Abstract
We sought to evaluate the effectiveness of the antibiotic treatment administered for infections caused by carbapenemase-producing Enterobacteriaceae. The PubMed and Scopus databases were systematically searched. Articles reporting the clinical outcomes of patients infected with carbapenemase-producing Enterobacteriaceae according to the antibiotic treatment administered were eligible. Twenty nonrandomized studies comprising 692 patients who received definitive treatment were included. Almost all studies reported on Klebsiella spp. In 8 studies, the majority of infections were bacteremia, while pneumonia and urinary tract infections were the most common infections in 12 studies. In 10 studies, the majority of patients were critically ill. There are methodological issues, including clinical heterogeneity, that preclude the synthesis of the available evidence using statistical analyses, including meta-analysis. From the descriptive point of view, among patients who received combination treatment, mortality was up to 50% for the tigecycline-gentamicin combination, up to 64% for tigecycline-colistin, and up to 67% for carbapenem-colistin. Among the monotherapy-treated patients, mortality was up to 57% for colistin and up to 80% for tigecycline. Certain regimens were administered to a small number of patients in certain studies. Three studies reporting on 194 critically ill patients with bacteremia showed individually significantly lower mortality in the combination arm than in the monotherapy arm. In the other studies, no significant difference in mortality was recorded between the compared groups. Combination antibiotic treatment may be considered the optimal option for severely ill patients with severe infections. However, well-designed randomized studies of specific patient populations are needed to further clarify this issue.
INTRODUCTION
Carbapenemase-producing Enterobacteriaceae have been steadily spreading worldwide during the last decade. Production of Klebsiella pneumoniae carbapenemase (KPC) enzymes is the most common mechanism of resistance among carbapenemase-producing Enterobacteriaceae, while these enzymes are most commonly encountered among K. pneumoniae isolates. Outbreaks due to KPC-producing K. pneumoniae have been recorded in many countries around the world (1); however, these infections have become endemic in the United States, Greece, Israel, and China (1). Carbapenems have been successfully, until recently, used for the treatment of infections caused by Enterobacteriaceae, including those producing extended-spectrum beta-lactamases (2). However, carbapenemases confer resistance to broad-spectrum antibiotics, usually including carbapenems, and therefore, the majority of carbapenemase-producing Enterobacteriaceae are carbapenem resistant (CRE). According to recent data from the Centers for Disease Control and Prevention in the United States, the percentage of CRE increased from 1.2% in 2001 to 4.2% in 2011 (3). The highest increase in proportion, from 1.6% to 10.4%, was observed for Klebsiella spp. during the same period (3).
Antibiotic treatment options for these multidrug-resistant infections are limited. Tigecycline, which was approved by the Food and Drug Administration in 2005, the “old” antibiotics colistin and fosfomycin, which have been revived (4, 5), and aminoglycosides are among the remaining treatment options for clinicians to battle these difficult-to-treat infections. An important question which still remains unanswered among clinicians regarding antibiotic treatment is whether combination or monotherapy antibiotic regimens are more effective. With regard to published literature, the major problem is that most studies reporting on treatment include retrospective data and a rather small number of patients. Besides, it has been shown that patients with infections due to carbapenemase-producing Enterobacteriaceae or CRE experience high mortality (6–8). Therefore, collection and analysis of the current published literature on the effectiveness of the antibiotic treatment used against these infections are a necessity.
In this context, we aimed to systematically review the available evidence in order to evaluate the effectiveness of the antibiotic treatment commonly administered to patients with infections caused by carbapenemase-producing Enterobacteriaceae and CRE.
MATERIALS AND METHODS
Literature search.
A systematic search was performed in the PubMed and Scopus databases during February and March 2013. The following search term was applied to the PubMed database: “(CRE or carbapenem-resistant or KPC or carbapenemase-producing or VIM or NDM or OXA or IMP) and (escherichia or klebsiella or enterobacter or proteus or serratia or citrobacter or salmonella or shigella) and (treatment).” A shorter search term was applied to Scopus: “(CRE or carbapenem-resistant or KPC or carbapenemase-producing or VIM or NDM or OXA or IMP) and (escherichia or klebsiella or enterobacter or enterobacteriaceae) and (treatment).” The bibliographies of all eligible studies were searched by hand in an effort to identify additional potentially eligible studies. Only articles published in English, German, French, Spanish, Italian, or Greek were evaluated.
Study selection.
Any article providing the clinical outcomes of patients treated for infections caused by carbapenemase-producing Enterobacteriaceae or CRE was considered eligible for inclusion in the review. Studies reporting on the treatment and clinical outcomes of colonized patients with carbapenemase-producing Enterobacteriaceae or CRE were excluded. When the clinical outcomes of the infected patients were presented separately from the outcomes of the colonized patients, only the outcomes of the infected patients were extracted. Case reports and case series including fewer than 10 infected patients were excluded from the review.
Data extraction.
The extracted data consisted of the main characteristics of a study (first-author name, year of publication, country, study period, and design), main characteristics and underlying diseases of the study population, number of patients with infections due to carbapenemase-producing Enterobacteriaceae or CRE, the causative pathogen(s), sites of infections, and antibiotic treatment (combination therapy or monotherapy). Clinical outcomes (mortality, treatment failure) of patients in each treatment group were recorded as well.
Definitions and outcomes.
The interpretation of the antimicrobial susceptibility patterns was performed according to the breakpoints used by the investigators of the individual studies.
The primary outcome of the review was 30-day mortality, while the secondary outcome was treatment failure. When 30-day mortality was unavailable, other types of mortality were extracted. Treatment failure was defined according to the definitions used by the investigators of the included studies.
RESULTS
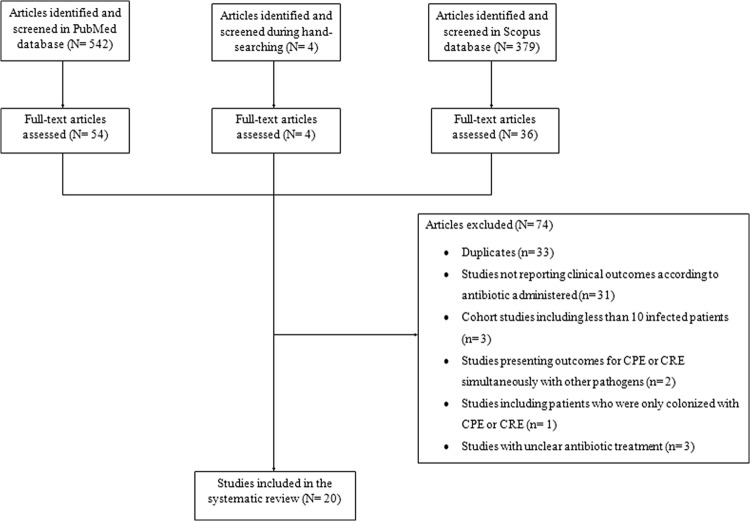
A total of 925 articles were retrieved during the search process in both databases (542 from PubMed, 379 from Scopus, 4 from searching by hand). Twenty studies met the inclusion criteria (9–28). The detailed search process and study selection are depicted in Fig. 1. Thirty-one studies were excluded because they did not present the clinical outcomes according to the antibiotic treatment administered. Among the included studies, 18 provided data on mortality, including for 651 patients who received definitive antibiotic treatment (9–13, 15–19, 21–28), while two other studies provided data on treatment failure, including for 41 patients who received definitive antibiotic treatment (14, 20). The characteristics and outcomes of the included studies according to the studied outcomes are presented in Table 1 and Table 2.
FIG 1.
Flow diagram of the detailed search process and study selection. CPE, carbapenemase-producing Enterobacteriaceae.
TABLE 1.
Mortality of infections caused by carbapenemase-producing Enterobacteriaceae or CRE among different antibiotic treatment regimens
| Organisms | First author, yr of study (reference) | Study design; period, country | Population characteristics; most common underlying diseases | Site of infection (% of total population) | No. of infected patients who received definitive antibiotic treatment | Causative pathogen(s) | Susceptibility breakpoints used (agent), yrj | Mortality assessed | Antibiotic treatment administered, no. of patients (% mortality) |
|
|---|---|---|---|---|---|---|---|---|---|---|
| Combination therapy | Monotherapy | |||||||||
| KPC-producing Klebsiella spp. | Capone, 2013 (11) | MC prospective cohort; 2010–2011, Italy | Inpatients (48.4% were ICU patients); DM, COPD, chronic kidney or liver disease, malignancy | BSI (37.4), UTI (31.9), septic shock (16.5), LRTI (15.4), SSTI (12.1)b | 67 (appropriate antibiotic treatment was known for 58) | KPC-producing Klebsiella pneumoniae | EUCAST, NR | In hospital | Coli-Tige, 16 (25) | Gen, 16 (6.3) |
| Tige-Fos, 6 (33) | Coli, 10 (40) | |||||||||
| Coli-Fos, 5 (0) | ||||||||||
| Coli-Gen, 5 (40) | ||||||||||
| Alexander, 2012 (9) | SC retrospective cohort; 2006–2008, USA | Inpatients | UTI, 2 patients developed bacteremia | 14 | KPC-producing K. pneumoniae and Citrobacter freundii | CLSI, 2006 | In hospital | NRd | Gen, 5 (40) | |
| FDA (Tige)c | Cipro, 4 (0) | |||||||||
| Dox, 1 (0) | ||||||||||
| Ntf, 1 (0) | ||||||||||
| Bergamasco, 2012 (10) | SC retrospective cohort; 2009–2010, Brazil | Solid-organ transplant recipients; DM, cardiovascular disease, liver disease, renal disease | BSI (33.3), UTI (33.3), SSI (16.7), pneumonia (16.7) | 12 | KPC-producing K. pneumoniae | CLSI, 2009 | At 30 days | Carba-PoB, 3 (67) | Carba, 2 (100) | |
| FDA (Tige)c | Tige-PoB, 3 (0) | PoB, 3 (33) | ||||||||
| Carba-Tige, 1 (0) | ||||||||||
| Qureshi, 2012 (19) | SC retrospective cohort; 2005–2009, USA | Inpatients (53.7% were ICU patients at enrollment); DM, cardiovascular disease, CRF, renal dialysis, COPD, malignancy (51.2% had an Apache II score of ≥20) | Bacteremia, the sources for which were pneumonia (24.4), line related (31.7), UTI (17.1), primary (14.6) | 34 | KPC-producing K. pneumoniae | CLSI, 2011 | At 28 days | Coli-Carba, 5 (20) | Coli, 7 (57) | |
| Coli-Tige, 1 (0) | Tige, 5 (80) | |||||||||
| Coli-FQ, 1 (0) | Carba, 4 (50) | |||||||||
| Tige-Carba, 3 (0) | Gen, 1 (0) | |||||||||
| Tige-AG, 2 (0) | A-S, 1 (0) | |||||||||
| Carba-FQ, 1 (100) | Tzp, 1 (100) | |||||||||
| Azt-FQ, 1 (0) | ||||||||||
| Cfpm-Gen, 1 (0) | ||||||||||
| Tumbarello, 2012 (25) | MC retrospective cohort; 2010–2011, Italy | Inpatients (13.6% were in shock); DM, heart failure, CRF, malignancy | BSI, the sources for which were LRTI, CVC, UTI, other, and unknown | 125 | KPC-producing K. pneumoniae | CLSI, 2011 | At 30 days | Tige-Coli, 23 (30) | Tige, 19 (53) | |
| FDA (Tige)c | Tige-Gen, 12 (50) | Coli, 22 (50) | ||||||||
| Coli-Gen, 7 (57) | Gen, 5 (80) | |||||||||
| Other 2-drug combinations, 14 (43) | ||||||||||
| Tige-Coli-Carba, 16 (13) | ||||||||||
| Tige-Gen-Carba, 6 (17) | ||||||||||
| Coli-Gen-Carba, 1 (100) | ||||||||||
| Zarkotou, 2011 (28) | SC prospective cohort; 2008–2010, Greece | Inpatients (71.7% were in the ICU at admission) | BSI, the sources for which were primary (43.4) and catheter related (22.6) | 35 | KPC-producing K. pneumoniae | CLSI, 2010 | Infection related | Tige-Coli, 9 (0) | Coli, 7 (57) | |
| EUCAST, 2010 (Coli) | Tige-Gen, 3 (0) | Tige, 5 (40) | ||||||||
| Tige-Coli-Carba, 2 (0) | Gen, 2 (0) | |||||||||
| Tige-Coli-Gen, 1 (0) | Carba, 1 (100) | |||||||||
| Tige-Carba, 1 (0) | ||||||||||
| Tige-Amk, 1 (0) | ||||||||||
| Coli-Gen, 2 (0) | ||||||||||
| Carba-Gen, 1 (0) | ||||||||||
| Souli, 2010 (22) | SC retrospective cohort; 2007–2008, Greece | Inpatients (61% were ICU patients); DM, cardiovascular disease, COPD, renal failure, malignancy | BSI (77.8), SSI (11), UTI (5.6), HAP (5.6) | 17 | KPC-producing K. pneumoniae | CLSI, 2009 EUCAST, 2009 (Fos, Coli) FDA (Tige)c |
Overall | Carba-Coli, 6 (67) Coli-Tige, 2 (0) Carba-Coli-Gen, 1 (100) Carba-Coli-Cipro, 1 (0) Carba-Coli-Tige, 1 (100) Carba-Coli-Tige-Amk, 1 (100) Coli-Tige-Amk-Tzp, 1 (100) Tzp-Cipro-Coli-Gen and then Tige-Amk, 1 (100) Tzp-Amk and then Tige, 1 (100) |
Carba, 1 (0) Tzp and then Tige, 1 (0) |
|
| Weisenberg, 2009 (26) | SC retrospective cohort; 2006, USA | Inpatients | Bacteremia (19), pneumonia (23.8), UTI (9.5), urosepsis (15.3), other RTIs (19), CSF infection (4.8), wound infection (4.8), line-related infection (4.8) | 21 | KPC-producing K. pneumoniae | NR | Undetermined | Tige-Gen, 1 (0) Tige-Carba, 1 (100) |
Carba, 11 (9) Tige, 5 (0) Gen, 2 (0) Amk, 1 (0) |
|
| MBL- or OXA-producing Klebsiella spp. | Navarro-San Francisco, 2013 (17) | SC prospective cohort; 2010–2012, Spain | Elderly patients; septic shock or severe sepsis (60%), malignancy (57.5%) | BSI, the sources for which were UTI (30), deep IAI/SSI (25), primary (17.5), catheter related (10), other (17.5) | 34 | OXA-48-producing Enterobacteriaceae (K. pneumoniae, Escherichia coli) | CLSI, 2012 FDA (Tige)c |
At 30 days | ≥2 active drugs (Carba not included), 21 (52.4) ≥2 active drugs (Carba included), 6 (33) |
Amk, 3 (33) Tige, 2 (0) Coli, 1 (0) Carba, 1 (100) |
| Sanchez-Romero, 2012 (21) | SC retrospective cohort; 2009, Spain | ICU patients | Pneumonia (29.2),e other LRTI (20.8), UTI (12.5), meningitis (8.3), CAB (25),e IAI (8.3), soft tissue (4.2) | 24 | VIM-1-producing K. pneumoniae | CLSI, 2011 EUCAST, 2011 (Tige) |
Undetermined | Tige-Coli, 11 (64) Amk-Tige-Coli, 1 (0) |
Tige, 9 (44) Amk, 1 (0) Mero, 1 (0) Erta, 1 (0) |
|
| Mouloudi, 2010 (16) | SC retrospective case-control study; 2007–2008, Greece | ICU patients; DM, cardiovascular, disease, respiratory disease, liver disease, trauma, transplant recipient | BSI | 59 | KPC- or MBL-producing K. pneumoniae | CLSI, 2007 | In hospital | Coli-Gen, 18 (61)f | Coli, 35 (51)f | |
| EUCAST, 2010 (Coli) | ||||||||||
| FDA (Tige)c | ||||||||||
| Daikos, 2009 (12) | MC prospective cohort; 2004–2006, Greece | Inpatients | BSI | 67 | VIM-1-producing K. pneumoniae | CLSI, 2004 | At 14 days | Carba-Coli, 8 (0) | Carba, 14 (21) | |
| Carba-AG, 4 (25) | Coli, 15 (27) | |||||||||
| AG, 8 (38) | ||||||||||
| No active drug, 18 (28)i | ||||||||||
| Souli 2008 (23) | SC retrospective cohort; 2003–2006, Greece | Inpatients (58.8% were ICU patients); congestive heart failure, renal failure, malignancy, DM | BSI (88.2), VAP (11.8) | 17 | VIM-1 MBL-producing Enterobacteriaceae (Klebsiella spp., Enterobacter spp.) | CLSI, 2006 | Overall | Carba-Coli, 6 (50) | Coli, 3 (33) | |
| BSAC (Coli)g | Coli-Tzp-Gen, 1 (100) | Tige, 1 (100) | ||||||||
| FDA (Tige)c | Carba-Coli-Lzd, 1 (0) | |||||||||
| Coli-Tzp-Lzd, 1 (100) | ||||||||||
| Carba-Coli-Amx-Gen-Van, 1 (100) | ||||||||||
| Carba-Coli-Tzp-Gen-Dox, 1 (100) | ||||||||||
| Coli-Cipro-Van-Dox, 1 (100) | ||||||||||
| Coli-Amk-Van-Caz, 1 (100) | ||||||||||
| Carbapenem-resistant Klebsiella spp. | Capone, 2013 (11) | MC prospective cohort; 2010–2011, Italy | Inpatients (48.4% were ICU patients); DM, COPD, chronic kidney or liver disease, malignancy | BSI (37.4), UTI (31.9), septic shock (16.5), LRTI (15.4), SSTI (12.1), IAI (3.3)b | 67 (appropriate antibiotic treatment was known for 58) | Carbapenem-resistant K. pneumoniae | EUCAST, NR | In hospital | Coli-Tige, 16 (25) | Gen, 16 (6.3) |
| Tige-Fos, 6 (33) | Coli, 10 (40) | |||||||||
| Coli-Fos, 5 (0) | ||||||||||
| Coli-Gen, 5 (40) | ||||||||||
| Huang 2012 (13) | SC prospective cohort; 2010–2011, Taiwan | NR | Undetermined | 33 | Carbapenem-resistant K. pneumoniae and E. coli | CLSI, 2009 | At 30 days | NR | Tige, 15 (73) | |
| Carba, 14 (50) | ||||||||||
| Coli, 4 (50) | ||||||||||
| Trevino, 2011 (24) | SC prospective cohort; 2009–2010, Spain | Patients submitted to surgery and/or who presented with severe underlying disease; cancer, transplant, cardiovascular disease, other | Miscellaneous infections | 10 | Carbapenem-resistant Klebsiella spp. | CLSI, 2010 | Undetermined | Amk-Carba, 4 (50) | Cipro, 1 (0) | |
| Tige-Amk, 2h | Amk, 1 (0) | |||||||||
| Fos-Amk, 1h | Tige, 1 (100) | |||||||||
| Amk-Carba-Lvf, 1 (0) | ||||||||||
| Michalopoulos, 2010 (15) | SC prospective cohort; 2008, Greece | ICU patients; DM, COPD | Hospital-acquired infections | 11 | Carbapenem-resistant K. pneumoniae | NR (Fos [the organism was susceptible when the zone of inhibition was ≥16 mm]) | In hospital | Fos-Coli, 6 (33) | Fos, 1 (0) | |
| Fos-Gen, 3 (0) | ||||||||||
| Fos-Tzp, 1 (0) | ||||||||||
| Nguyen, 2010 (18) | SC retrospective cohort; 2004–2008, USA | Inpatients (52.1% were ICU patients, 42% were solid organ transplant recipients, and 42% were in septic shock); DM, cardiovascular disease, malignancy, liver disease | Bacteremia | 48 | Carbapenem-resistant K. pneumoniae | CLSI, NR | At 30 days | PoB-Tige, 13 (31) | PoB, 9 (44) | |
| Tige, 10 (70) | ||||||||||
| Other, 9 (22)i | ||||||||||
| No potentially active treatment, 7 (43)i | ||||||||||
| Other carbapenemase-producing Enterobacteriaceae | Yan, 2013 (27) | SC retrospective cohort; NR, Taiwan | Inpatients (37.8% had a stay in the ICU); malignancy, DM, chronic renal disease | BSI, the sources for which were pneumonia (29.7), wound infection (21.6), peritonitis (16.2), UTI (13.5), unknown (13.5), catheter related (2.7), liver or biliary tract infection (2.7) | 32 | IMP-8-producing Enterobacteriaceae (Enterobacter cloacae, K. pneumoniae, E. coli, C. freundii) | CLSI, 2010 | At 28 days | NR | Carba, 20 (10) |
| Non-Carba (extended-spectrum cephalosporins, 6; FQ, 5; Azt, 1), 12 (16.7) | ||||||||||
CRE, carbapenem-resistant Enterobacteriaceae; KPC, Klebsiella pneumoniae carbapenemase; SC, single center; MC, multicenter; NR, not reported; CLSI, Clinical and Laboratory Standards Institute; EUCAST, European Committee on Antimicrobial Susceptibility Testing; FDA, Food and Drug Administration; BSAC, British Society for Antimicrobial Chemotherapy; DM, diabetes mellitus; COPD, chronic obstructive pulmonary disease; BSI, bloodstream infection; UTI, urinary tract infection; RTI, respiratory tract infection; LRTI, lower RTI; SSTI, skin and soft tissue infection; IAI, intra-abdominal infection; CVC, central venous catheter; ICU, intensive care unit; HAP, hospital-acquired pneumonia; SSI, surgical-site infection; CRF, chronic renal failure; CSF, cerebrospinal fluid; CAB, catheter-associated bacteremia; VAP, ventilator-associated pneumonia; Carba, carbapenem; Coli, colistin; PoB, polymyxin B; Tige, tigecycline; Ntf, nitrocefin; Mero, meropenem; Erta, ertapenem; Fos, fosfomycin; Gen, gentamicin; Amk, amikacin; A-S, ampicillin-sulbactam; AG, aminoglycoside; Azt, aztreonam; FQ, fluoroquinolone; Cipro, ciprofloxacin; Caz, ceftazidime; Lvf, levofloxacin; Cfpm, cefepime; Tzp, piperacillin-tazobactam; Van, vancomycin; Lzd, linezolid; Dox, doxycycline; Amx, amoxicillin; Tob, tobramycin.
In this study, certain patients had more than one site of infection.
The susceptibility breakpoint for tigecycline according to the FDA was ≤2 μg/ml.
In this study, 3 patients were excluded because it was unclear whether the antibiotic treatment was combination or monotherapy.
Two patients had both pneumonia and catheter-associated bacteremia.
The antibiotic treatment was unclear for six patients in this study; however, they did not receive either colistin in combination with gentamicin or colistin monotherapy.
The susceptibility breakpoint for colistin according to the BSAC was ≤ 4 μg/ml.
Two patients received the combination tigecycline-amikacin; one was cured, but the final outcome of the other was unknown at the time of the follow-up. One patient received the combination fosfomycin-amikacin, but the final outcome was also unknown at the time of the follow-up.
In these studies, it was unclear whether the treatment regimens were combination therapy or monotherapy.
The CLSI and EUCAST susceptibility breakpoints used in the included studies were the following: for CLSI, 2004, Carba ≤ 4; CLSI, 2006, Gen ≤ 4, Cipro ≤ 1, Dox ≤ 4, Ntf ≤ 32, Amx ≤ 8, and Tzp ≤ 16 and ≤ 4; CLSI, 2007, Gen ≤ 4; CLSI, 2009, Carba ≤ 4, Gen ≤ 4, Cipro ≤ 1, Amk ≤ 16, and Tzp ≤ 16 and ≤ 4; CLSI, 2010, Carba ≤ 4, Gen ≤ 4, Amk ≤ 16, Cipro ≤ 1, Lvf ≤ 2, Azt ≤ 4, Cfpm ≤ 8, and Tob ≤ 4; CLSI, 2011, Carba ≤ 1, Erta ≤ 0.25, Gen ≤ 4, Amk ≤ 16, A-S ≤ 8 and ≤ 4, Azt ≤ 4, and Tzp ≤ 16 and ≤ 4; CLSI, 2012, Carba ≤ 1 and Amk ≤ 16; EUCAST, 2009, Coli ≤ 2 and Fos ≤ 32; EUCAST, 2010, Coli ≤ 2; and EUCAST, 2011, Tige ≤ 1.
TABLE 2.
Treatment failure of infections caused by carbapenemase-producing Enterobacteriaceae among different antibiotic treatment regimensa
| First author, yr (reference) | Study design; period, country | Population characteristics; most common underlying diseases | Sites of infection (% of total population) | No. of infected patients who received definitive treatment | Causative pathogen(s) | CLSI yr of susceptibility breakpoints usedb | Antibiotic treatment administered, no. of patients (% treatment failure) |
|
|---|---|---|---|---|---|---|---|---|
| Combination therapy | Monotherapy | |||||||
| Rihani, 2012 (20) | SC retrospective cohort; 2008–2009, USA | 77% were in the ICU at enrollment | Blood, RTI, tissue, wound, drainage, UTI | 22 | Carbapenemase-producing Enterobacteriaceae (K. pneumoniae, E. coli, Enterobacter spp.) | 2010 | Coli-Carba, 4 | Coli, 4 |
| Coli-Tige, 2 | Carba, 3 | |||||||
| Carba-Amk, 2 | Other, 3 | |||||||
| Coli-Rifa, 1 | ||||||||
| Tob-Cfpm, 1 | Total (40) | |||||||
| Tige-Amk-Cfpm, 1 | ||||||||
| Coli-Carba-Tige-Amk, 1 | ||||||||
| Total (17) | ||||||||
| Maltezou, 2009 (14) | SC retrospective cohort; 2007–2008, Greece | 76.2% were ICU patients; DM, COPD, cardiovascular disease | Pneumonia (61.9), SSI (19), bacteremia (9.5), UTI (4.8), peritonitis (4.8) | 19 | KPC-producing K. pneumoniae | 2007 | Coli-Tige, 7 (43) | Gen, 1 (0) |
| Coli-Gen, 3 (0) | ||||||||
| Coli-Tige-Gen, 2 (0) | Unknown | |||||||
| Treatment, 6 (33)c | ||||||||
KPC, Klebsiella pneumoniae carbapenemase; SC, single center; CLSI, Clinical and Laboratory Standards Institute; DM, diabetes mellitus; COPD, chronic obstructive pulmonary disease; UTI, urinary tract infection; RTI, respiratory tract infection; ICU, intensive care unit; SSI, surgical-site infection; Carba, carbapenem; Coli, colistin; Tige, tigecycline; Gen, gentamicin; Amk, amikacin; Azt, aztreonam; Cipro, ciprofloxacin; Lvf, levofloxacin; Cfpm, cefepime; Rifa, rifampin.
The CLSI susceptibility breakpoints used in the included studies are the following: for 2007, Gen ≤ 4, and for 2010, Carba ≤ 4, Gen ≤ 4, Amk ≤ 16, Cipro ≤ 1, Lvf ≤ 2, Azt ≤ 4, Cfpm ≤ 8, and Tob ≤ 4.
In these studies, it was unclear whether the treatment regimens were combination or monotherapy.
Seven out of 21 studies were prospective cohort studies (11–13, 15, 17, 24, 28), 12 were retrospective cohort studies (9, 10, 14, 18–23, 25–27), and 1 was a retrospective case-control study (16). Fifteen studies reported on carbapenemase-producing Enterobacteriaceae (9, 10, 12, 14, 16, 17, 19–23, 25–28) and five others on carbapenem-resistant Enterobacteriaceae (11, 13, 15, 18, 24). One study included infections due to carbapenem-resistant K. pneumoniae isolates, mainly isolates that produced KPC (11). Klebsiella spp. were the sole causative pathogens in 14 studies (10–12, 14–16, 18, 19, 21, 22, 24–26, 28), while they were the predominant causative pathogens in 5 other studies (9, 13, 17, 20, 23). In one study, the major causative pathogen was Enterobacter cloacae (27). In 8 out of 20 studies, the total or the majority (>50% of the included infections) of the included infections were bacteremia (16–19, 22, 23, 25, 27, 28). Pneumonia and urinary tract infections were the most common infections among the remaining 12 studies. In 10 out of 20 studies, the majority of patients were critically ill (14–16, 18–23, 28).
Mortality.
In seven studies, the 28- or 30-day mortality was provided (10, 13, 17–19, 25, 27), in one, the 14-day mortality was provided (12), in four, the in-hospital mortality was provided (9, 11, 15, 16), in two, the overall mortality was provided (22, 23), in one, the infection-related mortality was provided (28), and in three, the type of mortality provided was not determined (21, 24, 26).
Carbapenemase-producing Klebsiella spp. as KPC, MBL, or OXA.
Eight studies (316 patients) reported data on KPC-producing Klebsiella spp. (9–11, 19, 22, 25, 26, 28), while five other studies (201 patients) reported data regarding metallo-β-lactamase (MBL)- or OXA-producing Klebsiella spp. (12, 16, 17, 21, 23). The mortality of the most commonly administered antibiotic treatment regimens among the included studies was recorded. Due to the fact that some treatment regimens were administered to very few patients in certain studies, only regimens reporting on more than 3 patients from each study were taken into account.
With regard to patients who received combination treatment, mortalities varied from 0% to 30% among 51 patients (4 studies) (10, 11, 25, 28) who received the tigecycline-colistin combination, from 0% to 50% among 15 patients (2 studies) (25, 28) who received the tigecycline-gentamicin combination, from 0% to 67% for 25 patients (4 studies) (12, 19, 22, 23) treated with a carbapenem-colistin combination, and from 40% to 61% for 30 patients (3 studies) (11, 16, 25) treated with a colistin-gentamicin combination. In a study including only intensive care unit (ICU) patients, the 11 patients with infections due to VIM-1-producing isolates who were treated with the tigecycline-colistin combination had 64% mortality (21). Among the 28 patients who were treated with the carbapenem-colistin combination, 16 were infected with carbapenem-susceptible strains (10, 12, 23), while the remaining 12 patients had infections due to carbapenem-resistant strains (19, 22). In addition, 67% mortality for patients receiving the carbapenem-colistin combination was recorded in two studies that included solid-organ transplant recipients (10) and ICU patients for the most part (22).
Regarding patients who received monotherapy, mortality varied from 9% to 50% for 29 patients (3 studies) (12, 19, 26) who received carbapenem, from 0% to 53% for 38 patients (4 studies) (21, 25, 26, 28) who received tigecycline, from 33% to 57% for 102 patients (8 studies) (10–12, 16, 19, 23, 25, 28) who received colistin, and from 6.3% to 80% among 26 patients (3 studies) (9, 11, 25) who received gentamicin. In a study including mainly ICU patients, patients who received monotherapy with tigecycline had 80% mortality (19). Among the 26 patients who were treated with gentamicin monotherapy, at least 19 patients had urinary tract infection, which was uncomplicated in most cases (9, 11). Twenty-five out of 29 patients who received carbapenem monotherapy were infected with carbapenem-susceptible strains (12, 26), while 3 patients were infected with strains with intermediate susceptibility to carbapenems (2 ≤ MIC ≤4) (19).
Finally, in a study including 34 elderly patients who received definitive antibiotic treatment for bloodstream infection due to OXA-48-producing Enterobacteriaceae, which were predominantly Klebsiella spp., all the antibiotics included in the combination treatment regimens were not precisely determined. Specifically, the 30-day mortality was 52.4% (11/21 patients) among patients who were treated with ≥2 active drugs, not including a carbapenem, while the 30-day mortality was 33.3% (2/6) among patients who were treated with ≥2 active drugs, including carbapenems (17). Patients who received monotherapy with amikacin had 33.3% (1/3) mortality.
Carbapenem-resistant Klebsiella spp.
Five studies (160 patients) reported data on carbapenem-resistant Klebsiella spp. (11, 13, 15, 18, 24). In two studies, rates of mortality were 25% and 31% among 16 and 13 patients, respectively, who received the tigecycline-colistin combination (11, 18). In one study, the majority of patients had infections caused by CRE K. pneumoniae (13). Mortality at day 30 was 50%, 50%, and 73% among patients who received monotherapy with a carbapenem, colistin, and tigecycline, respectively. There was no significant difference in 30-day mortality rates between the 15 patients treated with tigecycline and the other 18 patients treated with colistin, imipenem, or meropenem (P = 0.31). In another study including 10 patients, mortality was 50% (2/4) for patients who were treated with amikacin combined with a carbapenem (24). Finally, in the last study, 11 ICU patients with hospital-acquired infections received intravenous fosfomycin either combined with other antibiotics or alone (15). Two of the patients who received fosfomycin in combination with colistin died while in the hospital.
Other carbapenemase-producing Enterobacteriaceae.
One study reported on the definitive antibiotic treatment that was administered to 32 patients with bloodstream infections due to IMP-8-producing Enterobacteriaceae (mainly Enterobacter cloacae) (27). Patients who received treatment with carbapenems had a 10% 28-day mortality (2/20), whereas those who received non-carbapenem treatment had a 16.7% 28-day mortality (2/12). Approximately half the patients were infected with carbapenem-resistant strains.
Treatment failure.
Two studies (41 patients) reported relevant data on infections caused by carbapenemase-producing K. pneumoniae (14, 20). In the one study, including mainly ICU patients, treatment failure was 16.7% (2/12) among patients who received combination treatment, while treatment failure was 40% (4/10) among those who received monotherapy (P = 0.35) (20). The other study, also reporting mainly on ICU patients, showed that patients who received the tigecycline-colistin combination as well as those receiving the colistin-tigecycline-gentamicin combination had 42.9% (3/7) and 0% (0/2) treatment failures, respectively (14).
In total, in the majority of the studies, statistically significant differences in mortality and treatment failure were not detected between patients who received combination antibiotic treatment and those who received monotherapy. However, three studies, reporting on in total 194 patients, showed significantly lower mortality in the combination treatment arms than in the monotherapy arms (19, 25, 28). These studies reported on bloodstream infections in critically ill patients. In addition, another study showed numerically but not statistically significantly higher incidences of clinical cure and microbiological eradication among patients treated with a combination of antibiotics than among those treated with monotherapy (20). This study reported on critically ill patients, as well.
DISCUSSION
Methodological issues, including clinical heterogeneity, that have been detected among the included studies precluded the synthesis of the evidence using statistical analyses, including meta-analysis. However, among critically ill patients with bacteremia due to carbapenemase-producing Klebsiella spp., a combination antibiotic treatment may result in lower mortality than monotherapy.
Tigecycline in combination with colistin, carbapenem in combination with colistin, and tigecycline in combination with gentamicin were the commonly administered antibiotic treatment regimens among the included studies and might result in lower mortality than other combinations of antibiotics. An effectiveness similar to that of the aforementioned combinations was observed among patients treated with monotherapy with colistin, tigecycline, and carbapenems. However, the available data for the majority of the studied treatment regimens (both the combination regimens and the monotherapy regimens) was derived from fewer than 50 patients, and great variation existed with respect to site and severity of infection. Among patients treated with the same antibiotic treatment, either combination therapy or monotherapy, mortality exceeded 60% for patients in a critical care setting, while it was below 50% for non-ICU patients.
Carbapenems were, overall, administered to patients infected with strains for which the MICs were low. Interestingly, three of the included studies mention an important increase in survival when a carbapenem was administered in combination with another drug (12, 19, 25). In one of them, it is mentioned that the survival of patients treated with the colistin-tigecycline combination significantly increased when meropenem was added to the scheme (25). The authors commented that the survival benefit is possibly associated with meropenem because that was the antibiotic most commonly added to the colistin-tigecycline combination. It is also interesting that an increase in survival was observed both among patients infected with isolates for which the meropenem MIC was ≤4 and among patients infected with isolates for which MICs were elevated. Likewise, another study suggested that colistin-tigecycline along with a carbapenem was the most successful combination, even among patients infected with isolates resistant to carbapenems, possibly due to potential synergy between colistin and carbapenems, but results may be affected by the small number of cases studied and by the fact that carbapenems were very commonly included in the combination treatment regimens (19, 29). Last, in the remaining study, it is reported that the lowest mortality was noted among patients who received two active drugs, one of which was a carbapenem. However, all the combination regimens in this study included a carbapenem, precluding comparisons with other combination treatment regimens (12).
Gentamicin monotherapy was preferred in patients with urinary tract infections, and this may account for the high clinical success observed. There were few cases of successful treatment of bacteremia with gentamicin monotherapy as well as few cases of pneumonia, but monotherapy with an aminoglycoside is against the guidelines for these serious infections (30, 31). Also, catheter removal from patients with catheter-related bacteremia was probably the main factor leading to cure in those cases (11). With regard to tigecycline, it was administered either as monotherapy or in combination with colistin or gentamicin among the included studies. In the majority of these studies, tigecycline treatment regimens were not administered for approved indications (i.e., complicated intra-abdominal infections, complicated skin and soft tissue infections) but instead for bloodstream infection, pneumonia, and urinary tract infection. The use of tigecycline in an off-label manner is widespread due to the scarcity of approved effective alternative antibiotics for multidrug-resistant infections (32). Attention should be paid by clinicians, because tigecycline was associated with higher mortality than comparator antibiotics (33–35). However, a recent meta-analysis showed that the drug was not associated with significantly higher mortality than comparator antibiotics and was as effective as comparators when the analysis was restricted to patients who received tigecycline for approved indications (36).
The option between a combination of antibiotics and monotherapy can vary among different sites of infection, causative pathogens, antimicrobial susceptibility patterns, or patient comorbidity. In clinical practice, combinations of antibiotics are commonly administered to patients with severe infections either empirically to broaden the coverage or definitively for polymicrobial infections (37, 38). Evidence derived from clinical and in vitro studies reveals that a combination of antibiotics might prevent the emergence of resistant strains during therapy (39, 40). However, clinical data do not clearly support the idea that antibiotic combinations reduce the emergence of resistance (41). Synergy is another potential benefit arising from the use of antibiotic combinations (42–44), but in vitro synergy of specific antibiotics may not always translate into clinical effectiveness. Similarly, inactive in vitro antibiotics may potentially be useful when administered as adjuncts with an active antibiotic in cases when alternative antibiotics are not available.
Tigecycline with colistin, colistin with a carbapenem, fosfomycin with a carbapenem, fosfomycin with an aminoglycoside, and a carbapenem with an aminoglycoside have been reported as antibiotic combinations effectively administered to series of patients infected with carbapenemase-producing Enterobacteriaceae (45). In addition, there is clinical evidence suggesting that the tigecycline-colistin combination may be superior to colistin monotherapy in terms of emergence of colistin resistance during therapy for infection due to carbapenem-resistant K. pneumoniae (39). In contrast, a meta-analysis focusing on beta-lactams showed that a beta-lactam combined with an aminoglycoside was not superior to beta-lactam monotherapy with regard to emergence of resistance (41). Administration of colistin in combination with an aminoglycoside has also been reported (11, 14, 16, 25); however, attention should be paid during therapy since both agents can cause nephrotoxicity. In general, combinations of antibiotics should always be administered cautiously, bearing in mind the potential additive toxicity of the drugs. Among aminoglycosides, gentamicin has been suggested as the one with the highest in vitro activity against KPC- and VIM-producing Enterobacteriaceae (22, 46). Combination treatment including a carbapenem could be considered in infections due to carbapenemase-producing Enterobacteriaceae for which MICs are low and when extended or continuous but not short-term infusions are used (45, 47). Similarly, once-daily (extended-interval) dosing should be considered when aminoglycosides are administered, since this regimen provides optimal pharmacokinetics/pharmacodynamics for those drugs (48). Last, preliminary assays with in vitro and animal models support the idea that double-carbapenem therapy might be considered for treatment of infections caused by KPC-producing K. pneumoniae (49, 50).
On the other hand, treatment with a single antimicrobial agent, mainly colistin and tigecycline, has also been a choice (11, 13, 16, 18, 25). However, a recent review showed that treatment failure was more common among patients who were treated with monotherapy than among those who were treated with a combination of antibiotics for infections due to KPC-producing K. pneumoniae (51). The effectiveness of colistin monotherapy has been challenged due to low plasma concentrations owing to suboptimal dosing of the drug, especially in critically ill patients with impaired renal function (52). However, an increase in the daily dose of the antibiotic is risky due to colistin-induced nephrotoxicity. Also, low levels in serum are achieved with the usual dosing of tigecycline. Furthermore, colistin heteroresistance has emerged among Klebsiella species isolates (53), while outbreaks due to colistin-resistant carbapenemase-producing Enterobacteriaceae (54–57) and resistance to tigecycline (58) have also been recorded in some countries. Resistance to both antibiotics has been associated with prior exposure to the drugs (59, 60). Therefore, combination with another antibiotic might be the optimal option when patients are treated with colistin or tigecycline. Last, until further studies clarify the issue of the emergence of resistance during therapy with fosfomycin, fosfomycin should not be administered as monotherapy.
Our study should be interpreted in view of certain limitations. The major one is that the scarcity of evidence on how to treat these potentially severe infections forced us to present together different patient populations, different sites of infections, different genotypes of a pathogen (i.e., KPC, VIM, OXA), and different assessments of mortality. In addition, the interpretation of the findings should be done in view of the fact that lower antimicrobial susceptibility breakpoints were proposed for carbapenems by the Clinical and Laboratory Standards Institute (CLSI) in June 2010, while older breakpoints were used in some of the included studies. Another important limitation is that all included studies were nonrandomized, and the majority of them had a retrospective design. Furthermore, specific conclusions were drawn from studies with a small number of patients. Another important issue is that the administrations of the antibiotics differed among the studies with regard to the duration of infusion or the total daily dose. Accordingly, these differences might influence the clinical outcomes. With regard to the safety of the administered treatment regimens, the included studies did not provide relevant data. Last, the matter of the emergence of resistance during therapy was not raised by any of the included studies.
In conclusion, the available evidence, which came entirely from nonrandomized studies, suggests that combination antibiotic treatment may offer a comparative advantage over monotherapy with regard to the mortality of critically ill patients with severe infections due to carbapenemase-producing Klebsiella spp. Well-designed randomized controlled trials in specific patient populations are required to address this crucial question of everyday clinical practice.
ACKNOWLEDGMENTS
M.E.F. has participated in the advisory boards of Achaogen, Astellas, AstraZeneca, Bayer, and Pfizer. He also has received lecture honoraria from Angelini, Astellas, AstraZeneca, Glenmark, Merck, and Novartis and research support from Angelini, Astellas, and Rokitan. All of the other authors have nothing to disclose.
No funding was provided for this study.
Footnotes
Published ahead of print 30 September 2013
REFERENCES
- 1.Nordmann P, Cuzon G, Naas T. 2009. The real threat of Klebsiella pneumoniae carbapenemase-producing bacteria. Lancet Infect. Dis. 9:228–236. 10.1016/S1473-3099(09)70054-4 [DOI] [PubMed] [Google Scholar]
- 2.Vardakas KZ, Tansarli GS, Rafailidis PI, Falagas ME. 2012. Carbapenems versus alternative antibiotics for the treatment of bacteraemia due to Enterobacteriaceae producing extended-spectrum beta-lactamases: a systematic review and meta-analysis. J. Antimicrob. Chemother. 67:2793–2803. 10.1093/jac/dks301 [DOI] [PubMed] [Google Scholar]
- 3.CDC, CfDCaP 2013. Vital signs: carbapenem-resistant enterobacteriaceae. MMWR Morb. Mortal. Wkly. Rep. 62:165–170 [PMC free article] [PubMed] [Google Scholar]
- 4.Falagas ME, Kasiakou SK. 2005. Colistin: the revival of polymyxins for the management of multidrug-resistant gram-negative bacterial infections. Clin. Infect. Dis. 40:1333–1341. 10.1086/429323 [DOI] [PubMed] [Google Scholar]
- 5.Falagas ME, Kopterides P. 2007. Old antibiotics for infections in critically ill patients. Curr. Opin. Crit. Care 13:592–597. 10.1097/MCC.0b013e32827851d7 [DOI] [PubMed] [Google Scholar]
- 6.Ben-David D, Kordevani R, Keller N, Tal I, Marzel A, Gal-Mor O, Maor Y, Rahav G. 2012. Outcome of carbapenem resistant Klebsiella pneumoniae bloodstream infections. Clin. Microbiol. Infect. 18:54–60. 10.1111/j.1469-0691.2011.03478.x [DOI] [PubMed] [Google Scholar]
- 7.Chang HJ, Hsu PC, Yang CC, Kuo AJ, Chia JH, Wu TL, Lee MH. 2011. Risk factors and outcomes of carbapenem-nonsusceptible Escherichia coli bacteremia: a matched case-control study. J. Microbiol. Immunol. Infect. 44:125–130. 10.1016/j.jmii.2010.06.001 [DOI] [PubMed] [Google Scholar]
- 8.Patel G, Huprikar S, Factor SH, Jenkins SG, Calfee DP. 2008. Outcomes of carbapenem-resistant Klebsiella pneumoniae infection and the impact of antimicrobial and adjunctive therapies. Infect. Control Hosp. Epidemiol. 29:1099–1106. 10.1086/592412 [DOI] [PubMed] [Google Scholar]
- 9.Alexander BT, Marschall J, Tibbetts RJ, Neuner EA, Dunne WM, Jr, Ritchie DJ. 2012. Treatment and clinical outcomes of urinary tract infections caused by KPC-producing Enterobacteriaceae in a retrospective cohort. Clin. Ther. 34:1314–1323. 10.1016/j.clinthera.2012.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bergamasco MD, Barroso Barbosa M, de Oliveira Garcia D, Cipullo R, Moreira JC, Baia C, Barbosa V, Abboud CS. 2012. Infection with Klebsiella pneumoniae carbapenemase (KPC)-producing K. pneumoniae in solid organ transplantation. Transpl. Infect. Dis. 14:198–205. 10.1111/j.1399-3062.2011.00688.x [DOI] [PubMed] [Google Scholar]
- 11.Capone A, Giannella M, Fortini D, Giordano A, Meledandri M, Ballardini M, Venditti M, Bordi E, Capozzi D, Balice MP, Tarasi A, Parisi G, Lappa A, Carattoli A, Petrosillo N. 2013. High rate of colistin resistance among patients with carbapenem-resistant Klebsiella pneumoniae infection accounts for an excess of mortality. Clin. Microbiol. Infect. 19:E23–E30. 10.1111/1469-0691.12070 [DOI] [PubMed] [Google Scholar]
- 12.Daikos GL, Petrikkos P, Psichogiou M, Kosmidis C, Vryonis E, Skoutelis A, Georgousi K, Tzouvelekis LS, Tassios PT, Bamia C, Petrikkos G. 2009. Prospective observational study of the impact of VIM-1 metallo-beta-lactamase on the outcome of patients with Klebsiella pneumoniae bloodstream infections. Antimicrob. Agents Chemother. 53:1868–1873. 10.1128/AAC.00782-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang SR, Liu MF, Lin CF, Shi ZY. 28 November 2012. Molecular surveillance and clinical outcomes of carbapenem-resistant Escherichia coli and Klebsiella pneumoniae infections. J. Microbiol. Immunol. Infect. 10.1016/j.jmii.2012.08.029 [DOI] [PubMed] [Google Scholar]
- 14.Maltezou HC, Giakkoupi P, Maragos A, Bolikas M, Raftopoulos V, Papahatzaki H, Vrouhos G, Liakou V, Vatopoulos AC. 2009. Outbreak of infections due to KPC-2-producing Klebsiella pneumoniae in a hospital in Crete (Greece). J. Infect. 58:213–219. 10.1016/j.jinf.2009.01.010 [DOI] [PubMed] [Google Scholar]
- 15.Michalopoulos A, Virtzili S, Rafailidis P, Chalevelakis G, Damala M, Falagas ME. 2010. Intravenous fosfomycin for the treatment of nosocomial infections caused by carbapenem-resistant Klebsiella pneumoniae in critically ill patients: a prospective evaluation. Clin. Microbiol. Infect. 16:184–186. 10.1111/j.1469-0691.2009.02921.x [DOI] [PubMed] [Google Scholar]
- 16.Mouloudi E, Protonotariou E, Zagorianou A, Iosifidis E, Karapanagiotou A, Giasnetsova T, Tsioka A, Roilides E, Sofianou D, Gritsi-Gerogianni N. 2010. Bloodstream infections caused by metallo-beta-lactamase/Klebsiella pneumoniae carbapenemase-producing K. pneumoniae among intensive care unit patients in Greece: risk factors for infection and impact of type of resistance on outcomes. Infect. Control Hosp. Epidemiol. 31:1250–1256. 10.1086/657135 [DOI] [PubMed] [Google Scholar]
- 17.Navarro-San Francisco C, Mora-Rillo M, Romero-Gomez MP, Moreno-Ramos F, Rico-Nieto A, Ruiz-Carrascoso G, Gomez-Gil R, Arribas-Lopez JR, Mingorance J, Pano-Pardo JR. 2013. Bacteraemia due to OXA-48-carbapenemase-producing Enterobacteriaceae: a major clinical challenge. Clin. Microbiol. Infect. 19:E72–E79. 10.1111/1469-0691.12091 [DOI] [PubMed] [Google Scholar]
- 18.Nguyen M, Eschenauer GA, Bryan M, O'Neil K, Furuya EY, Della-Latta P, Kubin CJ. 2010. Carbapenem-resistant Klebsiella pneumoniae bacteremia: factors correlated with clinical and microbiologic outcomes. Diagn. Microbiol. Infect. Dis. 67:180–184. 10.1016/j.diagmicrobio.2010.02.001 [DOI] [PubMed] [Google Scholar]
- 19.Qureshi ZA, Paterson DL, Potoski BA, Kilayko MC, Sandovsky G, Sordillo E, Polsky B, Adams-Haduch JM, Doi Y. 2012. Treatment outcome of bacteremia due to KPC-producing Klebsiella pneumoniae: superiority of combination antimicrobial regimens. Antimicrob. Agents Chemother. 56:2108-2113. 10.1128/AAC.06268-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rihani DS, Wallace MR, Sieger BE, Waite RA, Fox M, Brown SA, Deryke CA. 2012. Over-treatment of carbapenemase-producing Enterobacteriaceae. Scand. J. Infect. Dis. 44:325–329. 10.3109/00365548.2011.638318 [DOI] [PubMed] [Google Scholar]
- 21.Sanchez-Romero I, Asensio A, Oteo J, Munoz-Algarra M, Isidoro B, Vindel A, Alvarez-Avello J, Balandin-Moreno B, Cuevas O, Fernandez-Romero S, Azanedo L, Saez D, Campos J. 2012. Nosocomial outbreak of VIM-1-producing Klebsiella pneumoniae isolates of multilocus sequence type 15: molecular basis, clinical risk factors, and outcome. Antimicrob. Agents Chemother. 56:420–427. 10.1128/AAC.05036-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Souli M, Galani I, Antoniadou A, Papadomichelakis E, Poulakou G, Panagea T, Vourli S, Zerva L, Armaganidis A, Kanellakopoulou K, Giamarellou H. 2010. An outbreak of infection due to beta-lactamase Klebsiella pneumoniae carbapenemase 2-producing K. pneumoniae in a Greek University Hospital: molecular characterization, epidemiology, and outcomes. Clin. Infect. Dis. 50:364–373. 10.1086/649865 [DOI] [PubMed] [Google Scholar]
- 23.Souli M, Kontopidou FV, Papadomichelakis E, Galani I, Armaganidis A, Giamarellou H. 2008. Clinical experience of serious infections caused by Enterobacteriaceae producing VIM-1 metallo-beta-lactamase in a Greek University Hospital. Clin. Infect. Dis. 46:847–854. 10.1086/528719 [DOI] [PubMed] [Google Scholar]
- 24.Trevino M, Navarro D, Barbeito G, Garcia-Riestra C, Crespo C, Regueiro BJ. 2011. Molecular and epidemiological analysis of nosocomial carbapenem-resistant Klebsiella spp. using repetitive extragenic palindromic-polymerase chain reaction and matrix-assisted laser desorption/ionization-time of flight. Microb. Drug Resist. 17:433–442. 10.1089/mdr.2010.0182 [DOI] [PubMed] [Google Scholar]
- 25.Tumbarello M, Viale P, Viscoli C, Trecarichi EM, Tumietto F, Marchese A, Spanu T, Ambretti S, Ginocchio F, Cristini F, Losito AR, Tedeschi S, Cauda R, Bassetti M. 2012. Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: importance of combination therapy. Clin. Infect. Dis. 55:943–950. 10.1093/cid/cis588 [DOI] [PubMed] [Google Scholar]
- 26.Weisenberg SA, Morgan DJ, Espinal-Witter R, Larone DH. 2009. Clinical outcomes of patients with Klebsiella pneumoniae carbapenemase-producing K. pneumoniae after treatment with imipenem or meropenem. Diagn. Microbiol. Infect. Dis. 64:233–235. 10.1016/j.diagmicrobio.2009.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yan JJ, Lee NY, Chen HM, Wang MC, Ko WC, Tsai LH, Wu JJ. 2013. Bloodstream infections caused by IMP-8-producing Enterobacteriaceae isolates: the need for clinical laboratory detection of metallo-beta-lactamases? Eur. J. Clin. Microbiol. Infect. Dis. 32:345–352. 10.1007/s10096-012-1748-x [DOI] [PubMed] [Google Scholar]
- 28.Zarkotou O, Pournaras S, Tselioti P, Dragoumanos V, Pitiriga V, Ranellou K, Prekates A, Themeli-Digalaki K, Tsakris A. 2011. Predictors of mortality in patients with bloodstream infections caused by KPC-producing Klebsiella pneumoniae and impact of appropriate antimicrobial treatment. Clin. Microbiol. Infect. 17:1798–1803. 10.1111/j.1469-0691.2011.03514.x [DOI] [PubMed] [Google Scholar]
- 29.Jernigan MG, Press EG, Nguyen MH, Clancy CJ, Shields RK. 2012. The combination of doripenem and colistin is bactericidal and synergistic against colistin-resistant, carbapenemase-producing Klebsiella pneumoniae. Antimicrob. Agents Chemother. 56:3395–3398. 10.1128/AAC.06364-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.American Thoracic Society, Infectious Diseases Society of America 2005. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am. J. Respir. Crit. Care Med. 171:388–416. 10.1164/rccm.200405-644ST [DOI] [PubMed] [Google Scholar]
- 31.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb S, Beale RJ, Vincent JL, Moreno R. 2013. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 39:165–228. 10.1007/s00134-012-2769-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tansarli GS, Rafailidis PI, Kapaskelis A, Falagas ME. 2012. Frequency of the off-label use of antibiotics in clinical practice: a systematic review. Expert Rev. Anti Infect. Ther. 10:1383–1392. 10.1586/eri.12.137 [DOI] [PubMed] [Google Scholar]
- 33.FDA 2010. FDA drug safety communication: increased risk of death with Tygacil (tigecycline) compared to other antibiotics used to treat similar infections. FDA, Rockville, MD: http://www.fda.gov/drugs/drugsafety/ucm224370.htm [Google Scholar]
- 34.Prasad P, Sun J, Danner RL, Natanson C. 2012. Excess deaths associated with tigecycline after approval based on noninferiority trials. Clin. Infect. Dis. 54:1699–1709. 10.1093/cid/cis270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yahav D, Lador A, Paul M, Leibovici L. 2011. Efficacy and safety of tigecycline: a systematic review and meta-analysis. J. Antimicrob. Chemother. 66:1963–1971. 10.1093/jac/dkr242 [DOI] [PubMed] [Google Scholar]
- 36.Vardakas KZ, Rafailidis PI, Falagas ME. 2012. Effectiveness and safety of tigecycline: focus on use for approved indications. Clin. Infect. Dis. 54:1672–1674. 10.1093/cid/cis239 [DOI] [PubMed] [Google Scholar]
- 37.Paul M, Benuri-Silbiger I, Soares-Weiser K, Leibovici L. 2004. Beta lactam monotherapy versus beta lactam-aminoglycoside combination therapy for sepsis in immunocompetent patients: systematic review and meta-analysis of randomised trials. BMJ 328:668. 10.1136/bmj.38028.520995.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vardakas KZ, Tansarli GS, Bliziotis IA, Falagas ME. 2013. β-Lactam plus aminoglycoside or fluoroquinolone combination versus beta-lactam monotherapy for Pseudomonas aeruginosa infections: a meta-analysis. Int. J. Antimicrob. Agents 41:301–310. 10.1016/j.ijantimicag.2012.12.006 [DOI] [PubMed] [Google Scholar]
- 39.Lee J, Patel G, Huprikar S, Calfee DP, Jenkins SG. 2009. Decreased susceptibility to polymyxin B during treatment for carbapenem-resistant Klebsiella pneumoniae infection. J. Clin. Microbiol. 47:1611–1612. 10.1128/JCM.02466-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Souli M, Galani I, Boukovalas S, Gourgoulis MG, Chryssouli Z, Kanellakopoulou K, Panagea T, Giamarellou H. 2011. In vitro interactions of antimicrobial combinations with fosfomycin against KPC-2-producing Klebsiella pneumoniae and protection of resistance development. Antimicrob. Agents Chemother. 55:2395–2397. 10.1128/AAC.01086-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bliziotis IA, Samonis G, Vardakas KZ, Chrysanthopoulou S, Falagas ME. 2005. Effect of aminoglycoside and beta-lactam combination therapy versus beta-lactam monotherapy on the emergence of antimicrobial resistance: a meta-analysis of randomized, controlled trials. Clin. Infect. Dis. 41:149–158. 10.1086/430912 [DOI] [PubMed] [Google Scholar]
- 42.Elemam A, Rahimian J, Doymaz M. 2010. In vitro evaluation of antibiotic synergy for polymyxin B-resistant carbapenemase-producing Klebsiella pneumoniae. J. Clin. Microbiol. 48:3558–3562. 10.1128/JCM.01106-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pournaras S, Vrioni G, Neou E, Dendrinos J, Dimitroulia E, Poulou A, Tsakris A. 2011. Activity of tigecycline alone and in combination with colistin and meropenem against Klebsiella pneumoniae carbapenemase (KPC)-producing Enterobacteriaceae strains by time-kill assay. Int. J. Antimicrob. Agents 37:244–247. 10.1016/j.ijantimicag.2010.10.031 [DOI] [PubMed] [Google Scholar]
- 44.Samonis G, Maraki S, Karageorgopoulos DE, Vouloumanou EK, Falagas ME. 2012. Synergy of fosfomycin with carbapenems, colistin, netilmicin, and tigecycline against multidrug-resistant Klebsiella pneumoniae, Escherichia coli, and Pseudomonas aeruginosa clinical isolates. Eur. J. Clin. Microbiol. Infect. Dis. 31:695–701. 10.1007/s10096-011-1360-5 [DOI] [PubMed] [Google Scholar]
- 45.Falagas ME, Karageorgopoulos DE, Nordmann P. 2011. Therapeutic options for infections with Enterobacteriaceae producing carbapenem-hydrolyzing enzymes. Future Microbiol. 6:653–666. 10.2217/fmb.11.49 [DOI] [PubMed] [Google Scholar]
- 46.Castanheira M, Sader HS, Jones RN. 2010. Antimicrobial susceptibility patterns of KPC-producing or CTX-M-producing Enterobacteriaceae. Microb. Drug Resist. 16:61–65. 10.1089/mdr.2009.0031 [DOI] [PubMed] [Google Scholar]
- 47.Falagas ME, Tansarli GS, Ikawa K, Vardakas KZ. 2013. Clinical outcomes with extended or continuous versus short-term intravenous infusion of carbapenems and piperacillin/tazobactam: a systematic review and meta-analysis. Clin. Infect. Dis. 56:272–282. 10.1093/cid/cis857 [DOI] [PubMed] [Google Scholar]
- 48.Freeman CD, Nicolau DP, Belliveau PP, Nightingale CH. 1997. Once-daily dosing of aminoglycosides: review and recommendations for clinical practice. J. Antimicrob. Chemother. 39:677–686. 10.1093/jac/39.6.677 [DOI] [PubMed] [Google Scholar]
- 49.Bulik CC, Nicolau DP. 2011. Double-carbapenem therapy for carbapenemase-producing Klebsiella pneumoniae. Antimicrob. Agents Chemother. 55:3002–3004. 10.1128/AAC.01420-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hong JH, Clancy CJ, Cheng S, Shields RK, Chen L, Doi Y, Zhao Y, Perlin DS, Kreiswirth BN, Nguyen MH. 2013. Characterization of porin expression in Klebsiella pneumoniae carbapenemase (KPC)-producing K. pneumoniae identifies isolates most susceptible to the combination of colistin and carbapenems. Antimicrob. Agents Chemother. 57:2147-2153. 10.1128/AAC.02411-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee GC, Burgess DS. 2012. Treatment of Klebsiella pneumoniae carbapenemase (KPC) infections: a review of published case series and case reports. Ann. Clin. Microbiol. Antimicrob. 11:32. 10.1186/1476-0711-11-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Garonzik SM, Li J, Thamlikitkul V, Paterson DL, Shoham S, Jacob J, Silveira FP, Forrest A, Nation RL. 2011. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob. Agents Chemother. 55:3284–3294. 10.1128/AAC.01733-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meletis G, Tzampaz E, Sianou E, Tzavaras I, Sofianou D. 2011. Colistin heteroresistance in carbapenemase-producing Klebsiella pneumoniae. J. Antimicrob. Chemother. 66:946–947. 10.1093/jac/dkr007 [DOI] [PubMed] [Google Scholar]
- 54.Kontopoulou K, Protonotariou E, Vasilakos K, Kriti M, Koteli A, Antoniadou E, Sofianou D. 2010. Hospital outbreak caused by Klebsiella pneumoniae producing KPC-2 beta-lactamase resistant to colistin. J. Hosp. Infect. 76:70–73. 10.1016/j.jhin.2010.03.021 [DOI] [PubMed] [Google Scholar]
- 55.Mammina C, Bonura C, Di Bernardo F, Aleo A, Fasciana T, Sodano C, Saporito MA, Verde MS, Tetamo R, Palma DM. 16 August 2012. Ongoing spread of colistin-resistant Klebsiella pneumoniae in different wards of an acute general hospital, Italy, June to December 2011. Euro urveill. 17(33):pii20248. [PubMed] [Google Scholar]
- 56.Marchaim D, Chopra T, Pogue JM, Perez F, Hujer AM, Rudin S, Endimiani A, Navon-Venezia S, Hothi J, Slim J, Blunden C, Shango M, Lephart PR, Salimnia H, Reid D, Moshos J, Hafeez W, Bheemreddy S, Chen TY, Dhar S, Bonomo RA, Kaye KS. 2011. Outbreak of colistin-resistant, carbapenem-resistant Klebsiella pneumoniae in metropolitan Detroit, Michigan. Antimicrob. Agents Chemother. 55:593–599. 10.1128/AAC.01020-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Valencia R, Arroyo LA, Conde M, Aldana JM, Torres MJ, Fernandez-Cuenca F, Garnacho-Montero J, Cisneros JM, Ortiz C, Pachon J, Aznar J. 2009. Nosocomial outbreak of infection with pan-drug-resistant Acinetobacter baumannii in a tertiary care university hospital. Infect. Control Hosp. Epidemiol. 30:257–263. 10.1086/595977 [DOI] [PubMed] [Google Scholar]
- 58.Karageorgopoulos DE, Kelesidis T, Kelesidis I, Falagas ME. 2008. Tigecycline for the treatment of multidrug-resistant (including carbapenem-resistant) Acinetobacter infections: a review of the scientific evidence. J. Antimicrob. Chemother. 62:45–55. 10.1093/jac/dkn165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Matthaiou DK, Michalopoulos A, Rafailidis PI, Karageorgopoulos DE, Papaioannou V, Ntani G, Samonis G, Falagas ME. 2008. Risk factors associated with the isolation of colistin-resistant gram-negative bacteria: a matched case-control study. Crit. Care Med. 36:807–811. 10.1097/CCM.0B013E3181652FAE [DOI] [PubMed] [Google Scholar]
- 60.Sun Y, Cai Y, Liu X, Bai N, Liang B, Wang R. 2013. The emergence of clinical resistance to tigecycline. Int. J. Antimicrob. Agents 41:110–116. 10.1016/j.ijantimicag.2012.09.005 [DOI] [PubMed] [Google Scholar]