Abstract
Increased susceptibility to genital herpes in medroxyprogesterone-treated mice may provide a surrogate of increased HIV risk and a preclinical biomarker of topical preexposure prophylaxis safety. We evaluated tenofovir disoproxil fumarate (TDF) in this murine model because an intravaginal ring eluting this drug is being advanced into clinical trials. To avoid the complications of surgically inserting a ring, hydroxyethylcellulose (HEC)-stable formulations of TDF were prepared. One week of twice-daily 0.3% TDF gel was well tolerated and did not result in any increase in HSV-2 susceptibility but protected mice from herpes simplex virus 2 (HSV-2) disease compared to mice treated with the HEC placebo gel. No significant increase in inflammatory cytokines or chemokines in vaginal washes or change in cytokine, chemokine, or mitochondrial gene expression in RNA extracted from genital tract tissue was detected. To further evaluate efficacy, mice were treated with gel once daily beginning 12 h prior to high-dose HSV-2 challenge or 2 h before and after viral challenge (BAT24 dosing). The 0.3% TDF gel provided significant protection compared to the HEC gel following either daily (in 9/10 versus 1/10 mice, P < 0.01) or BAT24 (in 14/20 versus 4/20 mice, P < 0.01) dosing. In contrast, 1% tenofovir (TFV) gel protected only 4/10 mice treated with either regimen. Significant protection was also observed with daily 0.03% TDF compared to HEC. Protection was associated with greater murine cellular permeability of radiolabeled TDF than of TFV. Together, these findings suggest that TDF is safe, may provide substantially greater protection against HSV than TFV, and support the further clinical development of a TDF ring.
INTRODUCTION
The majority of new human immunodeficiency virus (HIV) infections are transmitted through heterosexual contact, with young women bearing a disproportionate burden. However, efforts to prevent transmission in this population have met with limited success. Oral preexposure prophylaxis (PrEP) was effective in serodiscordant partners (where one partner is seropositive for HIV-1 infection and one is seronegative) (1) but not in other female populations, presumably reflecting difficulties with adherence (2, 3). Adherence has also been a problem in vaginal gel PrEP trials. The CAPRISA 004 trial, a randomized, placebo-controlled trial evaluating 1% tenofovir (TFV) vaginal gel for the prevention of HIV when it was applied before and after sexual intercourse (but no more than two doses in a 24-h period; referred to as BAT24 dosing), was the first topical PrEP study to demonstrate significant efficacy (4). TFV gel reduced HIV acquisition by an estimated 39% overall and by 54% in women with high gel adherence. Although the CAPRISA 004 trial was not designed to evaluate whether TFV gel protected against herpes simplex virus 2 (HSV-2), a 51% reduction in HSV-2 incidence was observed in the tenofovir arm (5). However, a subsequent study with daily TFV gel (Microbicide Trials Network study MTN 003) failed to show protection, a result that was most likely linked to low adherence, as evidenced by analysis of plasma drug levels (3).
The development of delivery systems that overcome some of the barriers to adherence, such as intravaginal rings (IVRs), is a priority for HIV prevention (6). To address this need, we recently developed an IVR formulation of tenofovir disoproxil fumarate (TDF) (7). We selected TDF, a prodrug of tenofovir (TFV), because it is more potent than TFV in vitro, reflecting greater and more rapid cellular permeability, which results in increased intracellular accumulation of the active metabolite, TFV-diphosphate (TFV-DP) (8). TDF inhibits HIV-1 and HSV-2 infection in cell and tissue culture models at ∼100-fold lower concentrations than TFV, suggesting that it may be an excellent candidate for prevention (9). Moreover, we recently demonstrated that the TDF IVR provided 100% protection against 16 weekly intravaginal challenges with simian-human immunodeficiency virus (SHIV) in macaques (7). TDF showed no in vitro toxicity in cell and tissue culture and, unlike other microbicides, had little effect on the epithelial barrier in a polarized dual-chamber cell culture model (9, 10).
Topical delivery of drugs has resulted in unanticipated toxicities, which have been linked to an increased risk of HIV acquisition in large clinical trials. For example, nonoxynol-9, which has been safely used for decades as a contraceptive, was found to increase the risk of HIV, possibly reflecting disruption of the epithelial barrier and induction of an inflammatory response (11–15). Similarly, 6% cellulose sulfate gel was also associated with a trend toward increased HIV risk, which resulted in early termination of the clinical trial (16). We subsequently found that mice were significantly more susceptible to low doses of HSV-2 administered 12 h after 7-daily intravaginal gel applications of 6% cellulose sulfate or various formulations of nonoxynol-9 than mice that received a hydroxyethylcellulose (HEC) placebo gel (17, 18). The increased susceptibility was associated with modest increases in inflammatory mediators in the genital tract and disruption of the epithelial barrier. In contrast, 1% TFV gel, 0.5% PRO 2000, and 0.1% griffithsin gel did not increase the risk of HSV infection in this model (15, 17, 19). These findings suggest that this relatively inexpensive small-animal model may provide a biomarker of microbicide safety.
Thus, the current studies were designed to test topically delivered TDF in the murine safety model. To avoid the difficulties in designing a device that could provide an allometrically scaled dose of TDF from our IVR technology (7) and the epithelial damage caused by surgically attaching a scaled version of a TDF IVR into the mice, we formulated different concentrations of TDF gel in HEC and stored the products at −80°C until immediately prior to vaginal application. Storage at −80°C prevents TDF from being hydrolyzed to TFV. In order to provide a stringent model of safety, mice were dosed with 0.3% TDF gel twice daily, a dose which is designed to deliver to the mouse vagina 10 times the estimated maximum daily dose released from the TDF IVR in humans, scaled to relative surface areas.
MATERIALS AND METHODS
Vaginal gels.
TDF was formulated in the HEC placebo gel (20). The TDF gel contained 0.3, 0.03, or 0.003 weight percent (wt%) TDF (Gilead, Foster City CA), 2.7 wt% Natrosol 250 HX Pharm HEC (Ashland Covington, KY), 0.85 wt% NaCl USP, and 0.1 wt% sorbic acid NF (Spectrum Chemicals, New Brunswick, NJ). The TDF concentration in the gel and TDF chemical stability were confirmed by extraction and high-performance liquid chromatography (HPLC) analysis (9). Conrad (Arlington, VA) provided 1% TFV and HEC placebo gels.
Murine model.
The studies were performed according to Albert Einstein College of Medicine IACUC-approved protocols. Female BALB/c mice (6 to 8 weeks old) were pretreated subcutaneously with 2.5 mg of medroxyprogesterone (MPA) (Sicor Pharmaceuticals, Irvine, CA) 5 days before gel application. To assess whether the gel increased the susceptibility to HSV-2, 30 μl of 0.3% TDF or HEC was delivered intravaginally twice daily for 7 days. Twelve hours after the seventh application, groups of 5 mice were inoculated with 103 and 104 PFU/mouse of the clinical isolate HSV-2 (4674) delivered in a volume of 30 μl. These doses typically induce lethal disease in 30% and 90% (LD30 and LD90, respectively) of mice (18). Mice were evaluated daily for evidence of erythema, edema, genital ulcers, hair loss around the perineum, and hind-limb paralysis and were euthanized if symptoms of severe ulceration, hair loss, or hind-limb paralysis developed (21).
Additional studies were conducted in the absence of viral challenge to further assess the mucosal response. Medroxyprogesterone-treated mice were treated twice daily with 30 μl of the 0.3% TDF or placebo gel or were untreated (no gel) for up to 7 days. Vaginal washes using normal saline (150 μl) were collected for detection of cytokines and chemokines on days 4 and 8. In addition, 6 untreated mice, 7 TFV-treated mice, and 8 TDF-treated mice were euthanized on days 4 and 8, and genital tracts, which included tissue from the external opening to the bifurcation of the uterus, were excised and processed for extraction of RNA for quantitative real-time reverse transcription-PCR (RT-qPCR). For determination of relative mitochondrial DNA copy numbers, five additional mice treated twice daily with 0.3% TDF or HEC gel were sacrificed on day 8, and the genital tract tissues were processed for DNA extraction.
To evaluate efficacy, MPA-treated mice received either a single 30-μl dose of 0.3% TDF, 1% TFV, or HEC gel 1 h before and 1 h after (BAT24) intravaginal inoculation with an LD90 (105 PFU/mouse) of virus or 30 μl of TDF (0.3%, 0.03%, or 0.003%), 1% TFV, or HEC intravaginally daily, with the first dose being administered 12 h prior to infection.
Cytokine and chemokine analysis.
Protease inhibitors (complete protease inhibitor cocktail; Roche Applied Science, Indianapolis, IN) were added to each vaginal wash sample before centrifugation at 210 × g for 10 min at 4°C. The supernatants were stored at −80°C, and samples pooled from two mice were assayed for interleukin-6 (IL-6), gamma interferon (IFN-γ), IL-1β, monocyte chemotactic protein 1 (MCP-1), macrophage inflammatory protein 1β (MIP-1β), MIP-2, RANTES, and tumor necrosis factor alpha (TNF-α), using a Milliplex MAP mouse cytokine/chemokine immunoassay (Millipore, Danvers, MA) and a Luminex 100 system and analyzed with StarStation (version 2.0; Applied Cytometry Systems).
RT-PCR.
Genital tract tissue was homogenized, and total RNA was extracted using an Absolutely RNA Miniprep Kit (Stratagene). Reverse transcription was performed using 400 ng of RNA and a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems). Quantitative real-time PCR was conducted in duplicate with 25 ng of cDNA and with 0.5 μl of 20× 6-carboxyfluorescein (FAM)-labeled probes in a 10-μl final reaction volume of ABsolute Blue qPCR master mix (Thermo Scientific). Probes were purchased from Applied Biosystems. PCR cycling conditions on an ABI Prism 7700 instrument as follows: 1 cycle at 50°C for 2 min, 1 cycle at 95°C for 15 min, 45 cycles of 95°C for 15 s, and 1 cycle at 60°C for 1 min. Relative expression levels were calculated using the comparative threshold cycle (CT) method (2−ΔΔCT)), where RNA from TDF- or HEC-treated mouse tissue was compared with RNA from untreated mouse tissue, and the CT values of both groups were normalized to β-actin RNA levels as an endogenous housekeeping gene.
Determination of relative mitochondrial DNA (mtDNA) copy numbers was assessed as previously described (22). Total DNA from genital tract tissue was extracted with a DNeasy Blood and Tissue Kit (Qiagen), and quantitative PCR was performed under conditions as described above. Reactions were performed with 5 ng of DNA per reaction in triplicate with probes for β-actin or ATP6, both purchased from Applied Biosystems. Relative mtDNA copy numbers were calculated with the ΔCT for ATP6 and β-actin, and the formula 2(2ΔCT). Results for HEC-treated mice were arbitrarily assigned the value of 1.
H&E staining.
Genital tract tissue was harvested after 3 or 7 days of twice-daily drug exposure, embedded in 22-oxacalcitriol (OCT), flash frozen in liquid nitrogen, and stored at −80°C. Samples were sectioned using a cryostat and stained with hematoxylin-eosin (H&E). Stained sections were evaluated in a blinded fashion for potential inflammatory cell infiltration or disruption of tissue.
Measurement of TDF and TFV intracellular accumulation.
The radiolabeled analogs [3H]TDF (1.3 Ci/mmol) and [3H]TFV (17.1 Ci/mmol) were purchased from Moravek Biochemicals (Brea, CA). 3T3 cells (murine fibroblasts obtained from ATCC) were seeded at 4.0 × 105 cells/well in 24-well plates and cultured for 24 h. Confluent cell monolayers were exposed to serum-free Dulbecco's modified Eagle's medium (DMEM) containing 0.1 μM and 1 μM [3H]TDF or [3H]TFV for 1 h at 37°C. To remove extracellular drug, the cells were then washed three times with ice-cold phosphate-buffered saline (PBS) and solubilized in 0.5 ml/well of 1% Triton X-100 for 30 min. Intracellular radioactivity in a 0.3-ml aliquot from each sample was measured after addition of 3.5 ml of Optiphase Supermix Cocktail (PerkinElmer, Waltham, MA) in a Tri-Carb 2900RT liquid scintillation analyzer. The protein content of each sample was determined using a Micro BCA Protein Assay Kit with bovine serum albumin (BSA) as a standard (Pierce Biotechnology, Rockford, IL). The intracellular drug concentrations were calculated and expressed as picomoles per milligram of total protein after the difference in specific activity between the two drugs was taken into account.
Statistical analysis.
GraphPad Prism (version 6; GraphPad Software) was used for statistical analysis. Concentrations of cytokines/chemokines and gene expression levels were compared using one-way analysis of variance (ANOVA) with Tukey's test for multiple comparisons. Kaplan-Meier survival curves were assessed by a log rank test. A P value of <0.05 was considered to be significant.
RESULTS
Repeat exposure to TDF gel does not increase susceptibility to HSV-2 infection.
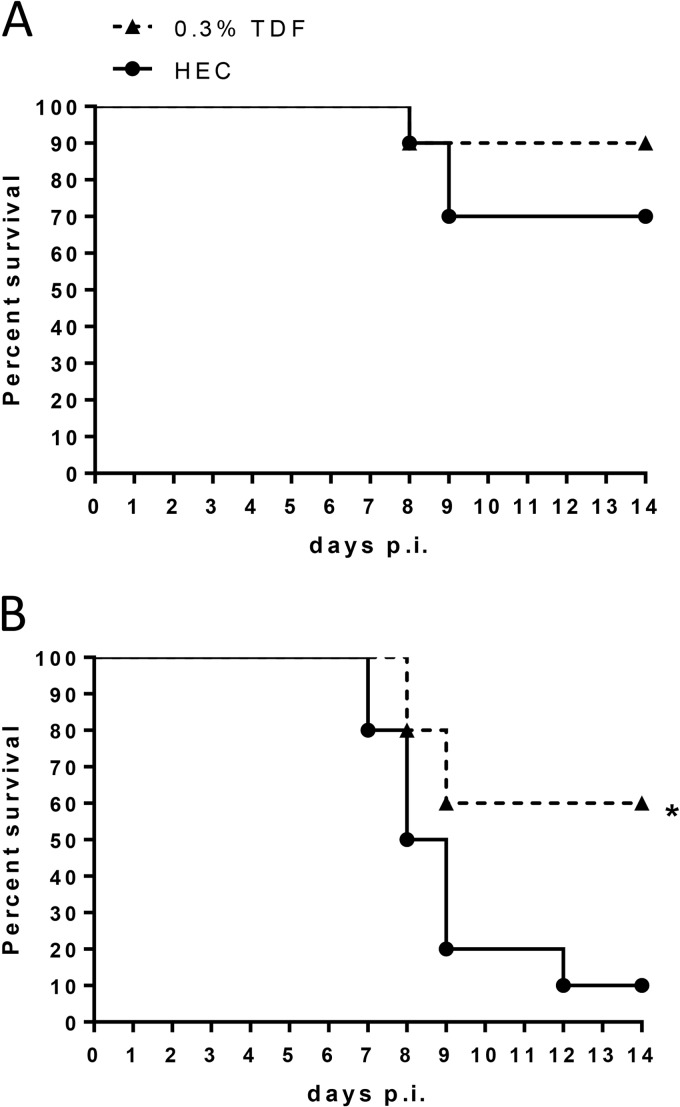
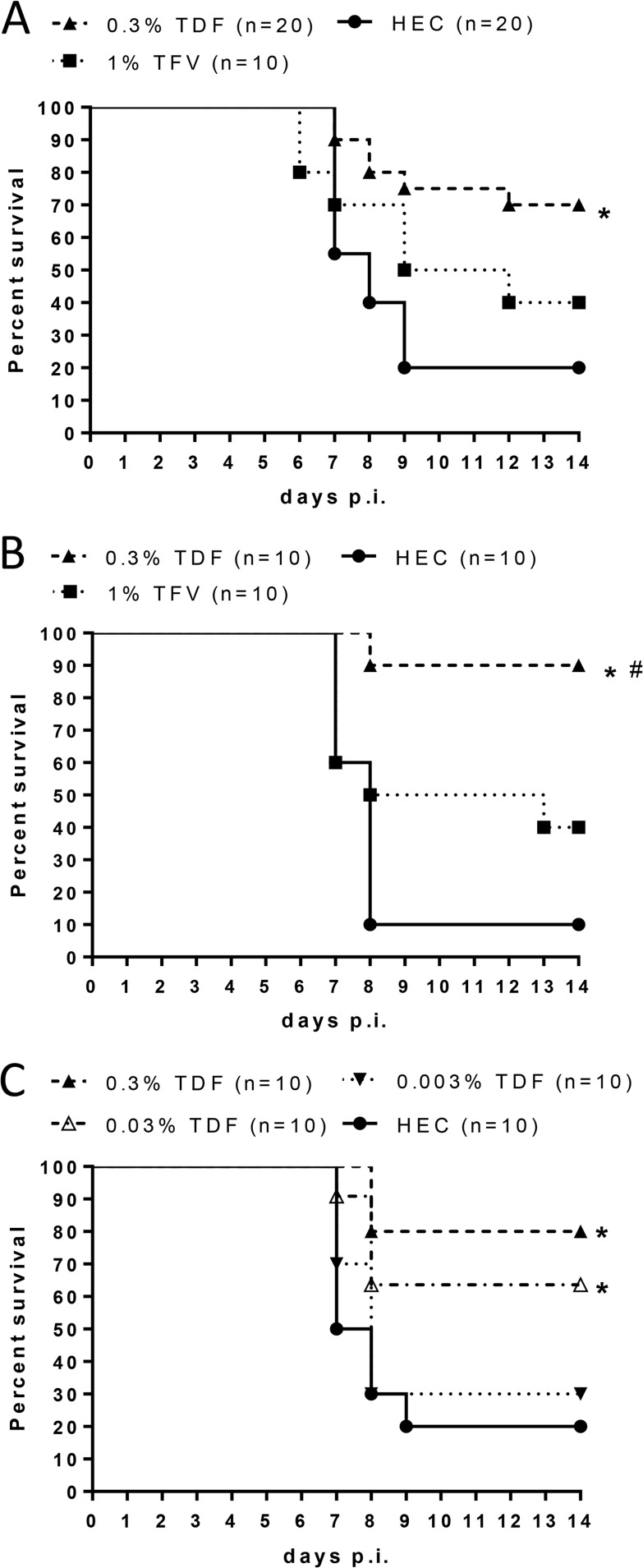
Mice administered a 0.3% TDF or placebo gel twice daily for 7 days were subsequently challenged with an LD30 or LD90 of HSV-2 intravaginally 12 h after the last gel dose to test for potential toxicities that manifest as increased susceptibility to HSV-2 infection. No increase in susceptibility was observed, but, rather, mice pretreated with 0.3% TDF gel were protected from genital herpes, and this protection reached statistical significance in response to an LD90 of HSV-2 (Fig. 1), suggesting that high enough levels of drug persist in epithelial cells to inhibit HSV-2 replication.
FIG 1.
Repeat exposure to TDF gel does not increase susceptibility to HSV-2 vaginal infection. Medroxyprogesterone-pretreated mice received twice-daily dosing of 30 μl of TDF or HEC gel for 7 days. On day 8, mice were challenged intravaginally with an LD30 (A) or LD90 (B) of virus. Mice were scored daily for disease and were euthanized for severe disease scores (n = 10 mice per group). The asterisk indicates significance compared to HEC treatment using a log rank test (P < 0.05).
No inflammatory response was detected in response to TDF in mice.
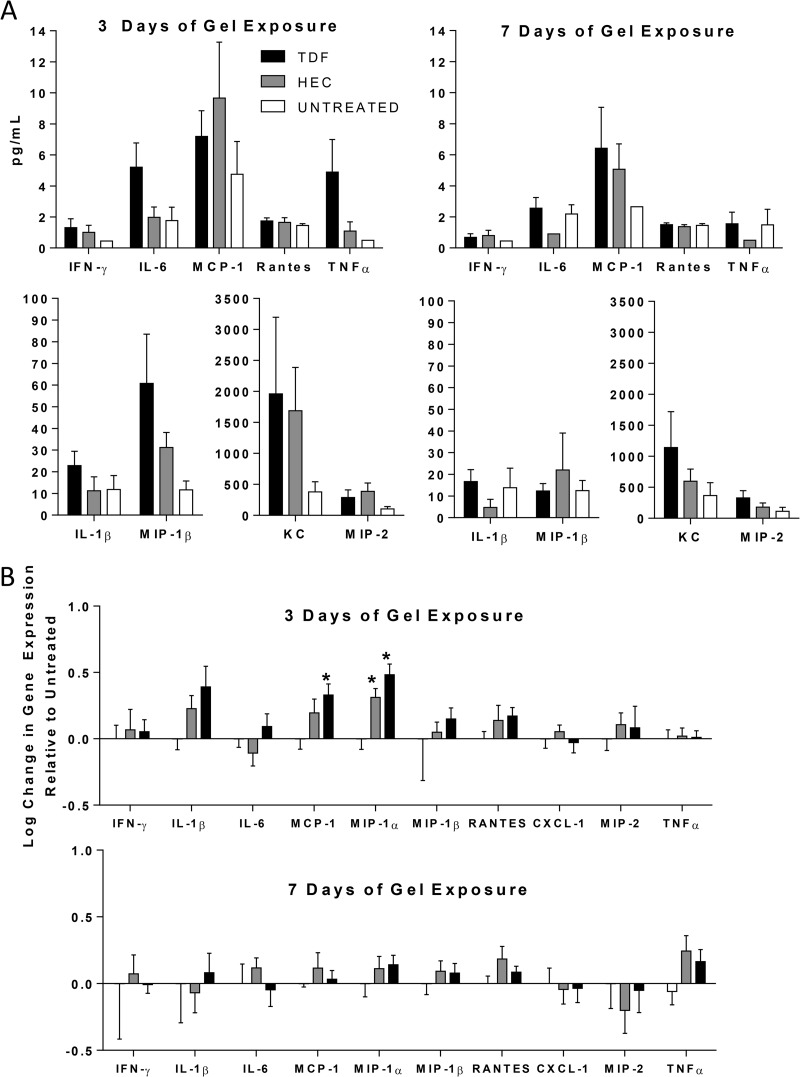
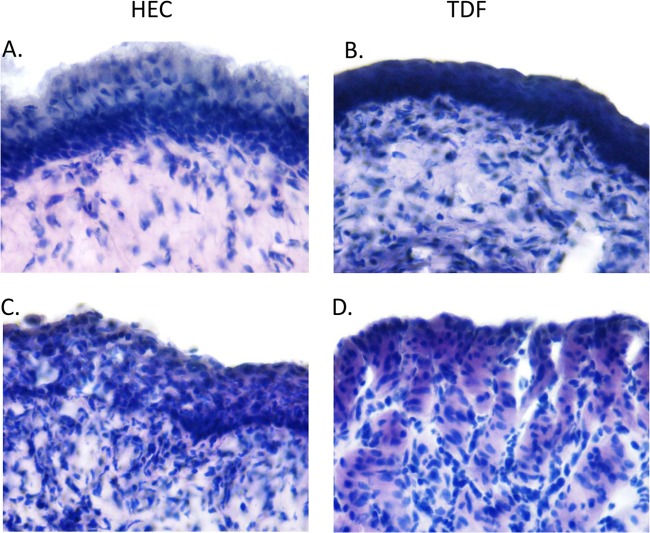
Concentrations of cytokines and chemokines were measured in vaginal washes obtained from mice treated with the 0.3% TDF or placebo gel twice daily for 3 or 7 days and compared to those of untreated mice from the appropriate day post-medroxyprogesterone treatment. There were no statistically significant differences in the concentrations of cytokines or chemokines recovered using one-way ANOVA (Fig. 2A). These findings were supported by RT-qPCR studies with the same panel of cytokines and chemokines using RNA extracted from genital tract tissue at the same time points (Fig. 2B). Relative to untreated animals, TDF and HEC placebo gels induced a small increase in MCP-1 and MIP-1α RNA levels on day 3 but not day 7, suggesting that gel application itself and/or the application procedure may induce a transient inflammatory response (Fig. 2B). In addition, genital tract tissue samples were stained and evaluated in a blinded fashion for inflammatory cell infiltration and histological evidence of disruption. There were no differences in the H&E staining between groups, and only one HEC-treated mouse (day 7) and one 0.3% TDF-treated mouse (day 3) exhibited focal signs of inflammation and/or disruption (Fig. 3 and Table 1).
FIG 2.
TDF does not trigger an inflammatory cytokine or chemokine response in the female genital tract. Mice pretreated with medroxyprogesterone received 30 μl of gel intravaginally twice daily for three or seven consecutive days. (A) Protein levels were measured in vaginal washes by a Luminex assay. Washes were grouped into pools of 2 mice, and five to eight pools per treatment arm were evaluated. The data are presented as means plus standard errors of the means. (B) RNA was extracted from genital tract tissue, reverse transcribed, and analyzed by qPCR for the indicated cytokine or chemokine gene. Data are presented as means plus standard errors of the means from 6 to 8 mice per group. Asterisks indicate significance compared to untreated mice using one-way ANOVA, with Tukey's test for multiple comparisons (P < 0.05). There were no statistically significant differences between HEC- and TDF-treated groups.
FIG 3.
H&E staining of genital tract tissue following TDF or HEC gel treatment. H&E-stained genital tract tissue sections were evaluated in a blinded fashion for inflammation and focal changes in architecture. Panels A and B show representative 60× images of sections obtained from HEC- or TDF-treated mouse tissue scored as showing no histological abnormalities. Panels C and D show sections from the only mice that showed histological changes: one mouse was treated with HEC gel for 7 days (C), and one was treated with TDF for 3 days (D). All other samples were histologically similar to those shown in panels A and B.
TABLE 1.
H&E staining of genital tract tissue obtained from mice treated twice daily with no gel, HEC placebo gel, or 0.3% TDF gel
| Treatment (n)a | No. of days treatedb | No. of mice with genital tract inflammation or disruption/no. of mice evaluated |
|---|---|---|
| None (2) | 3 | 0/2 |
| 7 | 0/2 | |
| HEC (3) | 3 | 0/3 |
| 7 | 1/3 | |
| 0.3% TDF (6) | 3 | 1/6 |
| 7 | 0/6 |
n, number of mice.
For untreated mice, the appropriate day post-medroxyprogesterone treatment was used.
Structural protein gene expression and mitochondrial DNA copy numbers are not impacted by repeated TDF gel exposures.
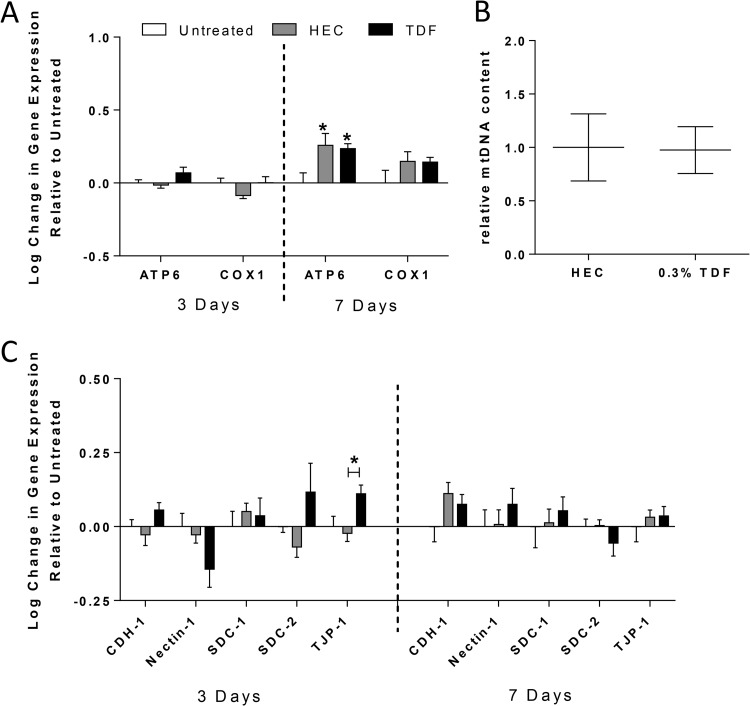
To further investigate potential toxicity of TDF, expression of mitochondrial and structural proteins was examined in the genital tract tissue. We focused on mitochondrial proteins because some of the adverse effects attributed to systemic therapy with reverse transcriptase inhibitors have been attributed to mitochondrial toxicity and junctional proteins because some of the toxicity associated with other topical PrEP drugs has been attributed to disruption of the epithelial barrier (10, 17). We also focused on the heparan sulfate proteoglycans, syndecans, because they serve as attachment receptors for HSV (23) and may also bind HIV gp120, capturing virus and subsequently transferring the virus to susceptible immune target cells (24, 25). There was no significant decrease in the expression of ATP6, a key component of the mitochondrial membrane ATP synthase, or COX1, which encodes the cytochrome c oxidase subunit involved in mitochondrial oxidative phosphorylation (Fig. 4A). Rather, a small but statistically significant increase in ATP6 was observed in response to both the TDF and HEC gels. We further assessed the potential mitochondrial toxicity of TDF by evaluating relative mtDNA copy numbers. No difference in mtDNA copy numbers in the TDF-treated mice compared to placebo was observed after 7 days of TDF treatment (Fig. 4B). Moreover, repeated exposure to the TDF gel had no impact on expression of syndecans or the junctional proteins cadherin-1 and nectin-1 (Fig. 4C). However, a statistically significant increase in tight junction protein 1 (TJP-1) expression was observed in TDF-treated compared to HEC-treated mice; this was detected only on day 3. The significance of this transient increase is unclear.
FIG 4.
TDF gel does not induce significant changes in mitochondrial RNA or DNA or in expression of structural proteins. RNA was extracted from genital tract tissue harvested 3 or 7 days after twice-daily gel dosing, reverse transcribed to cDNA, and analyzed using qPCR for expression of mitochondrial proteins (A) and structural proteins (C). Data are presented as means plus standard errors of the means relative to untreated mice, with 6 to 8 mice per group. In addition, the relative ratio of mitochondrial to nuclear DNA was analyzed in HEC- and TDF-treated mice (B). Asterisks indicate significance from untreated mice or between the TDF and HEC treatment groups where indicated (TJP-1, day 3), using one-way ANOVA with Tukey's test for multiple comparisons (P < 0.05).
TDF provides greater protection against HSV-2 than the TFV gel.
Repeated vaginal application of 0.3% TDF (Fig. 1) or, as previously shown, 1% TFV gel (17) did not increase the susceptibility to HSV-2 in mice, indicating the safety of the treatment, but the results differed in that only TDF provided partial protection against HSV-2 genital tract disease in this low-dose challenge model. To further explore this observation, additional experiments were conducted in which mice were treated either with a single dose of gel 1 h before and 1 h after infection to simulate BAT24 dosing (Fig. 5A) or daily beginning 12 h before infection and then challenged with an LD90 of HSV-2 (Fig. 5B). The 0.3% TDF gel but not the TFV gel provided significant protection against HSV-2 disease compared to HEC-treated mice with either dosing regimen.
FIG 5.
TDF provides greater protection than TFV gel against genital herpes. Medroxyprogesterone-pretreated female BALB/c mice received 30 μl intravaginally of the indicated concentration of the TDF gel, 1% TFV gel, or HEC gel at 1 h before and after challenge with an LD90 of HSV-2 (105 PFU/mouse) (A) or daily with the first dose applied 12 h prior to viral challenge (B and C). Mice were scored daily for disease and were euthanized when scores reached 4. Asterisks indicate significance compared to HEC-treated animals; the number sign (#) indicates significance compared to TFV gel-treated mice (P < 0.05, using a log rank test).
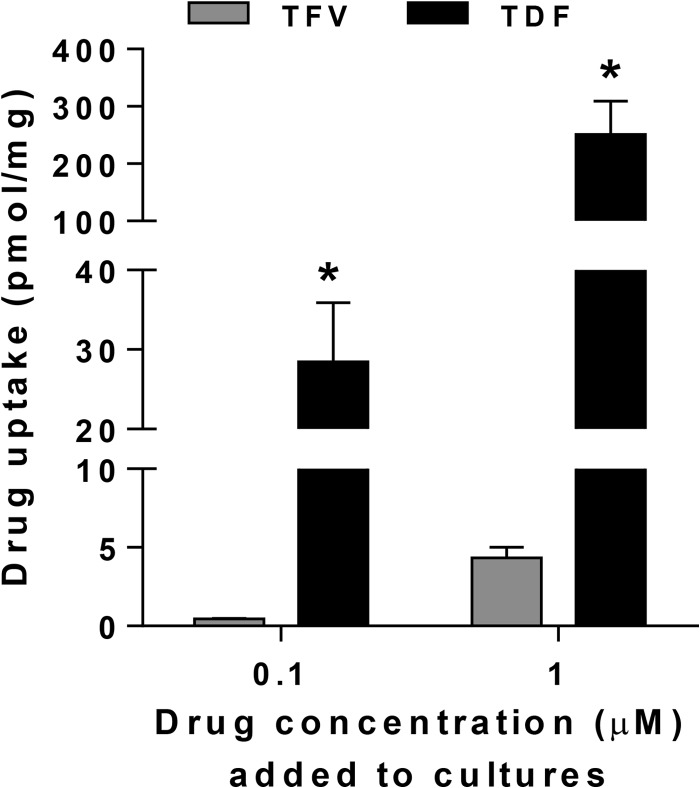
To further assess the potential for TDF to protect against HSV-2 infection, we tested additional concentrations of TDF gel in a daily dosing model. Both 0.3% and 0.03% gels provided significant protection against HSV disease (Fig. 5C). The increased protection observed with these low doses of TDF compared to the 1% TFV gel likely reflects increased cellular uptake. This is supported by the detection of increased intracellular levels of radiolabeled TDF compared to TFV in murine fibroblasts (3T3 cells) following a 1-h exposure (Fig. 6), which is consistent with studies in human peripheral blood mononuclear cells (PBMCs) (8).
FIG 6.
Increased uptake of radiolabeled TDF compared to that of TFV by murine cells. 3T3 cells (murine fibroblasts) were exposed to 0.1 or 1 μM [3H]TDF or [3H] TFV for 1 h at 37°C, washed three times to remove extracellular drug, and then solubilized, and intracellular radioactivity was measured. The protein content of each sample was determined in parallel, and results are presented as means plus standard deviations from two independent experiments conducted in duplicate. The asterisks indicate significant differences between TDF and TFV uptake by a t test (P < 0.05).
DISCUSSION
Development and validation of preclinical assays of topical PrEP safety that are predictive of what happens in clinical trials are a major research priority that has proven to be a formidable task. From a toxicology perspective, the rabbit vaginal irritation (RVI) model is used because the abdominal vaginal epithelium of the rabbit is columnar, and through multiple applications, the test product remains in contact with the tissue for an extended time, thereby increasing the potential to detect mucosal responses. However, experiences with nonoxynol-9, C31G, and cellulose sulfate, all of which were deemed nonirritating in subacute RVI exposure experiments but were associated with at least a trend toward increased risk of HIV acquisition in clinical studies, indicate that the RVI model is not sufficiently predictive of trial outcomes.
Subsequent studies suggest that murine models of genital herpes susceptibility might provide additional data on HIV risk by probing more subtle changes in the epithelia and the mucosal barrier. While HSV and HIV target different cell types (epithelial and immune, respectively) and engage different receptors and coreceptors, disruption of mucosal barriers likely facilitates infection by either pathogen. Disruption may make it easier for HIV to reach the submucosa, where greater numbers of activated T cells reside (26), and may promote access of HSV to nectin-1, an adherens junction protein that serves as the primary HSV coreceptor (17, 27).
While there is no standardized HSV murine susceptibility model and while investigators have used different strains of mice, hormonal treatments, viruses, and endpoints, the findings from multiple studies have yielded consistent results and provide a basis for understanding how different products failed to protect against HIV despite potent activity in vitro (12, 15, 17, 18, 28). In the murine studies conducted here, mice were pretreated with medroxyprogesterone, which may provide a more rigorous model by making the mucosa more susceptible to viral invasion. During the progesterone-dominant diestrous phase, the murine cervical epithelium is thinner and is composed of only a few layers of cuboidal nucleated cells, with cell junctions near the surface, as detected by electron microscopy, that render the mice more susceptible to any disruptive effects of topically administered drugs (29). In addition, mice are more susceptible to genital herpes during diestrous, in part because the structural changes include increased access to nectin-1 (30).
Building on in vitro preclinical safety studies demonstrating that drug released from a TDF IVR (or TDF itself) was not cytotoxic and had no impact on the ability of cells to form and maintain tight junctions, as measured by transepithelial electrical resistance in a polarized culture system (9), we tested the safety of a 0.3% TDF gel in a low-dose HSV susceptibility model in medroxyprogesterone-treated mice. We selected a gel formulation to avoid the complications of surgically suturing a small IVR-like device into the mice. The twice-daily dosing for this safety study was designed to deliver to the mouse vagina 10 times the estimated maximum daily dose released from the TDF IVR in humans, scaled to relative surface areas.
Twice-daily dosing of the gel was well tolerated and did not result in any increase in susceptibility to HSV-2 but, rather, protected the mice from HSV-2 disease compared dosing with a placebo gel. Moreover, there were no signs of irritation or erythema by naked-eye examination or histology and no significant increase in inflammatory cytokines or chemokines in vaginal washes or in RNA extracted from excised genital tract tissue. There was also no decrease in the expression of structural proteins (although a transient increase in TJP-1 expression of unclear biological significance was observed) and no decrease in the expression of mitochondrial genes or in mtDNA copy numbers in genital tract tissue. A small increase in ATP6 expression was observed in response to both the HEC and TDF gels. This observation contrasts with data obtained from a recent phase 1 clinical study of the rectal safety of a 1% TFV gel (31). Using a microarray approach, the investigators found that 505 genes were downregulated in response to seven doses of a 1% TFV gel, including ATP6 and other genes associated with mitochondrial function (F. Hladik, L. Flemming, J, McElrath, and I. McGowan, presented at the Microbicide Trials Network Annual Meeting, Bethesda, MD, 10 to 13 February 2013). In the same study nonoxynol-9 had no effect on ATP6. Whether the downregulation of mitochondrial genes was in response to the drug or the formulation, the clinical significance of the observed changes requires further study. Similar microarray studies have not been conducted with vaginal or cervical biopsy specimens. The differences observed in the phase 1 rectal microarray study and the murine model might reflect differences in species (mouse versus human), anatomic compartment (rectal versus vaginal), or formulation (the 1% tenofovir gel formulation is relatively hyperosmolar) (32).
In the murine safety studies, TDF gel was applied twice daily for 7 days prior to HSV exposure, but no doses were administered after HSV-2 challenge. To further explore the potential for TDF to protect against HSV-2, additional studies were conducted with once-daily dosing to simulate what might happen in the setting of a TDF IVR. Daily dosing of both 0.03% and 0.3% TDF gels significantly protected mice from a lethal challenge with HSV-2 and was more effective than the 1% TFV gel. These findings, combined with the protection against HIV observed in a nonhuman primate study (7), suggest that the TDF IVR may provide protection against both HIV and HSV and may be more efficacious than TFV gel because the prodrug has greater cell permeability (Fig. 6) and because the IVR formulation may overcome some of the obstacles to adherence that have been observed with gel studies. Both TDF and TFV are converted intracellularly to tenofovir diphosphate and block HIV and HSV replication by competing with intracellular pools of deoxynucleotides for viral DNA replication (33).
The results of these murine safety and efficacy studies, combined with other preclinical safety studies (9), clinical experience with oral formulations of TDF, protection against SHIV in macaque studies (7), and the potential for greater adherence with a ring support the advancement of a TDF IVR into the clinic.
ACKNOWLEDGMENTS
We thank Conrad for providing hydroxyethylcellulose and tenofovir gels and Gilead Sciences for providing tenofovir disoproxil fumarate.
This work was supported by NIH grants AI076980, A1065309, and AI03461.
Footnotes
Published ahead of print 9 December 2013
REFERENCES
- 1.Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, Tappero JW, Bukusi EA, Cohen CR, Katabira E, Ronald A, Tumwesigye E, Were E, Fife KH, Kiarie J, Farquhar C, John-Stewart G, Kakia A, Odoyo J, Mucunguzi A, Nakku-Joloba E, Twesigye R, Ngure K, Apaka C, Tamooh H, Gabona F, Mujugira A, Panteleeff D, Thomas KK, Kidoguchi L, Krows M, Revall J, Morrison S, Haugen H, Emmanuel-Ogier M, Ondrejcek L, Coombs RW, Frenkel L, Hendrix C, Bumpus NN, Bangsberg D, Haberer JE, Stevens WS, Lingappa JR, Celum C, Partners PrEP Study Team 2012. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N. Engl. J. Med. 367:399–410. 10.1056/NEJMoa1108524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Damme L, Corneli A, Ahmed K, Agot K, Lombaard J, Kapiga S, Malahleha M, Owino F, Manongi R, Onyango J, Temu L, Monedi MC, Mak'Oketch P, Makanda M, Reblin I, Makatu SE, Saylor L, Kiernan H, Kirkendale S, Wong C, Grant R, Kashuba A, Nanda K, Mandala J, Fransen K, Deese J, Crucitti T, Mastro TD, Taylor D. 2012. Preexposure prophylaxis for HIV infection among African women. N. Engl. J. Med. 367:411–422. 10.1056/NEJMoa1202614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marrazzo JM, Ramjee G, Nair G, Palanee T, Mkhize B, Nakabito C, Taljaajrd M, Piper J, Gomez Feliciano K, Chirenje M, VOICE Study Team 2013. Pre-exposure prophylaxis for HIV in women: daily oral tenofovir, oral tenofovir/emtricitabine, or vaginal tenofovir gel in the VOICE Study (MTN 003), abstr 26LB. Abstr. 20th Conf. Retrovir. Oppor. Infect., Atlanta, GA, 3 to 6 March 2013 [Google Scholar]
- 4.Abdool Karim Q, Abdool Karim SS, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, Kharsany AB, Sibeko S, Mlisana KP, Omar Z, Gengiah TN, Maarschalk S, Arulappan N, Mlotshwa M, Morris L, Taylor D. 2010. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 329:1168–1174. 10.1126/science.1193748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Celum C, Baeten JM. 2012. Tenofovir-based pre-exposure prophylaxis for HIV prevention: evolving evidence. Curr. Opin. Infect. Dis. 25:51–57. 10.1097/QCO.0b013e32834ef5ef [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kiser PF, Johnson TJ, Clark JT. 2012. State of the art in intravaginal ring technology for topical prophylaxis of HIV infection. AIDS Rev. 14:62–77 [PubMed] [Google Scholar]
- 7.Smith JM, Rastogi R, Teller RS, Srinivasan P, Mesquita PM, Nagaraja U, McNicholl JM, Hendry RM, Dinh CT, Martin A, Herold BC, Kiser PF. 2013. Intravaginal ring eluting tenofovir disoproxil fumarate completely protects macaques from multiple vaginal simian-HIV challenges. Proc. Natl. Acad. Sci. U. S. A. 110:16145–16150. 10.1073/pnas.1311355110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robbins BL, Srinivas RV, Kim C, Bischofberger N, Fridland A. 1998. Anti-human immunodeficiency virus activity and cellular metabolism of a potential prodrug of the acyclic nucleoside phosphonate 9-R-(2-phosphonomethoxypropyl)adenine (PMPA), bis(isopropyloxymethylcarbonyl)PMPA. Antimicrob. Agents Chemother. 42:612–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mesquita PM, Rastogi R, Segarra TJ, Teller RS, Torres NM, Huber AM, Kiser PF, Herold BC. 2012. Intravaginal ring delivery of tenofovir disoproxil fumarate for prevention of HIV and herpes simplex virus infection. J. Antimicrob. Chemother. 67:1730–1738. 10.1093/jac/dks097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mesquita PM, Cheshenko N, Wilson SS, Mhatre M, Guzman E, Fakioglu E, Keller MJ, Herold BC. 2009. Disruption of tight junctions by cellulose sulfate facilitates HIV infection: model of microbicide safety. J. Infect. Dis. 200:599–608. 10.1086/600867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fichorova RN, Tucker LD, Anderson DJ. 2001. The molecular basis of nonoxynol-9-induced vaginal inflammation and its possible relevance to human immunodeficiency virus type 1 transmission. J. Infect. Dis. 184:418–428. 10.1086/322047 [DOI] [PubMed] [Google Scholar]
- 12.Phillips DM, Zacharopoulos VR. 1998. Nonoxynol-9 enhances rectal infection by herpes simplex virus in mice. Contraception 57:341–348. 10.1016/S0010-7824(98)00040-7 [DOI] [PubMed] [Google Scholar]
- 13.Van Damme L, Chandeying V, Ramjee G, Rees H, Sirivongrangson P, Laga M, Perriens J. 2000. Safety of multiple daily applications of COL-1492, a nonoxynol-9 vaginal gel, among female sex workers. COL-1492 Phase II study Group. AIDS 14:85–88 [DOI] [PubMed] [Google Scholar]
- 14.Van Damme L, Ramjee G, Alary M, Vuylsteke B, Chandeying V, Rees H, Sirivongrangson P, Mukenge-Tshibaka L, Ettiegne-Traore V, Uaheowitchai C, Karim SS, Masse B, Perriens J, Laga M. 2002. Effectiveness of COL-1492, a nonoxynol-9 vaginal gel, on HIV-1 transmission in female sex workers: a randomised controlled trial. Lancet 360:971–977. 10.1016/S0140-6736(02)11079-8 [DOI] [PubMed] [Google Scholar]
- 15.Galen BT, Martin AP, Hazrati E, Garin A, Guzman E, Wilson SS, Porter DD, Lira SA, Keller MJ, Herold BC. 2007. A comprehensive murine model to evaluate topical vaginal microbicides: mucosal inflammation and susceptibility to genital herpes as surrogate markers of safety. J. Infect. Dis. 195:1332–1339. 10.1086/513279 [DOI] [PubMed] [Google Scholar]
- 16.Van Damme L, Govinden R, Mirembe FM, Guedou F, Solomon S, Becker ML, Pradeep BS, Krishnan AK, Alary M, Pande B, Ramjee G, Deese J, Crucitti T, Taylor D. 2008. Lack of effectiveness of cellulose sulfate gel for the prevention of vaginal HIV transmission. N. Engl. J. Med. 359:463–472. 10.1056/NEJMoa0707957 [DOI] [PubMed] [Google Scholar]
- 17.Wilson SS, Cheshenko N, Fakioglu E, Mesquita PM, Keller MJ, Herold BC. 2009. Susceptibility to genital herpes as a biomarker predictive of increased HIV risk: expansion of a murine model of microbicide safety. Antivir. Ther. 14:1113–1124. 10.3851/IMP1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Segarra TJ, Fakioglu E, Cheshenko N, Wilson SS, Mesquita PM, Doncel GF, Herold BC. 2011. Bridging the gap between preclinical and clinical microbicide trials: blind evaluation of candidate gels in murine models of efficacy and safety. PLoS One 6:e27675. 10.1371/journal.pone.0027675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nixon B, Stefanidou M, Mesquita PM, Fakioglu E, Segarra T, Rohan L, Halford W, Palmer KE, Herold BC. 2013. Griffithsin protects mice from genital herpes by preventing cell-to-cell spread. J. Virol. 87:6257–6269. 10.1128/JVI.00012-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tien D, Schnaare RL, Kang F, Cohl G, McCormick TJ, Moench TR, Doncel G, Watson K, Buckheit RW, Lewis MG, Schwartz J, Douville K, Romano JW. 2005. In vitro and in vivo characterization of a potential universal placebo designed for use in vaginal microbicide clinical trials. AIDS Res. Hum. Retroviruses 21:845–853. 10.1089/aid.2005.21.845 [DOI] [PubMed] [Google Scholar]
- 21.Hendrickson BA, Guo J, Brown I, Dennis K, Marcellino D, Hetzel J, Herold BC. 2000. Decreased vaginal disease in J-chain-deficient mice following herpes simplex type 2 genital infection. Virology 271:155–162. 10.1006/viro.2000.0303 [DOI] [PubMed] [Google Scholar]
- 22.Venegas VWJ, Dimmock D, Wong LJ. 2011. Real-time quantitative PCR analysis of mitochondrial DNA content., Curr. Protoc. Hum. Genet. Chapter 19:Unit 19.7. 10.1002/0471142905.hg1907s68 [DOI] [PubMed] [Google Scholar]
- 23.Cheshenko N, Liu W, Satlin LM, Herold BC. 2007. Multiple receptor interactions trigger release of membrane and intracellular calcium stores critical for herpes simplex virus entry. Mol. Biol. Cell 18:3119–3130. 10.1091/mbc.E07-01-0062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Witte L, Bobardt M, Chatterji U, Degeest G, David G, Geijtenbeek TB, Gallay P. 2007. Syndecan-3 is a dendritic cell-specific attachment receptor for HIV-1. Proc. Natl. Acad. Sci. U. S. A. 104:19464–19469. 10.1073/pnas.0703747104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bobardt MD, Chatterji U, Selvarajah S, Van der Schueren B, David G, Kahn B, Gallay PA. 2007. Cell-free human immunodeficiency virus type 1 transcytosis through primary genital epithelial cells. J. Virol. 81:395–405. 10.1128/JVI.01303-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haase AT. 2010. Targeting early infection to prevent HIV-1 mucosal transmission. Nature 464:217–223. 10.1038/nature08757 [DOI] [PubMed] [Google Scholar]
- 27.Galen B, Cheshenko N, Tuyama A, Ramratnam B, Herold BC. 2006. Access to nectin favors herpes simplex virus infection at the apical surface of polarized human epithelial cells. J. Virol. 80:12209–12218. 10.1128/JVI.01503-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cone RA, Hoen T, Wong X, Abusuwwa R, Anderson DJ, Moench TR. 2006. Vaginal microbicides: detecting toxicities in vivo that paradoxically increase pathogen transmission. BMC Infect. Dis. 6:90. 10.1186/1471-2334-6-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Corbeil LB, Chatterjee A, Foresman L, Westfall JA. 1985. Ultrastructure of cyclic changes in the murine uterus, cervix, and vagina. Tissue Cell 17:53–68. 10.1016/0040-8166(85)90015-1 [DOI] [PubMed] [Google Scholar]
- 30.Linehan MM, Richman S, Krummenacher C, Eisenberg RJ, Cohen GH, Iwasaki A. 2004. In vivo role of nectin-1 in entry of herpes simplex virus type 1 (HSV-1) and HSV-2 through the vaginal mucosa. J. Virol. 78:2530–2536. 10.1128/JVI.78.5.2530-2536.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGowan I, Hoesley C, Cranston RD, Andrew P, Janocko L, Dai JY, Carballo-Dieguez A, Ayudhya RK, Piper J, Hladik F, Mayer K. 2013. A phase 1 randomized, double blind, placebo controlled rectal safety and acceptability study of tenofovir 1% gel (MTN-007). PLoS One 8:e60147. 10.1371/journal.pone.0060147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rohan LC, Moncla BJ, Kunjara Na Ayudhya RP, Cost M, Huang Y, Gai F, Billitto N, Lynam JD, Pryke K, Graebing P, Hopkins N, Rooney JF, Friend D, Dezzutti CS. 2010. In vitro and ex vivo testing of tenofovir shows it is effective as an HIV-1 microbicide. PLoS One 5:e9310. 10.1371/journal.pone.0009310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andrei G, Lisco A, Vanpouille C, Introini A, Balestra E, van den Oord J, Cihlar T, Perno CF, Snoeck R, Margolis L, Balzarini J. 2011. Topical tenofovir, a microbicide effective against HIV, inhibits herpes simplex virus-2 replication. Cell Host Microbe 10:379–389. 10.1016/j.chom.2011.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]