Abstract
The effects of prior vancomycin exposure on ceftaroline and daptomycin therapy against methicillin-resistant Staphylococcus aureus (MRSA) have not been widely studied. Humanized free-drug exposures of vancomycin at 1 g every 12 h (q12h), ceftaroline at 600 mg q12h, and daptomycin at 10 mg/kg of body weight q24h were simulated in a 96-h in vitro pharmacodynamic model against three MRSA isolates, including one heteroresistant vancomycin-intermediate S. aureus (hVISA) isolate and one VISA isolate. A total of five regimens were tested: vancomycin, ceftaroline, and daptomycin alone for the entire 96 h, and then sequential therapy with vancomycin for 48 h followed by ceftaroline or daptomycin for 48 h. Microbiological responses were measured by the changes in log10 CFU during 96 h from baseline. Control isolates grew to 9.16 ± 0.32, 9.13 ± 0.14, and 8.69 ± 0.28 log10 CFU for MRSA, hVISA, and VISA, respectively. Vancomycin initially achieved ≥3 log10 CFU reductions against the MRSA and hVISA isolates, followed by regrowth beginning at 48 h; minimal activity was observed against VISA. The change in 96-h log10 CFU was largest for sequential therapy with vancomycin followed by ceftaroline (−5.22 ± 1.2, P = 0.010 versus ceftaroline) and for sequential therapy with vancomycin followed by ceftaroline (−3.60 ± 0.6, P = 0.037 versus daptomycin), compared with daptomycin (−2.24 ± 1.0), vancomycin (−1.40 ± 1.8), and sequential therapy with vancomycin followed by daptomycin (−1.32 ± 1.0, P > 0.5 for the last three regimens). Prior exposure of vancomycin at 1 g q12h reduced the initial microbiological response of daptomycin, particularly for hVISA and VISA isolates, but did not affect the response of ceftaroline. In the scenario of poor vancomycin response for high-inoculum MRSA infection, a ceftaroline-containing regimen may be preferred.
INTRODUCTION
Staphylococcus aureus is one of the most common causes of bloodstream infections, which are associated with increased mortality, lengths of stay, and health care-related costs (1). When methicillin-resistant S. aureus (MRSA) is suspected as the cause of the bloodstream infection, vancomycin is typically considered the first-line treatment and is often the initial antibiotic that patients receive upon arriving at an emergency department or when contracting the infection in a hospital. However, recent studies have suggested an increase in vancomycin MICs, making it increasingly difficult to successfully treat MRSA bloodstream infections with vancomycin based on the increasing drug exposures required (2–4). Reduced vancomycin susceptibility has been associated with increased rates of treatment failure and mortality, particularly in patients with S. aureus bacteremia. In addition to reduced susceptibilities, difficulties with providing adequate dosing while minimizing toxicity and the burdens of monitoring trough levels and obtaining optimal pharmacodynamic targets with vancomycin remain constant challenges.
Given the difficulties with vancomycin, other antimicrobials, such as daptomycin, linezolid, and telavancin, are sometimes used to treat MRSA infections when patients are not ideal candidates for vancomycin or have persistent infections despite treatment with vancomycin (1, 5). These agents can be particularly useful when treating less susceptible strains, such as heteroresistant vancomycin-intermediate S. aureus (hVISA) and VISA (6). Notably, daptomycin, a lipopeptide antibiotic with bactericidal activity against many Gram-positive organisms, has been approved for treating MRSA bacteremia and is often the first agent selected in the scenario of persistent infections in a patient receiving vancomycin (7). However, there are some studies that have suggested cross-resistance between vancomycin and daptomycin (8, 9). Ceftaroline fosamil is a cephalosporin with a broad spectrum of Gram-negative and Gram-positive activity, including against MRSA, due to its enhanced binding affinity to penicillin-binding protein (PBP) 2a. Ceftaroline displays potent in vitro activity against S. aureus strains with reduced vancomycin and daptomycin susceptibility (10) and has demonstrated bactericidal activity against MRSA in an in vivo rabbit endocarditis model (11). Ceftaroline fosamil has also been successfully used to treat some patients with endocarditis and bacteremia (12). With the increased awareness of MRSA persistence during treatment with vancomycin, newer therapies, such as daptomycin and ceftaroline fosamil, are often used, and the effect of prior vancomycin use on these therapies is unknown. Therefore, we evaluated human simulated regimens of vancomycin, daptomycin, and ceftaroline against various MRSA isolates, including hVISA and VISA strains, with the goal of studying the effects of prior vancomycin exposure on these other antimicrobial options.
MATERIALS AND METHODS
Bacterial strains and susceptibility testing.
Three clinical MRSA isolates with different phenotypic vancomycin profiles (1 vancomycin susceptible, 1 hVISA, and 1 VISA) were used. Vancomycin, daptomycin, and ceftaroline MICs were determined by broth microdilution using cation-adjusted Mueller-Hinton broth (CAMHB; Becton, Dickinson and Company, Sparks, MD) in accordance with Clinical and Laboratory Standards Institute (CLSI) recommendations (13). MIC calculations were performed in triplicate, and the modal MICs were reported. The phenotypic profiles are listed in Table 1. All isolates were susceptible to ceftaroline and daptomycin.
TABLE 1.
MICs of MRSA strains selected for use in the in vitro pharmacodynamic model
| Isolate no. (phenotypic classification) | MIC (μg/ml) |
||
|---|---|---|---|
| Vancomycin | Ceftaroline | Daptomycin | |
| 412 (MRSA) | 2 | 1 | 0.5 |
| 449 (hVISA) | 2 | 1 | 0.5 |
| 454 (VISA) | 8 | 1 | 1 |
Antibiotics.
Vancomycin (lot 7601063; expiration, December 2013; Pfizer Injectables) and daptomycin (lot ALTZ000; expiration, November 2013; Cubist Pharmaceuticals) clinical powder for injection were obtained from the Department of Pharmacy at Hartford Hospital in Hartford, CT. Analytical grade ceftaroline (lot FMD-CEF-051), the active component of ceftaroline fosamil, was provided by Forest Laboratories, Inc. (Jersey City, NJ).
Simulated drug exposures.
Free-drug concentrations for vancomycin at 1 g every 12 h (q12h), daptomycin at 10 mg/kg of body weight q24h, and ceftaroline-fosamil at 600 mg q12h were simulated in the in vitro experiments. Protein binding of 50%, 90%, and 20% were used for vancomycin, daptomycin, and ceftaroline, respectively, to derive free-drug exposures (14–16). The free-drug target area under the concentration-time curve from 0 to 12 h (AUC0–12) for vancomycin was 105 μg · h/ml with a target half-life of 9 h (17). Although the half-life of vancomycin is approximately 6 h in patients with normal renal function, the half-life simulated here was extended to achieve this specific AUC and maintain a free-drug trough concentration of 5 μg/ml. These vancomycin exposures were deliberately targeted to permit regrowth by 48 h in an effort to best simulate a clinical scenario in which a relevant human vancomycin exposure is not effective and will ultimately require a switch to another antibiotic. A high dose of daptomycin was selected based on current guidelines that recommend a dose of 10 mg/kg for patients with persistent bacteremia despite treatment with vancomycin (5). The free-drug target AUC0–24 for daptomycin was 108 μg · h/ml with a target half-life of 8 h (14). The target time that the free drug concentration remains above the MIC (fT>MIC) for ceftaroline at an MIC of 1 μg/ml was 60% with a target half-life of 2.6 h and a free peak concentration of 17.04 μg/ml (15, 18). A total of five treatment regimens were evaluated in the in vitro model: single-drug therapy with (i) vancomycin at 1 g q12h for 96 h (VAN), (ii) ceftaroline at 600 mg q12h for 96 h (CPT), and (iii) daptomycin at 10 mg/kg q24h for 96 h (DAP), and then sequential drug therapy with (iv) vancomycin at 1 g q12h for 48 h followed by ceftaroline at 600 mg q12h for 48 h (VAN-CPT), and (v) vancomycin at 1 g q12h for 48 h followed by daptomycin at 10 mg/kg q24h for 48 h (VAN-DAP).
In vitro pharmacodynamic model.
A one-compartment in vitro chemostat model was used for all experiments, as previously described (19). Briefly, each experiment consisted of three independent models (two experimental treatment models and one growth control model), which ran simultaneously for each isolate. The models were placed in a 37°C water bath for optimal temperature control. Magnetic stir bars were utilized to ensure adequate mixing of the contents of each model. A starting inoculum of 108 CFU/ml was used and prepared as previously described in order to simulate the high inoculum observed in bacteremia (19). Models were filled with brain heart infusion (BHI) broth (Becton, Dickinson and Company, Sparks, MD) and inoculated. For experiments with daptomycin, broth was supplemented with 50 mg/liter of calcium chloride (20). Previous experiments in our lab observed no difference in growth characteristics or MICs between BHI and CAMHB (data not shown). Antibiotic administration was started after 0.5 h of inoculation. Fresh broth was supplied via a peristaltic pump (Masterflex L/S model 7524-40; Cole-Palmer Instrument Company), which was set to achieve the human simulated half-life of the antimicrobial being tested. Each experiment was conducted for 96 h, and treatment models were performed in duplicate to ensure reproducibility.
Samples were obtained from each of the models at various time points throughout the 96-h experiment and were serially diluted in normal saline to assess changes in bacterial density over time. Aliquots were taken from each diluted sample and plated onto BHI agar plates and incubated at 37°C for 18 to 24 h for quantitative culture. Time-kill curves were constructed by plotting the log10 CFU/ml against time. The lower limit of detection for bacterial density was 1.7 log10 CFU/ml.
Antibiotic concentration determination.
To confirm drug concentrations, broth samples were simultaneously taken with bacterial density and assayed for vancomycin, daptomycin, and ceftaroline concentrations to ensure that the target exposures were achieved. All samples were immediately frozen and stored at −80°C until analysis. Daptomycin concentrations were determined by using a validated high-performance liquid chromatography (HPLC) method at the Center for Anti-Infective Research & Development, as described previously (21). Vancomycin concentrations were determined in the Hartford Hospital Clinical Laboratory with a clinically available fluorescence polarization immunoassay (FPIA) (Roche Diagnostic Corporation, Indianapolis, IN) by using a spectrophotometric detection method (Cobas c501; Roche Diagnostics Corporation, Indianapolis, IN). Ceftaroline samples were analyzed by Eurofins Medinet, Inc. (Chantilly, VA) using a validated liquid chromatography-tandem mass spectrometry (LC/MS-MS) assay.
Resistance.
The presence of resistant subpopulations was conducted by population analysis profiles (PAPs). Bacterial suspensions were plated on drug-containing BHI plates at 48 and 96 h and incubated for 48 h at 37°C. Vancomycin drug-containing plates with concentrations of 1, 2, 4, and 8 μg/ml were used for the hVISA and MRSA isolates, and concentrations of 8, 12, and 16 μg/ml were used for the VISA isolate. Ceftaroline drug-containing plates with concentrations of 0.5, 1, 2, 4, and 8 μg/ml and daptomycin drug-containing plates with concentrations of 0.5, 1, 2, 4, 8, and 16 μg/ml were used for all the isolates. Agar for daptomycin drug-containing plates was supplemented with 30 mg/liter of calcium chloride as previously described, as this concentration permitted the identification of a less susceptible population (8). The lower limit of quantification was 1.7 log10 CFU/ml. Additionally, organisms from quantitative culture plates at 48 and 96 h were frozen for broth microdilution MIC testing at the end of the experiment. Organisms were subcultured twice, and MICs were determined in triplicate by broth microdilution in accordance with CLSI methodology (13).
Statistics.
Changes in log10 CFU at 48 and 96 h were compared by analysis of variance with the Student-Newman-Keuls method for multiple comparisons. An a priori P value of <0.05 was considered statistically significant.
RESULTS
Pharmacokinetic analysis.
Targeted and confirmed exposures for each of the regimens are presented in Table 2. Confirmed concentrations, half-lives, and pharmacodynamic exposures were within 13% of the target with the exception of daptomycin; the observed daptomycin AUC exposure in the model was found to be 41% greater than the target AUC. This is a result of a modestly longer observed half-life than what was targeted.
TABLE 2.
Targeted and observed pharmacokinetics of all isolates against each regimen
| Antibiotic regimen | Pharmacodynamic target (AUC or fT>MICa) |
Peak (μg/ml) |
t1/2 (h) |
|||
|---|---|---|---|---|---|---|
| Target | Observed | Target | Observed | Target | Observed | |
| Vancomycin 1 g q12h | 105 | 106.5 ± 13.3 | 12.5 | 13.6 ± 1.6 | 9.0 | 9.6 ± 1.26 |
| Daptomycin 10 mg/kg q24h | 108 | 152.5 ± 33.9 | 13.9 | 12.6 ± 1.6 | 8.0 | 10.7 ± 2.03 |
| Ceftaroline 600 mg q12h | 60 | 61.8 ± 8.5 | 17.0 | 19.3 ± 1.9 | 2.7 | 2.5 ± 0.17 |
Shown are the AUC (μg · h/ml) of the dosing intervals for vancomycin and daptomycin and the fT>MIC (%) for ceftaroline.
Antibacterial results.
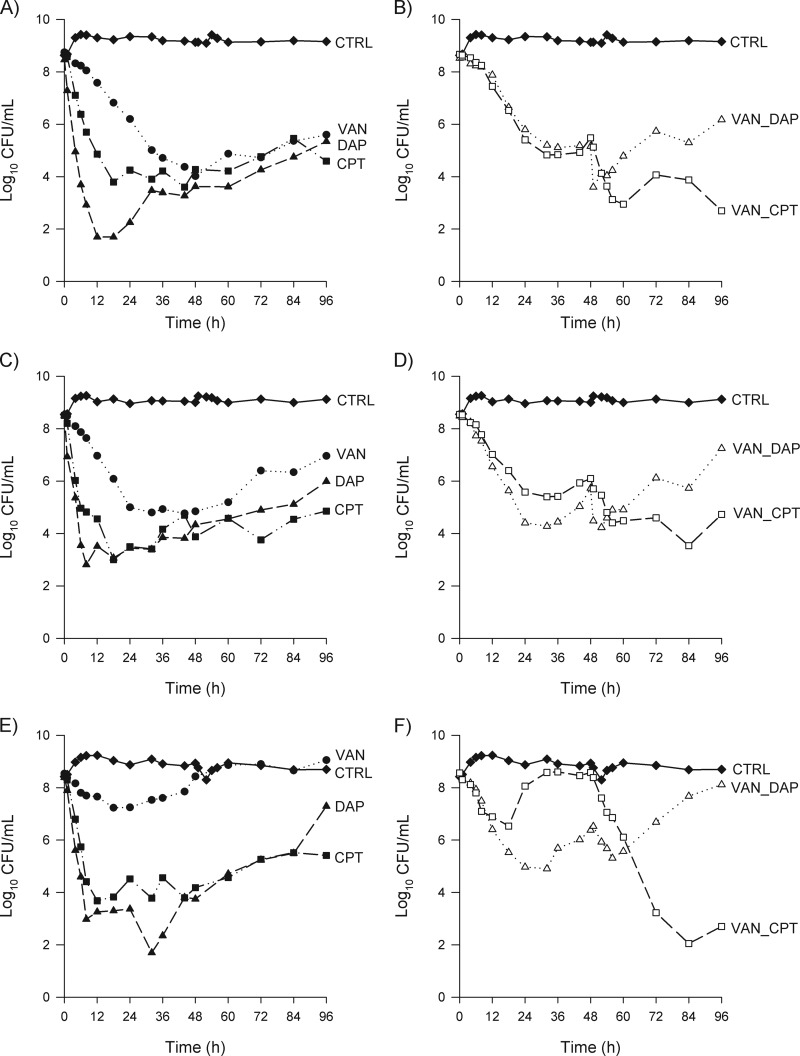
The average bacterial starting inocula for the MRSA (isolate 412), hVISA (isolate 449), and VISA (isolate 454) isolates were 8.6 ± 0.15, 8.5 ± 0.07, and 8.5 ± 0.18 log10 CFU/ml, respectively. Control models grew to 9.16 ± 0.32, 9.13 ± 0.14, and 8.69 ± 0.28 log10 CFU/ml for MRSA, hVISA, and VISA, respectively. Time-kill curves for all treatment regimens against each isolate are shown in Fig. 1A to F. Overall, greater bacterial reductions were observed against the MRSA isolate with all regimens tested than with the hVISA and VISA isolates.
FIG 1.
Mean bacterial densities over 96 h for single and sequential treatment regimens by isolate. (A) MRSA (isolate 412) single-treatment regimens; (B) MRSA (isolate 412) sequential treatment regimens; (C) hVISA (isolate 449) single-treatment regimens; (D) hVISA (isolate 449) sequential treatment regimens; (E) VISA (isolate 454) single-treatment regimens; (F) VISA (isolate 454) sequential treatment regimens. Closed diamonds, growth control; closed circles, vancomycin for 96 h; closed triangles, daptomycin for 96 h; closed squares, ceftaroline for 96 h; open triangles, vancomycin for 48 h followed by daptomycin for 48 h; open squares, vancomycin for 48 h followed by ceftaroline for 48 h.
By 48 h, all regimens that began with VAN (i.e., VAN, VAN-CPT, and VAN-DAP) had similar levels of regrowth (P > 0.05). The CPT and DAP regimens had the greatest reductions in CFU at 48 h (Fig. 1), so statistically, the largest reductions in the 48-h CFU were observed in the following order: CPT = DAP > VAN-CPT = VAN-DAP = VAN > control.
The log10 CFU/ml results at 96 h are provided in Table 3 for each isolate and collectively. Sequential therapy with vancomycin followed by ceftaroline showed a greater reduction in CFU at 96 h than that with all the other regimens tested. Ceftaroline therapy for 96 h displayed greater reductions than daptomycin therapy for 96 h (P = 0.037). At 96 h, there were no differences between VAN and daptomycin or sequential therapy with vancomycin followed by daptomycin (i.e., VAN-CPT > CPT > DAP = VAN-DAP = VAN > control) (Fig. 1; Table 3).
TABLE 3.
Mean observed change in log10 CFU for each treatment regimen against each isolate
| Regimen | Δ (log10 CFU at 96 h)a |
Mean Δ (log10 CFU at 96 h)b | ||
|---|---|---|---|---|
| MRSA | hVISA | VISA | ||
| Vancomycin | −3.15 ± 0.6 | −1.57 ± 1.0 | 0.52 ± 0.3 | −1.40 ± 1.8 |
| Ceftaroline | −4.12 ± 1.1 | −3.65 ± 0.1 | −3.03 ± 0.5 | −3.60 ± 0.6 |
| Daptomycin | −3.12 ± 0.6 | −2.51 ± 1.1 | −1.10 ± 0.1 | −2.24 ± 1.0 |
| Vancomycin, ceftaroline | −5.98 ± 1.2 | −3.82 ± 0.6 | −5.87 ± 1.0 | −5.22 ± 1.2 |
| Vancomycin, daptomycin | −2.35 ± 0.2 | −1.24 ± 0.0 | −0.36 ± 0.3 | −1.32 ± 1.0 |
Data presented are the mean ± standard deviation of two models during each experiment.
Using data from all isolates, the sequential treatment regimen of vancomycin followed by ceftaroline achieved statically greater reductions in 96-h CFU than single-drug therapy with ceftaroline (P = 0.010), single-drug therapy with ceftaroline achieved significantly greater reductions in 96-h CFU than single-drug therapy with daptomycin (P = 0.037), and all other regimens achieved CFU reductions that were statistically similar to those of single-drug therapy with daptomycin (P > 0.05).
Resistance.
During the PAP experiments, no resistance was observed at 48 h for any of the regimens. At 96 h, no additional vancomycin-resistant subpopulations were observed for VAN-containing regimens against hVISA and VISA relative to those of the control. No resistance was observed on ceftaroline-containing plates in any of the experiments, and no resistance was observed on daptomycin-containing plates against MRSA and hVISA. For daptomycin at 96 h against the VISA isolate, resistant subpopulations were identified up to a daptomycin concentration of 16 μg/ml during the DAP and VAN-DAP experiments. However, traditional broth microdilution MIC results from 48 and 96 h demonstrated no changes from baseline for any of the isolates (Table 1).
DISCUSSION
In situations where vancomycin is not performing optimally, therapy is often discontinued in favor of other available antibiotics with activity against MRSA. Other than the presence of cross-resistance between vancomycin and other lipopeptides in certain scenarios, the potential effects of prior therapy with vancomycin on newer antibiotics have not been well studied. In this in vitro pharmacodynamic experiment, prior therapy with vancomycin had no effect on ceftaroline reductions of CFU at 96 h. In contrast, sequential therapy with vancomycin followed by daptomycin diminished the initial bactericidal reductions observed when daptomycin was studied as a single-drug regimen.
In this study, three S. aureus isolates (1 MRSA, 1 hVISA, and 1 VISA) were tested. Clinical isolates with a vancomycin MIC of 2 μg/ml were chosen for the hVISA and MRSA, as this is considered a risk factor for poor vancomycin response. Notably, when these isolates were exposed to a low but clinically relevant dose of vancomycin at 1 g every 12 h, it was anticipated that regrowth would ultimately be observed against these MRSA, hVISA, and VISA isolates (2). Furthermore, in order to be able to uniformly assess the efficacy of these regimens, all the isolates were susceptible to daptomycin and ceftaroline. Daptomycin provided the greatest amount of initial killing (>5-log reductions); however, ceftaroline had more sustained activity, with little observed regrowth over the 96-h experiment (Fig. 1A, C, and E) against these three isolates. This is consistent with a number of other in vitro experiments in the literature (22–24).
The mean 24-h fAUC/MIC ratios observed for vancomycin exposure were 106 for the MRSA and hVISA isolates (MIC = 2 μg/ml) and 26 for the VISA isolate (MIC = 8 μg/ml). These ratios are much lower than the targeted total drug AUC/MIC ratio of ∼400, which is advocated for serious MRSA infections (3, 5). As a result, the regrowth observed here is to be expected and is similar to previous in vitro findings where comparable vancomycin exposures were tested (22, 23). This low exposure was deliberately targeted to simulate a scenario of poor vancomycin response and the need for transition to another antibiotic. This is consistent with studies that demonstrated that patients with infective endocarditis and positive blood cultures after 7 days of vancomycin therapy have a slower response to therapy and are bacteremic for a longer period of time than patients treated with beta-lactams (25–27). Additionally, while vancomycin has been the drug of choice for MRSA infections for a number of years, there have been studies that demonstrated that the ability of vancomycin to kill can be hindered at high inocula (19, 20, 23, 28, 29).
The activity of ceftaroline observed in this study is similar to that in a previous in vitro study by Steed and colleagues, which showed bactericidal activity for 96 h against S. aureus isolates, with MICs of 0.25 to 0.5, simulating a ceftaroline regimen of 600 mg every 12 h (24). Given that the cephalosporin pharmacodynamic target required for efficacy is around 30 to 40% in murine models, the activity seen with this regimen is not surprising, as the fT>MIC was 60% at an MIC of 1 μg/ml in our experiments (30). Additionally, an in vitro hollow-fiber model conducted by Vidaillac and colleagues showed no differences between a ceftaroline regimen equivalent to ceftaroline fosamil at 600 mg every 8 h and a ceftaroline regimen every 12 h, likely due to the fact that the fT>MIC obtained with each regimen far exceeded the cephalosporin pharmacodynamic target required for efficacy (31). The clinical use of ceftaroline fosamil at 600 mg every 8 h in the setting of persistent bacteremia treated with vancomycin or daptomycin resulted in favorable outcomes in a patient case series (12). Furthermore, a clinical trial assessing the safety and efficacy of ceftaroline fosamil at 600 mg every 8 h is under way (www.clinicaltrials.gov/ct2/show/NCT01701219).
In these studies, exposure to vancomycin did not seem to affect the activity of ceftaroline, as the initial CFU counts after ceftaroline initiation were similar or even further reduced compared with those after ceftaroline therapy for 96 h. These in vitro findings parallel those in a recently published case series of six bacteremic patients effectively treated with ceftaroline after the failure of vancomycin or daptomycin therapy (12). These patients had either persistent or recurrent bacteremia for which they had all received vancomycin therapy. Three of the patients also had endocarditis as the source of MRSA bacteremia, and all three had cleared their bacteremia on ceftaroline therapy within 48 h of switching therapy.
Daptomycin displayed rapid initial bactericidal killing against all three isolates, with approximately 5-log reductions in CFU within 8 h of therapy. The observed daptomycin exposures were slightly higher than the targets, resulting in 24-h fAUC/MIC ratios of 305 and 152 for MICs of 0.5 and 1 μg/ml, respectively. Despite the establishment of a target high dose of daptomycin of 10 mg/kg and obtaining even higher-than-targeted exposures, regrowth was eventually observed around 48 h against all three isolates. While some previous in vitro models showed greater and sustained CFU reductions with daptomycin than those observed in our study, it should be noted that some of these studies used a methodology to simulate drug concentrations that was different than the approach taken in the current study (22, 23). Many of these studies simulated total drug concentrations with the addition of albumin to the media, whereas we simulated free-drug concentrations directly. Previously published in vitro studies suggested that albumin may have the ability to enhance the activity of antimicrobials (32).
Notably, the initial rapid killing observed with daptomycin therapy against all isolates was significantly blunted after exposure to vancomycin (Fig. 1A to F). Additionally, despite initial MICs in the susceptible range, resistant subpopulations were identified for the VISA isolate at 96 h with both daptomycin-containing regimens (DAP for 96 h and sequential therapy with VAN-DAP). When the daptomycin MICs of the isolates collected at 96 h were retested via broth microdilution, however, there were no changes from baseline, suggesting that this observed resistance may not have been stable. In a similar study done by Rose and colleagues to determine the effects of daptomycin activity after vancomycin exposure, no changes in the MICs were detected for any MRSA isolates that were treated with daptomycin following vancomycin exposure for 4 days (33). Although increases in daptomycin MICs for the methicillin-susceptible S. aureus (MSSA) isolates were observed, upon further sequencing, it was noted that these strains did not demonstrate mutations in MprF and YycG, the amino acid substitutions believed to contribute to daptomycin nonsusceptibility (34). Although the activity of daptomycin after vancomycin exposure is not entirely clear in vitro, it has been suggested that prior failure of vancomycin therapy is one of the factors associated with diminished daptomycin efficacy (35).
While in vitro pharmacodynamic models provide valuable information in assessing an exposure-response relationship, it is important to recognize some limitations when interpreting the data. First, the in vitro chemostat system does not take into account the bactericidal effects of an immune system, which could greatly increase bacterial colony reductions; therefore, these experiments demonstrate the worst-case scenario for the antibiotics evaluated. In the current studies, we chose a high starting inoculum of 108 log10 CFU/ml in an attempt to simulate a more invasive S. aureus infection; increased antibacterial effects are typically seen at lower inocula, which may better represent less severe S. aureus infections. The 96-h experiment duration was selected to describe the earliest portion of the antibiotic effect; however, serious S. aureus infections are often treated for 14 days, if not longer, so the downstream effects on resistance development and total bacterial count reductions are not known. Finally, although the experiments were conducted in duplicate, only one isolate of each phenotype (MRSA, hVISA, and VISA) was included, and interorganism variability can result in different observations. Further experiments with additional S. aureus isolates and for extended durations would be the next step in addressing the effect of prior vancomycin exposure on ceftaroline and daptomycin activity.
To our knowledge, this is the first in vitro pharmacodynamic study to evaluate free-drug exposures of ceftaroline and daptomycin after prior vancomycin exposure against a high inoculum of MRSA isolates. Against these isolates, prior exposure to vancomycin did not reduce the activity of ceftaroline; it did, however, diminish the killing profile of daptomycin, which eventually resulted in regrowth. For serious infections caused by MRSA with a high bacterial burden, ceftaroline therapy may be an attractive option, especially for patients who fail vancomycin therapy. This agent deserves further attention in clinical trials with complicated MRSA bloodstream infections.
ACKNOWLEDGMENTS
This study was supported by an investigator-initiated research grant from the Forest Research Institute, Inc. (Jersey City, NJ). J.L.K. and D.P.N. are members of the Speakers Bureau for Forest Pharmaceuticals and have received research support from the Forest Research Institute and Cerexa, Inc., a subsidiary of Forest Pharmaceuticals. The other authors have nothing to disclose.
We acknowledge Maryanne Banevicius, Henry Christiansen, Christina Sutherland, and Pamela Tessier for their assistance with the conduct of the study and HPLC. We also thank George Sakoulas (Sharp Memorial Hospital, San Diego, CA), Thomas P. Lodise (Albany College of Pharmacy and Health Sciences, Albany, NY), and Gary E. Stein (Michigan State University School of Medicine, East Lansing, MI) for providing clinical S. aureus isolates.
Footnotes
Published ahead of print 11 November 2013
REFERENCES
- 1.Corey GR. 2009. Staphylococcus aureus bloodstream infections: definitions and treatment. Clin. Infect. Dis. 48(Suppl):S254–S259. 10.1086/598186 [DOI] [PubMed] [Google Scholar]
- 2.Han JH, Mascitti KB, Edelstein PH, Bilker WB, Lautenbach E. 2012. Effect of reduced vancomycin susceptibility on clinical and economic outcomes in Staphylococcus aureus bacteremia. Antimicrob. Agents Chemother. 56:5164–5170. 10.1128/AAC.00757-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kullar R, Davis SL, Levine DP, Rybak MJ. 2011. Impact of vancomycin exposure on outcomes in patients with methicillin-resistant Staphylococcus aureus bacteremia: support for consensus guidelines suggested targets. Clin. Infect. Dis. 52:975–981. 10.1093/cid/cir124 [DOI] [PubMed] [Google Scholar]
- 4.Moise PA, Sakoulas G, Forrest A, Schentag JJ. 2007. Vancomycin in vitro bactericidal activity and its relationship to efficacy in clearance of methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob. Agents Chemother. 51:2582–2586. 10.1128/AAC.00939-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, Rybak M, Talan DA, Chambers HF. 2011. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin. Infect. Dis. 52:e18–e55. 10.1093/cid/ciq146 [DOI] [PubMed] [Google Scholar]
- 6.Sader HS, Fritsche TR, Kaniga K, Ge Y, Jones RN. 2005. Antimicrobial activity and spectrum of PPI-0903M (T-91825), a novel cephalosporin, tested against a worldwide collection of clinical strains. Antimicrob. Agents Chemother. 49:3501–3512. 10.1128/AAC.49.8.3501-3512.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cha R, Brown WJ, Rybak MJ. 2003. Bactericidal activities of daptomycin, quinupristin-dalfopristin, and linezolid against vancomycin-resistant Staphylococcus aureus in an in vitro pharmacodynamic model with simulated endocardial vegetations. Antimicrob. Agents Chemother. 47:3960–3963. 10.1128/AAC.47.12.3960-3963.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakoulas G, Alder J, Thauvin-Eliopoulos C, Moellering RC, Eliopoulos GM. 2006. Induction of daptomycin heterogeneous susceptibility in Staphylococcus aureus by exposure to vancomycin. Antimicrob. Agents Chemother. 50:1581–1585. 10.1128/AAC.50.4.1581-1585.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cui L, Tominaga E, Neoh HM, Hiramatsu K. 2006. Correlation between reduced daptomycin susceptibility and vancomycin resistance in vancomycin-intermediate Staphylococcus aureus. Antimicrob. Agents Chemother. 50:1079–1082. 10.1128/AAC.50.3.1079-1082.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saravolatz L, Pawlak J, Johnson L. 2010. In vitro activity of ceftaroline against community-associated methicillin-resistant, vancomycin-intermediate, vancomycin-resistant, and daptomycin-non-susceptible Staphylococcus aureus isolates. Antimicrob. Agents Chemother. 54:3027–3030. 10.1128/AAC.01516-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacqueline C, Caillon J, Le Mabecque V, Miegeville AF, Hamel A, Bugnon D, Ge JY, Potel G. 2007. In vivo efficacy of ceftaroline (PPI-0903), a new broad-spectrum cephalosporin, compared with linezolid and vancomycin against methicillin-resistant and vancomycin-intermediate Staphylococcus aureus in a rabbit endocarditis model. Antimicrob. Agents Chemother. 51:3397–3400. 10.1128/AAC.01242-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ho TT, Cadena J, Childs LM, Gonzalez-Velez M, Lewis JS. 2012. Methicillin-resistant Staphylococcus aureus bacteraemia and endocarditis treated with ceftaroline salvage therapy. J. Antimicrob. Chemother. 67:1267–1270. 10.1093/jac/dks006 [DOI] [PubMed] [Google Scholar]
- 13.Clinical and Laboratory Standards Institute 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically: approved standard, 9th ed. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 14.Benvenuto M, Benziger DP, Yankelev S, Vigliani G. 2006. Pharmacokinetics and tolerability of daptomycin at doses up to 12 milligrams per kilogram of body weight once daily in healthy volunteers. Antimicrob. Agents Chemother. 50:3245–3249. 10.1128/AAC.00247-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forest Laboratories, Inc 2010. Teflaro package insert. Forest Laboratories, Inc., St. Louis, MO [Google Scholar]
- 16.Albrecht LM, Rybak MJ, Warbasse LH, Edwards DJ. 1991. Vancomycin protein binding in patients with infections caused by Staphylococcus aureus. DICP 25:713–715 [DOI] [PubMed] [Google Scholar]
- 17.Kuti JL, Kiffer CR, Mendes CM, Nicolau DP. 2008. Pharmacodynamic comparison of linezolid, teicoplanin and vancomycin against clinical isolates of Staphylococcus aureus and coagulase-negative staphylococci collected from hospitals in Brazil. Clin. Microbiol. Infect. 14:116–123. 10.1111/j.1469-0691.2007.01885.x [DOI] [PubMed] [Google Scholar]
- 18.Keel RA, Crandon JL, Nicolau DP. 2011. Efficacy of human simulated exposures of ceftaroline administered at 600 milligrams every 12 hours against phenotypically diverse Staphylococcus aureus isolates. Antimicrob. Agents Chemother. 55:4028–4032. 10.1128/AAC.00372-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hagihara M, Wiskirchen DE, Kuti JL, Nicolau DP. 2012. In vitro pharmacodynamics of vancomycin and cefazolin alone and in combination against methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 56:202–207. 10.1128/AAC.05473-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lamp KC, Rybak MJ, Bailey EM, Kaatz GW. 1992. In vitro pharmacodynamic effects of concentration, pH, and growth phase on serum bactericidal activities of daptomycin and vancomycin. Antimicrob. Agents Chemother. 36:2709–2714. 10.1128/AAC.36.12.2709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dandekar PK, Tessier PR, Williams P, Nightingale CH, Nicolau DP. 2003. Pharmacodynamic profile of daptomycin against Enterococcus species and methicillin-resistant Staphylococcus aureus in a murine thigh infection model. J. Antimicrob. Chemother. 52:405–411. 10.1093/jac/dkg337 [DOI] [PubMed] [Google Scholar]
- 22.Akins RL, Rybak MJ. 2001. Bactericidal activities of two daptomycin regimens against clinical strains of glycopeptide intermediate-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus faecium, and methicillin-resistant Staphylococcus aureus isolates in an in vitro pharmacodynamic model with simulated endocardial vegetations. Antimicrob. Agents Chemother. 45:454–459. 10.1128/AAC.45.2.454-459.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.LaPlante KL, Rybak MJ. 2004. Impact of high-inoculum Staphylococcus aureus on the activities of nafcillin, vancomycin, linezolid, and daptomycin, alone and in combination with gentamicin, in an in vitro pharmacodynamic model. Antimicrob. Agents Chemother. 48:4665–4672. 10.1128/AAC.48.12.4665-4672.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steed M, Vidaillac C, Rybak MJ. 2011. Evaluation of ceftaroline activity versus daptomycin (DAP) against DAP-nonsusceptible methicillin-resistant Staphylococcus aureus strains in an in vitro pharmacokinetic/pharmacodynamic model. Antimicrob. Agents Chemother. 55:3522–3526. 10.1128/AAC.00347-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lodise TP, Jr, McKinnon PS, Rybak M. 2003. Prediction model to identify patients with Staphylococcus aureus bacteremia at risk for methicillin resistance. Infect. Control Hosp. Epidemiol. 24:655–661. 10.1086/502269 [DOI] [PubMed] [Google Scholar]
- 26.Levine DP, Fromm BS, Reddy BR. 1991. Slow response to vancomycin or vancomycin plus rifampin in methicillin-resistant Staphylococcus aureus endocarditis. Ann. Intern. Med. 115:674–680. 10.7326/0003-4819-115-9-674 [DOI] [PubMed] [Google Scholar]
- 27.Gentry CA, Rodvold KA, Novak RM, Hershow RC, Naderer OJ. 1997. Retrospective evaluation of therapies for Staphylococcus aureus endocarditis. Pharmacotherapy 17:990–997 [PubMed] [Google Scholar]
- 28.Larsson AJ, Walker KJ, Raddatz JK, Rotschafer JC. 1996. The concentration-independent effect of monoexponential and biexponential decay in vancomycin concentrations on the killing of Staphylococcus aureus under aerobic and anaerobic conditions. J. Antimicrob. Chemother. 38:589–597. 10.1093/jac/38.4.589 [DOI] [PubMed] [Google Scholar]
- 29.Bowker KE, Noel AR, MacGowan AP. 2009. Comparative antibacterial effects of daptomycin, vancomycin and teicoplanin studied in an in vitro pharmacokinetic model of infection. J. Antimicrob. Chemother. 64:1044–1051. 10.1093/jac/dkp289 [DOI] [PubMed] [Google Scholar]
- 30.Andes D, Craig WA. 2006. Pharmacodynamics of a new cephalosporin, PPI-0903 (TAK-599), active against methicillin-resistant Staphylococcus aureus in murine thigh and lung infection models: identification of an in vivo pharmacokinetic-pharmacodynamic target. Antimicrob. Agents Chemother. 50:1376–1383. 10.1128/AAC.50.4.1376-1383.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vidaillac C, Leonard SN, Rybak MJ. 2009. In vitro activity of ceftaroline against methicillin-resistant Staphylococcus aureus and heterogeneous vancomycin-intermediate S. aureus in a hollow fiber model. Antimicrob. Agents Chemother. 53:4712–4717. 10.1128/AAC.00636-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cha R, Rybak MJ. 2004. Influence of protein binding under controlled conditions on the bactericidal activity of daptomycin in an in vitro pharmacodynamic model. J. Antimicrob. Chemother. 54:259–262. 10.1093/jac/dkh259 [DOI] [PubMed] [Google Scholar]
- 33.Rose WE, Leonard SN, Sakoulas G, Kaatz GW, Zervos MJ, Sheth A, Carpenter CF, Rybak MJ. 2008. Daptomycin activity against Staphylococcus aureus following vancomycin exposure in an in vitro pharmacodynamic model with simulated endocardial vegetations. Antimicrob. Agents Chemother. 52:831–836. 10.1128/AAC.00869-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Friedman L, Alder JD, Silverman JA. 2006. Genetic changes that correlate with reduced susceptibility to daptomycin in Staphylococcus aureus. Antimicrob. Agents Chemother. 50:2137–2145. 10.1128/AAC.00039-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moise PA, Amodio-Groton M, Rashid M, Lamp KC, Hoffman-Roberts HL, Sakoulas G, Yoon MJ, Schweitzer S, Rastogi A. 2013. Multicenter evaluation of the clinical outcomes of daptomycin with and without concomitant beta-lactams in patients with Staphylococcus aureus bacteremia and mild to moderate renal impairment. Antimicrob. Agents Chemother. 57:1192–1200. 10.1128/AAC.02192-12 [DOI] [PMC free article] [PubMed] [Google Scholar]