Abstract
PD 404,182 (PD) is a synthetic compound that was found to compromise HIV integrity via interaction with a nonenvelope protein viral structural component (A. M. Chamoun et al., Antimicrob. Agents Chemother. 56:672–681, 2012). The present study evaluates the potential of PD as an anti-HIV microbicide and establishes PD's virucidal activity toward another pathogen, herpes simplex virus (HSV). We show that the anti-HIV-1 50% inhibitory concentration (IC50) of PD, when diluted in seminal plasma, is ∼1 μM, similar to the IC50 determined in cell culture growth medium, and that PD retains full anti-HIV-1 activity after incubation in cervical fluid at 37°C for at least 24 h. In addition, PD is nontoxic toward vaginal commensal Lactobacillus species (50% cytotoxic concentration [CC50], >300 μM), freshly activated human peripheral blood mononuclear cells (CC50, ∼200 μM), and primary CD4+ T cells, macrophages, and dendritic cells (CC50, >300 μM). PD also exhibited high stability in pH-adjusted Dulbecco's phosphate-buffered saline with little to no activity loss after 8 weeks at pH 4 and 42°C, indicating suitability for formulation for transportation and storage in developing countries. Finally, for the first time, we show that PD inactivates herpes simplex virus 1 (HSV-1) and HSV-2 at submicromolar concentrations. Due to the prevalence of HSV infection, the ability of PD to inactivate HSV may provide an additional incentive for use as a microbicide. The ability of PD to inactivate both HIV-1 and HSV, combined with its low toxicity and high stability, warrants additional studies for the evaluation of PD's microbicidal candidacy in animals and humans.
INTRODUCTION
Since its discovery in humans in 1981, human immunodeficiency virus (HIV), the causative agent of AIDS, has infected over 60 million people worldwide and caused more than 25 million deaths (1). Although highly active antiretroviral therapy (HAART) can significantly reduce the viral load and prolong patients' life expectancy, these therapies are not curative (2). Worldwide, nearly half of all individuals living with HIV are women, most of whom acquire the virus after sexual intercourse with HIV-positive men. As receptive partners, women are twice as likely as their male counterparts to acquire HIV during sex (3). Despite the knowledge of effective prevention strategies, such as the ABC approach (abstinence, be faithful, and use of condoms), the rate of HIV transmission remains high in developing countries (4). Moreover, many women cannot reliably negotiate safe sex practices, leaving them vulnerable to sexually transmitted infections. Thus, the development of a safe, effective, and acceptable topical microbicide capable of retarding or preventing the sexual transmission of HIV could empower women to take personal responsibility to prevent HIV acquisition from their infected partners (5).
Topical microbicides are agents able to inhibit the transmission of viral infections when applied to the vagina, penis, and/or lower gastrointestinal (GI) tract via the rectum. An ideal anti-HIV microbicide should fulfill most or all of the following criteria: (i) inhibit transmission of wild-type and drug-resistant virus (6); (ii) be stable and potent in seminal fluids and vaginal secretions; (iii) lack toxicity to the vaginal epithelium and commensal bacterial flora; (iv) be able to interfere with multiple transmission modes (e.g., as cell-free virus versus cell-associated virus), given the unknowns in the exact mode of HIV transmission in vivo; (v) possess a high genetic barrier to resistance development; (vi) preferably act through a mode of action distinct from the modes of action of existing therapeutics (7); and (vii) lack proinflammatory activity and immunotoxicity. The last consideration derives from the presence of rare preexisting drug-resistant viral variants, as well as drug-resistant HIV variants, from patients who underwent previous antiretroviral treatment that can bypass the microbicidal barrier and transmit to target cells. Most current anti-HIV microbicide candidates in clinical trials are formulated on the basis of existing antiretroviral drugs and target well-studied viral proteins such as HIV protease (PR), reverse transcriptase, and HIV envelope protein (Env) (6–10). In the CAPRISA 004 clinical trial involving 1% tenofovir gel, HIV type 1 (HIV-1) acquisition was reduced by ∼38% in all woman and by 54% in woman who used the gel 80% or more of the time (11). Interestingly and unexpectedly, in the same trial, tenofovir gel was found to also inhibit HSV-2 acquisition by 51% (11). The recently FDA-approved anti-HIV prophylactic therapeutic Truvada comprises two nucleoside analogs, tenofovir and emtricitabine (12). Truvada offered a 44% reduction in HIV transmission during initial clinical trials (12). However, since both tenofovir and emtricitabine are currently used in the clinic for HIV treatment as part of a HAART drug cocktail, concerns were raised about the potential for the spread of drug-resistant variants when the drug is used by individuals with unknown or positive HIV status. This issue becomes more significant when the drug is used on a large scale, generating an extra incentive to identify new and specific anti-HIV microbicidal compounds with unique modes of action. In addition, a recently completed comprehensive HIV prevention trial among African women known as VOICE (Vaginal and Oral Interventions to Control the Epidemic) involving tenofovir failed to provide protection against HIV, underscoring the need for additional HIV prevention options that incentivize patient usage and adherence (6).
Recently, our laboratory discovered a synthetic small molecule, PD 404,182 (PD), that possesses virucidal activity toward retroviruses, including HIV (13). PD is structurally and mechanistically distinct from existing HIV microbicides (6, 7) and inhibits a broad range of primary isolates of HIV and simian immunodeficiency virus (SIV) at submicromolar to micromolar concentrations with minimal cytotoxicity to human cells (50% cytotoxic concentration [CC50]/50% inhibitory concentration [IC50], >300). Previously, we found that PD (i) is effective against a broad range of primary HIV-1 isolates as well as HIV-2 (IC50, ∼1 μM) in TZM-bl cells; (ii) is fully active in cervical fluids; (iii) exhibits low toxicity in 7 different human cell lines, including cervical cancer cells (CC50, >300 μM); (iv) is effective against both cell-free and cell-associated virus and inhibits the transmission of dendritic cell-associated HIV to T cells; (v) retains antiviral potency in cell culture for at least 8 h prior to the addition of HIV to the cells; and (vi) exhibits rapid antiviral action (HIV becomes >99% inactivated after a 5-min incubation with PD) (13).
In this study, we further evaluated the potential of PD as an anti-HIV microbicide and show that PD (vii) is stable and effective at both acidic and neutral pH for at least 48 h, (viii) remains fully active in the presence of seminal plasma and after incubation in cervical fluids for at least 24 h, (ix) retains full potency when stored in Dulbecco's phosphate-buffered saline (DPBS) under acidic pH at 42°C for at least 8 weeks, (x) can be formulated in hydroxyethyl cellulose (HEC) gel under acidic conditions, (xi) is nontoxic to the vaginal commensal bacterium Lactobacillus (CC50, >300 μM) and freshly activated peripheral blood mononuclear cells (PBMCs; CC50, >200 μM), (xii) is active in PBMCs against HIV-1 clinical isolates representing different viral subtypes and tropisms (average IC50 = 0.55 μM), and (xiii) does not foster the emergence of resistant variants when HIV-1-positive TZM-bl cells are passaged at subinhibitory concentrations of PD in cell culture for 60 days. Finally, we show that PD effectively inactivates human herpes simplex virus 1 (HSV-1) and HSV-2 at submicromolar concentrations (200 nM). In the United States alone, 16.2% of the population is estimated to be infected with HSV-2 (15). Infection with HSV-1 or -2 is an important risk factor for susceptibility to HIV-1 transmission in vitro (16). These new findings further underscore PD as a promising new HIV microbicide. In addition, PD is a small molecule that can be easily synthesized (17) and thus can potentially be manufactured at low cost on a large scale for use in developing countries.
MATERIALS AND METHODS
Cells, media, and reagents.
PD 404,182 was purchased from Sigma-Aldrich (St. Louis, MO). PD was dissolved in dimethyl sulfoxide (DMSO) to a final concentration of 30 to 40 mM, aliquoted, and stored at −20°C. DPBS and penicillin-streptomycin (pen-strep) were purchased from Thermo Scientific HyClone (Logan, UT) and Lonza (Walkersville, MD), respectively. Unless otherwise stated, the complete growth medium for all cell culture work was Dulbecco modified Eagle medium (DMEM) containing 4,500 mg/liter glucose, 4.0 mM l-glutamine, and 110 mg/liter sodium pyruvate (Thermo Scientific HyClone, Logan, UT) supplemented with 10% fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA) and 1× nonessential amino acids (Thermo Scientific HyClone, Logan, UT). 293T cells were from Life Technologies (Grand Island, NY). Vero cells were obtained from ATCC (Manassas, VA). The following reagents were obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH: TZM-bl from John C. Kappes, Xiaoyun Wu, and Tranzyme Inc.; HIV-1 isolates 92RW016, 92RW021, 92TH006, 92TH026, 93TH053, 93BR020, 93BR021, 93BR029, 98IN017, 98IN022, 92UG001, 92UG005, 92UG024, and 92UG029 from The UNAIDS Network for HIV Isolation and Characterization (18); HIV-1 92HT599 from Neal Halsey; HIV-1 96USNG31 from D. Ellenberger, P. Sullivan, and R.B. Lal (19); HIV-1 RU132 from A. Bobkov and Jonathon Weber; HIV-1 93IN101 from Robert Bollinger; and HIV-1 Jv1083 from Alash'le Abimiku (20). All primary HIV isolates were amplified in activated human PBMCs. HIV NL4-3 was obtained from the NIH AIDS Research and Reference Program. HSV-1 (strain Syn 17) and HSV-2 (strain 333) were obtained from Theo Geijtenbeek (21) and amplified, and titers were determined in Vero cells.
Lentiviral pseudoparticle production.
Pseudotyped lentiviruses were produced by cotransfecting 293T cells with plasmids carrying HIV gag-pol (22), a provirus (pV1-B1 [13], pTRIP-Gluc [23], or NL4-3.Luc [NIH AIDS Reagent Program]), and vesicular stomatitis virus glycoprotein (VSV-G) (22). TransIT reagent (Mirus, Madison, WI) was used to perform the transfection following the manufacturer's protocol. The supernatants containing the pseudoparticles were collected at 48 h posttransfection, filtered (0.45-μm pore size), and stored at −80°C until use.
PD stability.
As a gauge of compound stability, we determined the antiviral activity of PD after storage under different conditions for different periods of time. PD was diluted in buffered DPBS (pH 4, 6, 8, 10) or cervical fluids (a pool of fluids from 3 donors 5-fold diluted in DPBS; Lee Biosolutions, St. Louis, MO) to achieve a final concentration of 30 μM. DPBS was buffered to the desired pH using hydrochloric acid or sodium hydroxide. Diluted drug was incubated at the desired temperature for 0, 8, 24, or 48 h. After the temperature incubation, the drug mixture was further diluted to 1, 0.1, and 0.05 μM in complete growth medium and used for incubation with VSV-G lentiviral pseudoparticles (VSV-Gpp; harboring either pTRIP-Gluc or NL4-3.Luc viral supernatant diluted 500-fold in complete growth medium) at 37°C for 30 min. Huh-7.5 cells (2 × 104 cells/well) or 293T cells that had been seeded 24 h earlier were inoculated with the PD-treated virus at 4°C for 2 h, thoroughly washed to remove unbound viruses and drug, replenished with complete growth medium containing 1× pen-strep, and returned to 37°C in 5% CO2. Viral infectivity was quantified 48 h later by measuring supernatant Gaussia luciferase (Gluc) levels using a BioLux Gaussia luciferase assay kit (New England BioLabs, Ipswich, MA) or a firefly luciferase assay kit.
To study the long-term stability of PD, the compound was diluted to 5 μM in DPBS buffered at pH 4 and 7 using acetic acid (0.1%) and HEPES (2.5 mM), respectively, aliquoted, and incubated at 4°C, room temperature (RT), or 42°C. Each week an aliquot was removed and tested for antiviral activity as previously described above. Similar experiments were conducted with PD (5 μM) or vehicle control (0.02% DMSO) diluted in DPBS (adjusted to pH 4) containing 1.5% HEC.
PD stability in seminal plasma.
TZM-bl cells (105 cells/well, 50 μl) were seeded in a flat-bottom 96-well plate. On the next day, PD dilutions were prepared at a 2× concentration in seminal plasma (pooled from 10 HIV-seronegative donors [Lee Biosolutions] 2-fold diluted in DMEM), and 100 μl of the 2×-concentrated mixtures was added to the wells. Fifty microliters of a predetermined dilution of HIV stock (X4 NL4-3, 1 ng of p24, also in 50% seminal plasma) was placed in each test well. The cultures were incubated at 37°C in 5% CO2 for 4 h and washed with complete growth medium to remove unbound viruses and compound, fresh growth medium was replaced, and the cultures were returned to the incubator. Infection was scored 48 h later by β-galactosidase activity.
Anti-HIV efficacy evaluation in fresh human PBMCs.
Testing of the efficacy of PD against HIV-1 in PBMCs (Biological Specialty Corporation, Colmar, PA) was performed at the Southern Research Institute as described previously (24, 25). Briefly, phytohemagglutinin-stimulated cells from at least two healthy donors were mixed together, diluted in fresh medium to a final concentration of 1 × 106 cells/ml, and plated in a 96-well round-bottom microplate at 50 μl/well (5 × 104 cells/well). Test drug dilutions were prepared at a 2× concentration in microtiter tubes, and 100 μl of the 2×-concentrated mixtures was added to the wells. Fifty microliters of a predetermined dilution of virus stock was placed in each test well (final multiplicity of infection [MOI], ∼0.1). Separate plates were prepared identically without virus for drug cytotoxicity studies. The PBMC cultures were maintained for 7 days following infection at 37°C in 5% CO2. After this period, cell-free supernatant samples were collected for analysis of reverse transcriptase activity (26), and compound cytotoxicity was measured by addition of 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS; CellTiter 96 reagent; Promega) to the separate cytotoxicity plates for determination of cell viability. Wells were also examined microscopically, and any abnormalities were noted.
Lactobacillus toxicity testing.
Reference strains of Lactobacillus crispatus and L. jensenii were obtained from ATCC (Manassas, VA) and cultured on Columbia blood agar plates at 35°C in air enriched with 6% CO2. Bacterial suspensions were prepared in saline or ACES buffer (27) to a density of 2 McFarland units (∼2 × 108 bacteria/ml) and exposed to PD (300 μM) or DMSO (10%) for 30 min at 37°C. After incubation, the cells were serially diluted in ACES buffer, pH 7.0 (Sigma-Aldrich, St. Louis, MO), and plated on blood agar plates to quantify the number of CFU per ml (27).
Primary cell toxicity testing.
Primary cells were seeded at 6 × 103 cells/well in flat-bottom 96-well plates in triplicate in the presence of increasing concentrations of PD. We used the permeabilization agent saponin (0.1%) as a positive control. After 0, 7, and 14 days, the amounts of lactate dehydrogenase (LDH) in the cell culture medium were quantified using an LDH cytotoxicity assay kit (Cayman Chemical, Ann Arbor, MI).
Cell death can occur either by apoptosis or by necrosis. Necrosis is accompanied by mitochondrial swelling and increased plasma membrane permeability, whereas apoptosis involves an articulated breakdown of the cell into membrane-bound apoptotic bodies. LDH is a soluble cytosolic enzyme that is released into the culture medium following the loss of membrane integrity resulting from either apoptosis or necrosis. LDH activity, therefore, can be used as an indicator of cell membrane integrity and serves as a general means to assess cytotoxicity resulting from chemical compounds or environmental toxic factors. Cayman's LDH cytotoxicity assay kit measures the LDH activity present in the culture medium using a coupled two-step reaction. In the first step, LDH catalyzes the reduction of NAD+ to NADH and H+ by oxidation of lactate to pyruvate. In the second step of the reaction, diaphorase uses the newly formed NADH and H+ to catalyze the reduction of a tetrazolium salt [2-(4-iodophenyl)-3-(4-nitrophenyl)-5-phenyltetrazolium chloride] to highly colored formazan, which absorbs strongly at 490 to 520 nm.
HSV infection assays.
Vero cells (2 × 105 cells/well) were seeded in a 24-well plate. On the next day, these cells were infected with increasing titers (MOI range, 0.0001 to 1) of HSV-1 (strain Syn 17) or HSV-2 (strain 333) in the presence of PD (2 μM and 200 nM) or DMSO (0.01%) control prepared in DMEM in the absence of serum. At 2 days postinoculation, cells were harvested, fixed with 5% paraformaldehyde (PFA) in phosphate-buffered saline (PBS), stained with antibodies against HSV glycoprotein gD (Novus Biological, Littleton, CO), and analyzed by flow cytometry (21).
HSV sedimentation assay.
HSV was concentrated by loading 30 ml HSV-1-infected Vero cell supernatant on a 20% sucrose cushion and centrifuging in an SW 28 rotor at 20,000 rpm for 1 h at 4°C. Pelleted viruses (∼20 μg/ml) were resuspended in 1 ml PBS, exposed to PD (200 nM) or DMSO (0.01%) for 30 min at 37°C, and immediately loaded over a 20 to 70% sucrose density gradient (11 ml). After ultracentrifugation at 30,000 rpm for 24 h in an SW 41 T rotor at 4°C, fractions of 1 ml were collected and analyzed for HSV gB content by enzyme-linked immunosorbent assay (ELISA) using homemade rabbit polyclonal antibody. The density of each fraction from the sucrose gradient was determined by measuring the refractive index.
Statistical analysis.
Statistical significance between different samples was evaluated using a two-tailed Student's t test in the Microsoft Excel program. A P value of 0.05 was considered statistically significant.
RESULTS
Efficacy of PD in seminal plasma.
Previously, we showed that PD effectively inhibits several isolates of HIV-1 and SIV in TZM-bl cells at submicromolar to low micromolar concentrations (IC50, ∼1 μM) when diluted in DMEM or cervical fluid (13). It has been shown that seminal plasma can enhance HIV infectivity (28, 29) and protect HIV against the action of microbicides (30–32). We therefore sought to test the antiviral activity of PD in seminal fluids. Briefly, CD4+ CCR5+ HeLa cells (TZM-bl cells [33–37]) that produce β-galactosidase in response to HIV infection were exposed to 14 different clinical and laboratory isolates of HIV-1, representing various subtypes that use either coreceptor CCR5 (R5 viruses) or coreceptor CXCR4 (X4 viruses), in the presence of PD or DMSO prepared in 50% seminal plasma. After 4 h of incubation of the virus and compound with the cells, cells were washed and the infection was scored 48 h later by β-galactosidase activity. The IC50 and IC90 of PD against the tested subtypes of HIV-1 ranged from 0.42 to 1.96 μM and 1.58 to 7.19 μM, respectively (Table 1). These values are consistent with those of PD's anti-HIV activity determined in DMEM (0.33 to 1.80 μM for IC50 and 1.4 to 6.6 μM for IC90) and in cervical fluid (0.61 to 2.30 μM for IC50 and 1.80 to 7.50 μM for IC90) (13), indicating that seminal plasma does not negatively impact PD's anti-HIV activity.
TABLE 1.
PD's anti-HIV efficacy in seminal plasmaa
| HIV isolate | Clade | Coreceptor usage | IC50 (μM) | IC90 (μM) |
|---|---|---|---|---|
| 92RW021 | A | R5 | 0.58 ± 0.04 | 4.62 ± 0.32 |
| 92UG029 | A | X4 | 1.33 ± 0.02 | 4.71 ± 0.26 |
| 92TH026 | B | R5 | 0.43 ± 0.02 | 2.95 ± 0.18 |
| 92HT599 | B | X4 | 1.93 ± 0.03 | 6.31 ± 0.52 |
| 93IN101 | C | R5 | 1.76 ± 0.02 | 5.36 ± 0.37 |
| 98IN017 | C | X4 | 0.45 ± 0.05 | 2.09 ± 0.19 |
| 92UG005 | D | R5 | 1.32 ± 0.02 | 5.57 ± 0.44 |
| 92UG024 | D | X4 | 0.42 ± 0.03 | 1.58 ± 0.20 |
| 92TH006 | E | R5 | 1.96 ± 0.01 | 6.73 ± 0.51 |
| 93TH053 | E | X4 | 1.67 ± 0.02 | 5.56 ± 0.48 |
| 93BR029 | F | R5 | 0.85 ± 0.01 | 3.72 ± 0.26 |
| 93BR020 | F | X4 | 1.61 ± 0.02 | 7.19 ± 0.62 |
| RU132 | G | R5 | 0.74 ± 0.01 | 3.28 ± 0.22 |
| Jv1083 | G | R5 | 1.27 ± 0.01 | 4.22 ± 0.39 |
TZM-bl cells (1 × 105 cells/ml) were exposed to the indicated HIV isolates (1 ng of p24) in the presence of PD or DMSO diluted in 50% seminal plasma. Cells were washed at 4 h postinoculation, and fresh growth medium was added. Infection was scored 48 h later by β-galactosidase activity. Values and errors represent the means and standard deviations, respectively, of 2 independent experiments carried out in duplicate.
Efficacy and toxicity of PD evaluated using primary cells.
We previously evaluated the cytotoxicity of PD on 7 different human cell lines, including TZM-bl (HeLa) human cervical cells (13). In all cases, PD showed minimal cytotoxicity (CC50, >300 μM), giving a therapeutic index (CC50/IC50) of >300 for HIV-1. In the current study, freshly activated human PBMCs pooled from multiple donors were infected with 8 HIV-1 clinical isolates representing different viral subtypes and tropisms in the presence of different concentrations of PD. The supernatant reverse transcriptase activity was determined 7 days later and used as an indication of HIV infection. The toxicity of PD was determined under identical conditions in the absence of HIV infection and replication. As shown in Table 2, PD exhibited antiviral activity toward all the viral isolates tested, with an average IC50 of 0.55 μM (IC50 range, 0.14 μM with HIV-1 96USNG31 to 1.18 μM with HIV-1 92UG029). A 48% reduction in cell viability was observed at the highest tested PD concentration (200 μM), resulting in a CC50 of ∼200 μM, indicating that PD is relatively nontoxic to freshly activated human PBMCs. The therapeutic index of PD ranged from 170 (for HIV-1 92USNG31) to 1,425 (for HIV-1 96USNG31).
TABLE 2.
Toxicity and efficacy of PD in PBMCsa
| HIV isolate | Clade | Coreceptor usage | IC50 (μM) | IC90 (μM) | Therapeutic index |
|---|---|---|---|---|---|
| 92RW016 | A | R5 | 0.22 | 0.54 | 916 |
| 92UG029 | A | X4 | 1.18 | 4.67 | 170 |
| 92HT599 | B | X4 | 0.55 | 1.92 | 364 |
| 93BR021 | B | R5 | 0.6 | 4.26 | 334 |
| 96USNG31 | C | X4/R5 | 0.14 | 1.62 | 1,425 |
| 98IN022 | C | R5 | 0.4 | 1.48 | 506 |
| 92UG001 | D | X4/R5 | 1.11 | 1.89 | 181 |
| RU132 | G | R5 | 0.2 | 0.53 | 1,015 |
The CC50 was ∼200 μM for all isolates. Freshly activated PBMCs (5 × 104 cells/well) were infected with HIV isolates (MOI = 0.1) in the presence of different concentrations of PD. At 7 days after infection, supernatants were collected and analyzed for reverse transcriptase activity. Compound toxicity was determined using an MTS assay in the parallel uninfected plates. Values represent the means of triplicate samples from one experiment using zidovudine as the positive control (data not shown).
To evaluate the toxicity of PD against other primary cells, increasing concentrations of PD were incubated with CD4+ T lymphocytes, macrophages, and dendritic cells for up to 14 days. As shown in Fig. 1, minimum toxicity was observed in all three types of primary cells (CC50s, >300 μM), further pointing to the extremely low cytotoxicity of PD. We also conducted an 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide assay on these primary cells 7 days after PD exposure (200 μM) and did not observe any significant cytotoxic effect (data not shown).
FIG 1.
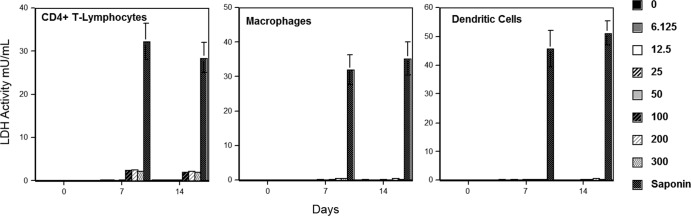
PD exhibits minimum toxicity against primary human cells. Increasing concentrations were incubated with primary CD4+ T lymphocytes, macrophages, and dendritic cells at 37°C, and the amounts of lactate dehydrogenase (LDH) present in the culture medium were quantified after 0, 7, and 14 days. Values and error bars represent the mean and standard deviation, respectively, from two independent experiments.
Toxicity of PD to Lactobacillus normal vaginal flora.
The vaginal microflora is a key component of the innate immune environment and plays an important role in reducing the risk of HIV infection (38–41). The dominant bacterial species in healthy woman is Lactobacillus, which produces lactic acid, hydrogen peroxide, bacteriocins, and other antimicrobial substances that inhibit the growth of pathogenic organisms in the vagina (38–42). PD was evaluated for toxicity toward three strains of Lactobacillus normally found in the vagina. These strains were incubated with 300 μM PD or the solvent DMSO (10%) at 37°C for 30 min and plated on blood agar plates to quantify the number of CFU per ml. A less than 1-log-unit difference between the control and test numbers of CFU is considered nontoxic (27). As shown in Table 3, no growth inhibition of any of the strains of bacteria was observed after incubation with PD at a concentration of 300 μM, indicating that PD is nontoxic to commensal Lactobacillus species.
TABLE 3.
Toxicity of PD toward commensal Lactobacillus bacteriaa
| Bacterial strain | No. of CFU (105)/ml |
Log(control)−log(PD) | |
|---|---|---|---|
| Control | PD | ||
| Lactobacillus crispatus ATCC 33197 | 75.8 ± 0.19 | 72.6 ± 0.55 | 0.019 |
| Lactobacillus jensenii ATCC 25258 | 99.8 ± 5.72 | 75.3 ± 16.8 | 0.122 |
| Lactobacillus jensenii LBP 28Ab | 105 ± 7.2 | 100 ± 0.124 | 0.021 |
Triplicate bacterial suspensions (∼2 × 108 bacteria/ml) were separately exposed to PD (300 μM) or 10% DMSO for 30 min at 37°C. After incubation, each suspension was serially diluted and plated on blood agar plates to quantify the number of CFU per ml. Values and errors represent the means and standard deviations, respectively, of triplicate samples.
PD short-term stability.
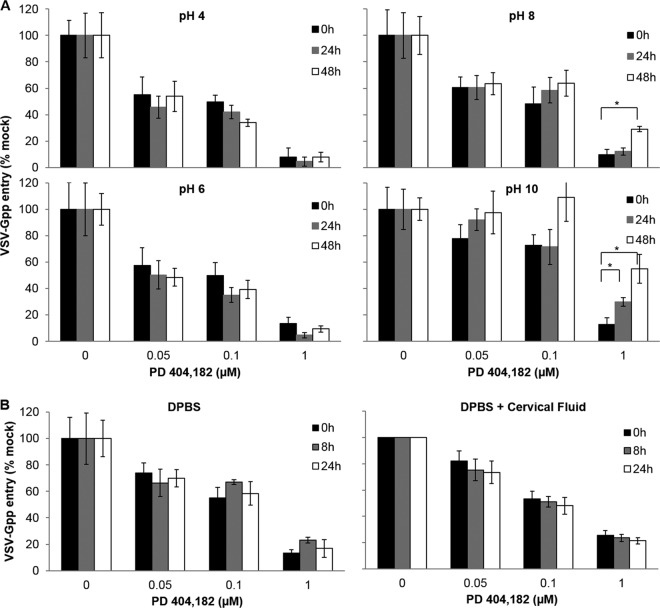
We sought to determine the short-term stability of PD under conditions that the compound is likely to encounter if used as a microbicide by measuring its antiviral activity at different times. The environment of the vagina is highly acidic (pH 3.5 to 4.9) due to the lactic acid produced by the commensal Lactobacillus bacteria (43). Exposure to seminal fluid (pH 7.2 to 8) (44) can raise the vaginal pH to 5.8 to 7.2 for several hours (45). Thus, we determined the stability of PD under different pHs at 37°C (Fig. 2A). To ensure that the loss of antiviral activity is not masked by an excess of compound, three different concentrations of PD were used, including two in the vicinity of the IC50. PD retained full activity in acidic buffer at pH 4 and 6 at 37°C. Since PD targets a nonenvelope protein HIV-1 structural component (13), we used HIV-1 pseudotyped with VSV-G (VSV-Gpp) for these studies because this virus is easy to generate to high titers and can be handled in a biosafety level 2 environment. A basic pH of 8 or 10 was observed to compromise PD's activity, but only after extended exposure. For example, PD lost ∼50% antiviral activity after incubation in pH 10 buffer for 48 h and lost ∼20% activity when exposed to pH 8 for 48 h at 37°C. In contrast, no activity loss was observed for PD after 24 h of exposure to pH 8 buffer, and minimal (∼20%) activity loss was seen after 24 h of exposure to pH 10. Taken together, these results indicate that PD will likely be stable in highly acidic cervical fluid and should remain active for at least several hours upon contact with seminal fluid.
FIG 2.
PD is stable and fully active at acidic pH and in cervical fluid. PD (30 μM) or DMSO (10%) was incubated at 37°C for 0, 8, 24, or 48 h in DPBS adjusted to pH 4, 6, 8, or 10 (A) or 20% cervical fluid (the diluent was DPBS) (B). These PD samples were then diluted to the desired concentration in complete growth medium containing VSV-Gpp (viral supernatant diluted 500-fold), and the PD-virus mixtures were incubated at 37°C for 30 min and used to inoculate naive Huh-7.5 or 293T cells at 4°C for 2 h prior to incubation at 37°C in 5% CO2. The infectivity (virus entry into cells) was quantified by measuring the supernatant luciferase reporter activity at 48 h postinfection. Values and error bars represent the means and standard deviations, respectively, of at least two independent experiments carried out in duplicate. Statistical significance was determined by Student's t test (*, P < 0.01).
Since cervical fluid is a complex mixture, we next determined the stability of PD in cervical fluid. As shown in Fig. 2B, no activity loss was observed after PD was incubated in 20% cervical fluid for 24 h at 37°C. These results indicate that once-a-day application of PD should be adequate to provide protection against HIV infection. The ability of PD to retain its antiviral potency at a nearly neutral pH suggests that PD may also be formulated as a rectal gel.
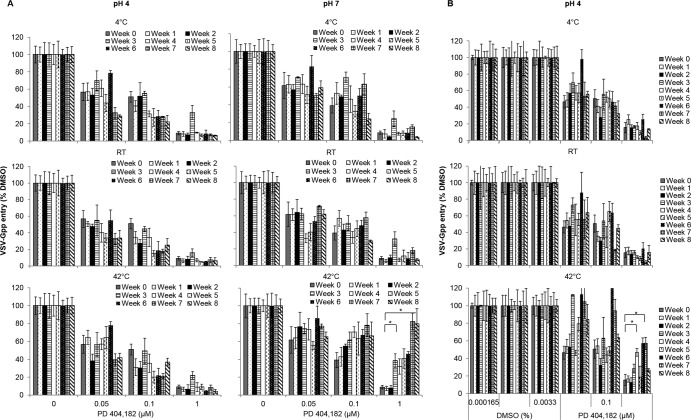
PD long-term stability.
To evaluate long-term stability, PD was diluted in DPBS buffered at pH 4 or 7 and incubated at 4°C, room temperature, or 42°C. An aliquot was taken every week for determination of antiviral activity. As shown in Fig. 3A, PD was extremely stable when stored in pH 4 buffer and retained full antiviral potency even after 8 weeks at 42°C. At pH 7, PD was stable only at room temperature and 4°C. Storage at 42°C and pH 7 significantly compromised PD activity after 2 weeks.
FIG 3.
Long-term stability of PD. PD (5 μM) or DMSO (1.67%) was diluted in pH-adjusted DPBS (pH 4 or 7) (A) or 1.5% HEC in DPBS (pH 4) (B) and stored at the indicated temperature for 8 weeks. An aliquot was removed each week, diluted to the desired concentration in complete growth medium containing VSV-Gpp (500-fold diluted), incubated at 37°C for 30 min, and used to infect naive Huh-7.5 cells at 4°C for 2 h prior to incubation at 37°C in 5% CO2. The viral infectivity was quantified by measuring the supernatant Gluc reporter activity at 48 h postinfection. Values and error bars represent the means and standard deviations, respectively, of duplicate samples. Statistical significance was determined by Student's t test (*, P < 0.05).
We also determined the stability of PD formulated in HEC gel at pH 4, as PD is not stable in the presence of HEC gel at pH 7 (data not shown). PD retained full potency at pH 4 in 1.5% HEC gel at 4°C and RT for at least 4 weeks. However, PD was not stable under the same buffer conditions if stored at 42°C (Fig. 3B) for more than 2 weeks, despite its stability in pH 4 DPBS buffer. Work is under way to determine other suitable formulation conditions for PD storage at high temperatures.
HIV-1 does not acquire resistance to PD after 60 days.
With the high rate of HIV mutation, an ideal microbicide should have a high threshold for viral resistance development. To gauge the ability of HIV-1 to acquire resistance to PD, TZM-bl cells were infected with HIV-1 at an estimated MOI of 0.001 to 0.005 and passaged in the presence of 1, 5, and 10 μM PD for 60 days. A similar method was previously employed to successfully evolve HIV-1 resistance against multiple HIV protease/reverse transcriptase inhibitors (46–48) and in resistance evolution of other viruses (49–53). For a classical HIV-1 protease and reverse transcriptase inhibitor with a known mechanism, the supernatant capsid p24 level is usually undetectable for the first 1 to 2 weeks but increases to 1 to 50 ng/ml after 4 weeks. No detectable p24 was measured in the supernatant even after 60 days (see Fig. S1 in the supplemental material), suggesting that no PD-resistant variants evolved during this time frame and also suggesting that PD has a high resistance barrier. The inability of HIV-1 to escape PD inactivation further underscores the potential of PD as an HIV-1 microbicide. We chose TZM-bl cells for the resistance study because these cells can be passaged for an extended period and remain viable for months even in the presence of viral replication. A similar experiment was performed using freshly activated human PBMCs (from two donors), and no emergence of viral resistance was observed (data no shown). However, HIV-1-infected PBMCs were cultured for only 12 days because significant cell death was observed after this period. We also cultured HSV-1-infected Vero cells in the presence of subinhibitory concentrations of PD for 2 weeks and were not able to detect any viral capsid by ELISA in the supernatant (data not shown).
PD inactivates HSV-1 and HSV-2.
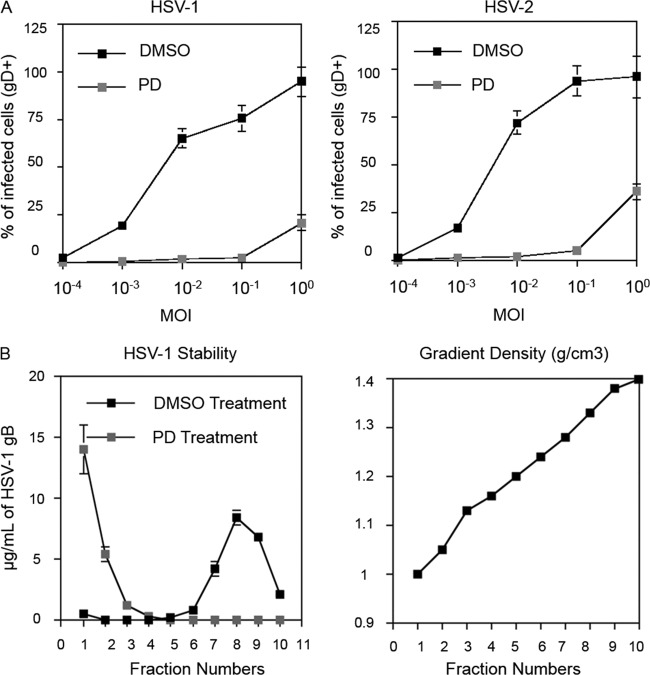
We investigated the ability of PD to inactivate HSV-1 and HSV-2. Vero cells were infected with HSV-1 and HSV-2 in the presence of PD (2 μM and 0.2 μM) or DMSO (0.01%). These concentrations were selected on the basis of their closeness to the in vitro IC50 of PD against HIV-1. Infection was quantified by the cell surface expression of HSV gD. PD was found to inhibit both HSV-1 and HSV-2 infection at low to intermediate MOIs (MOI range, 0.0001 to 0.1) and exhibited partial protection at an MOI of 1 (Fig. 4A). The data from the lowest concentration are presented. At 2 μM, similar results were obtained, except that the protection curve shifted to the right, leading to full protection at an MOI of 1 and partial protection at an MOI of 10.
FIG 4.
PD is effective against HSV. (A) PD blocks HSV infection. Vero cells were seeded in a 24-well plate and infected with HSV-1 (strain Syn 17) or HSV-2 (strain 333). PD (200 nM) or DMSO (0.01%) control was added to the target cells immediately after viral inoculation. Two days later, the cells were harvested, stained with antibody against HSV glycoprotein gD, and analyzed by flow cytometry. Error bars represent standard deviations of triplicate samples. Results are representative of two independent experiments. (B) PD destabilizes HSV particles. Concentrated HSV (∼20 μg/ml) was incubated with DMSO (0.01%) or PD (200 nM) for 30 min at 37°C, and the mixture was immediately loaded over a 20 to 70% sucrose density gradient. The amount of HSV glycoprotein gB in each fraction was analyzed by ELISA. The sucrose gradient density of each fraction was determined by measuring the refractive index. Results are representative of two independent experiments. Values and error bars represent the means and standard deviations, respectively, of triplicate samples.
Previously, we showed that PD compromises the integrity of retroviruses (13). We thus asked whether PD can disrupt the structure of HSV virions. Purified HSV-1 virions were resuspended in PBS and incubated with PD (0.2 μM) or DMSO (0.01%) for 30 min at 37°C. After the incubation, the mixture was immediately loaded over a 20 to 70% sucrose density gradient and centrifuged at 30,000 rpm in an SW 41 T rotor for 24 h. Each gradient fraction was analyzed for HSV glycoprotein gB by ELISA. DMSO-treated HSV-1 sediments at a density of 1.24 g/cm3. However, with PD-exposed HSV, all gB distributed to the top of the gradient (Fig. 4B). This result indicates that, like with HIV-1, PD inactivates HSV by compromising virion structural integrity.
DISCUSSION
Four major types of vaginal HIV microbicides have been developed with various degrees of clinical success: surfactants, entry inhibitors, vaginal milieu protectors, and reverse transcriptase inhibitors (6). Surfactants nonspecifically disrupt membranes and were the first molecules to enter clinical trials as candidate HIV microbicides. However, these surfactants were found to be toxic to the cervicovaginal mucosa and resulted in an increased rate of HIV infection in phase III clinical trials (54, 55). Entry inhibitors prevent HIV from binding to or entering cells and encompass a wide range of molecules, including CCR5 inhibitors (56–58) and fusion inhibitors (59, 60). Many polyanions have also been developed to inhibit HIV entry, and some have been extensively tested in phase III trials, including PRO 2000 (61), cellulose sulfate (62), and Carraguard (63). However, most of these candidates have failed to show in vivo efficacy in preventing HIV transmission, partly due to the complexity of the mucosal environment as well as the interference of semen (32, 64). Vaginal milieu protectors are designed to maintain or enhance the protective acidic pH of the vaginal environment through the use of strong buffering agents, such as Carbopol 974 (65), or genetically engineered lactobacilli (66). An agent that is being considered for HIV microbicidal applications in clinical trials, tenofovir, is a nucleotide analogue that inhibits the reverse transcriptase of HIV (12). A 1% tenofovir gel applied before and after sexual intercourse was 39% effective overall in preventing HIV infection in women and 54% effective among highly adherent users of the gel (11). These data are encouraging but nevertheless show that the efficacy of tenofovir microbicidal therapy alone is limited.
In contrast to existing anti-HIV microbicidal candidates, PD inactivates HIV via a novel mechanism. PD is the only nonsurfactant small molecule reported to physically compromise the integrity of HIV, thus rendering the extracellular virus noninfectious (Table 1 and 2). In addition, PD exhibits low toxicity toward several human cell lines (13), freshly activated PBMCs (Table 2), primary CD4+ T lymphocytes, macrophages, and dendritic cells (Fig. 1), and lactobacilli found in the normal vaginal flora (Table 3). It should be noted, nevertheless, that we have yet to test the cytotoxicity of PD under conditions that mimic the high level of basal inflammation typically seen in developing countries. For example, it has been reported that increased levels of immune activation were observed in the genital tract of healthy young women from sub-Saharan Africa (67).
The antiviral potency of PD is not affected by the presence of seminal plasma (Table 1) or exposure to cervical fluid at 37°C for 24 h (Fig. 2B), indicating the potential for a once-a-day application of PD for HIV prophylaxis. The very high stability of PD in acidic pH at both room temperature and 42°C and in neutral pH at room temperature (Fig. 3A) indicates that PD can be easily formulated for convenient transportation and storage in developing countries lacking refrigeration facilities. In the current study, we evaluated the stability of PD when formulated in 1.5% HEC gel. Surprisingly, PD was not stable when formulated in HEC gel at pH 7 (data not shown), although PD formulated in HEC gel at pH 4 retained full potency after 4 weeks at ambient temperature (Fig. 3B). Studies are under way to investigate the reason for PD's instability in HEC gel at neutral pH. One hypothesis that we are exploring is that PD reacts with the deprotonated hydroxyl group in HEC.
Genital herpes has been found to increase the vulnerability to HIV-1 infection by compromising the integrity of the mucosal barrier (68–70). Most genital herpes is caused by HSV-2 infection, although in some cases it can also be caused by HSV-1 (71–73). In one study, 50 to 90% of HIV-1-infected patients tested seropositive for HSV-2 (74), and HSV-2 infection was found to increase the rate of HIV-1 acquisition by 3-fold (72). Due to the synergy between HIV and HSV, a topical microbicide with dual action against both pathogens may more effectively reduce HIV transmission.
In addition to being an HIV microbicide, PD could also inactivate HSV at nanomolar concentrations. Genital herpes is one of the most prevalent sexually transmitted diseases worldwide and is the common cause of genital ulcers (75). Ulcerations can disrupt the mucosal barrier and abrogate the protective barrier function of the epithelium, allowing HIV-1 to reach the subepithelial dendritic cells susceptible to HIV-1 infection (16) and increasing the risk of HIV acquisition (76). Currently, there is no approved vaccine for HSV, and therapeutic treatment for genital herpes involves repeated dosing of antiviral drugs. Development of topical microbicides that are effective against both HIV and HSV may provide a more effective strategy to prevent HIV-1 infection/transmission. We showed that 0.2 μM PD physically compromises the integrity of extracellular HSV and effectively blocks the infection of both HSV-1 and 2 in vitro, in a manner similar to PD's action on HIV-1 (Fig. 4).
In the past 5 years, several broad-spectrum antiviral compounds have been discovered. For example, the rigid amphipathic fusion inhibitors (RAFIs) (77, 78) and lysophosphatidylcholine (79) inhibit virus-host fusion by inducing positive membrane curvature, while LJ001 compromises virion integrity by intercalating into the viral lipid membrane (80). There are others, like the C5A amphipathic peptide derived from HCV NS5A protein (81, 82) and alkylated porphyrins (83), whose mechanisms of virucidal action remain somewhat mysterious.
Finally, it is worth noting that a number of factors tend to raise the vaginal pH. For example, the seminal plasma can raise the vaginal pH to 7.2 to 8 after intercourse (44). Women diagnosed with Trichomonas vaginalis infection or bacterial vaginosis (BV) have vaginal pHs that range from 4 to 7 (84, 85). However, as shown in Fig. 2, PD retains most of its potency at pH 8 for over 24 h at 37°C. Thus, PD should remain effective in preventing HIV/HSV infection even in these individuals.
In summary, we demonstrated that the virucidal small molecule PD possesses several attributes that lend support to its use as a microbicide for combating HIV spread. These attributes include full activity and high stability in the fluids and pHs encountered physiologically, a lack of toxicity to freshly activated human PBMCs and vaginal commensal bacteria, and activity toward another virus—HSV—that exacerbates the pathogenicity of HIV. Future studies will focus on (i) formulation of PD into a topical form that promotes high PD activity and stability and (ii) evaluation of the toxicity and efficacy of PD in animals and humans.
Supplementary Material
ACKNOWLEDGMENTS
Funding for this study was provided by the Chemical Engineering Department, National Institutes of Health, grant 1R21AI083965-01 (to A.M.C.-E. and Z.C.), and U.S. Public Health Service grant AI087470 (to P.G). This project was conducted in part by the Southern Research Institute using federal funds from the Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health, under contract HHSN272200700041C, entitled Confirmatory In Vitro Evaluations of HIV Therapeutics.
Footnotes
Published ahead of print 11 November 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.02000-13.
REFERENCES
- 1.UNAIDS 2012, posting date HIV statistics: global fact sheet. UN Joint Programme on HIV/AIDS (UNAIDS). http://www.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2012/gr2012/20121120_FactSheet_Global_en.pdf [Google Scholar]
- 2.Yeni PG, Hammer SM, Hirsch MS, Saag MS, Schechter M, Carpenter CC, Fischl MA, Gatell JM, Gazzard BG, Jacobsen DM, Katzenstein DA, Montaner JS, Richman DD, Schooley RT, Thompson MA, Vella S, Volberding PA. 2004. Treatment for adult HIV infection: 2004 recommendations of the International AIDS Society—USA Panel. JAMA 292:251–265. 10.1001/jama.292.2.251 [DOI] [PubMed] [Google Scholar]
- 3.Naswa S, Marfatia YS, Prasad TL. 2012. Microbicides and HIV: a review and an update. Indian J. Sex. Transm. Dis. 33:81–90. 10.4103/0253-7184.102098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nuttall J, Romano J, Douville K, Galbreath C, Nel A, Heyward W, Mitchnick M, Walker S, Rosenberg Z. 2007. The future of HIV prevention: prospects for an effective anti-HIV microbicide. Infect. Dis. Clin. North Am. 21:219–239, x. 10.1016/j.idc.2007.01.009 [DOI] [PubMed] [Google Scholar]
- 5.Youle M, Wainberg MA. 2003. Pre-exposure chemoprophylaxis (PREP) as an HIV prevention strategy. J. Int. Assoc. Physicians AIDS Care (Chic.) 2:102–105. 10.1177/154510970300200302 [DOI] [PubMed] [Google Scholar]
- 6.Olsen JS, Easterhoff D, Dewhurst S. 2011. Advances in HIV microbicide development. Future Med. Chem. 3:2101–2116. 10.4155/fmc.11.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doncel GF, Clark MR. 2010. Preclinical evaluation of anti-HIV microbicide products: new models and biomarkers. Antiviral Res. 88(Suppl 1):S10–S18. 10.1016/j.antiviral.2010.09.018 [DOI] [PubMed] [Google Scholar]
- 8.Desai M, Iyer G, Dikshit RK. 2012. Antiretroviral drugs: critical issues and recent advances. Indian J. Pharmacol. 44:288–298. 10.4103/0253-7613.96296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vanpouille C, Arakelyan A, Margolis L. 2012. Microbicides: still a long road to success. Trends Microbiol. 20:369–375. 10.1016/j.tim.2012.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pozzetto B, Delezay O, Brunon-Gagneux A, Hamzeh-Cognasse H, Lucht F, Bourlet T. 2012. Current and future microbicide approaches aimed at preventing HIV infection in women. Expert Rev. Anti Infect. Ther. 10:167–183. 10.1586/eri.11.173 [DOI] [PubMed] [Google Scholar]
- 11.Abdool Karim Q, Abdool Karim SS, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, Kharsany AB, Sibeko S, Mlisana KP, Omar Z, Gengiah TN, Maarschalk S, Arulappan N, Mlotshwa M, Morris L, Taylor D, CAPRISA 004 Trial Group 2010. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 329:1168–1174. 10.1126/science.1193748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aschenbrenner D. 2012. Truvada: the first drug approved to prevent HIV infection. Am. J. Nurs. 112:20. 10.1097/01.NAJ.0000422248.42341.b1 [DOI] [Google Scholar]
- 13.Chamoun AM, Chockalingam K, Bobardt M, Simeon R, Chang J, Gallay P, Chen Z. 2012. PD 404,182 is a virocidal small molecule that disrupts hepatitis C virus and human immunodeficiency virus. Antimicrob. Agents Chemother. 56:672–681. 10.1128/AAC.05722-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenberg Z. 2011. Current advances in microbicides. Trop. Med. Int. Health 16:14–15 [Google Scholar]
- 15.Fatahzadeh M, Schwartz RA. 2007. Human herpes simplex virus infections: epidemiology, pathogenesis, symptomatology, diagnosis, and management. J. Am. Acad. Dermatol. 57:737–763. 10.1016/j.jaad.2007.06.027 [DOI] [PubMed] [Google Scholar]
- 16.Rebbapragada A, Wachihi C, Pettengell C, Sunderji S, Huibner S, Jaoko W, Ball B, Fowke K, Mazzulli T, Plummer FA, Kau R. 2007. Negative mucosal synergy between herpes simplex type 2 and HIV in the female genital tract. AIDS 21:589–598. 10.1097/QAD.0b013e328012b896 [DOI] [PubMed] [Google Scholar]
- 17.Mizuhara T, Oishi S, Fujii N, Ohno H. 2010. Efficient synthesis of pyrimido[1,2-c] [1,3]benzothiazin-6-imines and related tricyclic heterocycles by S(N)Ar-type C—S, C—N, or C—O bond formation with heterocumulenes. J. Org. Chem. 75:265–268. 10.1021/jo902327n [DOI] [PubMed] [Google Scholar]
- 18.Gao F, Yue L, Craig S, Thornton CL, Robertson DL, McCutchan FE, Bradac JA, Sharp PM, Hahn BH. 1994. Genetic variation of HIV type 1 in four World Health Organization-sponsored vaccine evaluation sites: generation of functional envelope (glycoprotein 160) clones representative of sequence subtypes A, B, C, and E. WHO Network for HIV Isolation and Characterization. AIDS Res. Hum. Retroviruses 10:1359–1368 [DOI] [PubMed] [Google Scholar]
- 19.Sullivan PS, Do AN, Ellenberger D, Pau CP, Paul S, Robbins K, Kalish M, Storck C, Schable CA, Wise H, Tetteh C, Jones JL, McFarland J, Yang C, Lal RB, Ward JW. 2000. Human immunodeficiency virus (HIV) subtype surveillance of African-born persons at risk for group O and group N HIV infections in the United States. J. Infect. Dis. 181:463–469. 10.1086/315254 [DOI] [PubMed] [Google Scholar]
- 20.Abimiku AG, Stern TL, Zwandor A, Markham PD, Calef C, Kyari S, Saxinger WC, Gallo RC, Robert-Guroff M, Reitz MS. 1994. Subgroup G HIV type 1 isolates from Nigeria. AIDS Res. Hum. Retroviruses 10:1581–1583. 10.1089/aid.1994.10.1581 [DOI] [PubMed] [Google Scholar]
- 21.de Witte L, Bobardt MD, Chatterji U, van Loenen FB, Verjans GM, Geijtenbeek TB, Gallay PA. 2011. HSV neutralization by the microbicidal candidate C5A. PLoS One 6:e18917. 10.1371/journal.pone.0018917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Evans MJ, von Hahn T, Tscherne DM, Syder AJ, Panis M, Wolk B, Hatziioannou T, McKeating JA, Bieniasz PD, Rice CM. 2007. Claudin-1 is a hepatitis C virus co-receptor required for a late step in entry. Nature 446:801–805. 10.1038/nature05654 [DOI] [PubMed] [Google Scholar]
- 23.Chockalingam K, Simeon RL, Rice CM, Chen Z. 2010. A cell protection screen reveals potent inhibitors of multiple stages of the hepatitis C virus life cycle. Proc. Natl. Acad. Sci. U. S. A. 107:3764–3769. 10.1073/pnas.0915117107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ptak RG, Gallay PA, Jochmans D, Halestrap AP, Ruegg UT, Pallansch LA, Bobardt MD, de Bethune MP, Neyts J, De Clercq E, Dumont JM, Scalfaro P, Besseghir K, Wenger RM, Rosenwirth B. 2008. Inhibition of human immunodeficiency virus type 1 replication in human cells by Debio-025, a novel cyclophilin binding agent. Antimicrob. Agents Chemother. 52:1302–1317. 10.1128/AAC.01324-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lanier ER, Ptak RG, Lampert BM, Keilholz L, Hartman T, Buckheit RW, Jr, Mankowski MK, Osterling MC, Almond MR, Painter GR. 2010. Development of hexadecyloxypropyl tenofovir (CMX157) for treatment of infection caused by wild-type and nucleoside/nucleotide-resistant HIV. Antimicrob. Agents Chemother. 54:2901–2909. 10.1128/AAC.00068-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buckheit RW, Swanstrom R. 1991. Characterization of an HIV-1 isolate displaying an apparent absence of virion-associated reverse-transcriptase activity. AIDS Res. Hum. Retroviruses 7:295–303. 10.1089/aid.1991.7.295 [DOI] [PubMed] [Google Scholar]
- 27.Moncla BJ, Pryke K, Rohan LC, Yang H. 2012. Testing of viscous anti-HIV microbicides using Lactobacillus. J. Microbiol. Methods 88:292–296. 10.1016/j.mimet.2011.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Introini A, Vanpouille C, Lisco A, Grivel JC, Margolis L. 2013. Interleukin-7 facilitates HIV-1 transmission to cervico-vaginal tissue ex vivo. PLoS Pathog. 9:e1003148. 10.1371/journal.ppat.1003148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Munch J, Rucker E, Standker L, Adermann K, Goffinet C, Schindler M, Wildum S, Chinnadurai R, Rajan D, Specht A, Gimenez-Gallego G, Sanchez PC, Fowler DM, Koulov A, Kelly JW, Mothes W, Grivel JC, Margolis L, Keppler OT, Forssmann WG, Kirchhoff F. 2007. Semen-derived amyloid fibrils drastically enhance HIV infection. Cell 131:1059–1071. 10.1016/j.cell.2007.10.014 [DOI] [PubMed] [Google Scholar]
- 30.Neurath AR, Strick N, Li YY. 2006. Role of seminal plasma in the anti-HIV-1 activity of candidate microbicides. BMC Infect. Dis. 6:150. 10.1186/1471-2334-6-150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abdool Karim SS. 2010. Results of effectiveness trials of PRO 2000 gel: lessons for future microbicide trials. Future Microbiol. 5:527–529. 10.2217/fmb.10.29 [DOI] [PubMed] [Google Scholar]
- 32.Patel S, Hazrati E, Cheshenko N, Galen B, Yang H, Guzman E, Wang R, Herold BC, Keller MJ. 2007. Seminal plasma reduces the effectiveness of topical polyanionic microbicides. J. Infect. Dis. 196:1394–1402. 10.1086/522606 [DOI] [PubMed] [Google Scholar]
- 33.Platt EJ, Bilska M, Kozak SL, Kabat D, Montefiori DC. 2009. Evidence that ecotropic murine leukemia virus contamination in TZM-bl cells does not affect the outcome of neutralizing antibody assays with human immunodeficiency virus type 1. J. Virol. 83:8289–8292. 10.1128/JVI.00709-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takeuchi Y, McClure MO, Pizzato M. 2008. Identification of gammaretroviruses constitutively released from cell lines used for human immunodeficiency virus research. J. Virol. 82:12585–12588. 10.1128/JVI.01726-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wei XP, Decker JM, Liu HM, Zhang Z, Arani RB, Kilby JM, Saag MS, Wu XY, Shaw GM, Kappes JC. 2002. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob. Agents Chemother. 46:1896–1905. 10.1128/AAC.46.6.1896-1905.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Derdeyn CA, Decker JM, Sfakianos JN, Wu X, O'Brien WA, Ratner L, Kappes JC, Shaw GM, Hunter E. 2000. Sensitivity of human immunodeficiency virus type 1 to the fusion inhibitor T-20 is modulated by coreceptor specificity defined by the V3 loop of gp120. J. Virol. 74:8358–8367. 10.1128/JVI.74.18.8358-8367.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Platt EJ, Wehrly K, Kuhmann SE, Chesebro B, Kabat D. 1998. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J. Virol. 72:2855–2864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kempf C, Jentsch P, Barre-Sinoussi FB, Poirier B, Morgenthaler JJ, Morell A, Germann D. 1991. Inactivation of human immunodeficiency virus (HIV) by low pH and pepsin. J. Acquir. Immune Defic. Syndr. 4:828–830 [PubMed] [Google Scholar]
- 39.Klebanoff SJ, Coombs RW. 1991. Viricidal effect of Lactobacillus acidophilus on human immunodeficiency virus type 1: possible role in heterosexual transmission. J. Exp. Med. 174:289–292. 10.1084/jem.174.1.289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hawes SE, Hillier SL, Benedetti J, Stevens CE, Koutsky LA, Wolner-Hanssen P, Holmes KK. 1996. Hydrogen peroxide-producing lactobacilli and acquisition of vaginal infections. J. Infect. Dis. 174:1058–1063. 10.1093/infdis/174.5.1058 [DOI] [PubMed] [Google Scholar]
- 41.Zheng HY, Alcorn TM, Cohen MS. 1994. Effects of H2O2-producing lactobacilli on Neisseria gonorrhoeae growth and catalase activity. J. Infect. Dis. 170:1209–1215. 10.1093/infdis/170.5.1209 [DOI] [PubMed] [Google Scholar]
- 42.Pavlova SI, Kilic AO, Kilic SS, So JS, Nader-Macias ME, Simoes JA, Tao L. 2002. Genetic diversity of vaginal lactobacilli from women in different countries based on 16S rRNA gene sequences. J. Appl. Microbiol. 92:451–459. 10.1046/j.1365-2672.2002.01547.x [DOI] [PubMed] [Google Scholar]
- 43.Owen DH, Katz DF. 1999. A vaginal fluid simulant. Contraception 59:91–95. 10.1016/S0010-7824(99)00010-4 [DOI] [PubMed] [Google Scholar]
- 44.Owen DH, Katz DF. 2005. A review of the physical and chemical properties of human semen and the formulation of a semen simulant. J. Androl. 26:459–469. 10.2164/jandrol.04104 [DOI] [PubMed] [Google Scholar]
- 45.Fox CA, Meldrum SJ, Watson BW. 1973. Continuous measurement by radio-telemetry of vaginal pH during human coitus. J. Reprod. Fertil. 33:69–75. 10.1530/jrf.0.0330069 [DOI] [PubMed] [Google Scholar]
- 46.Patick AK, Mo H, Markowitz M, Appelt K, Wu B, Musick L, Kalish V, Kaldor S, Reich S, Ho D, Webber S. 1996. Antiviral and resistance studies of AG1343, an orally bioavailable inhibitor of human immunodeficiency virus protease. Antimicrob. Agents Chemother. 40:292–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Patick AK, Rose R, Greytok J, Bechtold CM, Hermsmeier MA, Chen PT, Barrish JC, Zahler R, Colonno RJ, Lin PF. 1995. Characterization of a human immunodeficiency virus type 1 variant with reduced sensitivity to an aminodiol protease inhibitor. J. Virol. 69:2148–2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cherrington JM, Mulato AS, Fuller MD, Chen MS. 1996. Novel mutation (K70E) in human immunodeficiency virus type 1 reverse transcriptase confers decreased susceptibility to 9-[2-(phosphonomethoxy)ethyl]adenine in vitro. Antimicrob. Agents Chemother. 40:2212–2216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coburn GA, Fisch DN, Moorji SM, de Muys J-M, Murga JD, Paul D, Provoncha KP, Rotshteyn Y, Han AQ, Qian D, Maddon PJ, Olson WC. 2012. Novel small-molecule inhibitors of hepatitis C virus entry block viral spread and promote viral clearance in cell culture. PLoS One 7:e35351. 10.1371/journal.pone.0035351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Delogu I, Pastorino B, Baronti C, Nougairède A, Bonnet E, de Lamballerie X. 2011. In vitro antiviral activity of arbidol against Chikungunya virus and characteristics of a selected resistant mutant. Antiviral Res. 90:99–107. 10.1016/j.antiviral.2011.03.182 [DOI] [PubMed] [Google Scholar]
- 51.Tai CY, Escarpe PA, Sidwell RW, Williams MA, Lew W, Wu H, Kim CU, Mendel DB. 1998. Characterization of human influenza virus variants selected in vitro in the presence of the neuraminidase inhibitor GS 4071. Antimicrob. Agents Chemother. 42:3234–3241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pfeiffer JK, Kirkegaard K. 2003. A single mutation in poliovirus RNA-dependent RNA polymerase confers resistance to mutagenic nucleotide analogs via increased fidelity. Proc. Natl. Acad. Sci. U. S. A. 100:7289–7294. 10.1073/pnas.1232294100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Molla A, Kati W, Carrick R, Steffy K, Shi Y, Montgomery D, Gusick N, Stoll VS, Stewart KD, Ng TI. 2002. In vitro selection and characterization of influenza A (A/N9) virus variants resistant to a novel neuraminidase inhibitor, A-315675. J. Virol. 76:5380–5386. 10.1128/JVI.76.11.5380-5386.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mesquita PM, Cheshenko N, Wilson SS, Mhatre M, Guzman E, Fakioglu E, Keller MJ, Herold BC. 2009. Disruption of tight junctions by cellulose sulfate facilitates HIV infection: model of microbicide safety. J. Infect. Dis. 200:599–608. 10.1086/600867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hoffman IF, Taha TE, Padian NS, Kelly CW, Welch JD, Martinson FE, Kumwenda NI, Rosenberg ZF, Chilongozi DA, Brown JM, Chirenje M, Richardson BA. 2004. Nonoxynol-9 100 mg gel: multi-site safety study from sub-Saharan Africa. AIDS 18:2191–2195. 10.1097/00002030-200411050-00012 [DOI] [PubMed] [Google Scholar]
- 56.Veazey RS, Ketas TJ, Dufour J, Moroney-Rasmussen T, Green LC, Klasse PJ, Moore JP. 2010. Protection of rhesus macaques from vaginal infection by vaginally delivered maraviroc, an inhibitor of HIV-1 entry via the CCR5 co-receptor. J. Infect. Dis. 202:739–744. 10.1086/655661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lederman MM, Veazey RS, Offord R, Mosier DE, Dufour J, Mefford M, Piatak M, Jr, Lifson JD, Salkowitz JR, Rodriguez B, Blauvelt A, Hartley O. 2004. Prevention of vaginal SHIV transmission in rhesus macaques through inhibition of CCR5. Science 306:485–487. 10.1126/science.1099288 [DOI] [PubMed] [Google Scholar]
- 58.Neff CP, Kurisu T, Ndolo T, Fox K, Akkina R. 2011. A topical microbicide gel formulation of CCR5 antagonist maraviroc prevents HIV-1 vaginal transmission in humanized RAG-hu mice. PLoS One 6:e20209. 10.1371/journal.pone.0020209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Veazey RS, Klasse PJ, Schader SM, Hu Q, Ketas TJ, Lu M, Marx PA, Dufour J, Colonno RJ, Shattock RJ, Springer MS, Moore JP. 2005. Protection of macaques from vaginal SHIV challenge by vaginally delivered inhibitors of virus-cell fusion. Nature 438:99–102. 10.1038/nature04055 [DOI] [PubMed] [Google Scholar]
- 60.Tsai CC, Emau P, Jiang Y, Tian B, Morton WR, Gustafson KR, Boyd MR. 2003. Cyanovirin-N gel as a topical microbicide prevents rectal transmission of SHIV89.6P in macaques. AIDS Res. Hum. Retroviruses 19:535–541. 10.1089/088922203322230897 [DOI] [PubMed] [Google Scholar]
- 61.McCormack S, Ramjee G, Kamali A, Rees H, Crook AM, Gafos M, Jentsch U, Pool R, Chisembele M, Kapiga S, Mutemwa R, Vallely A, Palanee T, Sookrajh Y, Lacey CJ, Darbyshire J, Grosskurth H, Profy A, Nunn A, Hayes R, Weber J. 2010. PRO2000 vaginal gel for prevention of HIV-1 infection (Microbicides Development Programme 301): a phase 3, randomised, double-blind, parallel-group trial. Lancet 376:1329–1337. 10.1016/S0140-6736(10)61086-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Van Damme L, Govinden R, Mirembe FM, Guedou F, Solomon S, Becker ML, Pradeep BS, Krishnan AK, Alary M, Pande B, Ramjee G, Deese J, Crucitti T, Taylor D, CS Study Group 2008. Lack of effectiveness of cellulose sulfate gel for the prevention of vaginal HIV transmission. N. Engl. J. Med. 359:463–472. 10.1056/NEJMoa0707957 [DOI] [PubMed] [Google Scholar]
- 63.Skoler-Karpoff S, Ramjee G, Ahmed K, Altini L, Plagianos MG, Friedland B, Govender S, De Kock A, Cassim N, Palanee T, Dozier G, Maguire R, Lahteenmaki P. 2008. Efficacy of Carraguard for prevention of HIV infection in women in South Africa: a randomised, double-blind, placebo-controlled trial. Lancet 372:1977–1987. 10.1016/S0140-6736(08)61842-5 [DOI] [PubMed] [Google Scholar]
- 64.Keller MJ, Mesquita PM, Torres NM, Cho S, Shust G, Madan RP, Cohen HW, Petrie J, Ford T, Soto-Torres L, Profy AT, Herold BC. 2010. Postcoital bioavailability and antiviral activity of 0.5% PRO 2000 gel: implications for future microbicide clinical trials. PLoS One 5:e8781. 10.1371/journal.pone.0008781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Olmsted SS, Dubin NH, Cone RA, Moench TR. 2000. The rate at which human sperm are immobilized and killed by mild acidity. Fertil. Steril. 73:687–693. 10.1016/S0015-0282(99)00640-8 [DOI] [PubMed] [Google Scholar]
- 66.Vangelista L, Secchi M, Liu X, Bachi A, Jia L, Xu Q, Lusso P. 2010. Engineering of Lactobacillus jensenii to secrete RANTES and a CCR5 antagonist analogue as live HIV-1 blockers. Antimicrob. Agents Chemother. 54:2994–3001. 10.1128/AAC.01492-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cohen CR, Moscicki AB, Scott ME, Ma Y, Shiboski S, Bukusi E, Daud I, Rebbapragada A, Brown J, Kaul R. 2010. Increased levels of immune activation in the genital tract of healthy young women from sub-Saharan Africa. AIDS 24:2069–2074. 10.1097/QAD.0b013e32833c323b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Galvin SR, Cohen MS. 2004. The role of sexually transmitted diseases in HIV transmission. Nat. Rev. Microbiol. 2:33–42. 10.1038/nrmicro794 [DOI] [PubMed] [Google Scholar]
- 69.Kaul R, Pettengell C, Sheth PM, Sunderji S, Biringer A, MacDonald K, Walmsey S, Rebbapragada A. 2008. The genital tract immune milieu: an important determinant of HIV susceptibility and secondary transmission. J. Reprod. Immunol. 77:32–40. 10.1016/j.jri.2007.02.002 [DOI] [PubMed] [Google Scholar]
- 70.Celum CL. 2004. The interaction between herpes simplex virus and human immunodeficiency virus. Herpes 11(Suppl 1):36A–45A [PubMed] [Google Scholar]
- 71.Fleming DT, Wasserheit JN. 1999. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex. Transm. Infect. 75:3–17. 10.1136/sti.75.1.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. 2006. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS 20:73–83. 10.1097/01.aids.0000198081.09337.a7 [DOI] [PubMed] [Google Scholar]
- 73.Wald A, Link K. 2002. Risk of human immunodeficiency virus infection in herpes simplex virus type 2-seropositive persons: a meta-analysis. J. Infect. Dis. 185:45–52. 10.1086/338231 [DOI] [PubMed] [Google Scholar]
- 74.Strick LB, Wald A, Celum C. 2006. Management of herpes simplex virus type 2 infection in HIV type 1-infected persons. Clin. Infect. Dis. 43:347–356. 10.1086/505496 [DOI] [PubMed] [Google Scholar]
- 75.Looker KJ, Gamett GP, Schmid GP. 2008. An estimate of the global prevalence and incidence of herpes simplex virus type 2 infection. Bull. World Health Organ. 86:805–812. 10.2471/BLT.07.046128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mole L, Ripich S, Margolis D, Holodniy M. 1997. The impact of active herpes simplex virus infection on human immunodeficiency virus load. J. Infect. Dis. 176:766–770. 10.1086/517297 [DOI] [PubMed] [Google Scholar]
- 77.St Vincent MR, Colpitts CC, Ustinov AV, Muqadas M, Joyce MA, Barsby NL, Epand RF, Epand RM, Khramyshev SA, Valueva OA, Korshun VA, Tyrrell DL, Schang LM. 2010. Rigid amphipathic fusion inhibitors, small molecule antiviral compounds against enveloped viruses. Proc. Natl. Acad. Sci. U. S. A. 107:17339–17344. 10.1073/pnas.1010026107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Colpitts CC, Ustinov AV, Epand RF, Epand RM, Korshun VA, Schang LM. 2013. 5-(Perylen-3-yl)ethynyl-arabino-uridine (aUY11), an arabino-based rigid amphipathic fusion inhibitor, targets virion envelope lipids to inhibit fusion of influenza virus, hepatitis C virus, and other enveloped viruses. J. Virol. 87:3640–3654. 10.1128/JVI.02882-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gunther-Ausborn S, Praetor A, Stegmann T. 1995. Inhibition of influenza-induced membrane fusion by lysophosphatidylcholine. J. Biol. Chem. 270:29279–29285. 10.1074/jbc.270.49.29279 [DOI] [PubMed] [Google Scholar]
- 80.Wolf MC, Freiberg AN, Zhang T, Akyol-Ataman Z, Grock A, Hong PW, Li J, Watson NF, Fang AQ, Aguilar HC, Porotto M, Honko AN, Damoiseaux R, Miller JP, Woodson SE, Chantasirivisal S, Fontanes V, Negrete OA, Krogstad P, Dasgupta A, Moscona A, Hensley LE, Whelan SP, Faull KF, Holbrook MR, Jung ME, Lee B. 2010. A broad-spectrum antiviral targeting entry of enveloped viruses. Proc. Natl. Acad. Sci. U. S. A. 107:3157–3162. 10.1073/pnas.0909587107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bobardt MD, Cheng G, de Witte L, Selvarajah S, Chatterji U, Sanders-Beer BE, Geijtenbeek TB, Chisari FV, Gallay PA. 2008. Hepatitis C virus NS5A anchor peptide disrupts human immunodeficiency virus. Proc. Natl. Acad. Sci. U. S. A. 105:5525–5530. 10.1073/pnas.0801388105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cheng G, Montero A, Gastaminza P, Whitten-Bauer C, Wieland SF, Isogawa M, Fredericksen B, Selvarajah S, Gallay PA, Ghadiri MR, Chisari FV. 2008. A virocidal amphipathic {alpha}-helical peptide that inhibits hepatitis C virus infection in vitro. Proc. Natl. Acad. Sci. U. S. A. 105:3088–3093. 10.1073/pnas.0712380105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Guo H, Pan X, Mao R, Zhang X, Wang L, Lu X, Chang J, Guo JT, Passic S, Krebs FC, Wigdahl B, Warren TK, Retterer CJ, Bavari S, Xu X, Cuconati A, Block TM. 2011. Alkylated porphyrins have broad antiviral activity against hepadnaviuses, flaviviruses, filoviruses, and arenaviruses. Antimicrob. Agents Chemother. 55:478–486. 10.1128/AAC.00989-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hay PE. 2002. Bacterial vaginosis as a mixed infection, p 127–136 In Brogden KA, Guthmiller JM. (ed), Polymicrobial diseases. ASM Press, Washington, DC: [PubMed] [Google Scholar]
- 85.Cohen L. 1969. Influence of pH on vaginal discharges. Br. J. Vener. Dis. 45:241–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.