Abstract
Prolonged antimicrobial therapy is recommended for methicillin-susceptible Staphylococcus aureus (MSSA) bone and joint infections (BJI), but its safety profile and risk factors for severe adverse events (SAE) in clinical practice are unknown. We addressed these issues in a retrospective cohort study (2001 to 2011) analyzing antimicrobial-related SAE (defined according to the Common Terminology Criteria for Adverse Events) in 200 patients (male, 62%; median age, 60.8 years [interquartile range {IQR}, 45.5 to 74.2 years]) with MSSA BJI admitted to a reference regional center with acute (66%) or chronic arthritis (7.5%), osteomyelitis (9.5%), spondylodiscitis (16%), or orthopedic device-related infections (67%). These patients received antistaphylococcal therapy for a median of 26.6 weeks (IQR, 16.8 to 37.8 weeks). Thirty-eight SAE occurred in 30 patients (15%), with a median time delay of 34 days (IQR, 14.75 to 60.5 days), including 10 patients with hematologic reactions, 9 with cutaneomucosal reactions, 6 with acute renal injuries, 4 with hypokalemia, and 4 with cholestatic hepatitis. The most frequently implicated antimicrobials were antistaphylococcal penicillins (ASP) (13 SAE/145 patients), fluoroquinolones (12 SAE/187 patients), glycopeptides (9 SAE/101 patients), and rifampin (7 SAE/107 patients). Kaplan-Meier curves and stepwise binary logistic regression analyses were used to determine the risk factors for the occurrence of antimicrobial-related SAE. Age (odds ratio [OR], 1.479 for 10-year increase; 95% confidence interval [CI], 1.116 to 1.960; P = 0.006) appeared to be the only independent risk factor for SAE. In patients receiving ASP or rifampin, daily dose (OR, 1.028; 95% CI, 1.006 to 1.051; P = 0.014) and obesity (OR, 8.991; 95% CI, 1.453 to 55.627; P = 0.018) were associated with the occurrence of SAE. The high rate of SAE and their determinants highlighted the importance of the management and follow-up of BJI, with particular attention to be paid to older persons, especially for ASP dosage, and to rifampin dose adjustment in obese patients.
INTRODUCTION
Bone and joint infections (BJI), including septic arthritis, osteomyelitis, and orthopedic device-related infections (ODI), constitute difficult-to-treat clinical entities, as they chronically evolve and frequently cause a relapse in infection (1–4). Staphylococcus aureus is the leading cause of BJI, accounting for approximately 50% of cases, and it is particularly associated with treatment failure due to its various virulence factors, including its great ability to form biofilms (5–7). These concerns have led to the recommendation of a prolonged antimicrobial course in S. aureus BJI, frequently in combination to avoid the acquisition of drug resistance, with initial administration via the intravenous route and at a high dosage (8–10). It is well known that the prolonged use of antimicrobial combinations is associated with an increased toxicity risk (11), but only limited and conflicting data are found in the literature concerning the prevalence and predictive factors of antimicrobial-related adverse events (AE) occurring during the treatment of BJI. A recent meta-analysis concluded that the incidences of AE are 16.1% for mild AE and 7.7% for moderate and severe AE (12), but some studies found the incidence of AE can reach rates as high as 50% of the patients (13, 14). We aimed to describe the occurrence of and risk factors for antimicrobial-related AE in a retrospective cohort study that included patients admitted to a regional reference center with methicillin-susceptible S. aureus (MSSA) BJI.
MATERIALS AND METHODS
Ethics statements.
This study received the approval of the French South-East ethics committee with the reference number CAL2011-021. In accordance with French legislation, written informed patient consent was not required for any part of the study.
Inclusion criteria and data collection.
All patients with MSSA BJI who followed up in our institution between 2001 and 2011 were enrolled in a retrospective cohort study. Patients with diabetic foot- and decubitus ulcer-related BJI were excluded because of the particular pathophysiology proceeded by contiguity with bone exposition and implicating diabetes-associated vascular disease and peripheral neuropathy, leading to a specific management of these infections. For each patient, data were collected from medical records, nursing charts, and biological software in an anonymous standardized case report form.
Definitions.
BJI diagnosis was based upon the existence of clinical and biological evidences of infection and at least one reliable bacteriological sample that was positive for MSSA (i.e., percutaneous joint fluid aspiration, surgical sample, and/or blood culture). BJI were classified according to (i) the existence of an orthopedic implant (i.e., osteosynthetic material, joint prosthesis, or external fixator) and (ii) the time from the initiation of infection symptoms to diagnosis, with infections defined as acute (infection lasting for ≤4 weeks) or chronic.
The Charlson comorbidity index was calculated as previously described (15). Immunosuppression was defined as (i) steroid therapy of >10 mg of prednisone per day or the equivalent for ≥3 months, (ii) immunosuppressive drug therapy during the two months before BJI onset, or (iii) current chemotherapy or radiotherapy treatment.
Every adverse event (AE) occurring during follow-up was classified according to the Common Terminology Criteria for Adverse Events (CTCAE) (National Cancer Institute, 2003). Severe adverse events (SAE) corresponded to CTCAE grades 3 to 5. The role of the antimicrobial agents in AE occurrence was left to the judgment of the clinician, with the help of a pharmacovigilance specialist in doubtful cases. The risk factors analyzed for SAE included age, a Charlson comorbidity index of ≥2, obesity (body mass index [BMI] > 30 kg/m2), denutrition (BMI < 18 kg/m2), BJI type, mechanism, and presentation (i.e., acute or chronic, fever, fistula, abscess, biological inflammatory syndrome), time delay from diagnosis to infectious disease specialist referral, and the antibiotic administration characteristics (route, duration, and doses).
Statistical analysis.
Descriptive statistics were used to estimate the frequencies of the study variables, described as effectives (%) for dichotomous values and medians (interquartile range [IQR]) for continuous values. For the percentage calculation of each variable, the number of missing values was excluded from the denominator. Nonparametric statistical methods were used to compare the study groups (chi-square test, Fisher's exact test, or Mann-Whitney U test, as appropriate). Kaplan-Meier curves were compared between the groups using the log-rank test. Stepwise binary logistic regression analysis was used to determine the risk factors for the first antimicrobial-related SAE. After checking the variables for interactions, variables with medical meaning and with P values obtained in the univariate analysis of <0.15 were included in the final multivariate model, adopting a ratio of 10 events per independent variable to avoid overfitting. A P value of <0.05 was considered significant. All analyses were performed using SPSS software version 17.0 (SPSS, Chicago, IL).
RESULTS
Two hundred eleven patients with MSSA BJI were identified. After excluding diabetic foot-related (n = 3) and decubitus ulcer-related (n = 1) BJI and medical records with insufficient data (n = 7), 200 patients were included in the analysis.
Demographic characteristics, underlying conditions, and BJI presentation.
There were 124 male (62%) patients. The median age was 60.8 years (IQR, 45.5 to 74.2 years), with 103 patients (51.5%) >60 years of age. The mean Charlson comorbidity index was ≥2 in 61 patients (30.5%). The patient comorbidities are detailed in Table 1.
TABLE 1.
Demographic characteristics, underlying conditions, and BJI presentation of the 200 included patientsa
| Patient datab | Total (n = 200) | Patient group |
|
|---|---|---|---|
| Chronic BJI (n = 66) | ODIc (n = 134) | ||
| Sex (male) | 124 (62) | 46 (69.7) | 82 (61.2) |
| Age (median [IQR]) (yr) | 60.8 (45.5–74.2) | 55.8 (42.0–72.2) | 60.8 (44.5–75.3) |
| Comorbidity | |||
| Charlson score (IQR) | 0.0 (0.0–2.0) | 0.0 (0.0–2.0) | 0.0 (0.0–2.0) |
| Obesity (BMI > 30) | 39 (20) | 6 (9.4)d | 26 (20.0) |
| Denutrition (BMI < 18) | 9 (4.6) | 6 (9.4) | 6 (4.6) |
| Diabetes | 27 (13.5) | 8 (12.1) | 16 (11.9) |
| Immunodepression | 23 (11.5) | 4 (6.1) | 15 (11.2) |
| Nephropathy | 28 (14) | 9 (13.6) | 18 (13.4) |
| Hepatopathy | 5 (2.5) | 0 (0) | 3 (2.2) |
| Chronic pulmonary disease | 30 (15) | 5 (7.6)d | 18 (13.4) |
| Chronic heart failure | 23 (11.5) | 4 (6.1) | 18 (13.4) |
| Chronic inflammatory disease | 24 (12) | 6 (9.1) | 17 (12.7) |
| Neoplasm/hemopathy | 21 (10.5) | 5 (7.6) | 14 (10.4) |
| Dementia | 7 (3.5) | 1 (1.5) | 6 (4.5) |
| BJI type | |||
| Arthritis | 15 (7.5) | 2 (3.0) | NAe |
| Osteomyelitis | 19 (9.5) | 16 (24.2)d | NA |
| Vertebral osteomyelitis | 32 (16) | 10 (15.2) | NA |
| Orthopedic device infection | 134 (67) | 38 (57.6) | NA |
| Joint prosthesis | 76 (56.7) | 17 (44.7)d | 76 (56.7) |
| Osteosynthesis | 48 (35.8) | 20 (52.6) | 48 (35.8) |
| Vertebral osteosynthesis | 10 (7.5) | 1 (2.6) | 10 (7.5) |
| BJI mechanism | |||
| Hematogenous (for clinician) | 74 (37) | 15 (22.7)d | 34 (25.4)f |
| Inoculation | 121 (60.5) | 48 (72.7)d | 99 (73.9)f |
| Contiguity | 5 (2.5) | 3 (4.5) | 1 (0.7) |
| BJI diagnosis | |||
| Fever | 125 (62.5) | 20 (30.3)d | 82 (61.2) |
| Fistula | 86 (43.4) | 42 (63.6)d | 68 (50.7)f |
| Abscess | 78 (39) | 25 (37.9) | 45 (33.6)f |
| Chronic BJI (>4 wk) | 66 (33) | NA | 38 (28.4)f |
| Polymicrobial BJI | 31 (15.5) | 15 (22.7) | 21 (15.7) |
| Biological inflammatory syndrome | 188 (94) | 59 (89.4) | 130 (97.0)f |
| No. with positive blood culture/no. of total cultures (% positive) | 70/111 (63.1) | 8/23 (34.8)d | 35/65 (53.8)f |
| Initial hospitalization (median [IQR]) (wk) | 3.4 (1.6–7.0) | 2.0 (1.0–3.9)d | 2.9 (1.5–6.0) |
| Surgical treatment | 164 (82) | 54 (81.8) | 127 (94.8)f |
| Antibiotic use | |||
| Delay from diagnosis to specialist referral (median [IQR]) (wk) | 1.0 (0.0–3.0) | 0.0 (−0.3–3.0)d | 1.1 (0.0–2.8) |
| Total treatment duration (median [IQR]) (wk) | 26.6 (16.8–37.8) | 24.7 (17.7–40.5) | 26.0 (15.6–37.6) |
| Intravenous treatment | 182 (91) | 57 (86.4) | 123 (91.8) |
| Intravenous treatment duration (median [IQR]) (wk) | 7.4 (4.9–14.4) | 7.1 (4.6–14.7) | 7.4 (4.7–14.7) |
| Antimicrobial combination therapy | 200 (100) | 66 (100) | 134 (100) |
| Combination therapy duration (median [IQR]) (wk) | 24.6 (14.1–31.1) | 22.2 (14.2–31.0) | 23.0 (13.7–31.0) |
| Favorable clinical outcome | 117 (59.7) | 41 (62.1) | 68 (51.9)f |
The results are presented as no. (%) unless otherwise noted. For the percentage calculation of each variable, the number of missing values was excluded from the denominator. Nonparametric statistical methods were used to compare groups (chi-square test, Fisher's exact test, or Mann-Whitney U test, as appropriate).
IQR, interquartile range; BMI, body mass index; BJI, bone and joint infection.
ODI, orthopedic device infection.
Significant difference compared to acute bone and joint infections (P < 0.05).
NA, not applicable.
Significant difference compared to native bone and joint infections (P < 0.05).
There were 15 native joint infections (7.5%), 19 osteomyelitis cases (9.5%), 32 spondylodiscitis cases (16%), and 134 orthopedic device infections (67%). The orthopedic device infections included 76 prosthetic-joint infections (PJI) (consisting of 41 in the hip, 32 in the knee, 2 in the shoulder, and 1 in an ankle implant). The median time delay from symptoms to diagnosis was 1.3 weeks (IQR, 0.3 to 7.2 weeks), defining 66 (33%) cases as chronic BJI. Laboratory testing disclosed an inflammatory syndrome in 188 patients (94%), with a median maximal plasmatic C-reactive protein (CRP) level of 162.0 mg/liter (IQR, 80.0 to 298.9 mg/liter) and a white blood cell count of 10,810 cells/mm3 (IQR, 8,200 to 14,300 cells/mm3). Blood cultures were positive for MSSA in 70 of the 111 (63.1%) patients in whom they were realized, including primitive bacteremia with bone and joint metastatic septic focus and bacteremia resulting from BJI itself. Infection was considered by a clinician to be hematogenous in 74 cases (37%).
Treatments and outcomes.
All but 12 patients were hospitalized, with an initial mean stay of 3.4 weeks (IQR, 1.6 to 7.0 weeks). Surgical treatment was performed in 164 cases (82%), consisting of an orthopedic implant removal in 84 patients (65.6%), with no residual material in 70 cases (52.2%). The advice of an infectious disease specialist was sought in all cases, with a median time delay from microbiological diagnosis of 1.0 week (IQR, 0.0 to 3.0 weeks). The mean total duration of antibiotic therapy was 26.6 weeks (IQR, 16.8 to 37.8 weeks). All patients received initial combination therapy for a median duration of 24.6 weeks (IQR, 14.1 to 31.1 weeks). Intravenous administration of antibiotics was used in 182 patients (91%) for a median duration of 7.4 weeks (IQR, 4.9 to 14.4 weeks). The clinical outcome was favorable in 117 cases (59.7%), with a median follow-up time of 85.0 weeks (IQR, 49.0 to 154.4 weeks). Treatment failure rate was higher for those with an ODI (P < 0.001) (Table 1).
Description of antimicrobial-related adverse events and analysis of risk factors.
Ninety patients (45%) presented with at least one antimicrobial-related AE. Because of a declaration of bias toward mild side effects (CTCAE grade 1 to 2) due to the retrospective nature of the study, only the 38 SAE recorded in 30 patients (15%) were analyzed. They corresponded to 10 hematologic disorders (8 neutrophil count decreases, including 3 febrile neutropenia cases, 1 pancytopenia case, and 1 anemia case), 9 allergic reactions (5 generalized maculopapular rashes, 3 Stevens-Johnson syndrome cases, and 1 anaphylactic shock case), 6 acute renal injuries, 4 hypokalemia cases, 4 hepatic disorders (3 cholestatic hepatitis cases and 1 acute hepatic failure case), 3 upper digestive tract disorders (2 severe vomiting cases and 1 duodenal ulcer case), and 3 nervous system disorders (1 cognitive disturbance case and 1 ototoxicity case) (Table 2). They included 27 grade 3 SAE, 10 grade 4 SAE, and 1 grade 5 SAE. The most frequently involved antimicrobials were antistaphylococcal penicillins (n = 13), fluoroquinolones (n = 12), glycopeptides (n = 9), and rifampin (n = 7) (Table 3). In 14 cases (46.7%), these SAE were not attributed to a particular antibiotic. They occurred a median of 34.0 days (IQR, 14.75 to 60.5 days) after the initiation of the involved antimicrobial(s), during initial hospitalization stay (n = 23), in a rehabilitation center (n = 11), or after patients were discharged home (n = 4). Eighteen patients (60%) were admitted to the hospital or had a prolonged initial hospital stay due to the occurrence of SAE, with a median duration stay of 8.0 days (IQR, 4.0 to 29.0 days), that led to an increase in initial hospitalization stay (P = 0.003). Thirty SAE (78.9%) led to a disruption in treatment. In cases of acute renal failure or hypokalemia, a dose adjustment, hydration regimen, and/or potassium supplementation were started. The SAE resolved in all but two cases: one patient with acute renal failure due to a regimen of an antistaphylococcal penicillin with an aminoglycoside progressed to chronic renal failure, and one patient (80-year-old male with a Charlson comorbidity index of 6 and preexisting chronic renal failure) died from acute renal failure that occurred while receiving a combination therapy of ofloxacin and pristinamycin.
TABLE 2.
Description of the 38 SAEa occurring in 30 patients during antimicrobial course
| Type of SAE (n) | Subtype of SAE (n) | CTCAEb grade | Antimicrobial(s) involved (n) | Time from treatment initiation to SAE (median [IQR]) (days) |
|---|---|---|---|---|
| Hematologic disorders (10) | Neutrophil count decrease (5), febrile neutropenia (3), anemia (1), pancytopenia (1) | Grade 3 (5), grade 4 (5) | β-Lactams (7), fluoroquinolones (1), glycopeptides (1), fosfomycin (1), linezolid (1) | 26.0 (13.25–35.0) |
| Allergic reactions (9) | Maculopapular rash (5), Stevens-Johnson syndrome (3), anaphylactic shock (1) | Grade 3 (5), grade 4 (4) | Glycopeptides (5), fluoroquinolones (6), rifampin (4), β-lactams (3), macrolide group (1) | 20.0 (11.0–22.0) |
| Renal disorders (6) | Acute kidney injury (6) | Grade 3 (4), grade 4 (1), grade 5 (1) | β-Lactams (4), aminoglycosides (4), fluoroquinolones (4), glycopeptides (3), macrolide group (1) | 2.0 (1.25–7.25) |
| Metabolic disorders (4) | Hypokalemia (4) | Grade 3 (4) | Fosfomycin (4) | 21.0 (15.75–21.0) |
| Hepatobiliary disorders (4) | Blood bilirubin increase (2), blood GGTc increase (1), hepatic failure (1) | Grade 3 (4) | β-Lactams (3), rifampin (1), fluoroquinolones (1), fusidic acid (1), cotrimoxazole (1) | 43.5 (18.0–65.75 |
| Gastrointestinal disorders (3) | Vomiting (2), duodenal ulcer (1) | Grade 3 (3) | Rifampin (2), macrolide group (1) | 7.0 (4.0–86.0) |
| Nervous system disorders (2) | Cognitive disturbance (1), ototoxicity (1) | Grade 3 (2) | β-Lactams (1), fosfomycin (1) | 7.0 (4.5–9.5) |
SAE, severe adverse event.
CTCAE, Common Terminology Criteria for Adverse Events.
GGT, gamma glutamyl transpeptidases.
TABLE 3.
Description of the SAE observed for the main antimicrobials useda
| Antimicrobial(s) (n) | Type of SAEa (n) | CTCAEb grade (n) | Time from treatment initiation to SAE (median [IQR]) (days) |
|---|---|---|---|
| β-Lactams (17): ASPc (13), others (4) | Hematologic disorders (7), acute kidney injuries (4), allergic reactions (3), hepatobiliary disorders (2), cognitive disturbance (1) | Grade 3 (13), grade 4 (4) | 28.0 (7.0–63.0) |
| Fluoroquinolones (10) | Allergic reactions (6), acute kidney injuries (2), hematologic disorders (1), hepatobiliary disorders (1) | Grade 3 (3), grade 4 (6), grade 5 (1) | 20.0 (12.5–49.25) |
| Glycopeptides (9) | Allergic reactions (5), acute kidney injuries (3), hematologic disorders (1) | Grade 3 (5), grade 4 (3) | 20.0 (2.0–20.0) |
| Rifampin (7) | Allergic reactions (4), vomiting (2), blood bilirubin increase (1) | Grade 4 (3), grade 3 (4) | 20.0 (20.0–24.5) |
SAE, severe adverse event.
CTCAE, Common Terminology Criteria for Adverse Events.
ASP, antistaphylococcal penicillin.
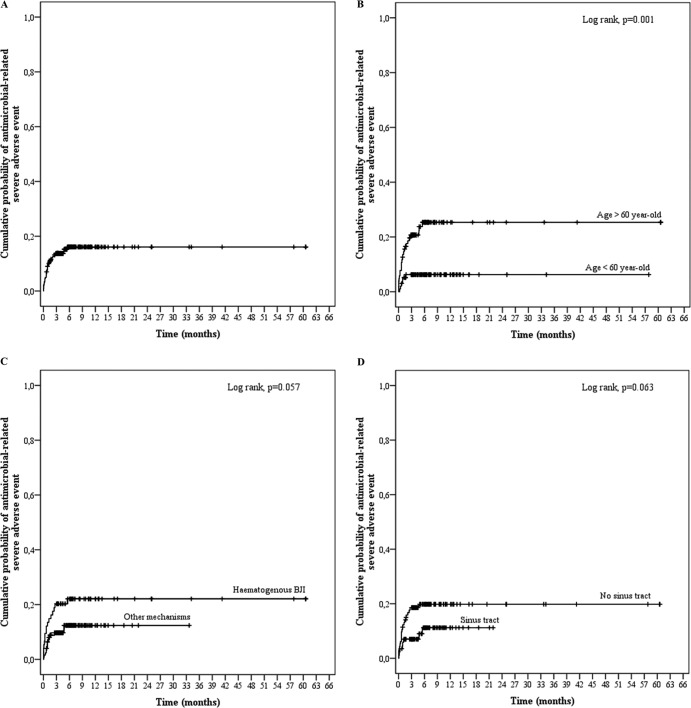
In univariate analysis, age (OR, 1.451 for a 10-year increase in age; 95% confidence interval [CI], 1.134 to 1.857; P = 0.003) and a hematogenous BJI mechanism (OR, 2.207; 95% CI, 1.007 to 4.835; P = 0.048) were significantly associated with the occurrence of antimicrobial-related SAE (Table 4). Clinically relevant and noninteracting factors with a P value of <0.15 in the univariate model were included in the multivariate analysis, which pinpointed age (OR, 1.383 for 10-year increase; 95% CI, 1.075 to 1.775; P = 0.011) as the only independent risk factor for antistaphylococcal therapy-related toxicity (Table 4 and Fig. 1).
TABLE 4.
Risk factor analysis for antimicrobial-related severe adverse eventsa
| Patient data | SAEb (n = 30) | No SAE (n = 170) | P | Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|---|---|---|
| OR (95% CI)c | P | OR (95% CI) | P | ||||
| Sex (male) | 15 (50) | 109 (64.1) | 0.142 | 0.560 (0.256–1.222) | 0.145 | 0.678 (0.300–1.533) | 0.350 |
| Age (median [IQR]) (yr) | 67.4 (62.1–80.1) | 57.4 (43.6–72.5) | 0.001 | 1.451 (1.134–1.857)d | 0.003 | 1.382 (1.075–1.775)d | 0.011 |
| Age > 60 yr | 24 (80) | 79 (46.5) | <0.001 | ||||
| Comorbidity | |||||||
| Charlson score ≥2 | 10 (33.3) | 51 (30.0) | 0.714 | 1.167 (0.510–2.667) | 0.715 | ||
| Obesity (BMI > 30) | 8 (26.7) | 31 (18.8) | 0.283 | 1.572 (0.640–3.860) | 0.324 | ||
| Denutrition (BMI < 18) | 1 (3.3) | 8 (4.7) | 0.885 | 0.677 (0.082–5.617) | 0.718 | ||
| Diabetes | 3 (10) | 24 (14.1) | 0.749 | 0.676 (0.190–2.403) | 0.545 | ||
| Immunodepression | 4 (13.3) | 19 (11.2) | 0.975 | 1.223 (0.385–3.884) | 0.733 | ||
| Nephropathy | 5 (16.7) | 23 (13.5) | 0.865 | 1.278 (0.445–3.675) | 0.649 | ||
| Hepatopathy | 2 (6.7) | 3 (1.8) | 0.341 | 3.976 (0.636–24.872) | 0.140 | 2.791 (0.429–18.147) | 0.283 |
| Chronic pulmonary disease | 8 (26.7) | 22 (12.9) | 0.052 | 2.446 (0.970–6.168) | 0.058 | ||
| Chronic heart failure | 4 (13.3) | 19 (11.2) | 0.975 | 1.223 (0.385–3.884) | 0.733 | ||
| Chronic inflammatory disease | 4 (13.3) | 20 (11.8) | 0.950 | 1.154 (0.365–3.649) | 0.808 | ||
| Neoplasm/hemopathy | 3 (10) | 18 (10.6) | 0.821 | 0.938 (0.259–3.405) | 0.923 | ||
| Dementia | 0 (0) | 7 (4.1) | 0.522 | NCd | NCd | ||
| BJI type | |||||||
| Arthritis | 3 (10) | 12 (70.6) | 0.852 | 1.463 (0.387–5.528) | 0.575 | ||
| Osteomyelitis | 3 (10) | 16 (9.4) | 0.813 | 1.069 (0.292–3.921) | 0.919 | ||
| Vertebral osteomyelitis | 6 (20) | 26 (15.3) | 0.705 | 1.385 (0.516–3.716) | 0.518 | ||
| Orthopedic device infection | 18 (60) | 116 (68.2) | 0.377 | 0.698 (0.314–1.552) | 0.378 | ||
| Prosthetic-joint infection | 15 (50) | 61 (35.9) | 0.142 | ||||
| Osteosynthesis | 1 (3.3) | 43 (25.3) | 0.015 | ||||
| Vertebral osteosynthesis | 2 (6.7) | 8 (4.7) | 1.000 | ||||
| External fixator | 0 (0) | 3 (1.8) | 0.933 | ||||
| Other | 0 (0) | 1 (0.6) | 0.356 | ||||
| Bone and joint infection mechanism | |||||||
| Hematogenous (for clinician) | 16 (53.3) | 58 (34.1) | 0.044 | 2.207 (1.007–4.835) | 0.048 | 1.372 (0.554–3.400) | 0.494 |
| Inoculation | 13 (43.3) | 108 (63.5) | 0.037 | 0.439 (0.200–0.964) | 0.040 | ||
| Contiguity | 1 (3.3) | 4 (2.4) | 0.751 | 1.431 (0.154–13.262) | 0.752 | ||
| BJI diagnosis | |||||||
| Fever | 23 (76.7) | 102 (60) | 0.082 | 2.190 (0.891–5.388) | 0.088 | ||
| Fistula | 8 (26.7) | 78 (46.4) | 0.050 | 0.429 (0.181–1.017) | 0.055 | 0.366 (0.233–1.710) | 0.0.366 |
| Abscess | 11 (36.7) | 67 (39.4) | 0.776 | 0.890 (0.398–1.988) | 0.776 | ||
| Chronic BJI (>4 wk) | 8 (26.7) | 58 (34.1) | 0.424 | 0.702 (0.294–1.675) | 0.425 | ||
| Polymicrobial BJI | 6 (20) | 25 (14.7) | 0.642 | 1.450 (0.539–3.903) | 0.462 | ||
| Biological inflammatory syndrome | 29 (96.7) | 159 (93.5) | 0.802 | 2.006 (0.249–16.140) | 0.513 | ||
| No. with positive blood culture/no. of total cultures (% positive) | 14/21 (66.7) | 56/90 (62.2) | 0.704 | 1.214 (0.446–3.309) | 0.704 | ||
| Initial hospitalization (median [IQR]) (wk) | 7.1 (3.4–9.3) | 3.0 (1.6–5.9) | 0.003 | ||||
| Surgical treatment | 24 (80.0) | 139 (81.8) | 0.818 | 0.892 (0.336–2.367) | 0.819 | ||
| Antibiotic use | |||||||
| Delay from diagnosis to specialist referral (median [IQR]) (wk) | 0.6 (0.0–1.6) | 1.0 (0.0–3.3) | 0.226 | 1.002 (0.998–1.006) | 0.282 | ||
| Intravenous treatment | 29 (96.7) | 153 (90) | 0.406 | 3.222 (0.413–25.167) | 0.265 | ||
| Antimicrobial combination therapy | 30 (100) | 170 (100) | NCe | NC | NC | ||
| Favorable clinical outcome | 18 (60) | 99 (59.6) | 0.856 | ||||
Results are presented as no. (%) unless otherwise noted. For the percentage calculation of each variable, the number of missing values was excluded from the denominator. Risk factors were assessed using a logistic binary regression model. Noninteracting variables with a P value of <0.15 in univariate analysis were included in the final model.
SAE, severe adverse events.
Odds ratio (95% confidence interval).
Odds ratio expressed for a 10-year increase in age.
NC, not calculable.
FIG 1.
Kaplan-Meier curves for the cumulative risk of antimicrobial-related severe adverse events (SAE) for all patients (A) and according to age (B), BJI mechanism (C), and the presence of a sinus tract (D).
Antistaphylococcal penicillin-related severe adverse events.
One hundred forty-five patients (72.5%) received intravenous antistaphylococcal penicillin at a median daily dose of 146.3 mg/kg of body weight (IQR, 131.6 to 171.4 mg/kg) for 44.0 days (IQR, 22.0 to 64.0 days). Sixty-six patients (45.5%) received a daily dose of >150 mg/kg, including 6 patients who were considered to be overdosed (received >200 mg/kg/day). The 13 SAE detailed in Table 3 occurred in a median time from initiation of antistaphylococcal penicillin treatment of 23.0 days (IQR, 5.0 to 52.0 days). Of note, another antimicrobial known to be nephrotoxic (i.e., an aminoglycoside and/or a glycopeptide) was associated with antistaphylococcal penicillin in the 4 observed cases of acute renal failure.
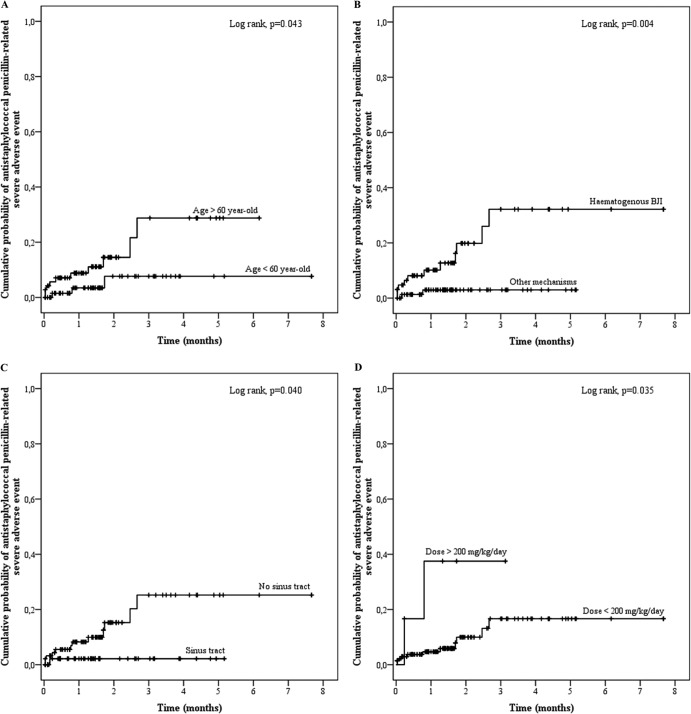
In the univariate analysis, the noninteracting factors associated with SAE occurrence were age (OR, 1.652 for a 10-year increase in age; 95% CI, 1.116 to 2.446; P = 0.012), a hematogenous BJI mechanism (OR, 8.198; 95% CI, 1.746 to 38.483; P = 0.008), and daily dose (OR, 1.016; 95% CI, 0.998 to 1.034; P = 0.087) (Fig. 2). All these factors were found to be independently associated with the occurrence of antistaphylococcal penicillin-related SAE in multivariate analysis, with ORs of 1.817 (95% CI, 1.170 to 2.820; P = 0.008), 5.927 (95% CI, 1.039 to 33.811; P = 0.045), and 1.028 (95% CI, 1.006 to 1.051; P = 0.014) for age, hematogenous BJI mechanism, and daily dose, respectively. Fistula, which was detected as a protective factor in univariate analysis (OR, 0.132; 95% CI, 0.017 to 1.049; P = 0.056), was not significantly associated with a risk of SAE in the final model.
FIG 2.
Kaplan-Meier curves for the cumulative risk of antistaphylococcal penicillin-related severe adverse events (SAE) according to age (A), BJI mechanism (B), the presence of a sinus tract (C), and the daily dose (D).
Glycopeptide-related severe adverse events.
Glycopeptides were used in 102 patients (51%) for a median of 23.0 days (IQR, 6.3 to 48.0 days), mostly as empirical therapy before a definitive bacteriological diagnosis was made (n = 33 [32.4%]), with the total duration of treatment being ≤15 days in all these cases. Other reasons to use glycopeptides were a previous allergic reaction to other antistaphylococcal antibiotics (n = 28 [27.5%]), a polymicrobial infection (n = 23 [22.5%]), and difficult venous access (n = 13 [12.7%]). The antibiotics teicoplanin (n = 63 [61.8%]) and vancomycin (n = 39, 38.2%) were administered at a median dose of 5.6 mg/kg/day (IQR, 4.7 to 6.7 mg/kg/day) and 25.6 mg/kg/day (IQR, 23.2 to 32.0 mg/kg/day), respectively. Nine SAE (8.8%) occurred in these patients (Table 3), with a median time delay of 20.0 days (IQR, 2.0 to 20.0 days). Of note, 5 presumed allergic reactions were reported in patients who presented with a diffuse cutaneous rash, which might correspond to a vancomycin-induced nonspecific histamine release (“red man syndrome”). In a univariate logistic regression model, only age tended to be associated with glycopeptide-related SAE (OR, 1.388; 95% CI, 0.901 to 2.139; P = 0.137). Baseline renal function, estimated by the glomerular filtration rate (Cockcroft-Gault formula) was not associated with glycopeptide-related SAE (OR, 1.008; 95% CI, 0.996 to 1.021; P = 0.205).
Fluoroquinolone-related severe adverse events.
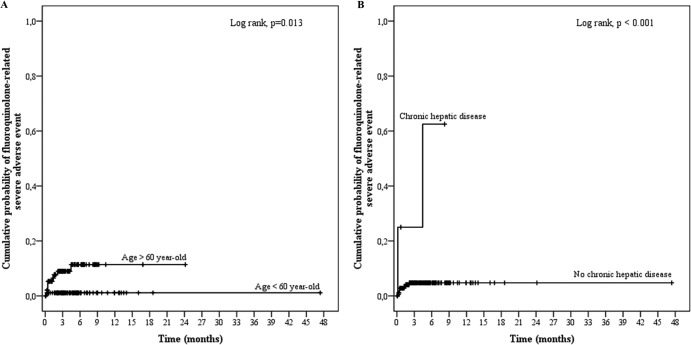
One hundred eighty-seven patients (93.5%) received fluoroquinolones for a median duration of 112.5 days (IQR, 65.8 to 184.5 days). The main antibiotic used was ofloxacin (n = 167 [89.3%]), mainly at a dose of 200 mg twice a day (n = 96 [57.5%]) or 200 mg three times a day (n = 58 [34.7%]), resulting in a median daily dose of 400.0 mg (IQR, 400 to 600 mg). Ten SAE occurred in these patients, with a median time delay of 20.0 days (IQR, 12.5 to 49.25 days) (Table 3 and Fig. 3). In the univariate analysis, the factors associated with fluoroquinolone-related SAE were age (OR, 1.445; 95% CI, 0.960 to 2.174; P = 0.078), a Charlson score of ≥2 (OR, 2.804; 95% CI, 0.750 to 10.489), and hepatopathy (OR, 14.500; 95% CI, 2.116 to 99.344; P = 0.006). None of these variables was significantly associated with SAE in the final multivariate model.
FIG 3.
Kaplan-Meier curves for the cumulative risk of fluoroquinolone-related severe adverse events (SAE) according to age (A) and the existence of chronic hepatic disease (B).
Rifampin-related SAE.
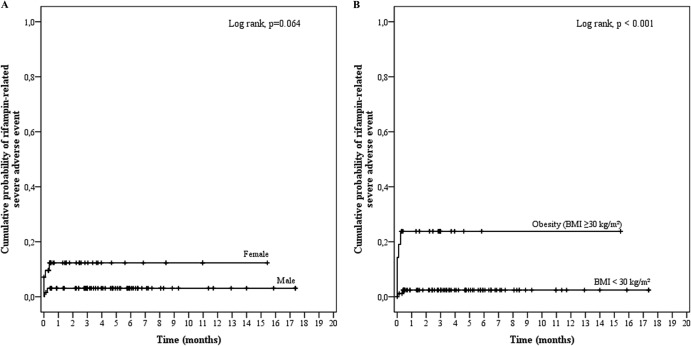
Rifampin was prescribed in 107 patients (53.5%) for a median of 101.0 days (IQR, 39.5 to 187.5 days). Rifampin was mainly used at a dose of 600 mg (n = 60 [56.1%]) or 900 mg (n = 23 [21.5%]) twice a day, for a median daily dose of 18.8 mg/kg (IQR, 16.2 to 21.2 mg/kg). Seven SAE occurred in these patients, with a time delay of 20.0 days (IQR, 20.0 to 24.5 days), and were associated with female sex (OR, 4.257; 95% CI, 0.786 to 23.054; P = 0.093) and obesity (OR, 12.969; 95% CI, 2.311 to 72.783; P = 0.004) (Table 3 and Fig. 4). After multivariate analysis, only obesity was found to be independently associated with rifampin-related SAE, with an OR of 8.991 (95% CI, 1.453 to 55.627; P = 0.018). Indeed, the weights of the patients suffering from rifampin-induced SAE (median, 90.0 kg [IQR, 44.0 to 104.0 kg]) were significantly higher than those receiving rifampin and were without SAE (71.5 kg [IQR, 63.0 to 81.6 kg]; P = 0.016). The daily dose of rifampin was significantly higher in the obese patients (1,500 mg; IQR, 1,200 to 1,800 mg) than in the normal-weight patients (1,200 mg; IQR, 1,200 to 1,500 mg; P = 0.021) but did not appear in the regression model analysis to be directly linked to the occurrence of SAE.
FIG 4.
Kaplan-Meier curves for the cumulative risk of rifampin-related severe adverse events (SAE) according to sex (A) and body mass index (BMI) (B).
DISCUSSION
This retrospective cohort study of 200 patients with MSSA BJI provides the largest published set of data regarding the tolerance of prolonged antimicrobial use for the treatment of BJI. The demographic characteristics of patients with BJI and the clinical presentation of BJI were similar to what is usually described, with a predominance of male patients and a median age of 60 years old (1–4, 16, 17). The main difference between our study and the current recommendations or recent published data is a longer duration of treatment, justified by the high rate of difficult-to-treat BJI, i.e., infections occurring in debilitated patients, the presence of bacteremia (>60%) or sinus tract or local abscess (39%), and for orthopedic device infections, a high rate of incomplete device removal (48%) or delayed surgical management (6, 18–20); also, these BJI were caused by S. aureus, which is known to be an independent risk factor of treatment failure (6, 7). The complicated nature of the included BJI was confirmed by the high rate of treatment failure (40%) despite prolonged antimicrobial combination therapy.
Our results pinpointed a high rate of SAE (15% of patients), consistent with the few other reports existing in the literature. In the Cochrane meta-analysis run in 2013 (12), the global incidence rates of mild and moderate-to-severe AE were 16.1% and 7.7%, respectively, with no difference between the oral and parenteral routes for antimicrobial administration. A similar antimicrobial-related toxicity rate was observed in the study of Pulcini et al. (14), including 129 patients receiving antimicrobial therapy for a mean of 205 days (SD, 200), initially intravenously administered for 133 days (SD, 100), with 18 patients (16%) experiencing antimicrobial-related adverse events.
In most cases, these antimicrobial-related SAE led to a disruption in treatment. Although our study failed to disclose any difference in terms of therapeutic success, treatment interruption can adversely affect patient outcomes due to the use of less suitable antibiotics or an earlier switch to oral therapy. In addition, the occurrence of AE led to patient hospitalization in 60% of cases for a median duration of 1 week, resulting in an increase in the overall cost of care.
Severe AE occurrence was independently associated with age and prolonged treatment duration. Since the age and underlying conditions of patients cannot be modified, these results stress the need to develop clinical trials aiming to reduce the treatment duration for these infections. Although our results failed to link the occurrence of SAE with an early referral to an infectious disease specialist, an early interaction between surgeons, microbiologists, and infectious disease clinicians might help reduce the incidence of antimicrobial-related SAE, in addition to being cost-efficient regarding the improvement of antibiotic therapy quality in BJI (21, 22). For instance, Pestotnik et al. (23) showed a decrease by 30% in the incidence of AE after the application of a computer-assisted antimicrobial prescription support based on clinician recommendations.
β-Lactams, and especially antistaphylococcal penicillins, were the most frequent antimicrobials involved in SAE occurrence, for which the daily dose appeared to be an independent risk factor. Allergic reactions (i.e., mucocutaneous events) were unexpectedly low (n = 3), but data collection and the CTCAE classification did not allow for specification of the mechanisms of the other observed AE. Antistaphylococcal penicillins are well known for inducing allergic interstitial nephritis characterized by acute and often severe renal failure, as well as dose-dependent hypersensitivity hepatitis (24–26), which can correspond to the acute renal failure cases (n = 4) and hepatobiliary disorders (n = 2) observed in our series. Hematologic reactions (n = 7) are also frequently immune-mediated and can consist of neutropenia, hemolytic anemia, or acute thrombopenia (25, 27).
Concerning rifampin, obesity was retained as the only independent risk factor for SAE incidence. Since rifampin daily dose alone was not linked with SAE, it appeared to be significantly higher in obese patients, probably due to a weight-guided dose adjustment. Little is known about the use of antimicrobials, especially rifampin, in obese patients, in whom drug pharmacokinetics may greatly vary due to differences in tissue distribution, protein binding, metabolism, and clearance of antimicrobials (28). The rifampin dose adjustment made for obese patients may point to the benefit of using the ideal body weight instead of the total weight, as has been described for many antimicrobials (29, 30).
Some limitations of our study should be addressed. Its retrospective observational design resulted in a reporting bias with a risk of underestimating minor AE. We tried to minimize this risk by studying SAE only. Another pitfall lies in the lack of information about other medications associated with antimicrobial therapy, as polymedication is a well-known risk factor for drug-related AE (31). In the same way, the heterogeneity of patient management and the frequent modifications of antimicrobial regimens during treatment prevented us from analyzing the risk of SAE associated with different antibiotics, which pertains to all patients in the study. Although a recent meta-analysis failed to disclose any difference in terms of AE occurrence between different antimicrobial regimens during the treatment of osteomyelitis (12), more powerful studies are needed.
In conclusion, the SAE rate was high (15%) in this cohort of patients with complicated MSSA BJI requiring long-term antimicrobial therapy. Clinicians should be aware of the risk of SAE in elderly and debilitated patients, especially when calculating antistaphylococcal penicillin dosages and rifampin dose adjustment in obese patients. These findings warrant the development of studies aimed at reducing the antimicrobial treatment length in BJI.
ACKNOWLEDGMENTS
The members of the Lyon Bone and Joint Infection Study Group are Florence Ader, François Biron, André Boibieux, Anissa Bouaziz, Evelyne Braun, Christian Chidiac, Fatiha Daoud, Tristan Ferry, Judith Karsenty, Johanna Lippman, Patrick Miailhes, Thomas Perpoint, Dominique Peyramond, Marie-Paule Vallat, and Florent Valour (physicians), Cédric Barrey, Pierre Breton, Fabien Boucher, Romain Desmarchelier, Michel-Henry Fessy, Olivier Guyen, Christophe Lienhart, Sébastien Lustig, Alain-Ali Mojallal, Philippe Neyret, Franck Trouillet, and Gualter Vaz (surgeons), Frédéric Laurent, Jean-Philippe Rasigade, and François Vandenesch (microbiologists), Emmanuel Deshayes, Francesco Giammarile, Marc Janier, and Isabelle Morelec (nuclear medicine specialists), Marie-Claude Gagnieu, Sylvain Goutelle, and Michel Tod (pharmacokinetics/pharmacodynamics specialists), and Marion Martinez (clinical research assistant).
We report no potential conflicts of interest.
Footnotes
Published ahead of print 18 November 2013
REFERENCES
- 1.Lew DP, Waldvogel FA. 2004. Osteomyelitis. Lancet 364:369–379. 10.1016/S0140-6736(04)16727-5 [DOI] [PubMed] [Google Scholar]
- 2.Mathews CJ, Weston VC, Jones A, Field M, Coakley G. 2010. Bacterial septic arthritis in adults. Lancet 375:846–855. 10.1016/S0140-6736(09)61595-6 [DOI] [PubMed] [Google Scholar]
- 3.Zimmerli W. 2010. Clinical practice. Vertebral osteomyelitis. N. Engl. J. Med. 362:1022–1029. 10.1056/NEJMcp0910753 [DOI] [PubMed] [Google Scholar]
- 4.Zimmerli W, Trampuz A, Ochsner PE. 2004. Prosthetic-joint infections. N. Engl. J. Med. 351:1645–1654. 10.1056/NEJMra040181 [DOI] [PubMed] [Google Scholar]
- 5.Brady RA, Leid JG, Calhoun JH, Costerton JW, Shirtliff ME. 2008. Osteomyelitis and the role of biofilms in chronic infection. FEMS Immunol. Med. Microbiol. 52:13–22. 10.1111/j.1574-695X.2007.00357.x [DOI] [PubMed] [Google Scholar]
- 6.Byren I, Bejon P, Atkins BL, Angus B, Masters S, McLardy-Smith P, Gundle R, Berendt A. 2009. One hundred and twelve infected arthroplasties treated with ‘DAIR' (debridement, antibiotics and implant retention): antibiotic duration and outcome. J. Antimicrob. Chemother. 63:1264–1271. 10.1093/jac/dkp107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee J, Kang CI, Lee JH, Joung M, Moon S, Wi YM, Chung DR, Ha CW, Song JH, Peck KR. 2010. Risk factors for treatment failure in patients with prosthetic joint infections. J. Hosp. Infect. 75:273–276. 10.1016/j.jhin.2010.03.012 [DOI] [PubMed] [Google Scholar]
- 8.Osmon DR, Berbari EF, Berendt AR, Lew D, Zimmerli W, Steckelberg JM, Rao N, Hanssen A, Wilson WR, Infectious Diseases Society of America 2013. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin. Infect. Dis. 56:e1–e25. 10.1093/cid/cis803 [DOI] [PubMed] [Google Scholar]
- 9.Société de Pathologie Infectieuse de Langue Française (SPILF) 2007. Primary infectious spondylitis, and following intradiscal procedure, without prothesis [sic]. Recommendations. Med. Mal Infect. 37:573–583 (In French.) 10.1016/j.medmal.2007.03.007 [DOI] [PubMed] [Google Scholar]
- 10.Société de Pathologie Infectieuse de Langue Française (SPILF), Collège des Universitaires de Maladies Infectieuses et Tropicales (CMIT), Groupe de Pathologie Infectieuse Pédiatrique (GPIP), Société Française d'Anesthésie et de Réanimation (SFAR), Société Française de Chirurgie Orthopédique et Traumatologique (SOFCOT), Société Française d'Hygiéne Hospitalière (SFHH), Société Française de Médecine Nucléaire (SFMN), Société Française de Médecine Physique et de Réadaptation (SOFMER), Société Française de Microbiologie (SFM), Société Française de Radiologie (SFR-Rad), Société Française de Rhumatologie (SFR-Rhu) 2009. Recommendations for clinical practice. Osteo-articular infection therapy according to materials used (prosthesis, implants, osteosynthesis). Med. Mal. Infect. 39:745–774 (In French.) [DOI] [PubMed] [Google Scholar]
- 11.Hagihara M, Crandon JL, Nicolau DP. 2012. The efficacy and safety of antibiotic combination therapy for infections caused by Gram-positive and Gram-negative organisms. Expert. Opin. Drug Saf. 11:221–233. 10.1517/14740338.2012.632631 [DOI] [PubMed] [Google Scholar]
- 12.Conterno LO, Turchi MD. 2013. Antibiotics for treating chronic osteomyelitis in adults. Cochrane Database Syst. Rev. 9:CD004439. 10.1002/14651858.CD004439.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoffman-Terry ML, Fraimow HS, Fox TR, Swift BG, Wolf JE. 1999. Adverse effects of outpatient parenteral antibiotic therapy. Am. J. Med. 106:44–49. 10.1016/S0002-9343(98)00362-3 [DOI] [PubMed] [Google Scholar]
- 14.Pulcini C, Couadau T, Bernard E, Lorthat-Jacob A, Bauer T, Cua E, Mondain V, Chichmanian RM, Dellamonica P, Roger PM. 2008. Adverse effects of parenteral antimicrobial therapy for chronic bone infections. Eur. J. Clin. Microbiol. Infect. Dis. 27:1227–1232. 10.1007/s10096-008-0570-y [DOI] [PubMed] [Google Scholar]
- 15.Charlson M, Szatrowski TP, Peterson J, Gold J. 1994. Validation of a combined comorbidity index. J. Clin. Epidemiol. 47:1245–1251. 10.1016/0895-4356(94)90129-5 [DOI] [PubMed] [Google Scholar]
- 16.Grammatico-Guillon L, Baron S, Gettner S, Lecuyer AI, Gaborit C, Rosset P, Rusch E, Bernard L. 2012. Bone and joint infections in hospitalized patients in France, 2008: clinical and economic outcomes. J. Hosp. Infect. 82:40–48. 10.1016/j.jhin.2012.04.025 [DOI] [PubMed] [Google Scholar]
- 17.Stengel D, Bauwens K, Sehouli J, Ekkernkamp A, Porzsolt F. 2001. Systematic review and meta-analysis of antibiotic therapy for bone and joint infections. Lancet Infect. Dis. 1:175–188. 10.1016/S1473-3099(01)00094-9 [DOI] [PubMed] [Google Scholar]
- 18.Brandt CM, Sistrunk WW, Duffy MC, Hanssen AD, Steckelberg JM, Ilstrup DM, Osmon DM. 1997. Staphylococcus aureus prosthetic joint infection treated with debridement and prosthesis retention. Clin. Infect. Dis. 24:914–919. 10.1093/clinids/24.5.914 [DOI] [PubMed] [Google Scholar]
- 19.Marculescu CE, Berbari EF, Hanssen AD, Steckelberg JM, Harmsen SW, Mandrekar JN, Osmon DR. 2006. Outcome of prosthetic joint infections treated with debridement and retention of components. Clin. Infect. Dis. 42:471–478. 10.1086/499234 [DOI] [PubMed] [Google Scholar]
- 20.Senneville E, Joulie D, Legout L, Valette M, Dezèque H, Beltrand E, Roselé B, d'Escrivan T, Loïez C, Caillaux M, Yazdanpanah Y, Maynou C, Migaud H. 2011. Outcome and predictors of treatment failure in total hip/knee prosthetic joint infections due to Staphylococcus aureus. Clin. Infect. Dis. 53:334–340. 10.1093/cid/cir402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bauer S, Bouldouyre MA, Oufella A, Palmari P, Bakir R, Fabrequettes A, Gros H. 2012. Impact of a multidisciplinary staff meeting on the quality of antibiotherapy prescription for bone and joint infections in orthopedic surgery. Med. Mal. Infect. 42:603–607. 10.1016/j.medmal.2012.09.005 [DOI] [PubMed] [Google Scholar]
- 22.Uçkay I, Vernaz-Hegi N, Harbarth S, Stern R, Legout L, Vauthey L, Ferry T, Lübbeke A, Assal M, Lew D, Hoffmeyer P, Bernard L. 2009. Activity and impact on antibiotic use and costs of a dedicated infectious diseases consultant on a septic orthopaedic unit. J. Infect. 58:205–212. 10.1016/j.jinf.2009.01.012 [DOI] [PubMed] [Google Scholar]
- 23.Pestotnik SL, Classen DC, Evans RS, Burke JP. 1996. Implementing antibiotic practice guidelines through computer-assisted decision support: clinical and financial outcomes. Ann. Intern. Med. 124:884–890. 10.7326/0003-4819-89-4-497. [DOI] [PubMed] [Google Scholar]
- 24.Onorato IM, Axelrod JL. 1978. Hepatitis from intravenous high-dose oxacillin therapy: findings in an adult inpatient population. Ann. Intern. Med. 89:497–500. 10.7326/0003-4819-89-4-497 [DOI] [PubMed] [Google Scholar]
- 25.Lagacé-Wiens P, Rubinstein E. 2012. Adverse reactions to β-lactam antimicrobials. Expert. Opin. Drug Saf. 11:381–399. 10.1517/14740338.2012.643866 [DOI] [PubMed] [Google Scholar]
- 26.Ditlove J, Weidmann P, Bernstein M, Massry SG. 1977. Methicillin nephritis. Medicine (Baltimore) 56:483–491 [DOI] [PubMed] [Google Scholar]
- 27.Olaison L, Belin L, Hogevik H, Alestig K. 1999. Incidence of beta-lactam-induced delayed hypersensitivity and neutropenia during treatment of infective endocarditis. Arch. Intern. Med. 159:607–615. 10.1001/archinte.159.6.607 [DOI] [PubMed] [Google Scholar]
- 28.Pai MP, Bearden DT. 2007. Antimicrobial dosing considerations in obese adult patients. Pharmacotherapy. 27:1081–1091. 10.1592/phco.27.8.1081 [DOI] [PubMed] [Google Scholar]
- 29.Wurtz R, Itokazu G, Rodvold K. 1997. Antimicrobial dosing in obese patients. Clin. Infect. Dis. 25:112–118. 10.1086/514505 [DOI] [PubMed] [Google Scholar]
- 30.Falagas ME, Karageorgopoulos DE. 2010. Adjustment of dosing of antimicrobial agents for bodyweight in adults. Lancet 375:248–251. 10.1016/S0140-6736(09)60743-1 [DOI] [PubMed] [Google Scholar]
- 31.Dequito AB, Mol PG, van Doormaal JE, Zaal RJ, ven den Bemt PM, Haaijer-Ruskamp FM, Kosterink JG. 2011. Preventable and non-preventable adverse drug events in hospitalized patients: a prospective chart review in the Netherlands. Drug Saf. 34:1089–1100. 10.2165/11592030-000000000-00000 [DOI] [PubMed] [Google Scholar]