Abstract
All cells need to protect themselves against the osmotic challenges of their environment by maintaining low permeability to ions across their cell membranes. This is a basic principle of cellular function, which is reflected in the interactions among ion transport and drug efflux genes that have arisen during cellular evolution. Thus, upon exposure to pore-forming antibiotics such as amphotericin B (AmB) or daptomycin (Dap), sensitive cells overexpress common resistance genes to protect themselves from added osmotic challenges. These genes share pathway interactions with the various types of multidrug resistance (MDR) transporter genes, which both preserve the native lipid membrane composition and at the same time eliminate disruptive hydrophobic molecules that partition excessively within the lipid bilayer. An increased understanding of the relationships between the genes (and their products) that regulate osmotic stress responses and MDR transporters will help to identify novel strategies and targets to overcome the current stalemate in drug discovery.
INTRODUCTION
Genes involved in antibiotic resistance have long been considered to have evolved from genes having other roles and functions (for a review, see reference 1). Among such genes can be included those encoding multidrug resistance (MDR) transport proteins that extrude antibiotics from cells. This minireview proposes that the evolution of specific genes that control antibiotic levels inside cells underlies a widespread resistance mechanism. This mechanism was a very early, primordial event associated with the fundamental need that all cells have to maintain at a very low level the permeability of cell membranes to ions. The principal function of such transporters was to prevent the futile ion cycling and energy loss that is produced by increased ion leakage induced by membrane-disrupting amphiphiles and hydrophobic molecules accumulating within the bilayer. I first address the “death by flooding” fate that all organisms encounter when energy-requiring ion transporters that maintain the osmotic balance across the membrane are absent or defective. A brief discussion follows of the postulated adverse hyperosmotic environments in which a robust metabolism emerging inside protocells evolved to allow the interplay of osmolyte and drug transporters. It is then shown how the defenses that have been established to protect cells from the disruption of the permeability barrier by the osmotic stress induced by pore-forming antibiotics such as amphotericin B (AmB) and daptomycin (Dap) are closely linked to the overexpression of MDR genes involved in the protection of membrane integrity.
THE “DEATH BY FLOODING” FATE CONFRONTED BY ALL CELLS
An increase in membrane permeability to sodium and chloride ions, which are the most abundant ions in the external environment of most cells, leads to the “death by flooding” fate. As initially formulated by Krogh (2), all cells suspended in an isosmotic solution of sodium chloride are under a permanent threat of osmotic lysis because they contain nonpermeable negatively charged molecules (proteins, nucleic acids, nucleotides, and small metabolites) while being immersed in an environment containing ions to which their membranes are somewhat permeable. As a consequence, all cells need to protect themselves against flooding and lysis, and cells expend considerable energy in maintaining the transport mechanisms that keep intracellular levels of permeable ions such as Na+ low.
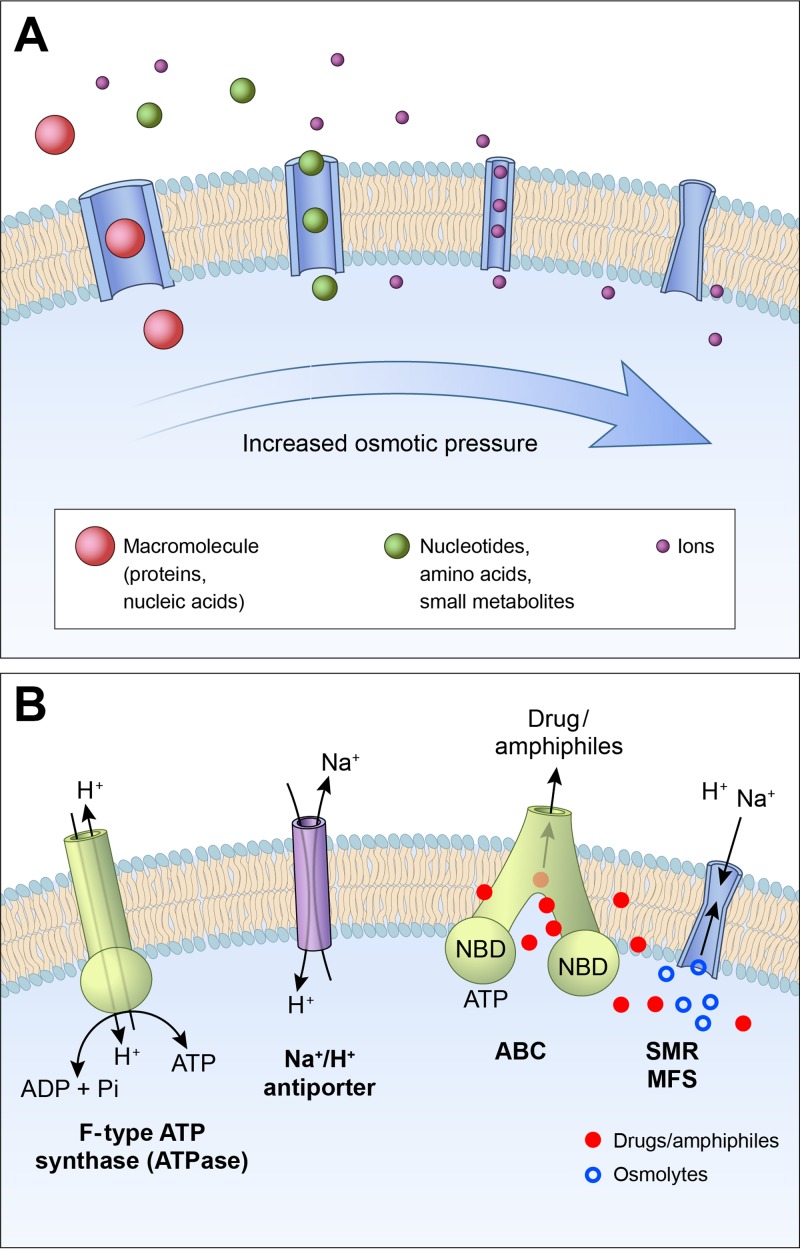
In protocells (3), passage of ions and large molecules may occur via the insertion into the phospholipid bilayers of aqueous pores with different pore sizes traversing the membrane (Fig. 1A). Under such conditions, there are no significant osmotic pressure differences across the membranes since the total number of compounds unable to enter the aqueous pores is negligible. However, with the relatively free passage of abiotically formed solutes such as nucleotides, amino acids, and sugars across the membranes, there is an increase in the synthesis of proteins and self-replicating RNA macromolecules, raising the intracellular levels of impermeable compounds. At this stage, only those protocells with limited membrane permeability to cations or/and anions were able to avoid the net influx of salt and the increased risk of flooding and loss of the membrane integrity. This selection process is postulated to have facilitated the evolution of membrane proteins from separate RNA helicases and membrane pores to the F- and V-type ATPases (4), as well as the subsequent formation of ATP-driven membrane pumps that remove abundant ions such as sodium ions from the cells (Fig. 1B).
FIG 1.
(A) Increase of the transmembrane osmotic pressure from protocells to cells (arrow). In protocells, the phospholipid membrane would have been embedded with aqueous channels of different pore diameters that prevent the development of a significant osmotic-pressure difference across the membrane. Cell membranes display narrow ion channels that are highly selective with respect to cations or anions of a certain size. (B) The main categories of protein transporters that are needed to maintain the osmotic equilibrium in a cell system in which energy is mainly provided by a chemiosmotic mechanism. The ATP production by synthases made possible the emergence of Na+ and H+ pumps (ATPases) that maintain the cation gradients in a steady state. The ATP-driven ABC pumps (ABC) prevent the accumulation of membrane-disrupting amphiphilic compounds within the bilayer. Secondary transporters of the small multidrug resistance superfamily (SMR) and major facilitator superfamily (MSF) protein families allow exchange of Na+/H+ ions with drug molecules as well as osmolytes. Members of the Nha family of Na+/H+ antiporters are also ubiquitously present in bacterial membranes.
Low permeability of the lipid bilayer to ions is a key factor in maintaining energy transduction at lower energy flux through proton or sodium pumping. In fact, in the evolution of protocells to archea and bacteria, maintaining a very low permeability to Na+ and H+ ions across the glycerol-1-phosphate (G1P) and the glycerol-3-phosphate (G3P) phospholipid membranes, respectively, was essential to reduce energy loss and maximize the use of the electrochemical gradients as sources of energy for the synthesis of ATP (5). In addition, the maintenance energy that is required to pump Na+ ions across cell membranes can be reduced by developing the capacity to withstand a positive osmotic pressure gradient across the plasma membrane, with external walls functioning as a protective counterbalancing force against the disruptive effect of the transmembrane osmotic pressure. In this regard, the cell walls developed by archea, bacteria, and some eukaryotes, such as fungi, allow these organisms to survive hypo-osmotic challenges, greatly expanding related capacities of adaptation to changes under environmental conditions.
THE PRESERVATION OF THE INTEGRITY OF THE LIPID BILAYER
All cells rapidly swell or shrink in the presence of a relatively small gradient of solute concentrations between the internal and the external media, primarily because cell membranes are composed of lipid bilayers, which have a much higher permeability to water and small hydrophilic nonelectrolytes such as urea or glycerol than to salts (6). As the maintenance of a lipid bilayer with low permeability to ions is essential for the functional organization of the cell, any type of chemical or osmotic disruption of this barrier represents a serious threat to cell integrity. The emergence of metabolism inside the protocells was likely facilitated by the availability of many abiotically formed organic compounds, but such compounds should not partition in the lipid bilayer, for to do so might affect the native low cation and proton permeability properties of membrane phospholipids (7). As a consequence, another universal mechanism of membrane protection that has emerged is based on keeping the levels of hydrophobic compounds in the bilayer at a minimum. This role is partially played by the family of ATP-binding cassette (ABC) transporters (Fig. 1B), which are found in all life forms and perform many functions. They might have originally evolved to remove small hydrophobic molecules from the bilayer (8) followed by the expansion of their role to cover removal of a broad variety of amphiphilic compounds entering cells (9). In this respect, the nucleotide-binding domains (NBD) of the ABC transporters (Fig. 1B) are among the most conserved phylogenetic DNA sequences (10). Of note, effective chemical compounds to be developed as antibiotics need to have drug-like characteristics for absorption that include the capacity to partition into the lipid membrane and a low (<500) molecular weight (MW) (11). The overlapping hydrophobic characteristics of drug-like compounds and MDR transporter substrates are among the most important factors contributing to the rapid development of antibiotic resistance (1) and remain a serious barrier to drug therapy.
AN ALTERNATIVE TO THE “DEATH BY FLOODING” FATE OF ALL LIVING ORGANISMS: EVOLUTION IN A HYPEROSMOTIC ENVIRONMENT
A postulated scenario explaining how protocells first evolved is that they flourished in a NaCl-poor external environment such as those present in geothermal fields enriched with K+ ions, metal sulfides, and Zn2+ and phosphorus compounds (12). This environment mimics the internal K+-enriched ionic composition of modern cells and is predicted to have preceded the evolution of membranes that were increasingly impermeable with respect to Na+ as protocells invaded seawater with its high NaCl concentration. The presence of this hyperosmotic environment would have allowed cells to develop without the threat of flooding, but it would have placed them in constant threat of water loss, which could lead to cell death by dehydration. So in order to promote survival in an external environment with a higher osmolarity, the emergence of membranes with increasing impermeability to Na+ and H+ ions was accompanied by the emergence of metabolic pathways that enhance the synthesis of small organic osmolytes capable of balancing osmotic pressure differences across the membranes. These metabolic pathways are well conserved in archea, bacteria, and eukaryotes and include the synthesis of small solutes such as amino acids and their derivatives, as well as glycerol and other polyhydric alcohols (13).
High osmolarity causes Escherichia coli to rapidly increase levels of negative DNA supercoiling, leading to the activation of a functionally significant set of genes involved in the osmotic response, including the primary active transporter for the osmolytes proline and glycine betaine (14). Such osmolytes are natural substrates of EmrE, a proton-driven member of the small multidrug resistance (SMR) family of transporters that confers resistance to a broad variety of quaternary ammonium compounds (15) (Fig. 1B). General stress regulators in E. coli such as RpoS, which is the sigma subunit of RNA polymerase, and quorum-sensing regulators cooperate in the responses to hyperosmotic stress and promote the overexpression of MDR transporters (16). High osmolarity is also a well-known inducer of porins, which are proteins located in the outer membrane (OM) of Gram-negative bacteria (17, 18). The porins are commonly found to be downregulated in MDR clinical isolates of enterobacteriaceae (18), an observation that is consistent with the function of porins as nonspecific channels for the passive penetration of antibiotics through the cell wall (19). TolC is another OM protein that is highly expressed under conditions of hyperosmotic stress (20). TolC works synergistically in the multicomponent transporters of the resistance-nodulation division (RND) superfamily, the major facilitator superfamily (MFS), and the ATP-binding cassette (ABC) superfamily of MDR efflux pumps (21). Of note, exposure of E. coli to pore-forming cationic antimicrobial peptides (CAMPs) (22, 23) led to the upregulation of the marRAB operon (24). This effect requires the presence of Rob, which may act as a signal of osmotic stress caused by membrane disruption (24). The marRAB operon activates MarA, Rob, and SoxS, which are transcriptional regulators of the RND proton/drug antiporter AcrAB and other TolC-dependent efflux pumps (25). The CAMPs are actively excreted by the AcrAB-TolC transporter system (24). These findings support the postulated complementary functional roles of the multidrug transporters in the protection of the integrity of the cell membrane when challenged with osmotic stresses.
After the emergence of broadly selective MDR membrane transporters, they could have acquired by selection other more specific physiological functions (Fig. 1B). Examples of MDR transporters with alternative functions include the MFS proton/drug antiporter MdfA and MdtM from E. coli, which have been found to function in alkaline pH homeostasis by exchanging Na+ or K+ for H+ (26, 27); PsmrAB, a SMR protein from halophilic bacteria that functions as a novel two-component Na+/H+ antiporter (28); and LmrP, an MFS proton/cationic drug antiporter from Lactococcus lactis that can also mediate calcium efflux (29).
THE FORMATION OF AQUEOUS PORES BY ANTIBIOTICS: THE CASE OF AMPHOTERICIN B
The formation of aqueous pores permeable to cations and anions across the cell membrane represents a primary threat to the integrity of the cell. As a consequence, such an action triggers signaling pathways responsible for sensing and responding to the osmotic stress. In the case of amphotericin B (AmB), a pore-forming natural antibiotic that has been used to treat systemic fungal infections for more than 50 years (30), the pathways that are activated include those involved in the control of the lipid composition and ergosterol content of the cell membrane (31, 32). Thus, AmB has been found to downmodulate genes that are involved in the synthesis of ergosterol, fatty acid elongases, and synthetases as well as ceramide, which are enzymes and lipid precursors required for the biosynthesis of sphingolipids. Sterols and shingolipids are important components of lipid rafts, the membrane microdomains that perform important roles in the targeting and activity of signaling proteins in eukaryote cells (33). Ergosterol is also an important factor involved in the mechanisms of resistance to AmB due to its critical role in the formation of the AmB aqueous pores (34).
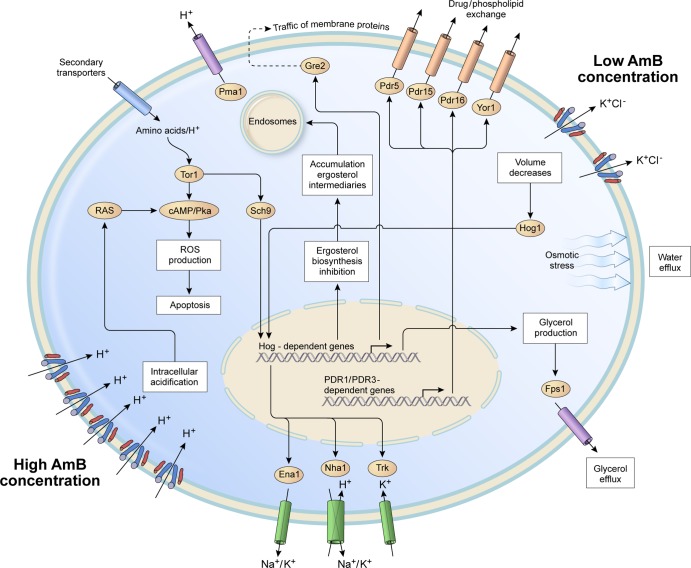
The addition of AmB to eukaryotic cells has several outcomes, depending on the concentration of antibiotic used and the ratio of impermeative to permeative osmolytes that are present in the medium in which cells are grown (34). For instance, when yeasts are cultured in yeast-peptone-dextrose (YPD) medium, which uses glucose as the main external osmolyte, the AmB-induced lysis is avoided and the cells shrink, leading to a loss of turgor that is followed by activation of the high-osmolarity glycerol (HOG) pathway (35) (Fig. 2, right). The HOG pathway controls osmotic pressure by increasing the synthesis of glycerol. In the presence of hyperosmotic stress, the terminal Hog1 kinase translocates into the nucleus, where it interacts directly with chromatin to regulate gene expression (36). Hog1 directly controls the transcription factors Sko1 and Hot1 as well as the redundant Msn2 and Msn4, which are responsible for environmental stress responses (ESR) (36). Remarkably, repression of ergosterol biosynthesis, which is also essential for stress resistance, is mediated by Hog1 via the transcriptional repressors Mot3 and Rox1 (37). Mutational defects in Mot3 and Rox1 produce hyperaccumulation of intracellular Na+ (37), an indication of changes in the activity of various ion transporters such as the Na+ ATPase (Ena1) and the Na+ K+/H+ antiporter (Nha1) and K+ uptake transporter (Trk). These ion transporters are encoded by HOG-dependent genes (38), and all of them are upregulated by AmB in fungi (31, 32) (Fig. 2). Other HOG-dependent genes that are strongly upregulated by AmB are ctt1 and hsp12 (32). Ctt1 is a fungal catalase that protects cells against oxidative stress, and Hsp12 is a small stress protein that is induced after yeasts are exposed to a short heat shock at sublethal temperatures (39). Hsp12 protects cells against ion leakage by decreasing the fluidity of the hydrocarbon chains upon binding into the lipid bilayer (39). In Cryptococcus neoformans, hsp12Δ mutants have been found to be hypersensitive to AmB action, indicating the important role of this protein in the preservation of the stability of the cell membrane under different stressful conditions (40).
FIG 2.
Expression of resistant genes and proteins in yeasts resulting from the action of AmB at low and high concentrations. Osmotic stress induced by AmB activates the protein kinase Hog1, which regulates several cellular components, including glycerol production and ion transport proteins. AmB action also leads to the activation of several members of the PDR network that are involved in multidrug resistance and other functions. High AmB concentrations lead to intracellular acidification, resulting in the activation of the Ras-cAMP-Pka pathway, ROS production, and cell death. See the text for descriptions of all proteins as well as of the main pathways represented in this figure.
Notably, under conditions in which no killing of cells occurred, exposure of yeast over hundreds of generations to increasing concentrations of AmB has yielded resistant strains with permanent changes in the expression of genes such as yor1 and pdr16 (41), which are members of the ATP-binding cassette (ABC) family of transporters (9). The activation of yor1 and pdr16 is controlled by the zinc finger transcription factors Pdr1 and Pdr3, which activate proteins involved in multidrug resistance and in the translocation of plasma membrane phospholipids (9). Among the stably overexpressed genes that also confer resistance to AmB (41) are ict1, which encodes a lysophosphatidic acid acyltransferase that is responsible for enhanced phospholipid synthesis and increased resistance to antifungal drugs, and ygr035C and ypl088, which are activated by Yrm1q and Yrr1, the yeast zinc finger transcription factors which are also controlled by the pleiotropic drug resistance (PDR) gene network (9).
Another yeast gene that is also stably overexpressed during long-term AmB treatment is gre2 (41), a HOG-dependent gene (38) that is homologous to mammalian 3-beta-hydroxysteroid dehydrogenases (42). When gre2Δ mutant yeasts were exposed to increasing AmB concentrations, their growth efficiency was reduced (42), an indication of the increased ATP expenditure required to maintain the AmB-induced alterations in the membrane properties of permeability to ions. Very similar defects in growth efficiency were observed when gre2Δ yeasts were treated with ergosterol or sphingolipid biosynthesis inhibitors as well as with exposure to brefeldin A (42), which is a fungal toxin that has a strong effect on the integrity of subcellular compartments, thereby inhibiting intracellular trafficking pathways (43). The available evidence suggests that Gre2 is involved in ergosterol metabolism, acting as a broad-specificity reductase that prevents the accumulation of ergosterol metabolic intermediaries in the endoplasmic reticulum and other intracellular membranes during inhibition of ergosterol biosynthesis (42). This role may be critically important to maintain the membrane trafficking of the PDR-network proteins to the lipid rafts (Fig. 2) because this transfer requires very specific sphingolipid and ergosterol combinations (33, 44). Notably, gre2 is one of the few yeast genes that have been clearly linked to osmotic responses and the PDR network (45). Both gre2 and ctt1 can be activated by hyperosmotic stress via a mechanism that involves the recruitment of Hog1 and Sch9 to chromatin-associated defense gene promoters (46). The signaling kinase Sch9 is a substrate of target of rapamycin complex 1 (TORC1) (47), a multiprotein complex that is predominantly localized in lysosomes and vacuoles (48), where it plays an important role in the integration of diverse signals elicited by growth factors, nutrients, energy status, and stressors (49) (Fig. 2). Yeast mutants of genes that encode proteins involved in vacuolar trafficking and/or function show growth sensitivities suggestive of defects in osmoregulation and stress survival in the presence of rapamycin (50). Of note, in parasitic protists, a Tor3 kinase has been shown to be involved in sensing of osmotic stress and energetic regulation (51, 52). In Trypanosoma brucei, high osmolarity causes Tor3 to relocate specifically from the acidocalcisome organelles to the cell periphery, where it may interacts with ion channels or/proteins directly involved in osmoregulation (52).
The HOG-dependent gre2 gene and the four PDR-regulated genes that confer stable yeast resistance to AmB are also significantly overexpressed by long-term treatment with fluconazole (FLC) (41). FLC is an antifungal drug that acts by inhibiting Erg11, a key enzyme in the biosynthesis of ergosterol, leading to accumulation of lanosterol and other ergosterol derivatives in intracellular membranes (53). It appears then that the membrane trafficking of ABC transport proteins that control the efflux of drugs such as FLC is also facilitated by the stable upregulation of gre2 in FLC-resistant cells. The role for Gre2 in the maintenance of membrane trafficking of transmembrane proteins is consistent with genome-wide data showing the growth fitness of yeast deletion strains that were grown in the presence of more than 300 chemical compounds, including AmB and FLC (54). Thus, examination of these extensive MDR gene data revealed that yeast deletion strains are highly enriched for certain gene ontology (GO) functions that are mainly associated with endosome transport, vacuolar degradation, and transcription (54).
Finally, it is important that at low doses of AmB, yeasts are able to maintain constant intracellular pH by increasing the activity of Pma1, the H+-ATPase pump (34). However, the uncoupling effect of high AmB concentrations makes H+ pumping an energetically expensive protective mechanism, leading to increased mitochondrial activity, reactive oxygen species (ROS) production, and apoptosis (55). Intracellular acidification induced by high AmB concentrations involves the activation of the Ras-cyclic AMP (cAMP)-Pka signaling pathway, and mutations that block this signaling pathway in yeast reduce or suppress the killing action induced by AmB (56) (Fig. 2, left).
GENES CONFERRING RESISTANCE TO AQUEOUS-PORE-FORMING ANTIBIOTICS IN BACTERIA
An antibiotic that acts by forming aqueous pores in Gram-positive bacteria, the cyclic lipopeptide daptomycin (Dap) (57), has also been shown to mediate the development of resistance by causing the overexpression of genes involved in osmotic regulation and maintenance of the native lipid membrane composition. Thus, Dap-resistant (Dap-R) bacterial strains express a number of mutated genes that are in different functional categories but are mainly involved in the biosynthesis of phospholipids responsible for maintaining membrane fluidity, phospholipid content, and bilayer asymmetry (58, 59). Among such genes are pgsA, a gene which is important for the production of phosphatidylglycerol (PG), and mprF, a membrane synthase that increases the overall synthesis of lysyl-PG from anionic PG and uses its flippase activity to translocate the positively charged lipid across the membrane, thus reducing the affinity for Dap and many other cationic antimicrobial peptides (59, 60). The deletion of mprF did not significantly affect lipid biosynthetic enzymes but led to a very distinct upregulation of a putative Na+/solute transporter as well as of a number of drug transporters and ABC transporter-like proteins (61). Dap-R strains also include mutated genes encoding proteins involved in glycine betaine transporter and accumulation, small transmembrane efflux proteins, and monovalent cation/H+ antiporters (59, 62) as well as genes encoding proteins involved in oxidative protection (62). However, as with AmB, sensitive bacterial strains exposed to increasing Dap concentrations exhibit rising energy demands for maintaining the membrane selectivity properties, leading to a rise in ROS production and cell death (63).
CONCLUSIONS
As water is the most abundant intracellular component, the control of its movement across cells is essential for life. This control is primarily exerted at the level of the cell membrane, as this structure determines the magnitude of the osmotic pressure differences arising from all cellular activities. The maintenance of low permeability of the cell membrane to ions is thus crucially important for life and must be reflected in the manner in which interactions among certain genes and proteins developed during cellular evolution. As discussed above, yeasts exposed to low concentrations of the pore-forming antibiotic AmB alter a specific array of genes involved in transcription and regulation of the membrane traffic of transmembrane ion transport proteins. Such resistance genes include the various overexpressed MDR transporters, which preserve the native low permeability of the lipid membrane to ions by eliminating hydrophobic molecules that accumulate excessively within the lipid bilayer. MDR transporters are also involved in the homeostasis of membrane lipidic components. In bacteria, the resistance genes whose expression is induced by Dap also include proline/betaine and ABC transporters, which are involved in the universal protective responses to osmotic challenge and in the homeostasis of membrane lipidic components.
The capacity of the active efflux of drugs by MDR transporters to act synergistically with other resistance mechanisms is a serious clinical concern (1, 64). The identification of a functional linkage between the genes that regulate essential cellular functions such as osmoregulation and MDR transporters—in both prokaryotic and eukaryotic organisms—emphasizes the importance of developing more-effective MDR pump inhibitors to overcome the current spread of antibiotic resistance in bacterial and fungal organisms (65). An increased understanding of the functional relationships between genomic/proteomic and metabolic pathways may also address the current need to develop more combined-drug approaches for treatment of infectious and noninfectious diseases.
ACKNOWLEDGMENTS
I am indebted to Gerald McLaughlin and Robert Gould for critically reading the manuscript before publication.
Footnotes
Published ahead of print 2 December 2013
REFERENCES
- 1.Baquero F, Blázquez J. 1997. Evolution of antibiotic resistance. Trends Ecol. Evol. 12:482–487. 10.1016/S0169-5347(97)01223-8 [DOI] [PubMed] [Google Scholar]
- 2.Krogh A. 1946. The active and passive exchanges of inorganic ions through the surfaces of living cells and through living membranes generally. Proc. R. Soc. Med. 133:140–200. 10.1098/rspb.1946.0008 [DOI] [PubMed] [Google Scholar]
- 3.Forterre P, Gribaldo S. 2007. The origin of modern terrestrial life. HFSP J. 1:156–168. 10.2976/1.2759103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mulkidjanian AY, Galperin MY, Koonin EV. 2009. Co-evolution of primordial membranes and membrane proteins. Trends Biochem. Sci. 34:206–215. 10.1016/j.tibs.2009.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valentine DL. 2007. Adaptations to energy stress dictate the ecology and evolution of the Archaea. Nat. Rev. Microbiol. 5:316–323. 10.1038/nrmicro1619 [DOI] [PubMed] [Google Scholar]
- 6.Cohen BE, Bangham AD. 1972. Diffusion of small non-electrolytes across liposome membranes. Nature 236:173–174. 10.1038/236173a0 [DOI] [PubMed] [Google Scholar]
- 7.Sikkema J, de Bont JA, Poolman B. 1995. Mechanisms of membrane toxicity of hydrocarbons. Microbiol. Rev. 59:201–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jin MS, Oldham ML, Zhang Q, Chen J. 2012. Crystal structure of the multidrug transporter P-glycoprotein from Caenorhabditis elegans. Nature 490:566–569. 10.1038/nature11448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prasad R, Goffeau A. 2012. Yeast ATP-binding cassette transporters conferring multidrug resistance. Annu. Rev. Microbiol. 66:39–63. 10.1146/annurev-micro-092611-150111 [DOI] [PubMed] [Google Scholar]
- 10.Isenbarger TA, Carr CE, Johnson SS, Finney M, Church GM, Gilbert W, Zuber MT, Ruvkun G. 2008. The most conserved genome segments for life detection on Earth and other planets. Orig. Life Evol. Biosph. 38:517–533. 10.1007/s11084-008-9148-z [DOI] [PubMed] [Google Scholar]
- 11.Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. 2001. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 46:3–26. 10.1016/S0169-409X(00)00129-0 [DOI] [PubMed] [Google Scholar]
- 12.Mulkidjanian AY, Bychkov AY, Dibrova DV, Galperin MY, Koonin EV. 2012. Origin of first cells at terrestrial, anoxic geothermal fields. Proc. Natl. Acad. Sci. U. S. A. 109:E821–E830. 10.1073/pnas.1117774109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burg MB, Ferraris JD. 2008. Intracellular organic osmolytes: function and regulation. J. Biol. Chem. 283:7309–7313. 10.1074/jbc.R700042200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheung KJ, Badarinarayana V, Selinger DW, Janse D, Church GM. 2003. A microarray-based antibiotic screen identifies a regulatory role for supercoiling in the osmotic stress response of Escherichia coli. Genome Res. 13:206–215. 10.1101/gr.401003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bay DC, Turner RJ. 2012. Small multidrug resistance protein EmrE reduces host pH and osmotic tolerance to metabolic quaternary cation osmoprotectants. J. Bacteriol. 194:5941–5948. 10.1128/JB.00666-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang S, Lopez CR, Zechiedrich EL. 2006. Quorum sensing and multidrug transporters in Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 103:2386–2391. 10.1073/pnas.0502890102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graeme-Cook KA, May G, Bremer E, Higgins CF. 1989. Osmotic regulation of porin expression: a role for DNA supercoiling. Mol. Microbiol. 3:1287–1294. 10.1111/j.1365-2958.1989.tb00279.x [DOI] [PubMed] [Google Scholar]
- 18.Dupont M, James CE, Chevalier J, Pagès JM. 2007. An early response to environmental stress involves regulation of OmpX and OmpF, two enterobacterial outer membrane pore-forming proteins. Antimicrob. Agents Chemother. 51:3190–3198. 10.1128/AAC.01481-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kojima S, Nikaido H. 2013. Permeation rates of penicillins indicate that Escherichia coli porins function principally as nonspecific channels. Proc. Natl. Acad. Sci. U. S. A. 110:E2629–E2634. 10.1073/pnas.1310333110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu C, Wang S, Ren H, Lin X, Wu L, Peng X. 2005. Proteomic analysis on the expression of outer membrane proteins of Vibrio alginolyticus at different sodium concentrations. Proteomics 5:3142–3152. 10.1002/pmic.200401128 [DOI] [PubMed] [Google Scholar]
- 21.Nikaido H. 2009. Multidrug resistance in bacteria. Annu. Rev. Biochem. 78:119–146. 10.1146/annurev.biochem.78.082907.145923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oren Z, Lerman JC, Gudmundsson GH, Agerberth B, Shai Y. 1999. Structure and organization of the human antimicrobial peptide LL-37 in phospholipid membranes: relevance to the molecular basis for its non-cell-selective activity. Biochem. J. 341:501–513. 10.1042/0264-6021:3410501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee CC, Sun Y, Qian S, Huang HW. 2011. Transmembrane pores formed by human antimicrobial peptide LL-37. Biophys. J. 100:1688–1696. 10.1016/j.bpj.2011.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Warner DM, Levy SB. 2010. Different effects of transcriptional regulators MarA, SoxS and Rob on susceptibility of Escherichia coli to cationic antimicrobial peptides (CAMPs): Rob-dependent CAMP induction of the marRAB operon. Microbiology 156:570–578. 10.1099/mic.0.033415-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barbosa TM, Levy SB. 2000. Differential expression of over 60 chromosomal genes in Escherichia coli by constitutive expression of MarA. J. Bacteriol. 182:3467–34674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewinson O, Padan E, Bibi E. 2004. Alkalitolerance: a biological function for a multidrug transporter in pH homeostasis. Proc. Natl. Acad. Sci. U. S. A. 101:14073–14078. 10.1073/pnas.0405375101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holdsworth SR, Law CJ. 2013. Multidrug resistance protein MdtM adds to the repertoire of antiporters involved in alkaline pH homeostasis in Escherichia coli. BMC Microbiol. 13:113. 10.1186/1471-2180-13-113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang J, Wang L, Zhang H, Wu H, Huang H, Yang L. 2013. Putative paired small multidrug resistance family proteins PsmrAB, the homolog of YvdSR, actually function as a novel two-component Na(+)/H(+) antiporter. FEMS Microbiol. Lett. 338:31–38. 10.1111/1574-6968.12008 [DOI] [PubMed] [Google Scholar]
- 29.Schaedler TA, Tong Z, van Veen HW. 2012. The multidrug transporter LmrP protein mediates selective calcium efflux. J. Biol. Chem. 287:27682–27690. 10.1074/jbc.M112.372334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loo AS, Muhsin SA, Walsh TJ. 2013. Toxicokinetic and mechanistic basis for the safety and tolerability of liposomal amphotericin B. Expert Opin. Drug Saf. 12:881–895. 10.1517/14740338.2013.827168 [DOI] [PubMed] [Google Scholar]
- 31.Liu TT, Lee RE, Barker KS, Lee RE, Wei L, Homayouni R, Rogers PD. 2005. Genome-wide expression profiling of the response to azole, polyene, echinocandin, and pyrimidine antifungal agents in Candida albicans. Antimicrob. Agents Chemother. 49:2226–2236. 10.1128/AAC.49.6.2226-2236.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Agarwal AK, Rogers PD, Baerson SR, Jacob MR, Barker KS, Cleary JD, Walker LA, Nagle DG, Clark AM. 2003. Genome-wide expression profiling of the response to polyene, pyrimidine, azole, and echinocandin antifungal agents in Saccharomyces cerevisiae. J. Biol. Chem. 278:34998–35015. 10.1074/jbc.M306291200 [DOI] [PubMed] [Google Scholar]
- 33.Guan XL, Souza CM, Pichler H, Dewhurst G, Schaad O, Kajiwara K, Wakabayashi H, Ivanova T, Castillon GA, Piccolis M, Abe F, Loewith R, Funato K, Wenk MR, Riezman H. 2009. Functional interactions between sphingolipids and sterols in biological membranes regulating cell physiology. Mol. Biol. Cell 20:2083–2095. 10.1091/mbc.E08-11-1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cohen BE. 2010. Amphotericin B membrane action: role for two types of ion channels in eliciting cell survival and lethal effects. J. Membr. Biol. 238:1–20. 10.1007/s00232-010-9313-y [DOI] [PubMed] [Google Scholar]
- 35.Reiser V, Raitt DC, Saito H. 2003. Yeast osmosensor Sln1 and plant cytokinin receptor Cre1 respond to changes in turgor pressure. J. Cell Biol. 161:1035–1040. 10.1083/jcb.200301099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saito H, Posas F. 2012. Response to hyperosmotic stress. Genetics 192:289–318. 10.1534/genetics.112.140863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Montañés FM, Pascual-Ahuir A, Proft M. 2011. Repression of ergosterol biosynthesis is essential for stress resistance and is mediated by the Hog1 MAP kinase and the Mot3 and Rox1 transcription factors. Mol. Microbiol. 79:1008–1023. 10.1111/j.1365-2958.2010.07502.x [DOI] [PubMed] [Google Scholar]
- 38.Capaldi AP, Kaplan T, Liu Y, Habib N, Regev A, Friedman N, O'Shea EK. 2008. Structure and function of a transcriptional network activated by the MAPK Hog1. Nat. Genet. 40:1300–1306. 10.1038/ng.235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Welker S, Rudolph B, Frenzel E, Hagn F, Liebisch G, Schmitz G, Scheuring J, Kerth A, Blume A, Weinkauf S, Haslbeck M, Kessler H, Buchner J. 2010. Hsp12 is an intrinsically unstructured stress protein that folds upon membrane association and modulates membrane function. Mol. Cell 39:507–520. 10.1016/j.molcel.2010.08.001 [DOI] [PubMed] [Google Scholar]
- 40.Maeng S, Ko YJ, Kim GB, Jung KW, Floyd A, Heitman J, Bahn YS. 2010. Comparative transcriptome analysis reveals novel roles of the Ras and cyclic AMP signaling pathways in environmental stress response and antifungal drug sensitivity in Cryptococcus neoformans. Eukaryot. Cell 9:360–378. 10.1128/EC.00309-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anderson JB, Sirjusingh C, Syed N, Lafayette S. 2009. Gene expression and evolution of antifungal drug resistance. Antimicrob. Agents Chemother. 53:1931–1936. 10.1128/AAC.01315-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Warringer J, Blomberg A. 2006. Involvement of yeast YOL151W/GRE2 in ergosterol metabolism. Yeast 23:389–398. 10.1002/yea.1363 [DOI] [PubMed] [Google Scholar]
- 43.Anders N, Jürgens G. 2008. Large ARF guanine nucleotide exchange factors in membrane trafficking. Cell. Mol. Life Sci. 65:3433–3445. 10.1007/s00018-008-8227-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bagnat M, Keränen S, Shevchenko A, Shevchenko A, Simons K. 2000. Lipid rafts function in biosynthetic delivery of proteins to the cell surface in yeast. Proc. Natl. Acad. Sci. U. S. A. 97:3254–3259. 10.1073/pnas.97.7.3254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.DeRisi J, van den Hazel B, Marc P, Balzi E, Brown P, Jacq C, Goffeau A. 2000. Genome microarray analysis of transcriptional activation in multidrug resistance yeast mutants. FEBS Lett. 470:156–160. 10.1016/S0014-5793(00)01294-1 [DOI] [PubMed] [Google Scholar]
- 46.Pascual-Ahuir A, Proft M. 2007. The Sch9 kinase is a chromatin-associated transcriptional activator of osmostress-responsive genes. EMBO J. 26:3098–3108. 10.1038/sj.emboj.7601756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Urban J, Soulard A, Huber A, Lippman S, Mukhopadhyay D, Deloche O, Wanke V, Anrather D, Ammerer G, Riezman H, Broach JR, De Virgilio C, Hall MN, Loewith R. 2007. Sch9 is a major target of TORC1 in Saccharomyces cerevisiae. Mol. Cell 26:663–674. 10.1016/j.molcel.2007.04.020 [DOI] [PubMed] [Google Scholar]
- 48.Sturgill TW, Cohen A, Diefenbacher M, Trautwein M, Martin DE, Hall MN. 2008. TOR1 and TOR2 have distinct locations in live cells. Eukaryot. Cell 7:1819–1830. 10.1128/EC.00088-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sengupta S, Peterson TR, Sabatini DM. 2010. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol. Cell 40:310–322. 10.1016/j.molcel.2010.09.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ricarte F, Menjivar R, Chhun S, Soreta T, Oliveira L, Hsueh T, Serranilla M, Gharakhanian E. 2011. A genome-wide immunodetection screen in S. cerevisiae uncovers novel genes involved in lysosomal vacuole function and morphology. PLoS One 6:e23696. 10.1371/journal.pone.0023696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Madeira da Silva L, Beverley SM. 2010. Expansion of the target of rapamycin (TOR) kinase family and function in Leishmania shows that TOR3 is required for acidocalcisome biogenesis and animal infectivity. Proc. Natl. Acad. Sci. U. S. A. 107:11965–11970. 10.1073/pnas.1004599107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.de Jesus TC, Tonelli RR, Nardelli SC, da Silva Augusto L, Motta MC, Girard-Dias W, Miranda K, Ulrich P, Jimenez V, Barquilla A, Navarro M, Docampo R, Schenkman S. 2010. Target of rapamycin (TOR)-like 1 kinase is involved in the control of polyphosphate levels and acidocalcisome maintenance in Trypanosoma brucei. J. Biol. Chem. 285:24131–24140. 10.1074/jbc.M110.120212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lupetti A, Danesi R, Campa M, Del Tacca M, Kelly S. 2002. Molecular basis of resistance to azole antifungals. Trends Mol. Med. 8:76–81. 10.1016/S1471-4914(02)02280-3 [DOI] [PubMed] [Google Scholar]
- 54.Hillenmeyer ME, Fung E, Wildenhain J, Pierce SE, Hoon S, Lee W, Proctor M, St Onge RP, Tyers M, Koller D, Altman RB, Davis RW, Nislow C, Giaever G. 2008. The chemical genomic portrait of yeast: uncovering a phenotype for all genes. Science 320:362–365. 10.1126/science.1150021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Phillips AJ, Sudbery I, Ramsdale M. 2003. Apoptosis induced by environmental stresses and amphotericin B in Candida albicans. Proc. Natl. Acad. Sci. U. S. A. 100:14327–14332. 10.1073/pnas.2332326100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Belenky P, Camacho D, Collins JJ. 2013. Fungicidal drugs induce a common oxidative-damage cellular death pathway. Cell Rep. 3:350–358. 10.1016/j.celrep.2012.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Straus SK, Hancock RE. 2006. Mode of action of the new antibiotic for Gram-positive pathogens daptomycin: comparison with cationic antimicrobial peptides and lipopeptides. Biochim. Biophys. Acta 1758:1215–1223. 10.1016/j.bbamem.2006.02.009 [DOI] [PubMed] [Google Scholar]
- 58.Mishra NN, Yang SJ, Sawa A, Rubio A, Nast CC, Yeaman MR, Bayer AS. 2009. Analysis of cell membrane characteristics of in vitro-selected daptomycin-resistant strains of methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 53:2312–2318. 10.1128/AAC.01682-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Peleg AY, Miyakis S, Ward DV, Earl AM, Rubio A, Cameron DR, Pillai S, Moellering RC, Jr, Eliopoulos GM. 2012. Whole genome characterization of the mechanisms of daptomycin resistance in clinical and laboratory derived isolates of Staphylococcus aureus. PLoS One 7:e28316. 10.1371/journal.pone.0028316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ernst CM, Peschel A. 2011. Broad-spectrum antimicrobial peptide resistances by MprF-mediated aminoacylation and flipping of phospholipids. Mol. Microbiol. 80:290–299. 10.1111/j.1365-2958.2011.07576.x [DOI] [PubMed] [Google Scholar]
- 61.Sievers S, Ernst CM, Geiger T, Hecker M, Wolz C, Becher D, Peschel A. 2010. Changing the phospholipid composition of Staphylococcus aureus causes distinct changes in membrane proteome and membrane-sensory regulators. Proteomics 10:1685–1693. 10.1002/pmic.200900772 [DOI] [PubMed] [Google Scholar]
- 62.Song Y, Rubio A, Jayaswal RK, Silverman JA, Wilkinson BJ. 2013. Additional routes to Staphylococcus aureus daptomycin resistance as revealed by comparative genome sequencing, transcriptional profiling, and phenotypic studies. PLoS One 8:e58469. 10.1371/journal.pone.0058469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu Y, Liu X, Qu Y, Wang X, Li L, Zhao X. 2012. Inhibitors of reactive oxygen species accumulation delay and/or reduce the lethality of several antistaphylococcal agents. Antimicrob. Agents Chemother. 56:6048–6050. 10.1128/AAC.00754-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li XZ, Nikaido H. 2009. Efflux-mediated drug resistance in bacteria: an update. Drugs 69:1555–1623. 10.2165/11317030-000000000-00000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.CDC 2013. Antibiotic resistance threats in the United States. http://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf [PubMed] [Google Scholar]