Abstract
Buruli ulcer, an ulcerating skin disease caused by Mycobacterium ulcerans infection, is common in tropical areas of western Africa. We determined the clinical and microbiological responses to administration of rifampin and streptomycin for 2 weeks followed by administration of rifampin and clarithromycin for 6 weeks in 43 patients with small laboratory-confirmed Buruli lesions and monitored for recurrence-free healing. Bacterial load in tissue samples before and after treatment for 6 and 12 weeks was monitored by semiquantitative culture. The success rate was 93%, and there was no recurrence after a 12-month follow-up. Eight percent had a positive culture 4 weeks after antibiotic treatment, but their lesions went on to heal. The findings indicate that rifampin and clarithromycin can replace rifampin and streptomycin for the continuation phase after rifampin and streptomycin administration for 2 weeks without any apparent loss of efficacy.
INTRODUCTION
Buruli ulcer (BU) is an ulcerating skin disease caused by Mycobacterium ulcerans infection that is common in tropical areas of western Africa, predominantly affecting children in the 5-to-15-year age group. The disease starts as a painless subcutaneous nodule or plaque and sometimes as a more aggressive edematous lesion. All these lesions ulcerate and enlarge progressively over the ensuing months. Until recently, the mainstay of treatment for Buruli ulcer was excision of lesions with a wide margin to ensure complete removal of visibly affected tissue. The recurrence rates after surgery ranged between 6% and 47% in Africa, depending on the type and extent of lesion and on the experience and skill of the surgeon (1–4). In Australia, where recurrence rates up to 35% have been reported, the presence of M. ulcerans-positive histological margins or immune suppression has been shown to be relevant (5). Recent evidence that antibiotics are effective has shifted the balance between surgery and antibiotics.
In vitro, M. ulcerans has been shown to be susceptible to rifampin (6), aminoglycosides (7), macrolides (8), and quinolones (9). A mouse footpad model has also been used to investigate the efficacy of antibiotics administered alone or in combination, and the combination of rifampin with amikacin or streptomycin was the most effective therapy with respect to preventing relapse after treatment for 12 weeks (10). Subsequently, bacterial killing was evaluated in the mouse footpad after 4 or 8 weeks of treatment with rifampin, rifapentine, streptomycin, clarithromycin, and moxifloxacin administered either alone or in combination (11, 12). The rifamycins and aminoglycosides appeared to be the strongest bactericidal drugs, and the combination of rifampin with a macrolide was also effective (13). The combination of rifampin with streptomycin (rifampin-streptomycin) has been evaluated for its ability to kill M. ulcerans in early human lesions of M. ulcerans disease. Treatment with rifampin at 10 mg/kg of body weight orally and streptomycin at 15 mg/kg intramuscularly daily was administered for 0, 2, 4, 8, or 12 weeks, and lesions were excised for quantitative culture. Cultures of M. ulcerans were positive at baseline and after treatment for 2 weeks but negative thereafter, and lesions became smaller during treatment (14). Based on these observations, the WHO Advisory Group on Buruli ulcer issued interim guidelines recommending the use of the rifampin-plus-streptomycin regimen described above for 8 weeks (RS8) (15). Subsequently, evidence from observational studies of a large number of patients offered antibiotic treatment with or without surgery in Benin and Ghana confirming the efficacy of this antibiotic regimen was published (16). A randomized controlled trial in which RS8 was compared with RS administered for 4 weeks followed by rifampin at 10 mg/kg and clarithromycin at 7.5 mg/kg orally for 4 weeks (RS4RC4) did not show a difference between the two arms (17).
The aim of the present pilot study was to determine the clinical and microbiological response to administration of rifampin-streptomycin for 2 weeks followed by rifampin-clarithromycin for 6 weeks (RS2RC6) in patients with Buruli lesions of less than 15 cm in maximum diameter.
MATERIALS AND METHODS
Patients.
Between July 2009 and July 2010, patients from three districts of Ghana where BU is endemic were recruited at Tepa Government Hospital, Agogo Presbyterian Hospital, and Nkawie Government Hospital after active case finding by district outreach teams. In this observational pilot study, patients were included if they were more than 5 years old and if they were willing to participate and met the WHO clinical case definition for M. ulcerans disease with a nodule, plaque, edema, or ulcer with a maximum diameter of less than 15 cm. Patients were excluded if they had tuberculosis, leprosy, or clinical and/or laboratory evidence of significant renal or hepatic impairment or auditory problems and if they were already under treatment with antibiotics or herbal preparations. Patients were also excluded if they were pregnant or known to be intolerant of the M. ulcerans active antibiotics. Patients were not tested for HIV infection. A detailed history was recorded, and clinical examination was performed. An informal assessment of functional limitation was performed before therapy was initiated. Ethical approval of the study protocol and consent forms was obtained from the Committee on Human Research Publications and Ethics of the Kwame Nkrumah University of Science and Technology and Komfo Anokye Teaching Hospital (CHRPE/05/18/09). Verbal and written informed consent was obtained from all participants and from parents, caretakers, or legal representatives of participants aged 18 years or younger.
Treatment administration and monitoring.
Patients with a clinical diagnosis of Buruli ulcer were administered rifampin (10 mg/kg)-streptomycin (15 mg/kg) for 2 weeks followed by rifampin and clarithromycin (7.5 mg/kg) for 6 weeks (RS2RC6). The doses of streptomycin and rifampin administered were based on WHO antibiotic guidance (13), whereas that for clarithromycin was based on those administered in a previous study where RS4RC4 was compared with RS8 (15). Clarithromycin was administered as a single dose at 7.5 mg/kg due to ease of administration. Upon initiation of therapy, patients returned daily to their nearest health center, where treatment was administered under the direct observation of an existing team of village health workers. Participants were educated not to use herbal medication. Clinical response was assessed fortnightly during antibiotic treatment to monitor the time to complete healing. Serial photographs were taken until healing was complete, and lesions were traced onto acetate paper by study nurses. The mean diameter was calculated by measuring the maximum diameter and that at right angles to it. The rate of reduction in mean diameter per week was calculated as the pretreatment mean diameter divided by the number of weeks to complete healing. Patients were questioned about side effects from the antibiotic treatment at each fortnightly clinical assessment and asked to report any problems to the health center between periodic reviews. Patients were questioned about symptoms of vertigo and abnormal hearing and examined for scleral icterus. Side effects were judged as mild (grade 1), moderate (grade 2), severe (grade 3), or life threatening (grade 4) according to the NIH/NCI Common Toxicity Criteria. Oral treatment with rifampin and clarithromycin was given in two weekly batches at study centers, and adherence was monitored by pill counts and by inspecting patient treatment cards. Patients who failed to appear at scheduled follow-up visits during the treatment period were traced by village health workers who assessed their compliance with therapy.
After completion of antibiotic treatment, daily saline dressing was continued until the ulcer healed and patients were reviewed monthly for a year after complete healing to detect recurrence. Surgical excision and grafting was offered to patients whose lesion had enlarged during or after treatment by more than 150% of the initial size or had not healed by week 52. New or paradoxical lesions occurring during the course of therapy or follow-up period were cultured to distinguish recurrent or new infection from sterile inflammatory lesions. Treatment failure was defined as failure of complete wound closure by week 52, enlargement of a lesion to more than 150% of its initial size during or after therapy requiring surgery, or loss to follow-up in a patient whose lesion had not yet healed at the last assessment. Paradoxical reaction was defined as an increase in inflammation with an increase in lesion size of greater than 100%, after initial improvement and decrease in size, and/or the appearance of a new lesion(s) following or during antimycobacterial treatment.
Diagnostic confirmation.
The diagnoses were confirmed by obtaining two fine-needle aspirates (FNA) for PCR from nonulcerative lesions or a swab from the undermined edge of ulcers which was examined for the presence of acid-fast bacilli (AFB), cultured for M. ulcerans, and tested by PCR for the IS2404 insertion sequence of M. ulcerans (18, 19). Before treatment, one 4-mm-diameter punch biopsy specimen was taken from nonulcerative lesions and from the margin of viable tissue in ulcerative lesions for AFB and semiquantitative culture. Punch biopsy specimens were also obtained at week 6 and week 12 if the lesion had not healed. Local anesthetic in the form of 1% lidocaine was applied before punch biopsy specimens were obtained. Additional swabs, punch biopsy specimens, or FNA were taken from any lesion deemed to be exhibiting a paradoxical reaction for culture and PCR in order to distinguish recurrent or new infection from sterile inflammatory lesions. All females above 10 years of age were tested for early pregnancy before recruitment (Guangzhou Wondfo Biotech Co., Ltd., China).
Microbiological procedures.
All specimens were processed at Komfo Anokye Teaching Hospital in Kumasi, Ghana. As described previously, 4-mm-diameter punch biopsy specimens were cut into pieces with a scalpel blade and homogenized in 2 ml of normal saline solution by the use of a sterile mortar and pestle for semiquantitative culture. Ziehl-Neelsen-stained smears of 10 μl of supernates were examined under a microscope in duplicate. Tissue homogenates were decontaminated by the sodium hydroxide (modified Petroff) method (20). An equal volume of 1 M NaOH (4% NaOH) was added to 1 ml of specimen at a final concentration of 0.5 M, and the mixture was left to stand for 10 min with occasional shaking and then neutralized with 1 ml of 1 M HCl (3.2% HCl). The mixture was centrifuged at 3,000 × g for 20 min, and the supernatants were removed. Sediments were diluted 10-fold to 106-fold, and 0.2 ml of undiluted samples and 10-fold dilutions was inoculated onto Lowenstein-Jensen (LJ) slopes in triplicate. All inoculated culture media were incubated at 31°C and checked weekly for 6 months. In positive cultures, colonies were enumerated. PCR targeting the IS2404 insertion sequence was performed as previously described (18, 19).
Statistical analyses.
The median time to complete healing and the cumulative rate of healing of lesions were represented in a survival analysis curve using GraphPad Prism 6 software. In addition, the proportion of lesions that were culture positive at 0, 6, and 12 weeks was determined.
RESULTS
Of 82 patients screened for M. ulcerans disease, 39 were excluded; of the 39, 17 did not meet clinical and or epidemiological criteria of M. ulcerans disease, 18 had large category III ulcers, 1 was pregnant, and 3 were below the age criteria for inclusion. The 3 below the age of inclusion were found to have had their ages misrepresented by their parents. Twenty-one participants who were too young or had large lesions received standard streptomycin and rifampin for 8 weeks (RS8). The pregnant participant was observed at the time of wound dressing until after delivery, when standard RS8 was administered. Diagnostic samples were obtained only from those who were clinically suspected of M. ulcerans disease. A total of 43 patients were recruited; all recruited patients had a positive PCR for M. ulcerans IS2404 and received RS2RC6. Table 1 shows the baseline characteristics of the study participants. All lesions were <15 cm in maximum diameter for single lesions; for 4 patients who had small multiple lesions, the lesions were classified as WHO category III ulcers. Three participants had mild to moderate functional limitation before treatment was commenced. Adherence to the treatment regimen was assessed by the use of directly observed therapy (DOT) forms, signed by health personnel at the health facilities. Adherence was assessed as 94%.
TABLE 1.
Demographics, clinical features, and diagnostic data of study participants with Buruli ulcer who received rifampin orally and streptomycin intramuscularly daily for 2 weeks followed by rifampin and clarithromycin orally each day for 6 weeksa
| Patient parameter | RS2RC6 value(s) |
|---|---|
| Total no. | 43 |
| Sex (no. of males/no. of females) | 18/25 |
| Median (range) age (yr) | 14 (5–70) |
| No. (%) with indicated site of lesion | |
| Head and neck | 1 (2) |
| Upper limbs | 21 (49) |
| Trunk | 1 (2) |
| Lower limbs | 20 (47) |
| No. (%) with indicated clinical form of lesion | |
| Nodule | 14 (32) |
| Plaque | 9 (21) |
| Ulcer | 20 (47) |
| No. (%) with indicated category of lesion | |
| I | 27 (63) |
| II | 12 (28) |
| III | 4 (9) |
| No. with indicated laboratory confirmation result | |
| AFB detectionb (+/−) | 11/21 |
| M. ulcerans culturec (+/−) | 14/22 |
| PCR for IS2404 (+/−) | 43/0 |
RS2RC6, administration of rifampin orally and streptomycin intramuscularly daily for 2 weeks followed by administration of rifampin and clarithromycin orally each day for 6 weeks. +, positive result; −, negative result.
Zeihl-Neelsen staining for acid-fast bacilli (AFB) was not performed at baseline in 11 patients.
M. ulcerans culture was not performed at baseline in 7 patients.
Healing after treatment with RS2RC6.
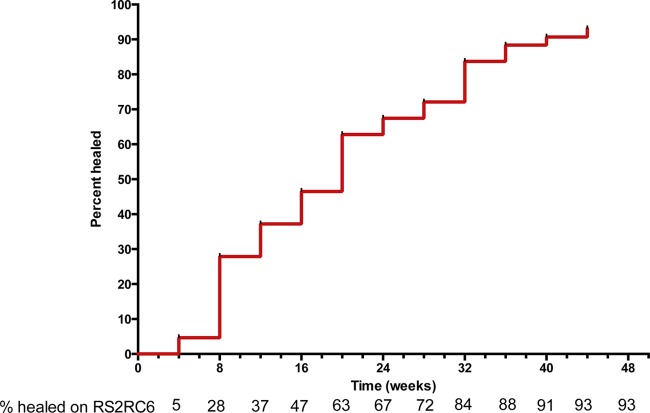
Figure 1 shows the proportions of patients healed after 4, 8, 12, 16, 20, and 24 weeks and up to 52 weeks. Forty patients had recurrence-free healing by 52 weeks without recourse to surgery. The success rate was 93%. There were 3 treatment failures. Two were lost to follow-up because they moved from the study area, one at week 4 and the other at week 14. In addition, 1 was offered skin grafting at week 32 due to poor healing (see Table 3). Lesions that had not healed upon completion of therapy subsequently went on to heal with observation and wound care. The median time to healing of lesions was 16 weeks (interquartile range [IQR], 4 to 36). The median (IQR) rate of reduction in the diameters of the lesions was 2.5 (1.1 to 5.1) mm/week, and there have been no recurrences after 12 months of follow-up.
FIG 1.
Survival analysis curve of cumulative complete healing of patients on rifampin and streptomycin for 2 weeks followed by rifampin and clarithromycin for 6 weeks (RS2RC6). Time to complete healing, defined as complete re-epithelialization with or without stable scab formation, was determined for each patient as well as the cumulative healing.
TABLE 3.
Summary of clinical outcomes and events of 43 patients with Buruli ulcer who received streptomycin and rifampin daily for 2 weeks followed by clarithromycin and rifampin administered orally for 6 weeks
| Outcome | No. (%) of patients with RS2RC6 treatment |
|---|---|
| Healed without surgery | 40 (93) |
| Healed with surgery | 1 (2) |
| Lost to follow-up | 2 (5) |
| Healed with functional limitation | 0 |
| Paradoxical reaction(s) | 4 (9) |
| Adverse event (vestibulocochlear toxicity) | 1 |
Paradoxical reactions were observed in 4 patients (Table 2). They occurred at a median time of 12 weeks (range, 4 to 32) and were associated with lesion enlargement in all cases. Lesions were painful in 2 of 4 cases, and there was pus requiring aspiration in 1 case. In 3 of 4 cases, the reaction occurred at the original site but one also occurred at a new site on the same limb. Culture was negative in 3 of 4 cases but positive from one ulcer that enlarged at week 4. In two cases, lesions which had completely healed by weeks 8 and 12 broke down at weeks 12 and 20, respectively. In one of these cases, the lesion healed at week 12, ulcerated at week 20, and improved but enlarged further at week 32. Paradoxical reactions were managed by observation, wound care, and aspiration of pus, but no additional antibiotics were administered to any patient. The median time to healing in patients who developed paradoxical reactions was 24 weeks (range, 18 to 39 weeks). In the two patients whose lesions completely healed before they further broke down, the time to healing was prolonged by 12 (week 8 to week 20) weeks and 27 (week 12 to week 39) weeks from the time of the initial healing.
TABLE 2.
Clinical features of Buruli ulcer patients with paradoxical reactions after administration of rifampin-streptomycin combination treatment daily for 2 weeks followed by rifampin-clarithromycin daily for 6 weeks
| Characteristic | Patient no. and result |
|||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Primary lesion form | Ulcer | Ulcer | Plaque | Nodule |
| Type of lesion reaction | Enlargement | Enlargement | Enlargement | Enlargement |
| Reaction site in relation to primary lesion | New site | Same site | Same site | Same site |
| Pus collection | No pus | No pus | No pus | Pus filled |
| Pain | Yes | No | No | Yes |
| Time of initial healing (no. of wks) | 8 | 12 | c | c |
| Onset(s) of reaction (no. of wks) | 12 | 20, 32b | 4 | 6 |
| M. ulcerans culture result | Negative | Negative | Positive | Negative |
| Treatment(s) administered | Wound care | Wound care | Wound care, antibioticsc | Pus aspiration, antibioticsc |
| Time to complete healing (no. of wks) | 20 | 39 | 30 | 18 |
The lesion broke down at week 20 and improved but further enlarged at week 32.
The lesion did not heal before the onset of the paradoxical reaction.
Routine antibiotics for treatment of Buruli ulcer were continued until week 8.
Three participants had functional limitation before therapy was initiated. All had a complete recovery of function with simple exercises during and after antibiotic treatment carried out according to WHO guidelines (15).
Treatment was well tolerated, but a 70-year-old man developed vestibulotoxicity in the second week of treatment that was assessed by the NIH/NCI common toxicity criteria as grade 3. His symptoms subsided when he was switched to the rifampin and clarithromycin combination. No dermatological or gastrointestinal intolerance or icterus (no clinically apparent hepatic toxicity) was observed among patients. Table 3 summarizes the main clinical outcomes.
Bacterial viability after treatment with RS2RC6.
Samples for M. ulcerans culture were obtained from 36 of 43 patients; of those samples, 14 (39%) were positive, with mean CFU per gram of tissue of 3.2 log10 (range, 1.6 to 4.3 log10). Further samples were taken from 16 lesions that had not healed or had nearly healed after antibiotic treatment for 6 weeks; of those samples, 10 (63%) were culture positive (3.0 log10 CFU/g; range, 2.0 to 4.0). Four weeks after the end of antibiotic treatment, 3 of 7 (43%) lesions sampled remained culture positive although the colony count was lower (1.6 log10 CFU/g; range, 1.0 to 2.3). All positive M. ulcerans cultures were confirmed by M. ulcerans-specific IS2404 PCR.
DISCUSSION
In this study, the clinical and microbiological response to administration of rifampin and streptomycin for 2 weeks followed by rifampin plus clarithromycin for 6 weeks (RS2RC6) in patients with Buruli lesions of less than 15 cm in maximum diameter was determined. Overall, treatment was successful in 93% of patients who had complete healing and there were no recurrences within 1 year of follow-up. These findings are comparable to recent observations, when patients administered a standard streptomycin and rifampin combination for 8 weeks achieved a 95% healing rate within 2 to 48 weeks (16). We recorded recovery of function with simple exercises in all patients who presented with functional limitation associated with Buruli ulcers.
One aim of this study was to investigate the microbiological response to treatment with RS2RC6. Experiments using the mouse footpad model of M. ulcerans infection showed that rifampin-streptomycin was the most powerful bactericidal combination for preventing recurrence (10, 21), but subsequent experiments in which bacteria were quantified after treatment for 8 weeks showed that the combination of rifampin with clarithromycin was equally successful in sterilizing footpads after 8 weeks (11) and that, in fact, treatment using rifampin alone was also successful (12, 13). We have shown previously that when cultures were taken from selected Buruli ulcers 4 to 6 weeks after the start of treatment with RS8, 9 of 49 (18%) yielded a positive culture result with 3.5 log10 CFU/g (range, 2.0 to 6.3 CFU/g) (16). Using the present treatment regimen, a higher proportion of cultures (10 of 16; 63%) were positive after 6 weeks but the mean CFU count was similar at 3.0 log10 CFU/g of tissue (range, 2.0 to 4.0 CFU/g). Thus, there was not a significant loss of bactericidal activity resulting from the shorter duration of streptomycin treatment, although the number of samples was small. It was possible to take more samples for culture at week 12 in the present study, and, as seen before, a surprising number (3 of 7) were positive, although the CFU count was lower than that at baseline or at 6 weeks. In this study, the dose of oral clarithromycin was 7.5 mg/kg, which is the same as that used in a previous study where clarithromycin plus rifampin administered during the second 4-week period after an initial 4 weeks of rifampin and streptomycin (RS4RC4) resulted in clinical outcomes similar to those seen after giving streptomycin and rifampin daily for 8 weeks despite the fact that this dose results in lower serum levels of clarithromycin (22).
All the lesions went on to heal without further antibiotic therapy or recurrence just as in the earlier study when RS8 was administered, suggesting that sterilization of the lesions may not be required for complete healing and that the immune response to M. ulcerans at that stage is sufficient to contain and even overcome the infection. However, culture positivity after administration of antibiotics may partially explain why some lesions heal slowly, as viable organisms may continue producing small amounts of mycolactone (16). At low concentrations, mycolactone inhibits the action of some cytokines and chemokines known to act as growth factors in healing (23).
The incidence and optimal management of paradoxical reactions are not certain at present (24–27). Paradoxical reactions have been defined as the presence of new inflammatory disease within or close to a Buruli ulcer leading to extension of the existing ulcer or new ulceration, usually with pus formation. However, diagnosis is challenging; a change in the size of ulcers on an uneven surface such as that of a limb is difficult to record, and erythema is easily missed on black African skin. By analogy with other diseases such as tuberculosis, these reactions are thought to result from increasing inflammation as a consequence of enhancement of the immune response during treatment (28–31). In the present study, paradoxical reactions were observed in 9% of patients with all forms of Buruli ulcer disease from week 4 during antibiotic treatment up to week 32, 24 weeks after treatment finished. Mycobacterium ulcerans was not cultured from reacting ulcers except in one that occurred at week 4 of antibiotic treatment. Although histological data are not available for that patient, the reaction is unlikely to have been due to treatment failure, as the culture result was negative 4 weeks after antibiotic completion and the lesion went on to heal at week 30 without recurrence after 2 months of follow-up. No additional antibiotics were administered to any of the patients, and paradoxical lesions occurring after antibiotic treatment were managed successfully by observation, wound care, and aspiration of pus. Others have given either a second course of RS8 (32) or corticosteroids (25). Our observations do not suggest that these interventions are necessary, but neither has been investigated in a controlled trial. Reactions such as these may account for the perception in the past that antibiotic treatment had not been successful in some patients. For example, surgical intervention was justified on the basis of treatment failure 4 weeks after antibiotic initiation in a number of patients in a recent study (33). These large ulcers were not thought by the authors to have developed paradoxical reactions, but in such circumstances, rapid enlargement after a gradual reduction in size may not have been apparent.
The treatment was clinically well tolerated, but unrecognized hepatic or vestibuloauditory toxicity assessment by audiometry or liver function tests was not performed in this study.
The findings from this study indicate that rifampin combined with clarithromycin can replace rifampin and streptomycin for the continuation phase after rifampin-streptomycin treatment for 2 weeks without any apparent loss of therapeutic effectiveness, with the implication that a controlled trial of fully oral therapy using rifampin and clarithromycin for 8 weeks (RC8) is justified. This is supported by evidence from 2 case reports (34, 35) as well as from a series of 30 patients in Benin with small, early lesions (<10 cm diameter) and 11 of 12 patients in Australia who healed after RC8 with no recurrences (36, 37). Administration of RC8 would be less painful for children, avoiding the need for injections, and it would be easier to implement in rural western Africa.
ACKNOWLEDGMENT
This work was supported by the European Foundation Initiative on Neglected Tropical Diseases (EFINTD) grant I/83994.
Footnotes
Published ahead of print 9 December 2013
REFERENCES
- 1.Revill WD, Morrow RH, Pike MC, Ateng J. 1973. A controlled trial of the treatment of Mycobacterium ulcerans infection with clofazimine. Lancet ii:873–877 [DOI] [PubMed] [Google Scholar]
- 2.Amofah G, Asamoah S, Afram-Gyening C. 1998. Effectiveness of excision of pre-ulcerative Buruli lesions in field situations in a rural district in Ghana. Trop. Doct. 28:81–83 [DOI] [PubMed] [Google Scholar]
- 3.Teelken MA, Stienstra Y, Ellen DE, Quarshie E, Klutse E, van der Graaf WT, van der Werf TS. 2003. Buruli ulcer: differences in treatment outcome between two centres in Ghana. Acta Trop. 88:51–56. 10.1016/S0001-706X(03)00170-0 [DOI] [PubMed] [Google Scholar]
- 4.Debacker M, Aguiar J, Steunou C, Zinsou C, Meyers WM, Portaels F. 2005. Buruli ulcer recurrence, Benin. Emerg. Infect. Dis. 11:584–589. 10.3201/eid1104.041000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O'Brien DP, Hughes AJ, Cheng AC, Henry MJ, Callan P, McDonald A, Holten I, Birrell M, Sowerby JM, Johnson PD, Athan E. 2007. Outcomes for Mycobacterium ulcerans infection with combined surgery and antibiotic therapy: findings from a south-eastern Australian case series. Med. J. Aust. 186:58–61 [DOI] [PubMed] [Google Scholar]
- 6.Havel A, Pattyn SR. 1975. Activity of rifampicin on Mycobacterium ulcerans. Ann. Soc. Belg. Med. Trop. 55:105–108 [PubMed] [Google Scholar]
- 7.Thangaraj HS, Adjei O, Allen BW, Portaels F, Evans MR, Banerjee DK, Wansbrough-Jones MH. 2000. In vitro activity of ciprofloxacin, sparfloxacin, ofloxacin, amikacin and rifampicin against Ghanaian isolates of Mycobacterium ulcerans. J. Antimicrob. Chemother. 45:231–233. 10.1093/jac/45.2.231 [DOI] [PubMed] [Google Scholar]
- 8.Portaels F, Traore H, De Ridder K, Meyers WM. 1998. In vitro susceptibility of Mycobacterium ulcerans to clarithromycin. Antimicrob. Agents Chemother. 42:2070–2073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stanford JL, Phillips I. 1972. Rifampicin in experimental Mycobacterium ulcerans infection. J. Med. Microbiol. 5:39–45. 10.1099/00222615-5-1-39 [DOI] [PubMed] [Google Scholar]
- 10.Bentoucha A, Robert J, Dega H, Lounis N, Jarlier V, Grosset J. 2001. Activities of new macrolides and fluoroquinolones against Mycobacterium ulcerans infection in mice. Antimicrob. Agents Chemother. 45:3109–3112. 10.1128/AAC.45.11.3109-3112.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Almeida D, Converse PJ, Ahmad Z, Dooley KE, Nuermberger EL, Grosset JH. 2011. Activities of rifampin, Rifapentine and clarithromycin alone and in combination against mycobacterium ulcerans disease in mice. PLoS Negl. Trop. Dis. 5:e933. 10.1371/journal.pntd.0000933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ji B, Chauffour A, Robert J, Jarlier V. 2008. Bactericidal and sterilizing activities of several orally administered combined regimens against Mycobacterium ulcerans in mice. Antimicrob. Agents Chemother. 52:1912–1916. 10.1128/AAC.00193-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ji B, Chauffour A, Robert J, Lefrancois S, Jarlier V. 2007. Orally administered combined regimens for treatment of Mycobacterium ulcerans infection in mice. Antimicrob. Agents Chemother. 51:3737–3739. 10.1128/AAC.00730-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Etuaful S, Carbonnelle B, Grosset J, Lucas S, Horsfield C, Phillips R, Evans M, Ofori-Adjei D, Klustse E, Owusu-Boateng J, Amedofu GK, Awuah P, Ampadu E, Amofah G, Asiedu K, Wansbrough-Jones M. 2005. Efficacy of the combination rifampin-streptomycin in preventing growth of Mycobacterium ulcerans in early lesions of Buruli ulcer in humans. Antimicrob. Agents Chemother. 49:3182–3186. 10.1128/AAC.49.8.3182-3186.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization 2004. Provisional guidance on the role of specific antibiotics in the management of Buruli ulcer. World Health Organization, Geneva, Switzerland: http://www.who.int/buruli/information/antibiotics/en [Google Scholar]
- 16.Sarfo FS, Phillips R, Asiedu K, Ampadu E, Bobi N, Adentwe E, Lartey A, Tetteh I, Wansbrough-Jones M. 2010. Clinical efficacy of combination of rifampin and streptomycin for treatment of Mycobacterium ulcerans disease. Antimicrob. Agents Chemother. 54:3678–3685. 10.1128/AAC.00299-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nienhuis WA, Stienstra Y, Thompson WA, Awuah PC, Abass KM, Tuah W, Awua-Boateng NY, Ampadu EO, Siegmund V, Schouten JP, Adjei O, Bretzel G, van der Werf TS. 2010. Antimicrobial treatment for early, limited Mycobacterium ulcerans infection: a randomised controlled trial. Lancet 375:664–672. 10.1016/S0140-6736(09)61962-0 [DOI] [PubMed] [Google Scholar]
- 18.Phillips R, Horsfield C, Kuijper S, Lartey A, Tetteh I, Etuaful S, Nyamekye B, Awuah P, Nyarko KM, Osei-Sarpong F, Lucas S, Kolk AH, Wansbrough-Jones M. 2005. Sensitivity of PCR targeting the IS2404 insertion sequence of Mycobacterium ulcerans in an Assay using punch biopsy specimens for diagnosis of Buruli ulcer. J. Clin. Microbiol. 43:3650–3656. 10.1128/JCM.43.8.3650-3656.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Phillips RO, Sarfo FS, Osei-Sarpong F, Boateng A, Tetteh I, Lartey A, Adentwe E, Opare W, Asiedu KB, Wansbrough-Jones M. 2009. Sensitivity of PCR targeting Mycobacterium ulcerans by use of fine-needle aspirates for diagnosis of Buruli ulcer. J. Clin. Microbiol. 47:924–926. 10.1128/JCM.01842-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palomino JC, Portaels F. 1998. Effects of decontamination methods and culture conditions on viability of Mycobacterium ulcerans in the BACTEC system. J. Clin. Microbiol. 36:402–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dega H, Bentoucha A, Robert J, Jarlier V, Grosset J. 2002. Bactericidal activity of rifampin-amikacin against Mycobacterium ulcerans in mice. Antimicrob. Agents Chemother. 46:3193–3196. 10.1128/AAC.46.10.3193-3196.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alffenaar JW, Nienhuis WA, de Velde F, Zuur AT, Wessels AM, Almeida D, Grosset J, Adjei O, Uges DR, van der Werf TS. 2010. Pharmacokinetics of rifampin and clarithromycin in patients treated for Mycobacterium ulcerans infection. Antimicrob. Agents Chemother. 54:3878–3883. 10.1128/AAC.00099-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phillips R, Sarfo FS, Guenin-Mace L, Decalf J, Wansbrough-Jones M, Albert ML, Demangel C. 2009. Immunosuppressive signature of cutaneous Mycobacterium ulcerans infection in the peripheral blood of patients with Buruli ulcer disease. J. Infect. Dis. 200:1675–1684. 10.1086/646615 [DOI] [PubMed] [Google Scholar]
- 24.O'Brien DP, Robson M, Friedman ND, Walton A, McDonald A, Callan P, Hughes A, Rahdon R, Athan E. 2013. Incidence, clinical spectrum, diagnostic features, treatment and predictors of paradoxical reactions during antibiotic treatment of Mycobacterium ulcerans infections. BMC Infect. Dis. 13:416. 10.1186/1471-2334-13-416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O'Brien DP, Robson ME, Callan PP, McDonald AH. 2009. “Paradoxical” immune-mediated reactions to Mycobacterium ulcerans during antibiotic treatment: a result of treatment success, not failure. Med. J. Aust. 191:564–566 [DOI] [PubMed] [Google Scholar]
- 26.Ruf MT, Chauty A, Adeye A, Ardant MF, Koussemou H, Johnson RC, Pluschke G. 2011. Secondary Buruli ulcer skin lesions emerging several months after completion of chemotherapy: paradoxical reaction or evidence for immune protection? PLoS Negl. Trop. Dis. 5:e1252. 10.1371/journal.pntd.0001252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nienhuis WA, Stienstra Y, Abass KM, Tuah W, Thompson WA, Awuah PC, Awuah-Boateng NY, Adjei O, Bretzel G, Schouten JP, van der Werf TS. 2012. Paradoxical responses after start of antimicrobial treatment in Mycobacterium ulcerans infection. Clin. Infect. Dis. 54:519–526. 10.1093/cid/cir856 [DOI] [PubMed] [Google Scholar]
- 28.Breen RA, Smith CJ, Bettinson H, Dart S, Bannister B, Johnson MA, Lipman MC. 2004. Paradoxical reactions during tuberculosis treatment in patients with and without HIV co-infection. Thorax 59:704–707. 10.1136/thx.2003.019224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bloch S, Wickremasinghe M, Wright A, Rice A, Thompson M, Kon OM. 2009. Paradoxical reactions in non-HIV tuberculosis presenting as endobronchial obstruction. Eur. Respir. Rev. 18:295–299. 10.1183/09059180.00003709 [DOI] [PubMed] [Google Scholar]
- 30.Wendel KA, Alwood KS, Gachuhi R, Chaisson RE, Bishai WR, Sterling TR. 2001. Paradoxical worsening of tuberculosis in HIV-infected persons. Chest 120:193–197. 10.1378/chest.120.1.193 [DOI] [PubMed] [Google Scholar]
- 31.Cheng VC, Ho PL, Lee RA, Chan KS, Chan KK, Woo PC, Lau SK, Yuen KY. 2002. Clinical spectrum of paradoxical deterioration during antituberculosis therapy in non-HIV-infected patients. Eur. J. Clin. Microbiol. Infect. Dis. 21:803–809. 10.1007/s10096-002-0821-2 [DOI] [PubMed] [Google Scholar]
- 32.Chauty A, Ardant MF, Adeye A, Euverte H, Guedenon A, Johnson C, Aubry J, Nuermberger E, Grosset J. 2007. Promising clinical efficacy of streptomycin-rifampin combination for treatment of Buruli ulcer (Mycobacterium ulcerans disease). Antimicrob. Agents Chemother. 51:4029–4035. 10.1128/AAC.00175-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kibadi K, Boelaert M, Kayinua M, Minuku JB, Muyembe-Tamfum JJ, Portaels F, Lefevre P. 2009. Therapeutic itineraries of patients with ulcerated forms of Mycobacterium ulcerans (Buruli ulcer) disease in a rural health zone in the Democratic Republic of Congo. Trop. Med. Int. Health 14:1110–1116. 10.1111/j.1365-3156.2009.02324.x [DOI] [PubMed] [Google Scholar]
- 34.Gordon CL, Buntine JA, Hayman JA, Lavender CJ, Fyfe JA, Hosking P, Starr M, Johnson PD. 2010. All-oral antibiotic treatment for Buruli ulcer: a report of four patients. PLoS Negl. Trop. Dis. 4:e770. 10.1371/journal.pntd.0000770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jenkin GA, Smith M, Fairley M, Johnson PD. 2002. Acute, oedematous Mycobacterium ulcerans infection in a farmer from far north Queensland. Med. J. Aust. 176:180–181 [DOI] [PubMed] [Google Scholar]
- 36.Chauty A, Ardant MF, Marsollier L, Pluschke G, Landier J, Adeye A, Goundote A, Cottin J, Ladikpo T, Ruf T, Ji B. 2011. Oral treatment for Mycobacterium ulcerans infection: results from a pilot study in Benin. Clin. Infect. Dis. 52:94–96. 10.1093/cid/ciq072 [DOI] [PubMed] [Google Scholar]
- 37.Friedman ND, Athan E, Hughes AJ, Khajehnoori M, McDonald A, Callan P, Rahdon R, O'Brien DP. 2013. Mycobacterium ulcerans disease: experience with primary oral medical therapy in an Australian cohort. PLoS Negl. Trop. Dis. 7:e2315. 10.1371/journal.pntd.0002315 [DOI] [PMC free article] [PubMed] [Google Scholar]