile MJ, Sniukiene V, Brass CA, Albrecht JK, Bronowicki JP, SPRINT-2 Investigators 2011. Boceprevir for untreated chronic HCV genotype 1 infection. N. Engl. J. Med. 364:1195–1206. 10.1056/NEJMoa1010494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, Bzowej NH, Marcellin P, Muir AJ, Ferenci P, Flisiak R, George J, Rizzetto M, Shouval D, Sola R, Terg RA, Yoshida EM, Adda N, Bengtsson L, Sankoh AJ, Kieffer TL, George S, Kauffman RS, Zeuzem S. 2011. Telaprevir for previously untreated chronic hepatitis C virus infection. N. Engl. J. Med. 364:2405–2416. 10.1056/NEJMoa1012912 [DOI] [PubMed] [Google Scholar]
- 12.Sherman KE, Flamm SL, Afdhal NH, Nelson DR, Sulkowski MS, Everson GT, Fried MW, Adler M, Reesink HW, Martin M, Sankoh AJ, Adda N, Kauffman RS, George S, Wright CI, Poordad F. 2011. Response-guided telaprevir combination treatment for hepatitis C virus infection. N. Engl. J. Med. 365:1014–1024. 10.1056/NEJMoa1014463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vierling JM, Flamm SL, Gordon SC, Lawitz EJ, Bronowicki JP, Davis M, Yoshida EM, Pedicone LD, Deng W, Treitel MA, Brass CA, Albrecht JK, Jacobson IM. 2011. Efficacy of boceprevir in prior null responders to peginterferon/ribavirin: the PROVIDE study. Hepatology 54(Suppl 1):796A–797A. 10.1002/hep.24666 [DOI] [Google Scholar]
- 14.Bacon BR, Gordon SC, Lawitz E, Marcellin P, Vierling JM, Zeuzem S, Poordad F, Goodman ZD, Sings HL, Boparai N, Burroughs M, Brass CA, Albrecht JK, Esteban R. 2011. Boceprevir for previously treated chronic HCV genotype 1 infection. N. Engl. J. Med. 364:1207–1217. 10.1056/NEJMoa1009482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weiss JJ, Alcorn MC, Rabkin JG, Dieterich DT. 2012. The critical role of medication adherence in the success of boceprevir and telaprevir in clinical practice. J. Hepatol. 56:503–504. 10.1016/j.jhep.2011.05.014 [DOI] [PubMed] [Google Scholar]
- 16.Gordon SC, Lawitz EJ, Bacon BR, Sulkowski MS, Yoshida EM, Davis M, Boparai N, Sniukiene V, Burroughs M, Brass CA, Albrecht JK, Reddy KR. 2011. Adherence to assigned dosing regimen and sustained virologic response among hepatitis C genotype 1 treatment-naive and PEG/ribavirin treatment-failures treated with boceprevir plus peginterferon alfa-2b/ribavirin. J. Hepatol. 54(Suppl 1):S173–S174. 10.1016/S0168-8278(11)60430-3 [DOI] [Google Scholar]
- 17.Poordad F, Dieterich D. 2012. Treating hepatitis C: current standard of care and emerging direct-acting antiviral agents. J. Viral Hepat. 19:449–464. 10.1111/j.1365-2893.2012.01617.x [DOI] [PubMed] [Google Scholar]
- 18.Seiwert SD, Andrews SW, Jiang Y, Serebryany V, Tan H, Kossen K, Rajagopalan PT, Misialek S, Stevens SK, Stoycheva A, Hong J, Lim SR, Qin X, Rieger R, Condroski KR, Zhang H, Do MG, Lemieux C, Hingorani GP, Hartley DP, Josey JA, Pan L, Beigelman L, Blatt LM. 2008. Preclinical characteristics of the hepatitis C virus NS3/4A protease inhibitor ITMN-191 (R7227). Antimicrob. Agents Chemother. 52:4432–4441. 10.1128/AAC.00699-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gane EJ, Rouzier R, Stedman C, Wiercinska-Drapalo A, Horban A, Chang L, Zhang Y, Sampeur P, Nájera I, Smith P, Shulman NS, Tran JQ. 2011. Antiviral activity, safety, and pharmacokinetics of danoprevir/ritonavir plus PEG-IFN alpha-2a/RBV in hepatitis C patients. J. Hepatol. 55:972–979. 10.1016/j.jhep.2011.01.046 [DOI] [PubMed] [Google Scholar]
- 20.Reddy MB, Chen Y, Haznedar JO, Fretland J, Blotner S, Smith P, Tran JQ. 2012. Impact of low-dose ritonavir on danoprevir pharmacokinetics: results of computer-based simulations and a clinical drug-drug interaction study. Clin. Pharmacokinet. 51:457–465. 10.2165/11599700-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 21.Everson GT, Cooper C, Hézode C, Shiffman ML, Yoshida EM, Beltran-Jaramillo T, Andreone P, Bruno S, Ferenci P, Zeuzem S, Brunda M, Le Pogam S, Nájera I, Zhou J, Navarro MT, Voulgari A, Shulman NS, Yetzer ES. 2012. High SVR24 rates with 12 to 24 weeks of ritonavir-boosted danoprevir plus Peg-IFNalfa-2a (40KD)/RBV in HCV genotype 1 or 4 patients in the DAUPHINE study. Hepatology 56(Suppl 1):552A. 10.1002/hep.26040 [DOI] [Google Scholar]
- 22.Goelzer P, Morcos PN, Tran JQ, Wen B, Shulman NS, Brennan BJ, Hammond J, Singer T, Smith P. 2012. Coadministration of ritonavir with the HCV protease inhibitor danoprevir substantially reduces reactive metabolite formation both in vitro and in vivo. Hepatology 56(Suppl 1):580A. 10.1002/hep.26040 [DOI] [Google Scholar]
- 23.Zeuzem S, Berg T, Gane E, Ferenci P, Foster GR, Fried MW, Hézode C, Hirschfield G, Jacobson I, Nikitin I, Pockros P, Poordad F, Lenz O, Peeters M, Sekar V, De Smedt G, Beumont-Mauviel M. 2012. TMC435 in HCV genotype 1 patients who have failed previous pegylated interferon/ribavirin treatment: final SVR24 results of the ASPIRE trial. J. Hepatol. 56(Suppl 2):S1–S2. 10.1016/S0168-8278(12)60016-622300459 [DOI] [Google Scholar]
- 24.Sulkowski MS, Bourlière M, Bronowicki J-P, Asselah T, Pawlotsky JM, Shafran SD, Pol S, Mauss S, Larrey D, Datsenko Y, Stern JO, Kukolj G, Scherer J, Nehmiz G, Steinmann GG, Böcher WO. 2013. Faldaprevir combined with peginterferon alfa-2a and ribavirin in chronic hepatitis C virus genotype-1 patients with prior nonresponse: SILEN-C2 trial. Hepatology 57:2155–2163. 10.1002/hep.26386 [DOI] [PubMed] [Google Scholar]
- 25.Sarrazin C, Kieffer TL, Bartels D, Hanzelka B, Muh U, Welker M, Wincheringer D, Zhou Y, Chu HM, Lin C, Weegink C, Reesink H, Zeuzem S, Kwong AD. 2007. Dynamic hepatitis C virus genotypic and phenotypic changes in patients treated with the protease inhibitor telaprevir. Gastroenterology 132:1767–1777. 10.1053/j.gastro.2007.02.037 [DOI] [PubMed] [Google Scholar]
- 26.Gane EJ, Roberts SK, Stedman CA, Angus PW, Ritchie B, Elston R, Ipe D, Morcos PN, Baher L, Nájera I, Chu T, Lopatin U, Berrey MM, Bradford W, Laughlin M, Shulman NS, Smith PF. 2010. Oral combination therapy with a nucleoside polymerase inhibitor (RG7128) and danoprevir for chronic hepatitis C genotype 1 infection (INFORM-1): a randomised, double-blind, placebo-controlled, dose-escalation trial. Lancet 376:1467–1475. 10.1016/S0140-6736(10)61384-0 [DOI] [PubMed] [Google Scholar]
- 27.Feld JJ, Jacobson IM, Jensen DM, Foster GR, Pol S, Tam E, Berak H, Vierling JM, Tavel J, Navarro MT, Shahdad S, Kulkarni R, Le Pogam S, Nájera I, Lim CY, Shulman NS, Yetzer ES. 2012. Up to 100% SVR4 rates with ritonavir-boosted danoprevir (DNVr), mericitabine (MCB) and ribavirin with or without peginterferon alfa-2a (40KD) in HCV genotype 1-infected partial and null responders: results from the MATTERHORN study. Hepatology 56(Suppl 1):231A–232A. 10.1002/hep.26040 [DOI] [Google Scholar]
- 28.Lok AS, Gardiner DF, Lawitz E, Martorell C, Everson GT, Ghalib R, Reindollar R, Rustgi V, McPhee F, Wind-Rotolo M, Persson A, Zhu K, Dimitrova DI, Eley T, Guo T, Grasela DM, Pasquinelli C. 2012. Preliminary study of two antiviral agents for hepatitis C genotype 1. N. Engl. J. Med. 366:216–224. 10.1056/NEJMoa1104430 [DOI] [PubMed] [Google Scholar]
- 29.Lok AS, Gardiner DF, Hézode C, Lawitz E, Bourlière M, Everson GT, Marcellin P, Rodriguez-Torres M, Pol S, Serfaty L, Eley T, Huang S, Wind-Rotolo M, McPhee F, Grasela DM, Pasquinelli C. 2012. Sustained virologic response in chronic HCV genotype (GT) 1-infected null responders with combination of daclatasvir (DCV; NS5A inhibitor) and asunaprevir (ASV; NS3 inhibitor) with or without peginterferon alfa-2a/ribavirin (PEG/RBV) (oral presentation 79). Hepatology 56(Suppl 1):230A–231A. 10.1002/hep.26040 [DOI] [Google Scholar]
- 30.Kowdley KV, Lawitz E, Poordad F, Cohen DE, Nelson D, Zeuzem S, Everson GT, Kwo P, Foster GR, Sulkowski M. 2012. A 12-week interferon-free treatment regimen with ABT-450/r, ABT-267, ABT-333 and ribavirin achieves SVR12 rates (observed data) of 99% in treatment-naive patients and 93% in prior null responders with HCV genotype 1 infection. Hepatology 56(Suppl 1):1515A–1516A. 10.1002/hep.26040 [DOI] [Google Scholar]
- 31.Abbott Laboratories 2012. Norvir (ritonavir) package insert. Abbott Laboratories, North Chicago, IL 60064 [Google Scholar]
- 32.Perelson AS, Deeks SG. 2011. Drug effectiveness explained: the mathematics of antiviral agents for HIV. Sci. Transl. Med. 13:91ps30. 10.1126/scitranslmed.3002656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marcellin P, Cooper C, Balart LA, Larrey DG, Box TD, Yoshida E, Lawitz E, Buggisch P, Ferenci P, Weltman M, Labriola-Tompkins E, Le Pogam S, Nájera I, Thomas D, Hooper G, Shulman NS, Zhang Y, Navarro MT, Lim CY, Brunda M, Terrault N, Yetzer ES. 2013. Randomized controlled trial of danoprevir plus peginterferon alfa-2a/ribavirin in treatment-naive HCV genotype 1 patients. Gastroenterology 145:790–800. 10.1053/j.gastro.2013.06.051 [DOI] [PubMed] [Google Scholar]
- 34.Tujios S, Fontana RJ. 2011. Mechanisms of drug-induced liver injury: from bedside to bench. Nat. Rev. Gastroenterol. Hepatol. 8:202–211. 10.1038/nrgastro.2011.22 [DOI] [PubMed] [Google Scholar]