Abstract
Onchocerciasis (river blindness), caused by the filarial nematode Onchocerca volvulus, is a major cause of visual impairment and dermatitis in sub-Saharan Africa. As O. volvulus contains an obligatory bacterial symbiont (Wolbachia), it is susceptible to antibiotic chemotherapy, although current regimens are considered too prolonged for community-level control programs. The aim of this study was to compare the efficacies of oxytetracycline and rifampin, administered separately or in combination, against a close relative of O. volvulus (Onchocerca ochengi) in cattle. Six animals per group were treated with continuous or intermittent oxytetracycline regimens, and effects on adult worm viability, dermal microfilarial loads, and Wolbachia density in worm tissues were assessed. Subsequently, the efficacies of 3-week regimens of oxytetracycline and rifampin alone and a combination regimen were compared, and rifampin levels in plasma and skin were quantified. A 6-month regimen of oxytetracycline with monthly dosing was strongly adulticidal, while 3-week and 6-week regimens exhibited weaker adulticidal effects. However, all three regimens achieved >2-log reductions in microfilarial load. In contrast, rifampin monotherapy and oxytetracycline-rifampin duotherapy failed to induce substantive reductions in either adult worm burden or microfilarial load, although a borderline effect on Wolbachia density was observed following duotherapy. Dermal rifampin levels were maintained above the MIC for >24 h after a single intravenous dose. We conclude that oxytetracycline-rifampin duotherapy is less efficacious against O. ochengi than oxytetracycline alone. Further studies will be required to determine whether rifampin reduces oxytetracycline bioavailability in this system, as suggested by human studies using other tetracycline-rifampin combinations.
INTRODUCTION
Onchocerciasis, or “river blindness,” is a vector-borne parasitic disease that continues to affect 26 million people, predominantly in sub-Saharan Africa, despite 4 decades of concerted control efforts (1). The etiological agent, Onchocerca volvulus, is a filarial worm that resides in subcutaneous nodules when mature and has a life span of >10 years (2). Although the nodules are generally benign, the female worm releases approximately 1,000 first-stage larvae (microfilariae [Mf]) per day (3), which accumulate in the skin and can induce severe pruritus and dermatitis. After several years, the Mf can also migrate into the eyes, where their death prompts local inflammatory reactions, visual impairment, and, ultimately, irreversible blindness. Whereas the original Onchocerciasis Control Programme was initially focused on vector control, the current African Programme for Onchocerciasis Control (APOC) is almost entirely dependent on mass drug administration (MDA) of the macrocyclic lactone ivermectin (4). This strategy has been in place in many regions of endemicity for approximately 2 decades and has been extremely successful in preventing blindness and onchodermatitis (5), since ivermectin rapidly clears Mf from the skin. Recent data have also demonstrated that ivermectin can eliminate onchocerciasis in areas of seasonal transmission (6), and this has led APOC to redefine itself as an elimination program with a target attainment date of 2025 (4). However, ivermectin MDA faces a number of challenges that may compromise the progress of APOC over the next decade. These include severe adverse events following ivermectin treatment in individuals heavily coinfected with Loa loa (a species of filarial worm endemic to Central Africa) (7), continued transmission of onchocerciasis in perennial-transmission zones despite 15 to 18 years of MDA (8, 9), evidence of decreased ivermectin susceptibility in some worm populations (10, 11), variable compliance with MDA within affected communities (12), and a lack of adulticidal efficacy against the parasite (13).
Until relatively recently, no safe adulticidal drug for onchocerciasis existed. This changed with the identification of Wolbachia endobacteria (order Rickettsiales) in filariae of medical importance in the late 1990s (14, 15), which prompted a series of experiments in animal models demonstrating that antibiotics (especially the tetracyclines) not only impede the growth and embryogenesis of filarial worms (16, 17) but also can kill the adult parasites (18). Such effects were not observed at clinically relevant doses in filarial species that naturally lack Wolbachia symbionts (16, 19). Clinical trials of doxycycline (DOX) for human onchocerciasis were implemented rapidly, which achieved sterilization of female worms using a regimen of 200 mg/day for 4 weeks (20) or 100 mg/day for 5 weeks (21). However, significant adulticidal activity (killing of 60 to 70% of female worms) required a regimen of 200 mg/day for 6 weeks (20). This relatively protracted course of treatment, coupled with contraindications in children below 8 years of age and in pregnant or lactating women, have prevented approval of DOX for MDA to date; this is despite the very high rates of compliance evident in a clinical trial that was conducted in a region of Cameroon where loiasis is endemic (22). Nevertheless, DOX has been applied in a small onchocerciasis focus in Venezuela to expedite elimination efforts (23).
Experiments performed in vitro using isolated worms or Wolbachia-infected cell lines (24–26), as well as in vivo trials in rodent models (27, 28), have indicated that rifampin (RIF) is at least as effective as the tetracyclines for symbiont depletion and, indeed, may be superior. However, two human trials of RIF for onchocerciasis failed to demonstrate that this bactericide could truncate the therapeutic duration significantly, as a 5-day regimen had no effect on either adult worms or microfilarial densities (29), whereas 2-week and 4-week regimens induced a partial embryostatic effect but were not adulticidal (30). Although these data were equivocal, there remains the possibility that a combination of a tetracycline and RIF substantially shortens the regimen required to achieve potent adulticidal effects.
A major challenge in onchocerciasis research is the inability of Onchocerca spp. to complete their life cycles in rodent models. However, in cattle, Onchocerca ochengi, which is the closest extant relative of O. volvulus (31), has been used extensively to investigate drug efficacy for onchocerciasis, and numerous bovine studies have displayed strong concordance with data obtained from human chemotherapeutic trials (18, 32, 33). Importantly, adult worms of O. ochengi reside in intradermal nodules with a histological structure highly similar to that of O. volvulus, and the parasites are fertile for many years (34). Here, we use this natural host-parasite system to determine whether the addition of RIF to an oxytetracycline (OXY) regimen with limited activity against adult worms can reduce the treatment duration for unequivocal parasite killing to 3 weeks.
MATERIALS AND METHODS
Drugs.
Oxytetracycline dihydrate was obtained as a commercially available, long-acting formulation (Terramycin LA, 200 mg/ml; Pfizer Animal Health, Tadworth, United Kingdom) that is licensed for use in cattle. RIF was kindly donated by Lupin Ltd. (Mumbai, India) in solid form as the unformulated active pharmaceutical ingredient. For administration to cattle, the powder was dissolved at 1% (wt/vol) in 25 mM HEPES buffer (pH 7.0 to 7.6), 20% (vol/vol) dimethyl sulfoxide (DMSO) solvent (both cell culture grade; Sigma-Aldrich, Gillingham, United Kingdom), and sterile normal saline in intravenous bags. Prior to injection, the RIF solution was warmed to 37°C in an incubator to ensure complete dissolution and was transported to the field protected from light.
Animals, chemotherapy, and sampling strategy.
Zebu cattle (Ngaoundéré Gudali breed), naturally infected with ≥20 palpable O. ochengi nodules per animal, were purchased from markets across the Adamawa Region of Cameroon (Vina Division) and assembled at the Institut de Recherche Agricole pour le Développement (IRAD), Regional Centre of Wakwa, where transmission of O. ochengi is negligible. The pretreatment recording of nodule position and the randomization of animals into treatment groups were performed as previously described (35). Evaluations of antibiotic efficacy were conducted in two consecutive experiments (Table 1); the first compared continuous 3-week or 6-week OXY monotherapy (OXY3 and OXY6, respectively) with a prolonged intermittent OXY regimen (PIR) (36), whereas the second was designed to determine whether a 3-week RIF-plus-OXY combination regimen (COM) was superior to 3 weeks of RIF or OXY monotherapy. These experiments utilized different animals, with the exception of two cows from the first control (CON-1) group, which were reused in the second control (CON-2) group. Four animals from experiment 1 and one animal from experiment 2 died before the studies were completed (Tables 1 and 2) from causes unrelated to either onchocerciasis or the drug treatments.
TABLE 1.
Sample sizes and treatment regimens for experiments 1 and 2
| Expt | Group designation | Original group size |
No. of animals lost during expt | Treatment regimen (duration)a | |
|---|---|---|---|---|---|
| Females | Males | ||||
| 1 | CON-1 | 6 | 0 | 1 | None |
| 1 | PIR | 6 | 0 | 1 | OXY at 20 mg/kg i.m. once/mo (6 mo) |
| 1 | OXY3-1 | 6 | 0 | 0 | OXY at 20 mg/kg i.m. twice/wk (3 wk) |
| 1 | OXY6 | 6 | 0 | 2 | OXY at 20 mg/kg i.m. twice/wk (6 wk) |
| 2 | CON-2 | 6 | 0 | 1 | DMSO at 220 mg/kg i.v. twice/wk (3 wk) |
| 2 | RIF3 | 5 | 1 | 0 | RIF at 10 mg/kg i.v. and DMSO 220 mg/kg i.v. twice/wk (3 wk) |
| 2 | OXY3-2 | 6 | 0 | 0 | OXY at 20 mg/kg i.m. twice/wk (3 wk) |
| 2 | COM | 5 | 1 | 0 | RIF at 10 mg/kg i.v., DMSO at 220 mg/kg i.v., and OXY at 20 mg/kg i.m. twice/wk (3 wk) |
OXY, oxytetracycline; i.m., intramuscularly; DMSO, dimethyl sulfoxide; i.v., intravenously; RIF, rifampin.
TABLE 2.
Effect of oxytetracycline, rifampin, and a combination regimen on adult worms of Onchocerca ochengi in naturally infected cattle
| Groupa | Time (wpt) | No. of cattle | No. of nodules |
No. of worms per nodule site (no. of viable worms onlyb) in: |
Median motility score inc: |
|||
|---|---|---|---|---|---|---|---|---|
| Intact | Degeneratedd | Males | Females | Males | Females | |||
| CON-1 | 0 | 6 | 6 | 0 | 1.7 | 1.3 | 2 | 2 |
| 4 | 6 | 6 | 0 | 3.3 | 1.3 | 1 | 2 | |
| 12 | 5 | 5 | 0 | 0.8 | 1.2 | 1.5 | 2 | |
| 24 | 5 | 5 | 0 | 1.4 | 1.0 (0.8) | 2 | 1 | |
| 36 | 5 | 5 | 0 | 2.2 (2.0) | 1.0 (0.8) | 1 | 1 | |
| 55 | 5 | 5 | 0 | 0.8 | 1.0 (0.8) | 2 | 2 | |
| PIR | 0 | 6 | 6 | 0 | 4 | 1.2 | 2 | 2 |
| 4 | 6 | 6 | 0 | 1.5 | 1.0 | 1 | 1.5 | |
| 12 | 5 | 5 | 0 | 0.8 | 1.0 | 2 | 1 | |
| 24 | 5 | 4 | 1 | 0.2 (1.0) | 1.2 | 2 | 1 | |
| 36 | 5 | 5 | 0 | 0.4 (0.0) | 1.0 (0.2) | 0 | 0 | |
| 55e | 5 | 2 | 3 | 0 | 0.4 (0.2) | —f | 1 | |
| OXY3-1 | 0 | 6 | 6 | 0 | 1.5 | 1.0 | 2 | 2 |
| 4 | 6 | 6 | 0 | 1.5 | 1.0 | 1 | 2 | |
| 12 | 6 | 6 | 0 | 0.8 | 1.0 | 2 | 1.5 | |
| 24 | 6 | 5 | 1 | 1.3 | 0.8 (0.7) | 1 | 1 | |
| 36 | 6 | 6 | 0 | 1.0 (0.3) | 1.0 (0.2) | 0 | 0 | |
| 55 | 6 | 2 | 4 | 0.2 | 0.3 | 2 | 1 | |
| OXY6 | 0 | 6 | 6 | 0 | 1.5 | 1.2 | 2 | 2 |
| 4 | 6 | 6 | 0 | 1.7 | 1.0 (0.8) | 2 | 1.5 | |
| 12 | 4 | 4 | 0 | 2.0 | 1.0 | 2 | 2 | |
| 24 | 4 | 4 | 0 | 3.0 (2.0) | 1.3 (1.0) | 1 | 1 | |
| 36 | 4 | 4 | 0 | 0.3 | 1.0 (0.5) | 1 | 0.5 | |
| 55e | 4 | 2 | 2 | 0 | 0.5 (0.3) | —f | 0.5 | |
| CON-2 | 0 | 6 | 6 | 0 | 0.8 | 1.0 | 2 | 2 |
| 4 | 6 | 6 | 0 | 1.2 | 1.0 | 2 | 2 | |
| 12 | 6 | 6 | 0 | 1.5 | 1.0 | 2 | 2 | |
| 24 | 6 | 6 | 0 | 1.5 | 1.0 (0.3) | 2 | 0 | |
| 36 | 6 | 5 | 1 | 1.5 | 0.8 | 2 | 2 | |
| 52 | 5 | 5 | 0 | 1.8 | 1.0 | 2 | 2 | |
| OXY3-2 | 0 | 6 | 6 | 0 | 1.7 | 1.0 | 2 | 2 |
| 4 | 6 | 6 | 0 | 0.3 | 1.2 (1.0) | 1.5 | 1 | |
| 12 | 6 | 6 | 0 | 0.3 | 1.0 | 1.5 | 1 | |
| 24 | 6 | 6 | 0 | 0.5 | 1.0 (0.7) | 2 | 1 | |
| 36 | 6 | 6 | 0 | 0.7 | 1.0 (0.8) | 2 | 1 | |
| 52e | 6 | 6 | 0 | 0.3 | 1.0 (0.5) | 1 | 0.5 | |
| RIF3 | 0 | 6 | 6 | 0 | 1.2 | 1.0 (0.8) | 2 | 2 |
| 4 | 6 | 6 | 0 | 2.0 | 1.0 | 1 | 1 | |
| 12 | 6 | 6 | 0 | 1.3 | 1.0 | 2 | 2 | |
| 24 | 6 | 6 | 0 | 0.5 | 1.0 (0.8) | 2 | 1 | |
| 36 | 6 | 6 | 0 | 0.3 | 1.0 (0.5) | 2 | 0.5 | |
| 52 | 6 | 6 | 0 | 0.8 (0.5) | 1.0 (0.8) | 1 | 1 | |
| COM | 0 | 6 | 6 | 0 | 0.8 | 1.0 | 2 | 1.5 |
| 4 | 6 | 6 | 0 | 1.3 | 1.0 | 2 | 2 | |
| 12 | 6 | 6 | 0 | 0.5 | 1.0 | 2 | 2 | |
| 24 | 6 | 6 | 0 | 1.3 | 1.0 (0.8) | 2 | 1 | |
| 36 | 6 | 5 | 1 | 1.0 | 0.8 | 1.5 | 2 | |
| 52 | 6 | 6 | 0 | 0.3 | 1.0 (0.7) | 1.5 | 2 | |
CON-1, no treatment; PIR, oxytetracycline once monthly for 6 months; OXY3-1 and OXY3-2, oxytetracycline twice weekly for 3 weeks; OXY6, oxytetracycline twice weekly for 6 weeks; CON-2, DMSO solvent only; RIF3, rifampin twice weekly for 3 weeks; COM; rifampin and oxytetracycline twice weekly for 3 weeks.
Worms were considered viable where the motility score was ≥1. Note that O. ochengi nodules normally contain a single female worm and a variable number of males (0 to >10).
Motility was scored on a 3-point scale after incubation of worms for 30 min at 37°C, as follows: 0, no movement; 1, sluggish movement; and 2, highly active.
“Degenerated” refers to worm remnants only or fragmented nodules from skin biopsy specimens (in which fragmentation was too advanced to allow sexing and motility scoring).
The total number of viable worms recovered at the end of the experiment was significantly reduced compared with the number in control animals (P < 0.05).
Data were missing due to the absence of male worms in all nodules.
Two nodules per animal were removed under local anesthesia in a randomized sequence at six predetermined time points, transferred to the laboratory in phosphate-buffered saline (PBS), and trimmed of excess tissue. One nodule was fixed by injection with 10% (vol/vol) neutral buffered formalin, while the diameter of the other was measured with calipers prior to parasitological analysis. Three superficial skin biopsy specimens per animal were also obtained from the ventral underside at each time point and transported to the laboratory in PBS for the determination of Mf density.
Ethical considerations.
This study was approved by the Ethics Committee of the Regional Centre of Wakwa, IRAD, and authorized by the Regional Programmes Committee of IRAD. All procedures performed on cattle in Cameroon were equivalent to those previously authorized by a Home Office Project License (Animals [Scientific Procedures] Act, 1986) for experimental infections of cattle in the United Kingdom and classified under the severity limit “mild.”
Parasitology.
Nodules were dissected in PBS, the male and female worms were separated, and their motility was scored on a 3-point scale after incubation at 37°C for 30 min as detailed previously (32). The Mf in skin biopsy specimens were quantified and normalized to densities per 100 mg skin as described previously (32).
Immunohistochemistry and semiquantification of Wolbachia.
For experiment 2, the formalin-fixed nodules were embedded in paraffin and 4-μm sections were probed with an anti-Wolbachia surface protein polyclonal antibody (kindly provided by M. Casiraghi, University of Milan, Italy) using the universal LSAB2 horseradish peroxidase kit (Dako United Kingdom Ltd., Ely, United Kingdom) as detailed previously (36). The sections were counterstained with Giemsa stain, and digital images were obtained on a Microphot-FX microscope (Nikon, Tokyo, Japan) using a DCM900 microscope camera with imaging software (ScopeTek, Hangzhou, China). Wolbachia staining of worm sections across the entirety of each slide was semiquantified on a 3-point scale, as follows: 0, no visible bacteria; 1, sparse bacteria (<50% of worm sections were positive); and 2, profuse bacteria (≥50% of worm sections were positive).
Quantification of RIF in bovine plasma and skin.
Four zebu cows that were extraneous to experiments 1 and 2 and did not receive other drugs were treated with a single dose of RIF at 10 mg/kg of body weight in DMSO at 220 mg/kg. A plasma sample and triplicate superficial skin biopsy specimens (mean weight, ∼140 mg) were obtained from each animal at 0, 1, 8, 24, 48, and 72 h after treatment and were stored at −80°C. The skin biopsy specimens were thawed, combined for each animal, and macerated (protected from light) in 2 ml PBS using a T10 basic Ultra-Turrax disperser (IKA, Staufen, Germany) with an 8-mm dispersing element. To comply with the stipulations of a United Kingdom Department of Environment, Food, and Rural Affairs import license, both the skin homogenates and the plasma samples were heat inactivated at 56°C for 30 min prior to transportation to the United Kingdom on dry ice. RIF was quantified in the samples using a previously published liquid chromatographic-tandem mass spectrometric method with a lower limit of quantification of 25 ng/ml (37). Calibration standards containing known amounts of RIF (Sigma-Aldrich) were prepared separately in drug-free bovine plasma and skin homogenates obtained from United Kingdom abattoir material.
Statistical analyses.
Fully quantitative time course data were analyzed by Friedman's two-way analysis of variance (ANOVA) by ranks, followed by all-pairwise comparisons (with P adjusted for multiple testing) if the significance of the omnibus test was <0.05. Only animals with complete data sets at all six time points were included in the Friedman analyses. Where within-group pairwise analyses were significant over time, medians between the treatment group and the respective control group at the equivalent time points were compared by the Mann-Whitney U test with exact significance. Fisher's exact test was used to analyze both the frequencies of viable and dead adult worms (both sexes combined) at the end of each experiment and the distributions of endobacterial density scores at 0, 24, and 36 weeks posttreatment start (wpt) in experiment 2. All analyses were performed with IBM SPSS Statistics v. 20 (IBM Corporation, Armonk, NY, USA), and differences were considered to be statistically significant at a critical probability of <0.05.
RESULTS
Effects on nodule diameter and adult worm recovery.
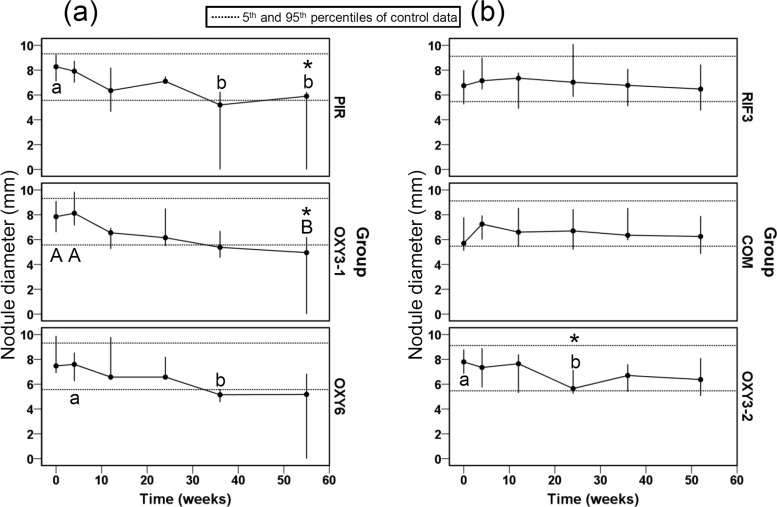
The objective of experiment 1 was to determine the simplest OXY regimen with partial adulticidal effects in order to facilitate the detection of modified efficacy when RIF was added in combination. Marked declines in nodule diameter were apparent in all three of the OXY-treated groups, although this became statistically significant at an earlier stage (36 wpt) in the PIR and OXY6 groups than in the OXY3-1 group (where 1 indicates experiment 1) (Fig. 1a). However, at the end of the experiment, only the PIR and OXY3-1 groups exhibited reduced median nodule diameters relative to those of the CON-1 group (Fig. 1a). Notably, one (OXY3-1, OXY6) or two (PIR) nodules per group had resolved completely at 55 wpt and were assigned a diameter of 0. For experiment 2, effects on nodule diameter were less pronounced than in the first experiment, with only the OXY3-2 group displaying a significant reduction in nodule diameter at 24 wpt compared with intragroup baseline data and CON-2 data at the equivalent time point (Fig. 1b). In addition, this reduction was not maintained at a statistically significant level at 36 and 52 wpt (Fig. 1b).
FIG 1.
Nodule diameters (medians and ranges) in Onchocerca ochengi-infected cattle treated with oxytetracycline delivered once monthly for 6 months (PIR; n = 5), twice weekly for 3 weeks (OXY3-1; n = 6), or twice weekly for 6 weeks (OXY6; n = 4) (a) and with rifampin alone twice weekly for 3 weeks (RIF3; n = 6), rifampin and oxytetracycline twice weekly for 3 weeks (COM; n = 6), or oxytetracycline alone twice weekly for 3 weeks (OXY3-2; n = 6) (b). Within groups, medians at time points annotated with different letters are significantly different at a P of <0.05 (lowercase letters) or a P of <0.01 (uppercase letters) by Friedman's two-way ANOVA by ranks. Between groups, medians at time points annotated with an asterisk are significantly different from medians for the control group (P < 0.05 by the Mann-Whitney U test).
The marked adulticidal effect of the PIR treatment was confirmed by analysis of adult worm recovery and viability at the end of experiment 1 (Table 2). This regimen induced a 75% reduction in the final yield of viable adult female worms relative to that of the CON-1 group, and no adult male worms were recovered; the combined effect on both sexes was statistically significant (P = 0.023). For the shorter OXY regimens, the reduction in the recovery of female worms was 63% at 55 wpt, and no male worms were obtained from OXY6 nodules at this time point (Table 2). However, the decrease in total adult worm recovery was not statistically significant for the OXY3-1 and OXY6 groups, although a strong trend was apparent for the latter (P = 0.052). In accordance with the nodule diameter data, significant adulticidal effects were not apparent in experiment 2 for the RIF3 and COM groups (Table 2). In contrast, the OXY3-2 regimen achieved reductions in loads of viable female and male worms of 50% and 83%, respectively, which was statistically significant in aggregate (P = 0.036).
Effects on microfilarial density.
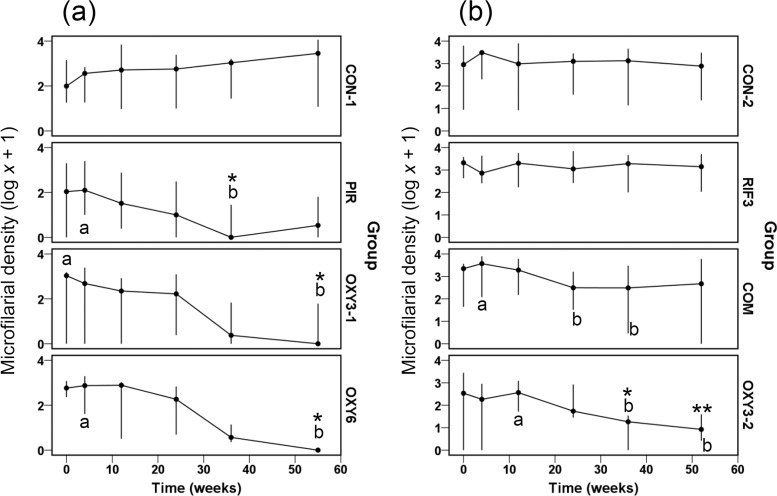
In experiment 1, statistically significant declines in Mf density of 2 to 3 logs were observed with all three OXY regimens by 36 to 55 wpt, although complete elimination of Mf was achieved only in the OXY6 group (Fig. 2a). The reductions in Mf load were also statistically significant compared with loads in the absence of treatment (CON-1) for the PIR group at 36 wpt and for the OXY3-1 and OXY6 regimens at 55 wpt (Fig. 2a). The lack of adulticidal effects exhibited by the RIF3 and COM groups in experiment 2 were also reflected by a limited impact on Mf, although a transient but statistically significant reduction in Mf density of ∼1 log was apparent in the COM group at 24 and 36 wpt (Fig. 2b). Conversely, the efficacy of the OXY3-2 regimen against adult worms was mirrored by an ∼2-log within-group decline in Mf density at 36 and 52 wpt; moreover, this was statistically significant relative to Mf densities in the CON-2 group at these time points (Fig. 2b).
FIG 2.
Microfilarial densities per 100 mg skin (medians and ranges, plotted on a log x + 1-transformed scale) in Onchocerca ochengi-infected cattle treated with oxytetracycline delivered once monthly for 6 months (PIR; n = 5), twice weekly for 3 weeks (OXY3-1; n = 6), or twice weekly for 6 weeks (OXY6; n = 4) (a) and with rifampin alone twice weekly for 3 weeks (RIF3; n = 6), rifampin and oxytetracycline twice weekly for 3 weeks (COM; n = 6), or oxytetracycline alone twice weekly for 3 weeks (OXY3-2; n = 6) (b). (a) Control animals (CON-1; n = 5) received no treatment; (b) control animals (CON-2; n = 5) received DMSO solvent at a dose equivalent to that of the RIF3 and COM groups. Within groups, medians at time points annotated with different letters are significantly different at a P of < 0.05 by Friedman's two-way ANOVA by ranks. Between groups, medians at time points annotated with asterisks are significantly different from those of the control group (P < 0.05 [*] or P < 0.01 [**] by the Mann-Whitney U test).
Effects on Wolbachia density in adult worms.
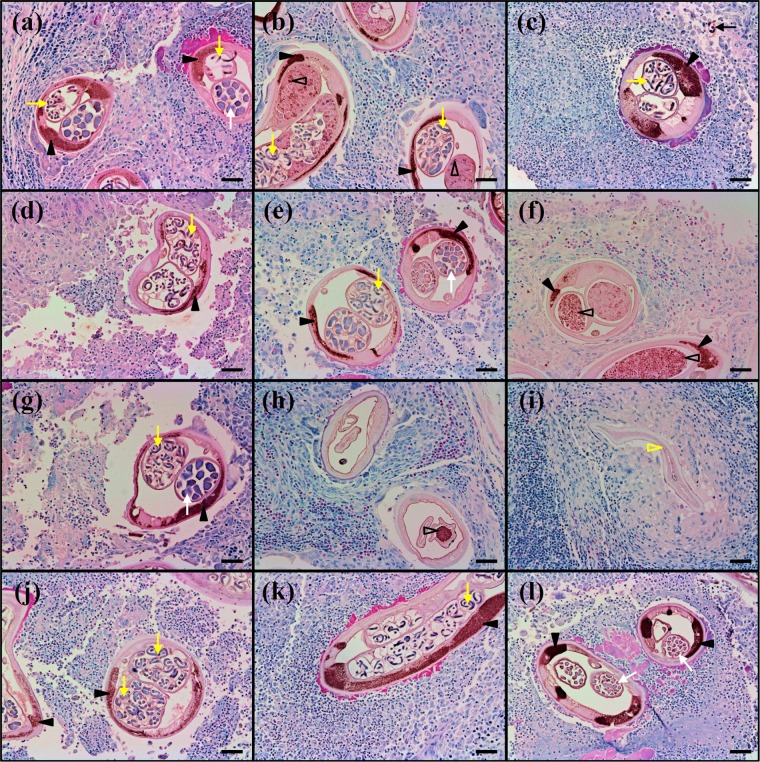
To determine whether the poor efficacy of the RIF3 and COM regimens in experiment 2 was associated with failure to clear Wolbachia populations within adult worms, we stained histological sections of bovine nodules from 0, 24, and 36 wpt with an anti-Wolbachia surface protein antibody. In O. ochengi and in filarial nematodes of medical importance, including O. volvulus, the distribution of Wolbachia conforms to a highly predictable pattern, with high densities of endobacteria in the somatic hypodermal cords of both adult males and females, whereas only females contain symbionts in their gonads and, consequently, in developing embryos. Thus, sections from CON-2 nodules showed intense, punctate staining of the bacteria in the hypodermal cords of the worms and finer stippling of the symbionts in the ovaries and intrauterine embryonic stages (Fig. 3a to c). In the RIF3 and COM groups, extensive Wolbachia populations remained clearly visible across the time course in both the hypodermal cords and the female reproductive tracts (Fig. 3d to f and j to l, respectively); however, oocytes and morulae tended to exhibit an abnormal, granular morphology at 36 wpt (Fig. 3f and l). The OXY3-2 regimen displayed the most pronounced effects, with complete loss of Wolbachia staining from the hypodermal cords in most sections and a marked reduction in uterine contents at 24 wpt, although endobacteria remained detectable occasionally in degenerated oocytes (Fig. 3 h). Importantly, by 36 wpt, almost-complete resolution of fragments from dead worms could be observed (Fig. 3i).
FIG 3.
Localization of Wolbachia endobacteria in nodule sections from Onchocerca ochengi-infected cattle treated with DMSO solvent only (a, b, c), rifampin alone twice weekly for 3 weeks (d, e, f), oxytetracycline alone twice weekly for 3 weeks (g, h, i), or rifampin and oxytetracycline twice weekly for 3 weeks (j, k, l) at 0 (a, d, g, j), 24 (b, e, h, k), and 36 (c, f, i, l) weeks posttreatment start. Yellow arrows, intrauterine microfilariae; filled black arrowheads, Wolbachia organisms in the hypodermal cords; white arrows, morulae; open black arrowheads, Wolbachia organisms in the female reproductive tract; black arrow, migrating microfilaria; open yellow arrowhead, partially resorbed worm section. Sections were probed with an anti-Wolbachia surface protein polyclonal antibody, which was visualized using 3,3′-diaminobenzidine precipitate, and counterstained with Giemsa stain. Bars, 50 μm.
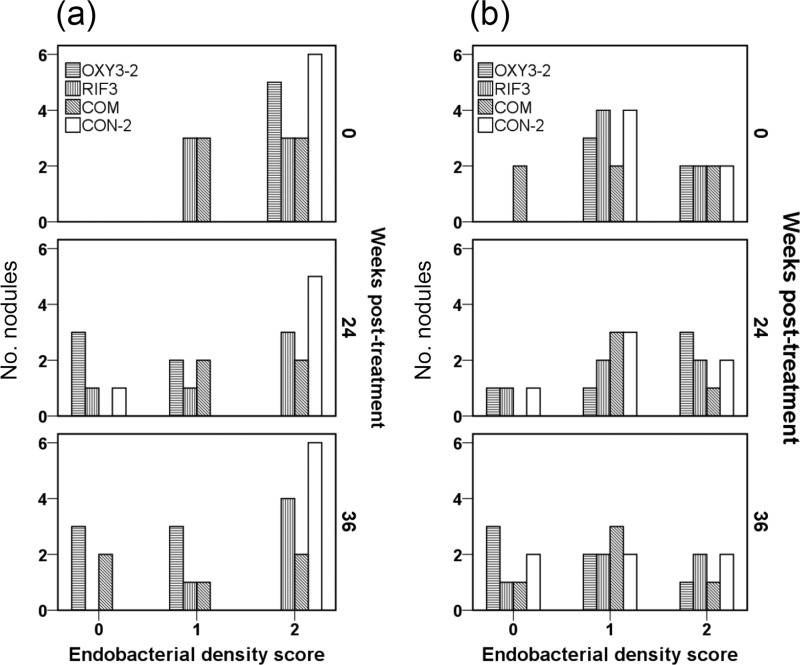
These immunohistochemical observations of Wolbachia density were verified using a semiquantitative scoring system, which revealed a statistically significant reduction in numbers of nodules containing ≥50% endobacterium-positive hypodermal cords within worm sections in the OXY3-2 group at both 24 (P = 0.015) and 36 (P = 0.002) wpt (Fig. 4a). This was not the case with either the RIF3 or the COM regimen, although a strong but nonsignificant (P = 0.061) pattern of reduced endobacterial density was observed at 36 wpt for the latter (Fig. 4a). In contrast, no statistically significant changes in Wolbachia staining within the female worm reproductive tract were apparent throughout experiment 2 (Fig. 4b).
FIG 4.
Endobacterial density scores in Onchocerca ochengi hypodermal cords (a) or O. ochengi female reproductive tracts (b) in nodular histological sections from infected cattle treated with DMSO solvent only (CON-2), rifampin alone twice weekly for 3 weeks (RIF3), rifampin and oxytetracycline twice weekly for 3 weeks (COM), or oxytetracycline alone twice weekly for 3 weeks (OXY3-2) at 0, 24, and 36 weeks posttreatment start. Wolbachia staining in worm sections across the entirety of each slide was semiquantified on a 3-point scale, as follows: 0, no visible bacteria; 1, sparse bacteria (<50% of worm sections were positive); and 2, profuse bacterial (≥50% of worm sections were positive). Note that data were not available for two OXY3-2 nodules, three COM nodules, and two RIF3 nodules due to complete resorption of the worms (n = 5) or misidentification of nodules caused by Demodex bovis (n = 2).
RIF concentrations in bovine plasma and skin.
To establish if the poor efficacy of the RIF3 regimen could be explained by the pharmacokinetics of this drug in cattle, we measured RIF concentrations in the plasma and skin of four animals treated with a regimen identical to that administered to the RIF3 group. The mean maximum concentration of RIF in plasma (Cmax) was ∼28 μg/ml and was maintained above the MIC for Wolbachia (24, 25) for at least 24 h (Fig. 5a) but was no longer detectable by 48 h. Similarly, RIF concentrations in skin, although approximately 10-fold lower than those achieved in plasma, remained above the MIC for ≥24 h (Fig. 5b) but were below the limit of quantification by 48 h.
FIG 5.
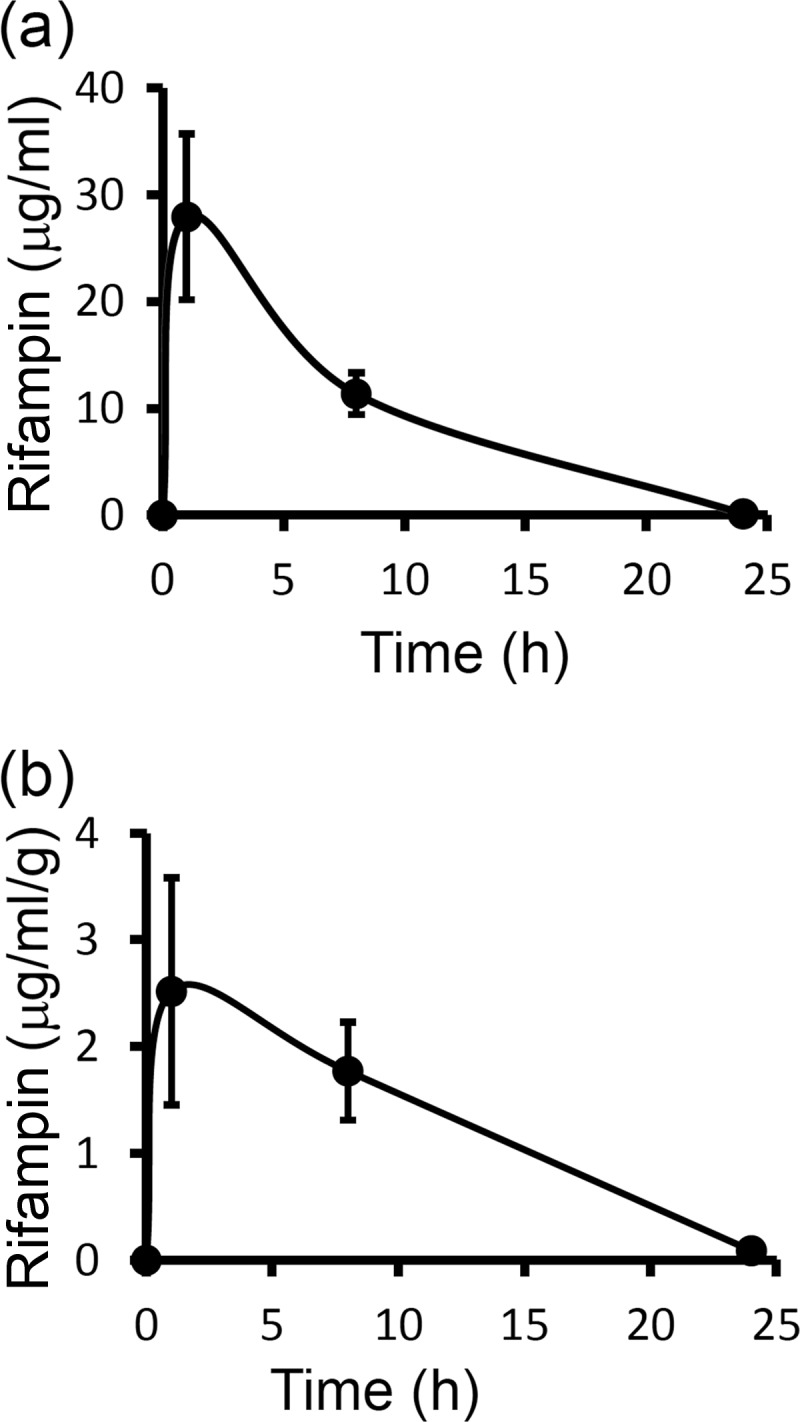
Rifampin concentration-time profiles (means ± standard deviations; n = 4) in bovine plasma (a) and bovine skin (b) following a single intravenous injection at 10 mg/kg.
DISCUSSION
The greatest barrier to the implementation of antibiotic chemotherapy for onchocerciasis has been the duration of the regimen required for permanent sterilization of female worms or potent adulticidal effects (23). Although DOX is clearly more efficacious than ivermectin, the latter can suppress Mf densities (and thus, disease manifestations) for up to 1 year following a single dose of 150 μg/kg, which makes it highly amenable to MDA programs (4). In a clinical trial in a area of Cameroon where loiasis is endemic, community-wide distribution of daily DOX therapy for 6 weeks was accompanied by very high rates of compliance (22), but to date, this has not resulted in wider application of DOX by APOC.
A lack of contraindication in children, coupled with RIF's bactericidal mode of action, has led it to be mooted as a more favorable alternative to DOX in this context. However, RIF alone has not proved to be strongly adulticidal in human trials, even if administered daily for 4 weeks (30). In the current study, we reasoned that a combination of a tetracycline and RIF might be more efficacious than either drug alone, as inhibition of both translation (by the former) and RNA synthesis (by the latter) is expected to induce synergistic effects. In addition, an earlier in vivo trial in the rodent filarial model Litomosoides sigmodontis revealed enhanced efficacy (prophylaxis, growth retardation, and embryostasis) for DOX and RIF duotherapy for 14 days compared with the efficacy of DOX monotherapy for 21 days (27). Moreover, an ∼50% adulticidal effect of 14-day duotherapy was observed in a human clinical trial against Wuchereria bancrofti (38), a pathogen responsible for lymphatic filariasis which also harbors Wolbachia. Surprisingly, not only did our data fail to support the superiority of duotherapy, they also consistently reflected an impaired efficacy of the combination relative to that of OXY alone. Thus, in experiment 2, only OXY monotherapy attained significant reductions in Mf loads, Wolbachia densities in the hypodermal cords, and the recovery of viable adult worms at the end of the trial.
Unfortunately, no commercial formulation of RIF licensed for administration to ruminants is available worldwide. For welfare reasons, we were unable to deliver RIF on a daily schedule due to the large volumes of intravenous solution required, and accordingly, drug concentrations fell below the MIC for Wolbachia (0.0625 mg/liter [24, 25]) between 24 and 48 h. As RIF levels are reduced by 20 to 25% by heat inactivation of plasma (37), the concentrations determined in our bovine samples may have been underestimated; nevertheless, it is likely that our regimen achieved a therapeutic dose in skin for only two periods slightly in excess of 24 h each week. Although this drug is generally considered to have good penetrance and residual activity in tissue, we are not aware of other studies that have quantified RIF in skin. However, in a case of human RIF overdose, discoloration of skin resolved after 24 h, while urine and tears remained red-orange for several days (39). Possibly, the subcutaneous location of human nodules renders O. volvulus more susceptible to RIF than is O. ochengi, especially as the drug is photosensitive (40). Conversely, the long-acting formulation of OXY is known to achieve therapeutic levels for 3 to 5 days following a single intramuscular injection (41); hence, a twice-weekly schedule is effectively a continuous regimen. While these considerations provide a superficial explanation for the inefficacy of RIF monotherapy relative to OXY monotherapy, intermittent regimens of OXY are generally superior to continuous administration in this system, as observed in the current study with PIR and in several previous trials (18, 36, 42). Moreover, the relatively rapid clearance of RIF in cattle does not explain why RIF-OXY duotherapy is apparently less efficacious than OXY alone.
In monogastric species, daily therapy with RIF is generally well tolerated, and in vivo trials against L. sigmodontis and Brugia pahangi in rodents used doses of 50 mg/kg (27) and up to 100 mg/kg (28), respectively. However, even clinical trials of “higher-dose” RIF for tuberculosis in humans have not exceeded regimens of 20 mg/kg daily (43), which raises questions regarding the interpretation of the rodent studies. Notably, 20 doses of RIF monotherapy at 100 mg/kg failed to achieve a significant adulticidal effect on B. pahangi transplanted into jirds (28), whereas the potent antifilarial properties of a 14-day combination regimen of RIF and DOX against L. sigmodontis in mice were manifested when chemotherapy was initiated simultaneously with infection using third-stage larvae (27). Thus, these effects against L. sigmodontis were not genuinely adulticidal. Combinations of RIF and DOX have rarely been used in human clinical trials for other conditions, although in the case of scrub typhus (caused by another member of the Rickettsiales, Orientia tsutsugamushi), duotherapy was inefficacious compared to either drug alone (44). The reasons for this failure were not determined, but in duotherapy for brucellosis, pharmacokinetic interference between DOX and RIF has been reported. Hence, plasma levels of DOX were reduced by 50% in brucellosis patients exhibiting metabolic induction of hepatic microsomal enzymes (45), while in a larger study, a statistically significant inverse correlation between DOX and RIF plasma concentrations was apparent, which resulted in two cases of therapeutic failure or relapse (46). Moreover, there was a trend for DOX levels to be lower in brucellosis patients also receiving RIF who had a “rapid acetylator” N-acetyltransferase-2 genotype (46). Although, to the best of our knowledge, no studies on liver function following RIF therapy have been performed on ruminants, a detrimental effect on OXY pharmacokinetics caused by metabolic induction is a plausible explanation for the poor efficacy of duotherapy in our study.
In conclusion, despite promising early indications in vitro and in small-animal studies in vivo, RIF (whether alone or in combination with a tetracycline) may not represent the breakthrough in adulticidal therapy for onchocerciasis that was hoped. In addition to having potentially unfavorable pharmacokinetic interactions with tetracyclines, RIF remains of critical importance in tuberculosis control in the countries where onchocerciasis is endemic (47), rendering RIF MDA for onchocerciasis extremely unlikely. In this context, the recent discovery that Wolbachia is highly susceptible to another RNA polymerase inhibitor, corallopyronin A, which lacks activity against mycobacteria, is timely (48). Moreover, the successful chemical synthesis of this natural product (49) provides an avenue for its clinical development and ultimate application to human filarial diseases (50).
ACKNOWLEDGMENTS
We are very grateful for the technical assistance of David Ekale and Henrietta Ngangyung at IRAD (Ngaoundéré, Cameroon) and of Catherine Hartley at the University of Liverpool. We also thank Shrikant Kulkarni and Mukul Jerath of Lupin Ltd. (Mumbai, India) for the kind donation of rifampin.
This study was supported by the European Commission (contracts INCO-CT-2006-032321 and HEALTH-F3-2010-242131).
We declare that we have no conflict of interest.
Footnotes
Published ahead of print 18 November 2013
REFERENCES
- 1.Anonymous. 2010. Onchocerciasis (river blindness), p 123–128 In Crompton DWT, Peters P. (ed), Working to overcome the global impact of neglected tropical diseases: first WHO report on neglected tropical diseases. World Health Organization, Geneva, Switzerland [Google Scholar]
- 2.Plaisier AP, Van Oortmarssen GJ, Remme J, Habbema JD. 1991. The reproductive lifespan of Onchocerca volvulus in West African savanna. Acta Trop. 48:271–284. 10.1016/0001-706X(91)90015-C [DOI] [PubMed] [Google Scholar]
- 3.Schulz-Key H. 1990. Observations on the reproductive biology of Onchocerca volvulus. Acta Leiden 59:27–44 [PubMed] [Google Scholar]
- 4.Crump A, Morel CM, Omura S. 2012. The onchocerciasis chronicle: from the beginning to the end? Trends Parasitol. 28:280–288. 10.1016/j.pt.2012.04.005 [DOI] [PubMed] [Google Scholar]
- 5.Tielsch JM, Beeche A. 2004. Impact of ivermectin on illness and disability associated with onchocerciasis. Trop. Med. Int. Health 9:A45–A56. 10.1111/j.1365-3156.2004.01213.x [DOI] [PubMed] [Google Scholar]
- 6.Traore MO, Sarr MD, Badji A, Bissan Y, Diawara L, Doumbia K, Goita SF, Konate L, Mounkoro K, Seck AF, Toe L, Toure S, Remme JH. 2012. Proof-of-principle of onchocerciasis elimination with ivermectin treatment in endemic foci in Africa: final results of a study in Mali and Senegal. PLoS Negl. Trop. Dis. 6:e1825. 10.1371/journal.pntd.0001825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gardon J, Gardon-Wendel N, Demanga N, Kamgno J, Chippaux JP, Boussinesq M. 1997. Serious reactions after mass treatment of onchocerciasis with ivermectin in an area endemic for Loa loa infection. Lancet 350:18–22. 10.1016/S0140-6736(96)11094-1 [DOI] [PubMed] [Google Scholar]
- 8.Katabarwa MN, Lakwo T, Habomugisha P, Agunyo S, Byamukama E, Oguttu D, Tukesiga E, Unoba D, Dramuke P, Onapa A, Tukahebwa EM, Lwamafa D, Walsh F, Unnasch TR. 2013. Transmission of Onchocerca volvulus continues in Nyagak-Bondo focus of northwestern Uganda after 18 years of a single dose of annual treatment with ivermectin. Am. J. Trop. Med. Hyg. 89:293–300. 10.4269/ajtmh.13-0037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katabarwa MN, Eyamba A, Nwane P, Enyong P, Kamgno J, Kuete T, Yaya S, Aboutou R, Mukenge L, Kafando C, Siaka C, Mkpouwoueiko S, Ngangue D, Biholong BD, Andze GO. 2013. Fifteen years of annual mass treatment of onchocerciasis with ivermectin have not interrupted transmission in the west region of Cameroon. J. Parasitol. Res. 2013:420928. 10.1155/2013/420928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Osei-Atweneboana MY, Eng Boakye JKDA, Gyapong JO, Prichard RK. 2007. Prevalence and intensity of Onchocerca volvulus infection and efficacy of ivermectin in endemic communities in Ghana: a two-phase epidemiological study. Lancet 369:2021–2029. 10.1016/S0140-6736(07)60942-8 [DOI] [PubMed] [Google Scholar]
- 11.Osei-Atweneboana MY, Awadzi K, Attah SK, Boakye DA, Gyapong JO, Prichard RK. 2011. Phenotypic evidence of emerging ivermectin resistance in Onchocerca volvulus. PLoS Negl. Trop. Dis. 5:e998. 10.1371/journal.pntd.0000998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brieger WR, Okeibunor JC, Abiose AO, Ndyomugyenyi R, Wanji S, Elhassan E, Amazigo UV. 2012. Characteristics of persons who complied with and failed to comply with annual ivermectin treatment. Trop. Med. Int. Health 17:920–930. 10.1111/j.1365-3156.2012.03007.x [DOI] [PubMed] [Google Scholar]
- 13.Awadzi K, Attah SK, Addy ET, Opoku NO, Quartey BT. 1999. The effects of high-dose ivermectin regimens on Onchocerca volvulus in onchocerciasis patients. Trans. R. Soc. Trop. Med. Hyg. 93:189–194. 10.1016/S0035-9203(99)90305-X [DOI] [PubMed] [Google Scholar]
- 14.Taylor MJ, Bilo K, Cross HF, Archer JP, Underwood AP. 1999. 16S rDNA phylogeny and ultrastructural characterization of Wolbachia intracellular bacteria of the filarial nematodes Brugia malayi, B. pahangi, and Wuchereria bancrofti. Exp. Parasitol. 91:356–361. 10.1006/expr.1998.4383 [DOI] [PubMed] [Google Scholar]
- 15.Henkle-Dührsen K, Eckelt VH, Wildenburg G, Blaxter M, Walter RD. 1998. Gene structure, activity and localization of a catalase from intracellular bacteria in Onchocerca volvulus. Mol. Biochem. Parasitol. 96:69–81. 10.1016/S0166-6851(98)00109-1 [DOI] [PubMed] [Google Scholar]
- 16.Hoerauf A, Nissen-Pähle K, Schmetz C, Henkle-Dührsen K, Blaxter ML, Büttner DW, Gallin MY, Al-Qaoud KM, Lucius R, Fleischer B. 1999. Tetracycline therapy targets intracellular bacteria in the filarial nematode Litomosoides sigmodontis and results in filarial infertility. J. Clin. Invest. 103:11–18. 10.1172/JCI4768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bandi C, McCall JW, Genchi C, Corona S, Venco L, Sacchi L. 1999. Effects of tetracycline on the filarial worms Brugia pahangi and Dirofilaria immitis and their bacterial endosymbionts Wolbachia. Int. J. Parasitol. 29:357–364. 10.1016/S0020-7519(98)00200-8 [DOI] [PubMed] [Google Scholar]
- 18.Langworthy NG, Renz A, Mackenstedt U, Henkle-Dührsen K, de Bronsvoort MB, Tanya VN, Donnelly MJ, Trees AJ. 2000. Macrofilaricidal activity of tetracycline against the filarial nematode Onchocerca ochengi: elimination of Wolbachia precedes worm death and suggests a dependent relationship. Proc. R. Soc. B Biol. Sci. 267:1063–1069. 10.1098/rspb.2000.1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brouqui P, Fournier PE, Raoult D. 2001. Doxycycline and eradication of microfilaremia in patients with loiasis. Emerg. Infect. Dis. 7:604–605. 10.3201/eid0707.017747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoerauf A, Specht S, Buttner M, Pfarr K, Mand S, Fimmers R, Marfo-Debrekyei Y, Konadu P, Debrah AY, Bandi C, Brattig N, Albers A, Larbi J, Batsa L, Taylor MJ, Adjei O, Buttner DW. 2008. Wolbachia endobacteria depletion by doxycycline as antifilarial therapy has macrofilaricidal activity in onchocerciasis: a randomized placebo-controlled study. Med. Microbiol. Immunol. 197:295–311. 10.1007/s00430-007-0062-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoerauf A, Specht S, Marfo-Debrekyei Y, Buttner M, Debrah AY, Mand S, Batsa L, Brattig N, Konadu P, Bandi C, Fimmers R, Adjei O, Buttner DW. 2009. Efficacy of 5-week doxycycline treatment on adult Onchocerca volvulus. Parasitol. Res. 104:437–447. 10.1007/s00436-008-1217-8 [DOI] [PubMed] [Google Scholar]
- 22.Wanji S, Tendongfor N, Nji T, Esum M, Che JN, Nkwescheu A, Alassa F, Kamnang G, Enyong PA, Taylor MJ, Hoerauf A, Taylor DW. 2009. Community-directed delivery of doxycycline for the treatment of onchocerciasis in areas of co-endemicity with loiasis in Cameroon. Parasit. Vectors 2:39. 10.1186/1756-3305-2-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taylor MJ, Hoerauf A, Townson S, Slatko BE, Ward SA. 18 July 2013. Anti-Wolbachia drug discovery and development: safe macrofilaricides for onchocerciasis and lymphatic filariasis. Parasitology 10.1017/S0031182013001108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hermans PG, Hart CA, Trees AJ. 2001. In vitro activity of antimicrobial agents against the endosymbiont Wolbachia pipientis. J. Antimicrob. Chemother. 47:659–663. 10.1093/jac/47.5.659 [DOI] [PubMed] [Google Scholar]
- 25.Fenollar F, Maurin M, Raoult D. 2003. Wolbachia pipientis growth kinetics and susceptibilities to 13 antibiotics determined by immunofluorescence staining and real-time PCR. Antimicrob. Agents Chemother. 47:1665–1671. 10.1128/AAC.47.5.1665-1671.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Townson S, Tagboto S, McGarry HF, Egerton GL, Taylor MJ. 2006. Onchocerca parasites and Wolbachia endosymbionts: evaluation of a spectrum of antibiotic types for activity against Onchocerca gutturosa in vitro. Filaria J. 5:4. 10.1186/1475-2883-5-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Volkmann L, Fischer K, Taylor M, Hoerauf A. 2003. Antibiotic therapy in murine filariasis (Litomosoides sigmodontis): comparative effects of doxycycline and rifampicin on Wolbachia and filarial viability. Trop. Med. Int. Health 8:392–401. 10.1046/j.1365-3156.2003.01040.x [DOI] [PubMed] [Google Scholar]
- 28.Townson S, Hutton D, Siemienska J, Hollick L, Scanlon T, Tagboto SK, Taylor MJ. 2000. Antibiotics and Wolbachia in filarial nematodes: antifilarial activity of rifampicin, oxytetracycline and chloramphenicol against Onchocerca gutturosa, Onchocerca lienalis and Brugia pahangi. Ann. Trop. Med. Parasitol. 94:801–816. 10.1080/00034980020027988 [DOI] [PubMed] [Google Scholar]
- 29.Richards FO, Jr, Amann J, Arana B, Punkosdy G, Klein R, Blanco C, Lopez B, Mendoza C, Dominguez A, Guarner J, Maguire JH, Eberhard M. 2007. No depletion of Wolbachia from Onchocerca volvulus after a short course of rifampin and/or azithromycin. Am. J. Trop. Med. Hyg. 77:878–882 [PubMed] [Google Scholar]
- 30.Specht S, Mand S, Marfo-Debrekyei Y, Debrah AY, Konadu P, Adjei O, Buttner DW, Hoerauf A. 2008. Efficacy of 2- and 4-week rifampicin treatment on the Wolbachia of Onchocerca volvulus. Parasitol. Res. 103:1303–1309. 10.1007/s00436-008-1133-y [DOI] [PubMed] [Google Scholar]
- 31.Morales-Hojas R, Cheke RA, Post RJ. 2006. Molecular systematics of five Onchocerca species (Nematoda: Filarioidea) including the human parasite, O. volvulus, suggest sympatric speciation. J. Helminthol. 80:281–290. 10.1079/JOH2006331 [DOI] [PubMed] [Google Scholar]
- 32.Renz A, Trees AJ, Achukwi D, Edwards G, Wahl G. 1995. Evaluation of suramin, ivermectin and CGP 20376 in a new macrofilaricidal drug screen, Onchocerca ochengi in African cattle. Trop. Med. Parasitol. 46:31–37 [PubMed] [Google Scholar]
- 33.Bronsvoort BM, Renz A, Tchakouté V, Tanya VN, Ekale DD, Trees AJ. 2005. Repeated high doses of avermectins cause prolonged sterilisation, but do not kill Onchocerca ochengi adult worms in African cattle. Filaria J. 4:8. 10.1186/1475-2883-4-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trees AJ, Wahl G, Klager S, Renz A. 1992. Age-related differences in parasitosis may indicate acquired immunity against microfilariae in cattle naturally infected with Onchocerca ochengi. Parasitology 104:247–252. 10.1017/S0031182000061680 [DOI] [PubMed] [Google Scholar]
- 35.Hansen RD, Trees AJ, Bah GS, Hetzel U, Martin C, Bain O, Tanya VN, Makepeace BL. 2011. A worm's best friend: recruitment of neutrophils by Wolbachia confounds eosinophil degranulation against the filarial nematode Onchocerca ochengi. Proc. R. Soc. B Biol. Sci. 278:2293–2302. 10.1098/rspb.2010.2367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gilbert J, Nfon CK, Makepeace BL, Njongmeta LM, Hastings IM, Pfarr KM, Renz A, Tanya VN, Trees AJ. 2005. Antibiotic chemotherapy of onchocerciasis: in a bovine model, killing of adult parasites requires a sustained depletion of endosymbiotic bacteria (Wolbachia species). J. Infect. Dis. 192:1483–1493. 10.1086/462426 [DOI] [PubMed] [Google Scholar]
- 37.Srivastava A, Waterhouse D, Ardrey A, Ward SA. 2012. Quantification of rifampicin in human plasma and cerebrospinal fluid by a highly sensitive and rapid liquid chromatographic-tandem mass spectrometric method. J. Pharm. Biomed. Anal. 70:523–528. 10.1016/j.jpba.2012.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Debrah AY, Mand S, Marfo-Debrekyei Y, Batsa L, Albers A, Specht S, Klarmann U, Pfarr K, Adjei O, Hoerauf A. 2011. Macrofilaricidal activity in Wuchereria bancrofti after 2 weeks treatment with a combination of rifampicin plus doxycycline. J. Parasitol. Res. 2011:201617. 10.1155/2011/201617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gross DJ, Dellinger RP. 1988. Red/orange person syndrome. Cutis 42:175–177 [PubMed] [Google Scholar]
- 40.Ali J, Ali N, Sultana Y, Baboota S, Faiyaz S. 2007. Development and validation of a stability-indicating HPTLC method for analysis of antitubercular drugs. Acta Chromatogr. 18:168–179 [Google Scholar]
- 41.Davey LA, Ferber MT, Kaye B. 1985. Comparison of the serum pharmacokinetics of a long acting and a conventional oxytetracycline injection. Vet. Rec. 117:426–429. 10.1136/vr.117.17.426 [DOI] [PubMed] [Google Scholar]
- 42.Nfon CK, Makepeace BL, Njongmeta LM, Tanya VN, Trees AJ. 2007. Lack of resistance after re-exposure of cattle cured of Onchocerca ochengi infection with oxytetracycline. Am. J. Trop. Med. Hyg. 76:67–72 [PubMed] [Google Scholar]
- 43.Diacon AH, Patientia RF, Venter A, van Helden PD, Smith PJ, McIlleron H, Maritz JS, Donald PR. 2007. Early bactericidal activity of high-dose rifampin in patients with pulmonary tuberculosis evidenced by positive sputum smears. Antimicrob. Agents Chemother. 51:2994–2996. 10.1128/AAC.01474-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Watt G, Kantipong P, Jongsakul K, Watcharapichat P, Phulsuksombati D, Strickman D. 2000. Doxycycline and rifampicin for mild scrub-typhus infections in northern Thailand: a randomised trial. Lancet 356:1057–1061. 10.1016/S0140-6736(00)02728-8 [DOI] [PubMed] [Google Scholar]
- 45.Garraffo A, Dellamonica P, Fournier JP, Lapalus P, Bernard E. 1988. The effect of rifampicin on the pharmacokinetics of doxycycline. Infection 16:297–298. 10.1007/BF01645076 [DOI] [PubMed] [Google Scholar]
- 46.Colmenero JD, Fernandez-Gallardo LC, Agundez JA, Sedeno J, Benitez J, Valverde E. 1994. Possible implications of doxycycline-rifampin interaction for treatment of brucellosis. Antimicrob. Agents Chemother. 38:2798–2802. 10.1128/AAC.38.12.2798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aristoff PA, Garcia GA, Kirchhoff PD, Hollis Showalter HD. 2010. Rifamycins—obstacles and opportunities. Tuberculosis (Edinb.) 90:94–118. 10.1016/j.tube.2010.02.001 [DOI] [PubMed] [Google Scholar]
- 48.Schiefer A, Schmitz A, Schaberle TF, Specht S, Lammer C, Johnston KL, Vassylyev DG, Konig GM, Hoerauf A, Pfarr K. 2012. Corallopyronin A specifically targets and depletes essential obligate Wolbachia endobacteria from filarial nematodes in vivo. J. Infect. Dis. 206:249–257. 10.1093/infdis/jis341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rentsch A, Kalesse M. 2012. The total synthesis of corallopyronin A and myxopyronin B. Angew. Chem. Int. Ed. Engl. 51:11381–11384. 10.1002/anie.201206560 [DOI] [PubMed] [Google Scholar]
- 50.Schaberle TF, Schiefer A, Schmitz A, Konig GM, Hoerauf A, Pfarr K. 2013. Corallopyronin A—a promising antibiotic for treatment of filariasis. Int. J. Med. Microbiol. 10.1016/j.ijmm.2013.08.010 [DOI] [PubMed] [Google Scholar]