Abstract
The activity of solithromycin was evaluated against clinical Legionella pneumophila serogroup 1 (Lp1) isolates (n = 196) collected in Ontario, Canada, from 1980 to 2011. Its in vitro activity was compared to that of azithromycin (AZM) using the broth microdilution method. Solithromycin had a MIC50 of ≤0.015 μg/ml and a MIC90 of 0.031 μg/ml, making its activity at least 8-fold to 32-fold higher than that of AZM (MIC50 and MIC90, 0.125 μg/ml and 1 μg/ml, respectively). Ninety-nine percent of the isolates had MICs for solithromycin ranging from ≤0.015 μg/ml to 0.031 μg/ml, whereas 83.6% of the isolates showed MICs for AZM ranging from 0.062 μg/ml to 0.25 μg/ml. Interestingly, 96.7% (30 out of 31 clinical isolates) identified with higher AZM MICs (0.5 μg/ml to 2 μg/ml) belonged to the clinically prevalent sequence type 1. To investigate the intracellular activity of solithromycin, in vitro invasion assays were also performed against a subset of representative Lp1 isolates internalized within human lung epithelial cells. Solithromycin and AZM both inhibited growth of all intracellular Lp1 isolates at 1× or 8× MICs, displaying bacteriostatic effects, as would be expected with protein synthesis inhibitor rather than bactericidal activity. Solithromycin demonstrated the highest in vitro and intracellular potency against all Lp1 isolates compared to AZM. Given the rapid spread of resistance mechanisms among respiratory pathogens and the reported treatment failures in legionellosis, the development of this new fluoroketolide, already in phase 3 oral clinical studies, constitutes a promising alternative option for the treatment of legionellosis.
INTRODUCTION
Legionellosis is a major public health concern in industrialized countries. Manifestations of the disease range from a mild respiratory illness (Pontiac fever) to a severe and rapidly fatal pneumonia (Legionnaires' disease) (1). The case fatality rate of legionellosis ranges between 40 and 80% in untreated immunosuppressed patients but can be reduced from 5 to 30% with appropriate case management (2). Macrolides and fluoroquinolones have become the preferred and recommended therapeutic agents to treat these infections (3). Legionellosis is acquired by inhaling airborne water droplets contaminated with Legionella bacteria (4). These bacterial species are found worldwide and are detected in up to 80% of freshwater sites (5). Among more than 54 species of Legionella, Legionella pneumophila is the major cause of outbreaks (91.5%), and serogroup 1 (Lp1) is the predominant serotype isolated from patients (84.2%) (6). Since human-to-human transfer of these bacteria has not been reported, to explain the higher incidence of Lp1 than of other Legionella species, it has been hypothesized that Lp1 is more virulent and/or more fit for survival in anthropogenic aquatic environments (7). In its natural environments, Legionella is presumably exposed to residual concentrations of antibiotics used in medical or veterinary practice (8). This could constitute a potential selective pressure for the acquisition of antimicrobial resistance or decreased susceptibility to antibiotics for L. pneumophila. Indeed, it has been shown that the environmental exposure of bacteria to low antibiotic concentrations may promote the selection of resistance mechanisms (9, 10). The in vitro acquisition of resistance to erythromycin, ciprofloxacin, and rifampin by L. pneumophila has been reported (11, 12). Although there are no data available suggesting the in vivo emergence of resistance against commonly used antimicrobial agents in patients, the hypothesis of resistance emergence in vivo would be consistent with the mortality rates of 10% to 30% that are usually reported for legionellosis and for fluoroquinolone treatment failures (13). Recently, Lp1 clinical isolates with MICs of 6 μg/ml for azithromycin (AZM) were reported to be outside the wild-type distribution among a large collection of isolates (14). Although there are no established macrolide CLSI breakpoints for Legionella, these isolates with reduced susceptibility to AZM represent a potential risk for patient treatment.
Solithromycin is a fluoroketolide that has demonstrated high in vitro potency against Gram-positive and some Gram-negative pathogens (15, 16) (Fig. 1). It is the first fluoroketolide under clinical development that has been well tolerated in phase I studies, with high plasma, tissue, and intracellular concentrations (17). Moreover, Oldach et al. reported recently that in a randomized, double-blind, phase 2 study, solithromycin had comparable efficacy and favorable safety relative to levofloxacin in the treatment of patients with community-acquired bacterial pneumonia (CABP) (18). The objective of our study was to evaluate the in vitro activity of solithromycin compared to that of AZM against a large, well-characterized population-based collection of clinical L. pneumophila isolates that was collected between 1980 and 2011 in Ontario, Canada (19). In addition, we also investigated the intracellular activity of solithromycin against a subset of selected isolates internalized within human lung epithelial cells (20).
FIG 1.
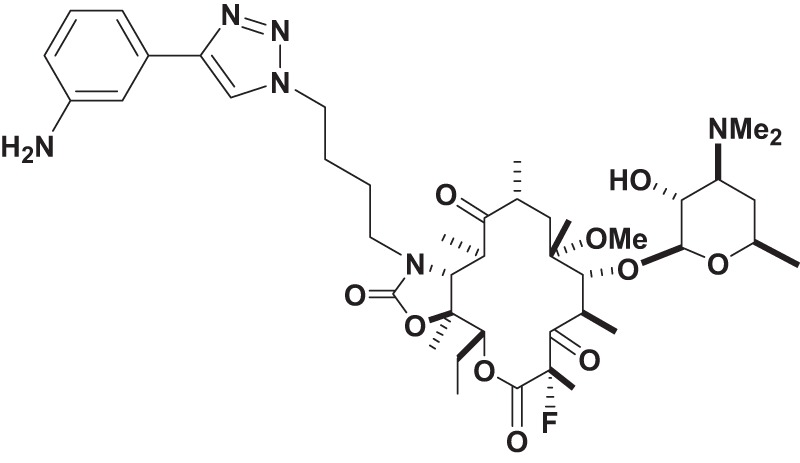
Chemical structure of solithromycin (CEM-101).
(Part of this study was presented at the 23rd European Congress of Clinical Microbiology and Infectious Diseases, Berlin, Germany, 27 to 30 April 2013 [poster 1640].)
MATERIALS AND METHODS
Bacterial strains.
Since 1978, the diagnosis of Legionella infections has been centralized at the Public Health Ontario Laboratory (PHOL), Toronto, Ontario, Canada, which serves as the reference laboratory and performs all testing for outbreak investigations and most testing of clinical specimens in Ontario. A total of 196 Lp1 clinical isolates collected from 1980 to 2011, representative of the strains isolated in Ontario in the past 3 decades and previously sequence base typed at the PHOL, were analyzed in this study (19). The isolates were stored in Trypticase soy broth supplemented with 5% horse blood at −80°C. Cultures from the frozen stock were prepared by inoculating buffered charcoal yeast extract (BCYE) plates, which were incubated for 3 days at 37°C in 5% CO2.
Broth microdilution susceptibility testing.
Colonies of all isolates were subcultured on BCYE plates for 3 days at 37°C in 5% CO2 before antimicrobial testing. The MICs of solithromycin and AZM were determined by the broth microdilution method in 96-well microtiter plates with minor modifications for L. pneumophila according to the Clinical and Laboratory Standards Institute guidelines (21) and the methods of Stout et al. (22). Briefly, suspensions of each isolate were prepared in buffered yeast extract broth (BYEB). The turbidities of the growing broth cultures were adjusted to an optical density equivalent to 0.5 McFarland standard (∼1 × 108 CFU/ml). The adjusted broth cultures were diluted to approximately 1 × 107 CFU/ml. Twofold serial dilutions of antibiotics were prepared in broth, and the wells were filled with a 50-μl antimicrobial solution. Then, the bacterial suspension was added to an equal volume (50 μl) in each well to reach a final volume of 100 μl per well and a bacterial concentration of approximately 5 × 106 CFU/ml. MICs were read as the first well showing no visible growth after 48 h of incubation at 37°C in 5% CO2. Staphylococcus aureus ATCC 29213 and Escherichia coli ATCC 25922 were used as quality control strains. No susceptibility-testing breakpoints are available for L. pneumophila. Correlation analysis between MICs and sequence-based molecular types was performed using BioNumerics software.
To determine if efflux pumps were involved in isolates with reduced susceptibility to AZM (MIC, 1 to 2 μg/ml), the broth microdilution method described above was used in the presence or absence of carbonyl cyanide m-chlorophenylhydrazone (CCCP) at a concentration of 0.031 μg/ml) (23). A reduction of at least 2-fold in the AZM MICs in the presence of CCCP was considered an indicator of efflux activity.
Analysis of macrolide targets in L. pneumophila.
The main targets involved in macrolide resistance were analyzed in 13 isolates belonging to sequence type 1 (ST1), including 4 isolates with wild-type MICs of AZM (≤0.625 μg/ml) and 9 isolates associated with higher MICs of AZM (1 to 2 μg/ml). Genomic DNAs were purified using a QIAamp DNA Mini Kit (Qiagen, Mississauga, ON, Canada). Ten to 50 ng of DNA was used as a template for each PCR.
Primers for detection of acquired macrolide resistance mechanisms were previously described (24). Moreover, the 3 copies of 23S rRNA rrl genes identified in Lp1 were analyzed by PCR amplification and DNA sequencing, using an approach previously described (25). Primers were designed from the genome of L. pneumophila Philadelphia (GenBank accession number NC_002942.5) (Table 1) (26). Long PCR amplifications of a 4-kb region from each of the three rrl gene copies were performed using Expand Long Range dNTPack (Roche, Montreal, QC, Canada) according to the manufacturer's instructions, with an annealing temperature (Ta) of 52°C. Each 4-kb amplicon was used as a DNA template for nested PCRs to specifically amplify domains V (the peptidyl-transferase loop) and II (hairpin 35, involved in the binding of solithromycin, but not AZM), using the primers listed in Table 1. Nested PCRs were performed by using the Platinum Taq DNA polymerase (Invitrogen, Burlington, ON, Canada) under standard amplification conditions (Ta = 57°C). Amplification of genes encoding the L4 and L22 riboproteins were also performed using Platinum Pfx DNA polymerase High Fidelity (Invitrogen) according to the manufacturer's instructions (Ta = 51°C).
TABLE 1.
Oligonucleotides used for amplification and sequencing of domains II and V of the 23S rRNA gene and riboproteins L4 and L22
| PCR and primer designation | Sequence (5′ to 3′) | Amplicon size |
|---|---|---|
| Primary allele PCR | ||
| Internal common primer | ||
| 23S common 741-744 | CTTACTGACCGATAGTGAACCAGTACCGTG | |
| External specific primer | ||
| 23S1-tufA1 | CGTGCCCACGTTTACGTGCGGCTTCTTACG | 4 kb |
| 23S2-lpl0613-R | TCATGATGGTCCTTATACTCCAGGCGGTAG | 4 kb |
| 23S3-lpl2670-R | AGATAATCACACTCTGAGTCAGCAATCCAG | 4 kb |
| Nested PCR 23S rRNA, domain V | ||
| 23S 2058 region | ||
| 23S common 2302-2332-F | CCGTAACTATAACGGTCCTAAGGTAGCGAA | 423 bp |
| 23S 3201-3350-R | CTTTACACTCTTGGTACGATGTCCGACCGT | |
| 23S 2611 region | ||
| 23S common 2302-2332-F | CCGTAACTATAACGGTCCTAAGGTAGCGAA | 928 bp |
| 23S 2604-2605-R | CTTCCACACCTTGCCTATCAACGTCGTAGT | |
| Nested PCR 23S rRNA, domain II | ||
| 23S 752 region | ||
| 23S region 741-744-F | CAGCGAGTTACTTTCAGTGGCGAGGTTAAC | 688 bp |
| 23S region741-744-R | CGTTACTCATGTCAGCATTCGCACTTCTGA | |
| L4 and L22 riboproteins | ||
| L4-F | GCAATTCCTGGTGCTCCAGGTTCTAGAGTA | 757 bp |
| L4-R | CTGTTTAAACTTATCGGCCATGACAGTTGC | |
| L22-F | ATGGTTGGTCATAAATTAGGTGAGTTTGCC | 463 bp |
| L22-R | CGTATACCTATTGGGTTTACTTTTTGTCCC |
Sequencing of all of the amplicons was performed using the same primers for amplification on a 3130xl Genetic Analyzer (Applied Biosystems, Streetsville, ON, Canada).
Intracellular activity of solithromycin versus that of AZM.
In vitro invasion assays were performed using monolayers of NCI-H292 human lung epithelial cells and 18 Lp1 clinical isolates displaying AZM susceptibility levels ranging from 0.0625 μg/ml to 2 μg/ml (see Table S1 in the supplemental material). NCI-H292 cells from human lung mucoepidermoid carcinoma (ATCC CRL-1848) were grown to 70 to 80% confluence (5 × 105 cells/well) in 24-well plates (Costar 24-well flat bottom plate; Corning Inc., Lowell, MA) containing 0.5 ml/well of RPMI 1640 medium (Life Technologies, Burlington, ON, Canada) supplemented with 10% heat-inactivated fetal calf serum and 1% glutamine (Life Technologies) and incubated at 37°C in 5% CO2 for 24 h. The cells were washed three times in serum-free RPMI 1640 prior to infection. Lp1 isolates were grown on BCYE agar plates and incubated for 4 days at 37°C in 5% CO2. Bacterial suspensions were obtained by resuspending colonies in 2 ml of phosphate-buffered saline (PBS). Cell suspensions were adjusted to an OD600 of 2 (approximately 1.5 × 109 CFU/ml) and added to the epithelial cells at a multiplicity of infection (MOI) of 30:1 in a volume of 0.5 ml per well (approximately 1.5 × 107 bacteria/well). Samples were subjected to centrifugation for 5 min at 500 rpm to promote bacterial association with the NCI-H292 cells. The cultures were incubated for 3 h at 37°C in 5% CO2. After 3 washes in serum-free RPMI to remove nonadherent bacteria, gentamicin was added (100-μg/ml final concentration) for 1 h to eradicate extracellular bacteria. The cells were washed three times in serum-free RPMI 1640 (to remove extracellular bacteria and gentamicin) and then incubated with solithromycin or AZM (8× and 1× the MIC for each strain) for 24 h and 48 h at 37°C in 5% CO2. Wells containing no antibiotic were used as growth controls at each time point (0, 24, and 48 h). RPMI 1640 does not sustain the extracellular growth of L. pneumophila, and all bacteria collected from the extracellular milieu resulted from intracellular growth (27). The supernatants of the intracellular assays were centrifuged to remove residual antimicrobial agent and resuspended in 0.5 ml of PBS. The lung epithelial cells were then lysed by osmotic shock using ice-cold filter-sterilized deionized water for 30 min. The lysed cells and their respective supernatants were pooled and homogenized by pipetting. Serial dilutions of bacterial suspension were plated on BCYE agar and incubated for 3 days at 37°C in 5% CO2. The intracellular activity of each antibacterial agent was determined by counting the CFU per milliliter on each plate. All assays were performed in quadruplicate with two independent biological duplicates.
The results were expressed as percent viability, defined as the total Legionella CFU at each time point (24 h and 48 h) with antibiotic divided by the total Legionella CFU at 0 h without antibiotic × 100. Values of ≥100% indicate absence of antimicrobial inhibition, whereas values of <100% indicate an inhibitory effect.
RESULTS
Susceptibility testing.
Solithromycin demonstrated the highest potency against all L. pneumophila isolates, displaying lower MIC values (≤0.015 to 0.0625 μg/ml) than AZM (≤0.0625 to 2 μg/ml) (Fig. 2). In other words, 99% of the isolates had solithromycin MICs of ≤0.031 μg/ml (only 2 isolates showed MICs of 0.0625 μg/ml), whereas 83.6% of the isolates had AZM MICs ranging from ≤0.0625 μg/ml to 0.25 μg/ml (Fig. 2). Solithromycin had an MIC50 of ≤0.015 μg/ml and an MIC90 of 0.031 μg/ml, making its activity at least 8-fold to 32-fold higher than that of AZM (MIC50, 0.125 μg/ml, and MIC90, 1 μg/ml) (Fig. 2).
FIG 2.
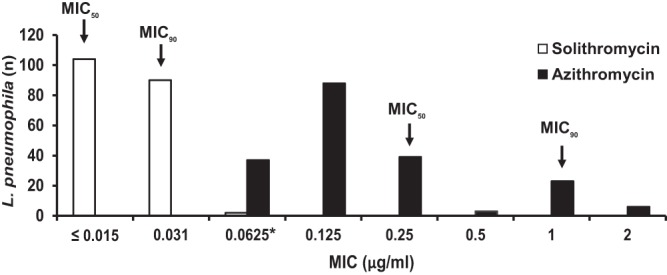
Distribution of solithromycin and azithromycin MICs for L. pneumophila serogroup 1 isolates. *, lowest concentration of azithromycin that was tested.
Association between STs and MICs.
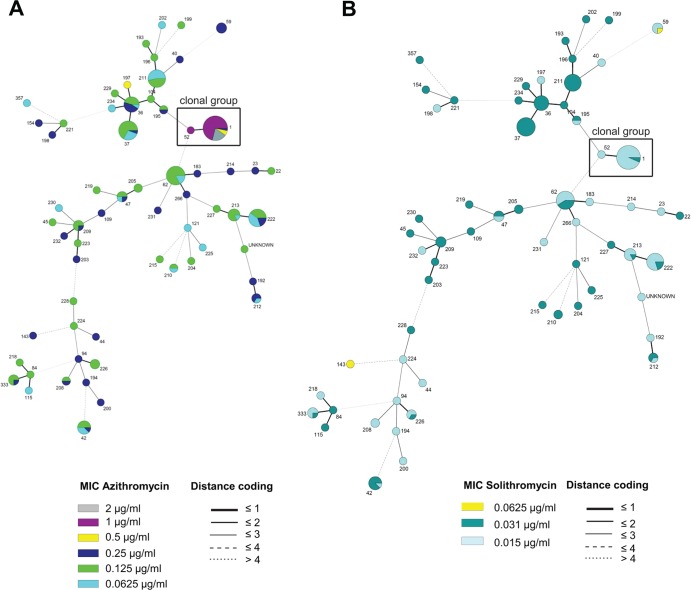
To evaluate a possible correlation between the MICs and the molecular-typing profiles of the Ontario Lp1 isolates, we performed a phylogenetic cluster analysis combining the susceptibility profiles with the previously reported sequence-based types of all isolates (19). Categorical clustering analysis of Lp1 based on STs showed that a large clonal group, including ST1 and ST52 isolates, was associated with the highest AZM MICs from our collection (Fig. 3A). Among the isolates of this clonal group, 93.7% of ST1 isolates showed MICs for AZM ranging from 0.5 to 2 μg/ml. In addition, the only isolate with ST52 showed an AZM MIC of 1 μg/ml (Fig. 3A). In contrast, isolates from this clonal group did not show solithromycin MICs higher than 0.031 μg/ml (Fig. 3B).
FIG 3.
Minimum spanning tree of L. pneumophila serogroup 1 (n = 196). A categorical clustering was performed based on STs. STs sharing the maximum number of single-locus variants were connected first. Each circle represents an ST, and its size is proportional to the number of isolates within that particular type. The colors of the circles indicate the MIC ranges for azithromycin (A) and solithromycin (B). Relationships between the STs are depicted by the lines connecting the STs and the relative lengths of the branches linking them. Distance coding enumerates the differences at a given typing locus. A distance code of greater than 2 implies a different clonal complex. The angles of the line connections and the overlapping circles have no significance.
Antimicrobial inhibition of intracellular growth of L. pneumophila.
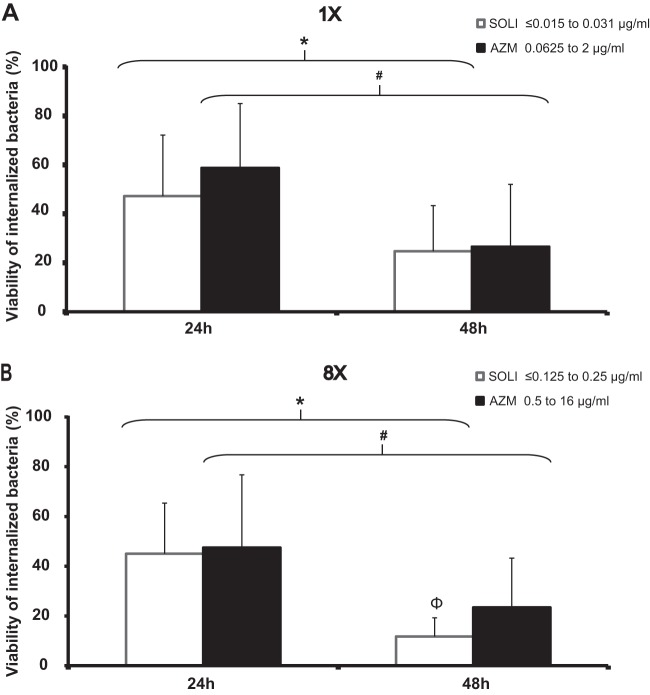
The intracellular susceptibilities to solithromycin and AZM of 18 selected Lp1 isolates displaying AZM MICs ranging from 0.0625 μg/ml to 2 μg/ml (solithromycin MICs of ≤0.015 to 0.031 μg/ml) were compared (see Table S1 in the supplemental material). Following an initial incubation with human NCI-H292 lung epithelial cells, all the isolates were internalized with similar efficiencies (data not shown). At 24 h and 48 h postinfection, all untreated control assays showed similar numbers of intracellular bacteria (1.62 × 1010 to 2.8 × 1011 CFU/ml and 4.05 × 1012 to 1.17 × 1014 CFU/ml, respectively [data not shown]). Twenty-four hours posttreatment, exposure to solithromycin or AZM at 1× the MIC resulted in the decline of the percentages of CFU per milliliter relative to the number of internalized bacteria pretreatment (to 47.2% and 58.8%, respectively) (Fig. 4A). Extension of the treatment to 48 h significantly reduced the viability of the Lp1 isolates exposed to solithromycin (to 24.7%) and AZM (to 26.6%). Although 4 to 64 times lower concentrations of solithromycin than of AZM were used in these assays, no significant difference in Lp1 viability was observed between the 2 antibiotic treatments (Fig. 4A). The effects of solithromycin and AZM on intracellular bacteria were next evaluated at 8× their respective MICs. Forty-eight hours posttreatment, the percentages of viable intracellular bacteria declined to 11.7% (solithromycin) and 23.4% (AZM). Overall, with 8× MICs, solithromycin inhibited the growth of intracellular Lp1 clinical isolates significantly more than AZM (P < 0.05).
FIG 4.
Intracellular activity of solithromycin compared to that of AZM against Lp1 clinical isolates (n = 18) displaying AZM MICs ranging from 0.0625 μg/ml to 2 μg/ml and solithromycin MICs ranging from ≤0.015 to 0.031 μg/ml. Lp1 isolates internalized within NCI-H292 lung epithelial cells were exposed to 2 concentrations of solithromycin and azithromycin. At the indicated time points, samples were harvested, and bacteria were enumerated by CFU assays. 8×, exposure to 8 times the MIC; 1×, exposure to 1× the MIC. The percent intracellular bacterial viability was calculated as the total number of Lp1 organisms at each time point with antibiotic divided by the total number of Lp1 organisms at time zero without antibiotic × 100. *, P < 0.01 at 24 h versus 48 h; #, P < 0.001 at 24 h versus 48 h; Φ, P < 0.05 for solithromycin versus AZM (Student's t test). The error bars indicate standard deviations.
Analyses of potential mechanisms involved in reduced susceptibility to macrolides.
To investigate the mechanisms involved in reduced susceptibility to AZM (MIC, 1 to 2 μg/ml) compared to the wild-type distribution (MIC, ≤0.0625 to 0.25 μg/ml), the most common targets and acquired mechanisms affecting macrolide susceptibility described in other microorganisms were analyzed (25). First, the rRNA and riboprotein genes were compared between nine ST1 isolates displaying AZM MICs ranging from 1 to 2 μg/ml versus four isolates displaying a low level of susceptibility to AZM (≤0.0625 μg/ml). The nine isolates with reduced susceptibility showed no difference in domains V and II in the 3 rrl gene copies or in the rplD and rplV genes (riboproteins L4 and L22) compared to the wild-type isolates. Acquired mechanisms of macrolide resistance (23S rRNA methylase genes, ermA, ermB, ermC, and ermF; efflux pump genes, mefA and mefE; and erythromycin esterase genes, ereA and ereB) were not detected in these isolates. Next, the role of efflux pumps in reduced susceptibility to AZM was evaluated using an inhibitor. None of the tested isolates showed a change in their AZM MIC levels in the presence of CCCP (data not shown).
DISCUSSION
L. pneumophila is a facultative intracellular pathogen. Hence, antimicrobial agents capable of achieving intracellular concentrations higher than the in vitro bacterial MICs (e.g., tetracyclines, macrolides, rifampin, and fluoroquinolones) are usually recommended for the treatment of legionellosis, rather than antibiotics with poor intracellular penetration (28). Despite the use of these antimicrobial agents and the improvement of diagnostic methods allowing early detection of infections, the mortality rate among legionellosis patients remains elevated (10 to 30%) (13). This situation is worsened by the fact that there are no in vitro antimicrobial breakpoints for L. pneumophila to define reduced susceptibility or resistance. Case reports of initial empirical treatment failures showed that final cures were achieved after changing the antimicrobial class during the treatment (29), pointing out the potential emergence of isolates with reduced susceptibility and the need for more careful use of the available antibiotics. In the present study, we evaluated the activity and potency of solithromycin against a collection of clinical Lp1 isolates. Solithromycin is a fluoroketolide with high affinity for bacterial ribosomes. In contrast to AZM, which binds only one site on the 23S ribosomal subunit, evidence suggests that solithromycin has three sites of interaction with the 23S ribosomal subunit (30), thereby enhancing its activity due to the higher number of binding sites. These multiple interactions may explain the better anchoring of solithromycin to the ribosome even in the presence of mutations at key positions (i.e., A2058-A2059 [E. coli numbering]) (31).
Our study shows that solithromycin has better in vitro activity than AZM against a variety of L. pneumophila clinical isolates, displaying AZM susceptibility levels ranging from ≤0.0625 μg/ml to 2 μg/ml (Fig. 2). This range of AZM MICs is consistent with previous studies reporting MIC distributions in clinical L. pneumophila serogroup 1 isolates using broth dilution and intracellular susceptibility assays (22, 32).
A possible correlation between the MICs and the molecular typing profiles of the Ontario Lp1 isolates was evaluated, and our data showed that most ST1-related isolates were associated with higher AZM MICs (0.5 to 2 μg/ml) (Fig. 3A), but they did not show increased solithromycin MICs (0.031 μg/ml) compared to other sequence types (Fig. 3B). ST1 isolates are most commonly associated with Legionnaires' disease (19), and they are also frequently recovered from environmental samples from various countries, including the United States and Canada (33, 34). This suggests that this prevalent L. pneumophila clonal group may have evolved to become less susceptible to macrolides such as AZM. No human-to-human transmission has been recorded to date for legionellosis patients, but other pathogens can become resistant during the course of antimicrobial therapy (24), increasing the potential for horizontal gene transfer between bacterial genera. Mechanisms of macrolide resistance remain unknown in L. pneumophila, but three different mechanisms have been well documented in other bacteria. These mechanisms include modifications of the ribosomal target by methylases (35), ribosomal modifications by point mutations in the macrolide targets (25, 36), and the overexpression of chromosomal or acquired efflux pump systems (37). The reduced AZM susceptibility observed in some isolates was investigated by addressing the possible acquisition of resistance mechanisms from other pathogens and by searching for mutations in macrolide targets. However, none of the acquired mechanisms screened were detected in the isolates, nor were the mutations in riboproteins L4 and L22 and the rrl gene (domains II and V, related to antimicrobial resistance in other species). Additionally, no MIC differences were observed in the presence of the efflux pump inhibitor CCCP. These results indicate that there are other mechanisms involved in the reduced susceptibility to AZM in L. pneumophila (e.g., permeability or other efflux pumps not affected by CCCP). Further studies are needed to elucidate these mechanisms. It is interesting, though, to note that the decreased susceptibilities to AZM in Lp1 isolates were not correlated with increased solithromycin MICs, suggesting that these unknown potential mechanisms do not affect solithromycin activity.
It is unlikely that the L. pneumophila clinical isolates with reduced susceptibility could reenter their natural aquatic reservoirs and spread to other human hosts. However, a growing concern for environmental pathogens is the possible selection and emergence of decreased antimicrobial susceptibilities due to the presence of residual macrolides in wastewater. It has been shown that 30 to 40% of macrolides are excreted in an unchanged active form via urine into wastewater, manure, and runoff water (38). Although these macrolides end up in a diluted form in various aquatic environments and/or soil, they could still be of great importance for the evolution of resistance (38).
Due to the intracellular location of L. pneumophila in alveolar macrophages, lung epithelial cells, and monocytes (39), antimicrobial susceptibility assays by standard methods, such as agar and broth dilutions, are only partially relevant. Therefore, we evaluated the activity of solithromycin against L. pneumophila isolates using an established intracellular model of infection. We showed that solithromycin and AZM inhibit the growth of Lp1 clinical strains independently of their sequence type. Solithromycin maintained the same efficiency as AZM after 24 h of exposure. Interestingly, solithromycin showed a much higher relative intracellular potency 48 h posttreatment against all clinical isolates (Fig. 4). This finding is in agreement with the study of Lemaire et al., who showed first that solithromycin has high intracellular activity against reference laboratory strains, such as S. aureus ATCC 25923, Listeria monocytogenes strain EGD, and L. pneumophila ATCC 33153, due to high intracellular accumulation (40). Here, in agreement with this preliminary study performed with one reference laboratory strain with a low AZM MIC, solithromycin demonstrated the highest in vitro and intracellular potency against a large population-based collection of clinical isolates compared to AZM. Interestingly, we show for the first time that solithromycin remains highly potent against isolates with increased AZM MICs. Legionella has also been recognized as a significant pathogen in CABP (41), and given the reported treatment failure in legionellosis, our findings suggest that solithromycin could be used in the treatment of legionellosis. In a phase 2 study, solithromycin was well tolerated, with efficacy comparable to that of levofloxacin in patients with CABP (18). Altogether, these results support the current development of this new fluoroketolide in phase 3 studies in patients with CABP.
Supplementary Material
ACKNOWLEDGMENTS
We thank Patrick Tang (Public Health Ontario) for providing the L. pneumophila isolates used in this study.
This work was supported by Cempra, Inc., Chapel Hill, NC.
P.F. is the CEO of Cempra, Inc. We have no other conflicts of interest to declare.
Footnotes
Published ahead of print 25 November 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.01639-13.
REFERENCES
- 1.Stout JE, Yu VL. 1997. Eradicating Legionella from hospital water. JAMA 278:1404–1405. 10.1001/jama.1997.03550170034023 [DOI] [PubMed] [Google Scholar]
- 2.Benin AL, Benson RF, Besser RE. 2002. Trends in legionnaires disease, 1980–1998: declining mortality and new patterns of diagnosis. Clin. Infect. Dis. 35:1039–1046. 10.1086/342903 [DOI] [PubMed] [Google Scholar]
- 3.Pedro-Botet ML, Garcia-Cruz A, Tural C, Mateu L, Sopena N, Roure S, Rey-Joly C, Sabria M. 2006. Severe Legionnaires' disease successfully treated with levofloxacin and azithromycin. J. Chemother. 18:559–561 [DOI] [PubMed] [Google Scholar]
- 4.Fields BS, Benson RF, Besser RE. 2002. Legionella and Legionnaires' disease: 25 years of investigation. Clin. Microbiol. Rev. 15:506–526. 10.1128/CMR.15.3.506-526.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fliermans CB, Cherry WB, Orrison LH, Smith SJ, Tison DL, Pope DH. 1981. Ecological distribution of Legionella pneumophila. Appl. Environ. Microbiol. 41:9–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu VL, Plouffe JF, Pastoris MC, Stout JE, Schousboe M, Widmer A, Summersgill J, File T, Heath CM, Paterson DL, Chereshsky A. 2002. Distribution of Legionella species and serogroups isolated by culture in patients with sporadic community-acquired legionellosis: an international collaborative survey. J. Infect. Dis. 186:127–128. 10.1086/341087 [DOI] [PubMed] [Google Scholar]
- 7.Piao Z, Sze CC, Barysheva O, Iida K, Yoshida S. 2006. Temperature-regulated formation of mycelial mat-like biofilms by Legionella pneumophila. Appl. Environ. Microbiol. 72:1613–1622. 10.1128/AEM.72.2.1613-1622.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McArdell CS, Molnar E, Suter MJ, Giger W. 2003. Occurrence and fate of macrolide antibiotics in wastewater treatment plants and in the Glatt Valley watershed, Switzerland. Environ. Sci. Technol. 37:5479–5486. 10.1021/es034368i [DOI] [PubMed] [Google Scholar]
- 9.D'Costa VM, McGrann KM, Hughes DW, Wright GD. 2006. Sampling the antibiotic resistome. Science 311:374–377. 10.1126/science.1120800 [DOI] [PubMed] [Google Scholar]
- 10.Hughes D, Andersson DI. 2012. Selection of resistance at lethal and non-lethal antibiotic concentrations. Curr. Opin. Microbiol. 15:555–560. 10.1016/j.mib.2012.07.005 [DOI] [PubMed] [Google Scholar]
- 11.Jonas D, Engels I, Hartung D, Beyersmann J, Frank U, Daschner FD. 2003. Development and mechanism of fluoroquinolone resistance in Legionella pneumophila. J. Antimicrob. Chemother. 51:275–280. 10.1093/jac/dkg054 [DOI] [PubMed] [Google Scholar]
- 12.Nielsen K, Bangsborg JM, Hoiby N. 2000. Susceptibility of Legionella species to five antibiotics and development of resistance by exposure to erythromycin, ciprofloxacin, and rifampicin. Diagn. Microbiol. Infect. Dis. 36:43–48. 10.1016/S0732-8893(99)00095-4 [DOI] [PubMed] [Google Scholar]
- 13.Onody C, Matsiota-Bernard P, Nauciel C. 1997. Lack of resistance to erythromycin, rifampicin and ciprofloxacin in 98 clinical isolates of Legionella pneumophila. J. Antimicrob. Chemother. 39:815–816. 10.1093/jac/39.6.815 [DOI] [PubMed] [Google Scholar]
- 14.Bruin JP, Diederen BM, Ijzerman EP, Den Boer JW, Mouton JW. 2013. Correlation of MIC value and disk inhibition zone diameters in clinical Legionella pneumophila serogroup 1 isolates. Diagn. Microbiol. Infect. Dis. 76:339–342. 10.1016/j.diagmicrobio.2013.03.001 [DOI] [PubMed] [Google Scholar]
- 15.Farrell DJ, Sader HS, Castanheira M, Biedenbach DJ, Rhomberg PR, Jones RN. 2010. Antimicrobial characterisation of CEM-101 activity against respiratory tract pathogens, including multidrug-resistant pneumococcal serogroup 19A isolates. Int. J. Antimicrob. Agents 35:537–543. 10.1016/j.ijantimicag.2010.01.026 [DOI] [PubMed] [Google Scholar]
- 16.Woosley LN, Castanheira M, Jones RN. 2010. CEM-101 activity against Gram-positive organisms. Antimicrob. Agents Chemother. 54:2182–2187. 10.1128/AAC.01662-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Still JG, Schranz J, Degenhardt TP, Scott D, Fernandes P, Gutierrez MJ, Clark K. 2011. Pharmacokinetics of solithromycin (CEM-101) after single or multiple oral doses and effects of food on single-dose bioavailability in healthy adult subjects. Antimicrob. Agents Chemother. 55:1997–2003. 10.1128/AAC.01429-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oldach D, Clark K, Schranz J, Das A, Craft JC, Scott D, Jamieson BD, Fernandes P. 2013. Randomized, double-blind, multicenter phase 2 study comparing the efficacy and safety of oral solithromycin (CEM-101) to those of oral levofloxacin in the treatment of patients with community-acquired bacterial pneumonia. Antimicrob. Agents Chemother. 57:2526–2534. 10.1128/AAC.00197-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tijet N, Tang P, Romilowych M, Duncan C, Ng V, Fisman DN, Jamieson F, Low DE, Guyard C. 2010. New endemic Legionella pneumophila serogroup I clones, Ontario, Canada. Emerg. Infect. Dis. 16:447–454. 10.3201/eid1603.081689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teruya H, Higa F, Akamine M, Ishikawa C, Okudaira T, Tomimori K, Mukaida N, Tateyama M, Heuner K, Fujita J, Mori N. 2007. Mechanisms of Legionella pneumophila-induced interleukin-8 expression in human lung epithelial cells. BMC Microbiol. 7:102. 10.1186/1471-2180-7-102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.CLSI 2009. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. M07-A8, 8th ed. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 22.Stout JE, Sens K, Mietzner S, Obman A, Yu VL. 2005. Comparative activity of quinolones, macrolides and ketolides against Legionella species using in vitro broth dilution and intracellular susceptibility testing. Int. J. Antimicrob. Agents 25:302–307. 10.1016/j.ijantimicag.2004.08.019 [DOI] [PubMed] [Google Scholar]
- 23.Viveiros M, Martins A, Paixao L, Rodrigues L, Martins M, Couto I, Fahnrich E, Kern WV, Amaral L. 2008. Demonstration of intrinsic efflux activity of Escherichia coli K-12 AG100 by an automated ethidium bromide method. Int. J. Antimicrob. Agents 31:458–462. 10.1016/j.ijantimicag.2007.12.015 [DOI] [PubMed] [Google Scholar]
- 24.Allen VG, Farrell DJ, Rebbapragada A, Tan J, Tijet N, Perusini SJ, Towns L, Lo S, Low DE, Melano RG. 2011. Molecular analysis of antimicrobial resistance mechanisms in Neisseria gonorrhoeae isolates from Ontario, Canada. Antimicrob. Agents Chemother. 55:703–712. 10.1128/AAC.00788-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farrell DJ, Douthwaite S, Morrissey I, Bakker S, Poehlsgaard J, Jakobsen L, Felmingham D. 2003. Macrolide resistance by ribosomal mutation in clinical isolates of Streptococcus pneumoniae from the PROTEKT 1999–2000 study. Antimicrob. Agents Chemother. 47:1777–1783. 10.1128/AAC.47.6.1777-1783.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chien M, Morozova I, Shi S, Sheng H, Chen J, Gomez SM, Asamani G, Hill K, Nuara J, Feder M, Rineer J, Greenberg JJ, Steshenko V, Park SH, Zhao B, Teplitskaya E, Edwards JR, Pampou S, Georghiou A, Chou IC, Iannuccilli W, Ulz ME, Kim DH, Geringer-Sameth A, Goldsberry C, Morozov P, Fischer SG, Segal G, Qu X, Rzhetsky A, Zhang P, Cayanis E, De Jong PJ, Ju J, Kalachikov S, Shuman HA, Russo JJ. 2004. The genomic sequence of the accidental pathogen Legionella pneumophila. Science 305:1966–1968. 10.1126/science.1099776 [DOI] [PubMed] [Google Scholar]
- 27.Edelstein PH, Beer KB, DeBoynton ED. 1987. Influence of growth temperature on virulence of Legionella pneumophila. Infect. Immun. 55:2701–2705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sabria M, Pedro-Botet ML, Gomez J, Roig J, Vilaseca B, Sopena N, Banos V, Legionnaires Disease Therapy Group 2005. Fluoroquinolones vs macrolides in the treatment of Legionnaires disease. Chest 128:1401–1405. 10.1378/chest.128.3.1401 [DOI] [PubMed] [Google Scholar]
- 29.Salord JM, Matsiota-Bernard P, Staikowsky F, Kirstetter M, Frottier J, Nauciel C. 1993. Unsuccessful treatment of Legionella pneumophila infection with a fluoroquinolone. Clin. Infect. Dis. 17:518–519. 10.1093/clinids/17.3.518 [DOI] [PubMed] [Google Scholar]
- 30.Llano-Sotelo B, Dunkle J, Klepacki D, Zhang W, Fernandes P, Cate JH, Mankin AS. 2010. Binding and action of CEM-101, a new fluoroketolide antibiotic that inhibits protein synthesis. Antimicrob. Agents Chemother. 54:4961–4970. 10.1128/AAC.00860-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mallegol J, Fernandes P, Seah C, Guyard C, Melano RG. 24 June 2013. Determination of in vitro activity of solithromycin at different pHs and its intracellular activity tested against clinical isolates of Neisseria gonorrhoeae from a laboratory collection. Antimicrob. Agents Chemother. 10.1128/AAC.00564-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bruin JP, Ijzerman EP, den Boer JW, Mouton JW, Diederen BM. 2012. Wild-type MIC distribution and epidemiological cut-off values in clinical Legionella pneumophila serogroup 1 isolates. Diagn. Microbiol. Infect. Dis. 72:103–108. 10.1016/j.diagmicrobio.2011.09.016 [DOI] [PubMed] [Google Scholar]
- 33.Cazalet C, Jarraud S, Ghavi-Helm Y, Kunst F, Glaser P, Etienne J, Buchrieser C. 2008. Multigenome analysis identifies a worldwide distributed epidemic Legionella pneumophila clone that emerged within a highly diverse species. Genome Res. 18:431–441. 10.1101/gr.7229808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ginevra C, Jacotin N, Diancourt L, Guigon G, Arquilliere R, Meugnier H, Descours G, Vandenesch F, Etienne J, Lina G, Caro V, Jarraud S. 2012. Legionella pneumophila sequence type 1/Paris pulsotype subtyping by spoligotyping. J. Clin. Microbiol. 50:696–701. 10.1128/JCM.06180-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roberts MC, Chung WO, Roe D, Xia M, Marquez C, Borthagaray G, Whittington WL, Holmes KK. 1999. Erythromycin-resistant Neisseria gonorrhoeae and oral commensal Neisseria spp. carry known rRNA methylase genes. Antimicrob. Agents Chemother. 43:1367–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ng LK, Martin I, Liu G, Bryden L. 2002. Mutation in 23S rRNA associated with macrolide resistance in Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 46:3020–3025. 10.1128/AAC.46.9.3020-3025.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luna VA, Cousin S, Jr, Whittington WL, Roberts MC. 2000. Identification of the conjugative mef gene in clinical Acinetobacter junii and Neisseria gonorrhoeae isolates. Antimicrob. Agents Chemother. 44:2503–2506. 10.1128/AAC.44.9.2503-2506.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wright GD. 2010. Antibiotic resistance in the environment: a link to the clinic? Curr. Opin. Microbiol. 13:589–594. 10.1016/j.mib.2010.08.005 [DOI] [PubMed] [Google Scholar]
- 39.Stout JE, Arnold B, Yu VL. 1998. Activity of azithromycin, clarithromycin, roxithromycin, dirithromycin, quinupristin/dalfopristin and erythromycin against Legionella species by intracellular susceptibility testing in HL-60 cells. J. Antimicrob. Chemother. 41:289–291. 10.1093/jac/41.2.289 [DOI] [PubMed] [Google Scholar]
- 40.Lemaire S, Van Bambeke F, Tulkens PM. 2009. Cellular accumulation and pharmacodynamic evaluation of the intracellular activity of CEM-101, a novel fluoroketolide, against Staphylococcus aureus, Listeria monocytogenes, and Legionella pneumophila in human THP-1 macrophages. Antimicrob. Agents Chemother. 53:3734–3743. 10.1128/AAC.00203-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, Dowell SF, File TM, Jr, Musher DM, Niederman MS, Torres A, Whitney CG, Infectious Diseases Society of America, American Thoracic Society 2007. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin. Infect. Dis. 44(Suppl 2):S27–S72. 10.1086/511159 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.