Abstract
Encapsulated Klebsiella pneumoniae has emerged as one of the most clinically relevant and more frequently encountered opportunistic pathogens in combat wounds as the result of nosocomial infection. In this report, we show that imipenem displayed potent activity against established K. pneumoniae biofilms under both static and flow conditions in vitro. Using a rabbit ear model, we also demonstrated that imipenem was highly effective against preformed K. pneumoniae biofilms in wounds.
TEXT
Klebsiella pneumoniae is a Gram-negative, encapsulated, nonmotile, rod-shaped opportunistic pathogen. This organism can cause a wide range of nosocomial infections, including pneumonia, bacteremia, and urinary tract infections, (1) in hosts with weakened immune systems. K. pneumoniae has emerged as one of the most clinically relevant and frequently isolated bacterial strains for its ability to cause nosocomial infections. A 12-year longitudinal study showed that K. pneumoniae is one of the most frequently isolated pathogens from intensive care unit patients and is responsible for approximately 15% of the Gram-negative infections (2). Clinical data from the burn center at the U.S. Army Institute of Surgical Research/Brooke Army Medical Center indicate that K. pneumoniae is also one of the top four organisms recovered from the blood of burn patients involved in combat operations overseas (3). Many of these K. pneumoniae clinical isolates are highly resistant to commonly used antibiotics, resulting in increased mortality (4). One major factor contributing to treatment resistance to antibiotics is the ability of the organism to form biofilms on medical devices, such as catheters, and biotic surfaces, such as wounds.
Biofilms are sessile microbial communities. In these communities, cells are surrounded by protective matrix containing extracellular polysaccharides, proteins, DNA, lipopeptides, and others. Some biofilm cells are metabolically inactive (5). Together, these properties make the biofilm cells antibiotic tolerant. Furthermore, biofilms are able to withstand several mechanisms of innate host defense, such as phagocytosis (6). The formation of pathogenic biofilms can result in chronic infections (7) and/or delay or alter the proper course of wound healing (8, 9). Thus, identifying a biofilm-destroying agent(s) is essential for controlling biofilm-caused chronic and/or recurring infections and improving wound healing. Herein, we report that imipenem displays potent activity both in vitro and in vivo against the established biofilms formed by the clinical isolate K. pneumoniae BAMC 07-18 (kindly provided by Clinton Murray of Brooke Army Medical Center, Fort Sam Houston, TX). This clinical isolate forms O-type capsule (data not shown) and is resistant to a number of different classes of antibiotics (Table 1), such as amoxicillin and piperacillin (β-lactam group), cefotaxime and ceftazidime (cephalosporin group), ciprofloxacin and levofloxacin (fluoroquinolone group), and gentamicin (aminoglycoside group).
TABLE 1.
MICs for K. pneumoniae BAMC 07-18
| Antimicrobial compound | MIC (μg/ml) |
|---|---|
| Amoxicillin-clavulanate | 16/8 |
| Ampicillin | >200 |
| Azithromycin | >200 |
| Cefepime | >16 |
| Cefotaxime | >32 |
| Ceftazidime | >200 |
| Cefuroxime | >16 |
| Cephalothin | >16 |
| Chloramphenicol | >200 |
| Ciprofloxacin | 6.2 |
| Clarithromycin | 200 |
| Erythromycin | 100 |
| Gatifloxacin | 4 |
| Gentamicin | 200 |
| Imipenem | 0.39 |
| Levofloxacin | >4 |
| Linezolid | >200 |
| Meropenem | 1 |
| Nitrofurantoin | >64 |
| Piperacillin | >64 |
| Rifampin | 25 |
| Streptomycin | 100 |
| Trimethoprim-sulfamethoxazole | 2/>38 |
| Tetracycline | 200 |
| Tobramycin | 8 |
To screen for and identify an effective K. pneumoniae biofilm-disrupting agent(s), we used a moderate-throughput in vitro BioFlux 200 system (Fluxion Biosciences, South San Francisco, CA) that is based on a microfluidic platform. The system enables us to grow and treat K. pneumoniae biofilms under physiologically relevant conditions with the continuous perfusion of medium and removal of metabolic end products at a low shear force. In combination with confocal laser inverted scanning microscopy (LSM), the entire process can be observed in real time. To produce the target biofilms, we precoated the flow channels in the 48-well BioFlux microplates with 10 μg/ml human collagen type I (BD Bioscience, Bedford, MA) overnight at 4°C to enhance the attachment of K. pneumoniae prior to medium priming and bacterial inoculation. The bacterium was grown to mid-log phase in Trypticase soy broth (TSB). Prior to use, the culture was first passed through a 5-μm syringe filter (Pall Corporation, Ann Arbor, MI) to minimize the presence of aggregates and adjusted to an optical density at a wavelength of 600 nm (OD600) of 0.1 for inoculation. After medium priming, each channel was inoculated with 50 μl K. pneumoniae suspension. Shear flow at 0.55 dyn/cm2 was initiated after 2 h of attachment for biofilm growth using brain heart infusion (BHI) medium supplemented with 1% glucose and 2% sodium chloride. Approximately 8-μm-thick K. pneumoniae biofilm was formed in each channel after overnight growth (about 15 h) at 37°C, and very few dead cells were present in the biofilms (Fig. 1). These biofilms were treated with different test agents at various concentrations continuously for 5 h with the same flow rate under the same growth temperature. The morphological changes were captured in real time during the treatment process using LSM710 (Carl Zeiss MicroImaging, Thornwood, NY). At the end of the treatment, the channels were stained with Live/Dead BacLight (Invitrogen) for 30 min to determine the live/dead status of bacteria in the remaining biofilms.
FIG 1.
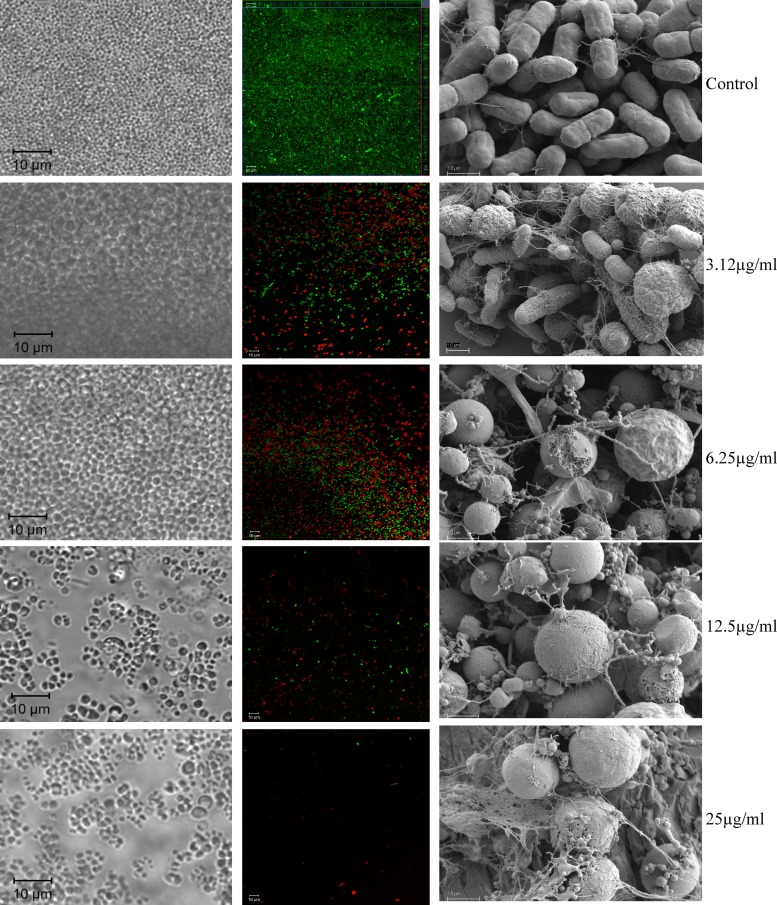
Activity of imipenem against overnight K. pneumoniae BAMC 07-18 biofilms grown in the BioFlux system (left and center columns). Biofilms were treated with different concentrations of imipenem (shown on the right) for 5 h, stained with Live (green)/Dead (red) BacLight, and observed by confocal microscopy. SEM images of K. pneumoniae BAMC 07-18 biofilms grown on the MBEC pegs and treated for 5 h with different concentrations of imipenem are shown in the right column. Note the morphological changes of imipenem-treated cells and the presence of significant amounts of cellular debris in the treated biofilms. Only a few individual live cells remained after the 5-hour treatment with 25 μg/ml imipenem.
Due to the multidrug-resistant property of K. pneumoniae BAMC 07-18, we investigated different categories of compounds for a potential biofilm-disrupting agent(s). Compounds included antibiotics, quorum-sensing inhibitors, antimicrobial peptides, surfactants, and d-amino acids. Some of these compounds have been shown to disrupt bacterial biofilms. Among all the compounds screened (Table 2), we identified a few compounds that were effective against established K. pneumoniae biofilms. However, imipenem was the most effective drug in terms of the concentration required to clear the K. pneumoniae biofilms. Imipenem starts to show some effect on the biofilm at a concentration of 3.12 μg/ml. At 25 μg/ml, imipenem completely destroyed the overnight Klebsiella biofilms, leaving only a few individual cells behind (within 5 h or less) (Fig. 1). Live/dead staining results indicated that some of these cells were alive, as shown by the cells stained green in the central panel of Fig. 1. The numbers of live cells in the channels treated with 25 μg/ml imipenem or more (up to 200 μg/ml) were similar (see Fig. S1 in the supplemental material), suggesting that these cells may represent biofilm persister cells, the dormant variants of regular cells that are highly tolerant to antibiotics (10). Treatment of biofilms with imipenem caused a significant number of K. pneumoniae biofilm cells to lose their typical morphology, changing from rod-shaped bacteria to spherical, and ultimately fragmented them and removed them from the channels by the flow of the medium (Fig. 1).
TABLE 2.
Effects of tested compounds on the K. pneumoniae BAMC 07-18 biofilms
| Group and compound | Concn used (μg/ml)a | Log reduction of viable cell countsb | Change(s) observed in BioFlux system |
|---|---|---|---|
| Antibiotics | |||
| Imipenem | ≥12.5 | 4–5 | Biofilms were cleared |
| Ciprofloxacin | 100 | 2 | Some dead cells |
| Rifampacin | 100 | 3 | Many dead cells |
| Tobramycin | 100 | 4–5 | Lots of dead cells, biofilms were not cleared |
| Peptides | |||
| KSL-W | 200 | 1 | Some dead cells |
| DJK-6 | 100 | 4–5 | Lots of dead cells, biofilms were not cleared |
| HHC10 | 100 | No effect | No change |
| HHC36 | 400 | 1–2 | Not tested |
| H53 | 200 | No effect | Not tested |
| Detergentsc | |||
| CTAB | 0.1% (vol/vol) | No effect | No change |
| CPC | 0.07% (vol/vol) | 3–4 | Many dead cells |
| SBMA | 1% (vol/vol) | No effect | No change |
| CAPB DC | 1% (vol/vol) | No effect | No change |
| Tristat P5 | 1% (vol/vol) | 3 | Lots of dead cells, biofilms were reduced but not cleared |
| Delmonpinol | 2,000 | No effect | No change |
| Dispersin B | 100 | No effect | No change |
| cis-2-Decenoic acid | 500 | No effect | No change |
| Quorum-sensing inhibitors | |||
| F-219 | 500 | 2 | Lots of dead cells |
| F-217 | 500 | No effect | Lots of dead cells |
| F-195 | 500 | No effect | No change |
| Furanone C-30 | 50 | No effect | No change |
| d-Amino acids | |||
| d-Methionene | 500 | 1 | No change |
| d-Tryptophan | 500 | No effect | No change |
| d-Leucine | 500 | No effect | No change |
| d-Tyrosine | 500 | No effect | No change |
Concentrations used are in μg/ml unless otherwise noted.
Based on MBEC assay.
CTAB, hexadecyltrimethylammonium bromide; CPC, cetylpyridinium chloride; SBMA, sulfobetaine methacrylate.
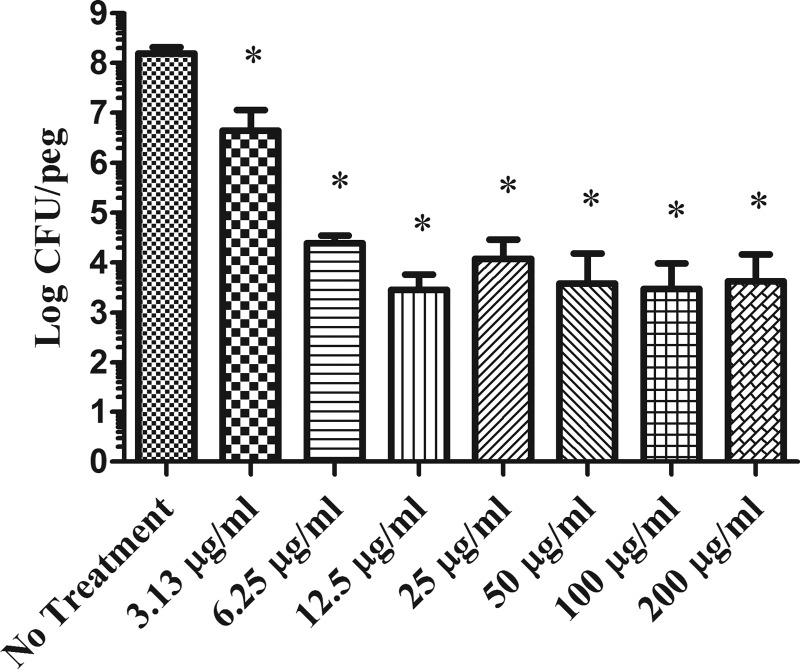
To quantify the killing effect of imipenem, we used the minimum biofilm eradication concentration (MBEC) assay (11) to determine the total number of viable cells in the treated versus nontreated K. pneumoniae biofilms. Like in the microfluidic system, the pegs from the MBEC plate were precoated with 10 μg/ml human collagen type I, followed by insertion of these coated pegs into wells, each of which contained 150 μl of mid-log-phase bacterial suspension at an OD600 of 0.05. After approximately 20 h of incubation at 37°C, the pegs were rinsed with sterile phosphate-buffered saline (PBS) to remove loosely attached planktonic cells and then placed in medium containing different concentrations of imipenem. Biofilms formed on each peg, treated and nontreated, were recovered by sonication, and the number of viable bacteria was determined by serial dilutions and plating. As shown in Fig. 2, approximately 4-log reduction was achieved when the imipenem concentration used was 6.25 μg/ml or higher. This is lower than the 25 μg/ml required to exhibit the maximum killing observed in the BioFlux system. The smaller amount of imipenem required in the MBEC system was most likely due to the difference in the biofilm thickness formed in the two different systems. However, similar to what was observed in the microfluidic system, increased concentrations of imipenem up to 200 μg/ml did not enhance more killing. Thus, these data confirmed that there was a small subpopulation of biofilm cells that were tolerant to imipenem. These remaining cells, which represented approximately 0.01% of the target biofilm cell population, were likely to be persister cells. Similar observations were reported with other microorganisms. For instance, there was a small subpopulation of cells in Pseudomonas aeruginosa biofilms which were completely tolerant to antibiotics (12, 13). It has also been shown that approximately 0.001% to 0.1% of the cells of an isogenic bacterial population display tolerance to prolonged treatment with high doses of bactericidal antibiotics (10, 14).
FIG 2.
Activity of imipenem against K. pneumoniae BAMC 07-18 biofilms grown on the MBEC pegs. Imipenem concentrations of 6.25 μg/ml or higher cause approximately 4-log reduction of viability counts of target biofilms. Compared to the untreated biofilms, t test shows that these reductions are statistically significant. *, P < 0.0001.
We used scanning electron microscopy (SEM) to determine the effect of imipenem treatment on the overall structure of biofilms grown on the MBEC pegs. Following previously described procedures (15), treated and untreated biofilm-containing pegs were fixed, dehydrated, sputter coated with gold/palladium (50%/50%), and observed under Sigma VP40 field emission SEM (Carl Zeiss MicroImaging). As shown in the right column of Fig. 1, before treatments, K. pneumoniae biofilms grown on the pegs displayed an organized community of rod-shaped bacteria embedded within some fibrillar materials. In contrast, the treatments caused significant changes in cell shape and disruption of the overall organization of the biofilm community (Fig. 1). Similar to the live/dead staining results of treated Klebsiella biofilms, some regular rod-shaped cells remained in the biofilms treated with up to 200 μg/ml imipenem (data not shown).
To determine if imipenem is active in vivo against established K. pneumoniae biofilms and to promote wound healing, we tested the ability of imipenem to reduce the K. pneumoniae viable cell count and to improve the healing parameters, such as epithelial and granulation gaps, using a rabbit ear biofilm-infected wound model (16). A total of 12 rabbits were used for this study under an approved protocol by the Animal Care and Use Committee at Northwestern University. Animal care, wound creation, wound treatment, wound harvesting, and histologic analysis were performed according to previously described methods (16, 17). This study has been conducted in compliance with the Animal Welfare Act, the implementing Animal Welfare Regulations, and the principles of the Guide for the Care and Use of Laboratory Animals. Briefly, six 6-mm-diameter, full-thickness dermal wounds were created on each rabbit ear and 106 CFU of K. pneumoniae BAMC 07-18 was inoculated into each wound on postoperative day (POD) 3. Free-floating planktonic bacteria were removed using the topical agent ciprofloxacin HCl (Ciloxan; Alcon, Fort Worth, TX) on POD 4. Treatments with imipenem were administered every other day starting at POD 6, the time at which a steady-state, predominantly biofilm infection is present. After harvesting at POD 12, each wound was examined for viable bacterial counts by a plating method and epithelial and granulation gaps by a digital analysis system.
For treatments, imipenem was mixed with Intrasite gel (Smith & Nephew, St. Petersburg, FL) for steady release of imipenem. The release kinetics was determined by the use of a six-station Franz diffusion cell (PermeGear Inc., Hellertown, PA). Release samples of 0.2 ml were removed at different intervals (0.5, 1, 2, 4, 6, 8, 12, and 24 h), and the level of released imipenem was determined by high-performance liquid chromatography (HPLC) (18) and confirmed by an activity assay. An average of 5% of imipenem was released from the gel after half an hour. The released imipenem percentage increased with time and reached a plateau after approximately 12 h at about 25% of imipenem incorporated in the gel (see Fig. S2 in the supplemental material). For comparison, we divided the wounds into the following 3 groups: wounds were treated with (i) Intrasite gel only (untreated control), (ii) an imipenem gel containing 0.04 mg/ml of imipenem with a release of imipenem at a range of approximately 2 to 10 μg/ml based on the release kinetics, and (iii) a gel containing 4 mg/ml of imipenem, with a release of approximately 200 to 1,000 μg/ml imipenem. We arbitrarily set these as low (2 to 10 μg/ml) and high (200 to 1,000 μg/ml) imipenem treatments with the goal of proving conceptually if imipenem is active against established K. pneumoniae biofilm in vivo.
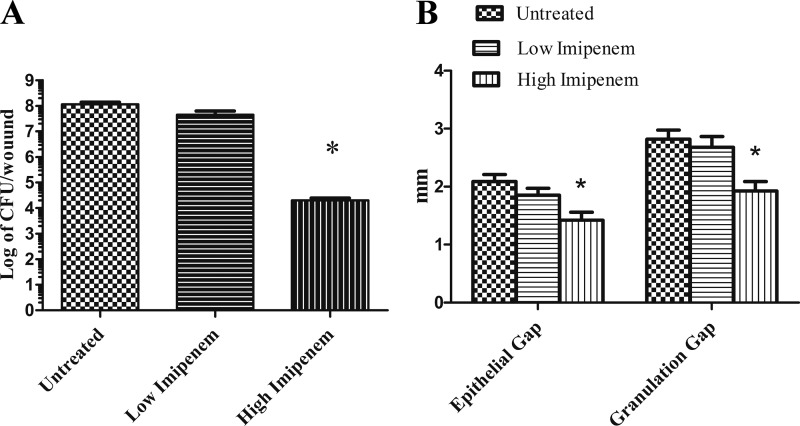
Each group consisted of 18 wounds. As shown in Fig. 3A, wounds treated with the gel containing the higher concentration of imipenem had significantly more reduction of bacterial burden (close to a 4-log reduction) at POD 12 than untreated wounds. An unpaired t test indicated that the difference is statistically significant with a P value of <0.0001. Further, both epithelial and granulation gaps were smaller in wounds treated with the gel containing the higher concentration of imipenem than in untreated wounds or wounds treated with the gel containing a lower concentration of imipenem (Fig. 3B). These differences were statistically significant with a P value of <0.0001. However, there were no significant statistical differences found between the wounds treated with gel containing the lower concentration of imipenem and the untreated wounds with respect to viable cell count or epithelial and granulation gaps. Thus, our data suggest that the gel containing the higher concentration of imipenem is effective at reducing K. pneumoniae biofilm burden; the imipenem gel improved healing of biofilm-infected wounds.
FIG 3.
Imipenem activity against established K. pneumoniae BAMC 07-18 biofilms in rabbit ear wounds. Biofilm-infected wounds were treated every other day starting at POD 6 and analyzed at POD 12. Imipenem treatment reduced the bacterial burden (viable K. pneumoniae cell counts) (A) and reduced the gaps of epithelialization and granulation (B). Untreated, Intrasite gel only. See the text for description of gels containing high or low concentrations of imipenem. t test shows that the differences between untreated and high-imipenem biofilms are statistically significant. *, P < 0.0001.
K. pneumoniae is an increasing threat to human health due to the emergence of multidrug-resistant strains. In this report, we have shown that imipenem has potent activity against multidrug-resistant strain K. pneumoniae BAMC 07-18 in the biofilm state both in vitro and in vivo. The cell shape was altered by the imipenem treatment, as determined by both SEM and confocal microscopy. The membranes of treated cells were compromised, as suggested by the SEM images. The formation of spheres with subsequent cell rupture among the imipenem-treated cells is consistent with imipenem's mechanism of action on cell wall synthesis (19).
Imipenem exhibits in vitro bactericidal activity on Acinetobacter baumannii sessile cells growing in the biofilm state (20) alone. The drug also possesses in vivo activity against biofilms formed by Staphylococcus aureus (21) or biofilms formed by Pseudomonas aeruginosa and Staphylococcus epidermidis (22) when combined with another agent. Although the imipenem treatment did not eliminate all K. pneumoniae biofilm cells both in vitro and in vivo, the higher concentration of imipenem used did cause significant reductions of viable K. pneumoniae cells in the biofilm-infected wounds. The reductions in bacterial burden may translate into a less sizable bioburden for the host innate immune response to control the infection. Thus, imipenem is potentially useful in controlling wound infections caused by K. pneumoniae biofilm.
Supplementary Material
ACKNOWLEDGMENTS
We thank Tao You for assistance with SEM and Hector Machado for assistance with imipenem release kinetics from Intrasite gel. We thank Robert E. W. Hancock for the kind gifts of peptides DJK-6, HHC10, and HHC36.
This work was supported by the U.S. Army Medical Research and Materiel Command, Combat Casualty Care Research Directorate.
The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Department of the Army or the Department of Defense.
Footnotes
Published ahead of print 18 November 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.01353-13.
REFERENCES
- 1.Podschun R, Ullmann U. 1998. Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin. Microbiol. Rev. 11:589–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lockhart SR, Abramson MA, Beekmann SE, Gallagher G, Riedel S, Diekema DJ, Quinn JP, Doern GV. 2007. Antimicrobial resistance among Gram-negative bacilli causing infections in intensive care unit patients in the United States between 1993 and 2004. J. Clin. Microbiol. 45:3352–3359. 10.1128/JCM.01284-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ressner RA, Murray CK, Griffith ME, Rasnake MS, Hospenthal DR, Wolf SE. 2008. Outcomes of bacteremia in burn patients involved in combat operations overseas. J. Am. Coll. Surg. 206:439–444. 10.1016/j.jamcollsurg.2007.09.017 [DOI] [PubMed] [Google Scholar]
- 4.Bennett JW, Robertson JL, Hospenthal DR, Wolf SE, Chung KK, Mende K, Murray CK. 2010. Impact of extended spectrum beta-lactamase producing Klebsiella pneumoniae infections in severely burned patients. J. Am. Coll. Surg. 211:391–399. 10.1016/j.jamcollsurg.2010.03.030 [DOI] [PubMed] [Google Scholar]
- 5.Lopez D, Vlamakis H, Kolter R. 2010. Biofilms. Cold Spring Harb. Perspect. Biol. 2(7):a000398. 10.1101/cshperspect.a000398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leid JG. 2009. Bacterial biofilms resist key host defenses. Microbe 4:66–70 [Google Scholar]
- 7.Donlan RM. 2008. Biofilms on central venous catheters: is eradication possible? Curr. Top. Microbiol. Immunol. 322:133–161 [DOI] [PubMed] [Google Scholar]
- 8.Bjarnsholt T, Kirketerp-Moller K, Jensen PO, Madsen KG, Phipps R, Krogfelt K, Hoiby N, Givskov M. 2008. Why chronic wounds will not heal: a novel hypothesis. Wound Repair Regen. 16:2–10. 10.1111/j.1524-475X.2007.00283.x [DOI] [PubMed] [Google Scholar]
- 9.Black CE, Costerton JW. 2010. Current concepts regarding the effect of wound microbial ecology and biofilms on wound healing. Surg. Clin. North Am. 90:1147–1160. 10.1016/j.suc.2010.08.009 [DOI] [PubMed] [Google Scholar]
- 10.Lewis K. 2010. Persister cells. Annu. Rev. Microbiol. 64:357–372. 10.1146/annurev.micro.112408.134306 [DOI] [PubMed] [Google Scholar]
- 11.Ceri H, Olson ME, Stremick C, Read RR, Morck D, Buret A. 1999. The Calgary Biofilm Device: new technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J. Clin. Microbiol. 37:1771–1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brooun A, Liu S, Lewis K. 2000. A dose-response study of antibiotic resistance in Pseudomonas aeruginosa biofilms. Antimicrob. Agents Chemother. 44:640–646. 10.1128/AAC.44.3.640-646.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spoering AL, Lewis K. 2001. Biofilms and planktonic cells of Pseudomonas aeruginosa have similar resistance to killing by antimicrobials. J. Bacteriol. 183:6746–6751. 10.1128/JB.183.23.6746-6751.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kint CI, Verstraeten N, Fauvart M, Michiels J. 2012. New-found fundamentals of bacterial persistence. Trends Microbiol. 20:577–585. 10.1016/j.tim.2012.08.009 [DOI] [PubMed] [Google Scholar]
- 15.Araujo JC, Téran FC, Oliveira RA, Nour EAA, Montenegro MAP, Campos JR, Vazoller RF. 2003. Comparison of hexamethyldisilazane and critical point drying treatments for SEM analysis of anaerobic biofilms and granular sludge. J. Electron Microsc. 52:429–433. 10.1093/jmicro/52.4.429 [DOI] [PubMed] [Google Scholar]
- 16.Gurjala AN, Geringer MR, Seth AK, Hong SJ, Smeltzer MS, Galiano RD, Leung KP, Mustoe TA. 2011. Development of a novel, highly quantitative in vivo model for the study of biofilm-impaired cutaneous wound healing. Wound Repair Regen. 19:400–410. 10.1111/j.1524-475X.2011.00690.x [DOI] [PubMed] [Google Scholar]
- 17.Seth AK, Geringer MR, Gurjala AN, Hong SJ, Galiano RD, Leung KP, Mustoe TA. 2012. Treatment of Pseudomonas aeruginosa biofilm-infected wounds with clinical wound care strategies: a quantitative study using an in vivo rabbit ear model. Plast. Reconstr. Surg. 129:262e–274e. 10.1097/PRS.0b013e31823aeb3b [DOI] [PubMed] [Google Scholar]
- 18.Fernandez-Torres R, Bello-Lopez MA, Callejon-Mochon M, Jimenez-Sanchez JC. 2008. Determination of imipenem and rifampicin in mouse plasma by high performance liquid chromatography-diode array detection. Anal. Chim. Acta 608:204–210. 10.1016/j.aca.2007.12.026 [DOI] [PubMed] [Google Scholar]
- 19.Rodloff AC, Goldstein EJ, Torres A. 2006. Two decades of imipenem therapy. J. Antimicrob. Chemother. 58:916–929. 10.1093/jac/dkl354 [DOI] [PubMed] [Google Scholar]
- 20.Vidal R, Dominguez M, Urrutia H, Bello H, Garcia A, Gonzalez G, Zemelman R. 1997. Effect of imipenem and sulbactam on sessile cells of Acinetobacter baumannii growing in biofilm. Microbios 91:79–87 [PubMed] [Google Scholar]
- 21.Yamasaki O, Akiyama H, Toi Y, Arata J. 2001. A combination of roxithromycin and imipenem as an antimicrobial strategy against biofilms formed by Staphylococcus aureus. J. Antimicrob. Chemother. 48:573–577. 10.1093/jac/48.4.573 [DOI] [PubMed] [Google Scholar]
- 22.Furuhata M, Iwamura M, Baba S, Inoue M. 2003. Combined effect of clarithromycin and imipenem/cilastatin against urinary biofilm infection after pyeloplasty. Int. J. Urol. 10:228–230. 10.1046/j.0919-8172.2003.00598.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.