Abstract
To provide support for in vitro susceptibility test interpretive criteria decisions for ceftaroline against Staphylococcus aureus and Streptococcus pneumoniae, as well as dose adjustment recommendations for renal impairment, pharmacokinetic-pharmacodynamic (PK-PD) target attainment was evaluated for simulated patients administered intravenous (i.v.) ceftaroline fosamil at 600 mg twice daily (q12h) and simulated patients with renal impairment administered various dosing regimens. Using a previously developed population PK model, Monte Carlo simulation was used to generate ceftaroline plasma concentration profiles for simulated patients with normal renal function or mild, moderate, or severe renal impairment. Using these profiles, the percentage of time during the dosing interval that free-drug concentrations remained above the MIC (f%T>MIC) for ceftaroline at steady state was calculated. Percentages of simulated patients achieving f %T>MIC targets for S. aureus and S. pneumoniae based on murine infection models were calculated by MIC. At MICs of 2 mg/liter for S. aureus and 1 mg/liter for S. pneumoniae, the percentages of simulated patients with normal renal function and mild renal impairment following administration of ceftaroline fosamil at 600 mg q12h, moderate renal impairment following administration of ceftaroline fosamil at 400 mg q12h, and severe renal impairment following administration of ceftaroline fosamil at 300 mg q12h achieving f %T>MIC targets (≥26 for S. aureus and ≥44 for S. pneumoniae) exceeded 90%. The results of these analyses, which suggested that in vitro susceptibility test interpretive criteria defining susceptible could be as high as MICs of ≤2 and ≤1 mg/liter for ceftaroline against S. aureus and S. pneumoniae, respectively, provide support for current FDA and CLSI criteria, which define susceptible as MICs of 1 and 0.5 mg/liter, respectively. Recommendations for dose adjustments for patients with renal impairment were also supported by the results of these analyses.
INTRODUCTION
When establishing in vitro susceptibility test interpretive criteria to guide effective antimicrobial therapy, a hierarchy of data is considered. This typically includes organism-drug susceptibility population statistics, pharmacokinetic-pharmacodynamic (PK-PD) data, and clinical response evaluations (1, 2, 3, 4). The application of PK-PD principles for this purpose involves the use of population PK models, which are developed using clinical PK data, and PK-PD targets for efficacy, which are based on data from preclinical infection models and/or infected patients. Using these data and Monte Carlo simulation, the impact of the PK variability on the probability of achieving PK-PD targets is assessed in the context of MIC distributions for the pathogens of interest. As described herein, such an assessment was carried out during the clinical development program for ceftaroline fosamil to support the determination of in vitro susceptibility test interpretive criteria and dosing regimens for patients with renal impairment.
Ceftaroline fosamil, a water-soluble prodrug of ceftaroline, is approved in the United States as an intravenous (i.v.) treatment for patients with acute bacterial skin and skin structure infections (ABSSSI) and community-acquired bacterial pneumonia (CABP) and in Europe for similar indications (5, 6, 7). Ceftaroline exhibits in vitro activity against a broad spectrum of Gram-positive and common Gram-negative pathogens associated with either CABP or ABSSSI, including methicillin-resistant Staphylococcus aureus (MRSA) and penicillin-resistant Streptococcus pneumoniae.
The objective of these analyses was to evaluate PK-PD target attainment by MIC in simulated patients following i.v. administration of ceftaroline fosamil at 600 mg twice daily (q12h) in order to provide support for in vitro susceptibility test interpretive criteria decisions for S. aureus and S. pneumoniae. These analyses also included the evaluation of various i.v. dosing regimens for administration to simulated patients with different categories of renal impairment to support recommendations for dose adjustments of ceftaroline fosamil.
MATERIALS AND METHODS
The analyses described herein were conducted using two steps, the details of which are described in the sections below. The first step involved using Monte Carlo simulation to generate four separate patient populations, one for each renal function category (normal renal function or mild, moderate, or severe renal impairment). Steady-state ceftaroline plasma concentration-time profiles following administration of different i.v. ceftaroline fosamil dosing regimens were simulated using previously developed population PK models for the prodrug, ceftaroline fosamil, and the active agent, ceftaroline (8). The percentage of time during the dosing interval that free-drug ceftaroline plasma concentrations remained above the MIC (f %T>MIC) was calculated for each simulated plasma concentration-time profile.
The second step of these analyses involved the determination of the percentage of simulated patients achieving f %T>MIC targets associated with efficacy for S. aureus and S. pneumoniae based on murine infection models (9) by MIC value, dosing regimen, and renal function category. These data were then evaluated in the context of MIC distributions for each of these pathogens.
Population PK model.
The population PK models for the ceftaroline fosamil and ceftaroline used for these analyses were developed using NONMEM version 6.2 (10) and plasma concentration-time data obtained from phase 1 subjects with various degrees of renal function and phase 2 and 3 patients with ABSSSI. The final population PK model was later validated using data from phase 3 patients with CABP to demonstrate that ceftaroline PKs are similar between patients with CABP and ABSSSI. The results of this analysis are presented in detail elsewhere (8).
In brief, the disposition of ceftaroline fosamil after i.v. administration was best described using a three-compartment model with zero-order input and rapid first-order conversion of the prodrug to ceftaroline. The disposition of ceftaroline was best described using a two-compartment model with parallel first-order and Michaelis-Menten elimination pathways. However, ceftaroline PKs could be adequately approximated by a two-compartment linear model within a ceftaroline fosamil dose range of 250 to 1,000 mg. The final ceftaroline fosamil and ceftaroline population PK model parameters after accounting for all statistically significant parameter-covariate relationships, as well as the magnitude of the remaining unexplained interindividual variability for each parameter, are provided in Tables 1 and 2. All model parameters were conditioned on the actual fraction of the prodrug converted to active ceftaroline (fm), which was not estimated. Creatinine clearance (CLCR) was identified as a clinically and statistically significant predictor of both the linear and intrinsic clearance terms for ceftaroline, as both parameters decreased with renal impairment. Intrinsic clearance also was shown to decrease to a lesser degree with age. Distribution clearance and peripheral volume were also increased for males relative to females. However, CLCR was the only clinically important covariate that required a dose adjustment (8).
TABLE 1.
Final population PK model for ceftaroline fosamila
| Parameterb | Estimate | % SEM |
|---|---|---|
| CLp (liters/h)c | 228 | 2.59 |
| CLp-CLCR power | 0.147 | 26.5 |
| Vc,p (liters)d | 10.8 | 8.35 |
| Vc,p shift for SEVj | 17.2 | 32.8 |
| Vc,p shift for POPj | −5.81 | 18.8 |
| CLd1p (liters/h) | 25.7 | 11.3 |
| Vp1 (liters) | 363 | 15.2 |
| CLd2p (liters/h) | 16.2 | 11.8 |
| Vp2 (liters) | 3.24 | 6.00 |
| F | 1.23 | 4.96 |
| ka (h−1) | 0.627 | 8.12 |
| FRC | 0.772 | 5.73 |
| tlag (h) | 0.462 | 7.36 |
| ω2ka | 26.4% CVe | 50.7 |
| ω2CLp | 25.7% CV | 13.2 |
| ω2Vc,p | 68.2% CV | 13.5 |
| ω2CLd1p | 70.3% CV | 24.4 |
| σ2 for studies 1, 2, and 3 | 34.2% CV | 4.91 |
| σ2 for all other studies | 36.6% CV | 5.80 |
Data were obtained from reference 8.
CLd1p, prodrug distribution clearance for the first peripheral compartment; CLd2p, prodrug distribution clearance for the second peripheral compartment; CLp, prodrug clearance; F, intramuscular bioavailability; FRC, fraction of prodrug dose in the first depot compartment; ka, first-order absorption rate constant; ω2, interindividual variability; σ2, residual variability; tlag, delay in the start of absorption from the second depot compartment; Vc,p, prodrug central volume of distribution; Vp1, prodrug volume of distribution for the first peripheral compartment; Vp2, prodrug volume of distribution for the second peripheral compartment.
Population mean CLp (liters/h) = 228 · (CLCRj/102)0.147.
Population mean Vc,p (liters/h) = 10.8 + 17.2 · SEVj − 5.81 · POPj, where SEVj is an indicator variable in the jth subject with a value of 1 if CLCR < 30 ml/min/1.73 m2 and 0 otherwise and POPj is an indicator variable in the jth subject with a value of 1 for phase 2/3 patients and 0 otherwise.
CV, coefficient of variation.
TABLE 2.
Final population PK model for ceftarolinea
| Parameterb | Estimate | % SEM | Bootstrap mean (90% confidence interval) |
|---|---|---|---|
| CLi (liters/h)c | 11.6 | 9.24 | 11.5 (9.61, 13.1) |
| CLi-CLCR power | 0.441 | 10.8 | 0.443 (0.361, 0.518) |
| CLi-age slope | −0.0883 | 17.0 | −0.0902 (−0.111, −0.0698) |
| CLi shift for POPj | 4.11 | 17.7 | 4.16 (3.11, 5.28) |
| Km (mg/liter) | 9.62 | 26.6 | 9.58 (6.79, 13.6) |
| CLlin (liters/h)d | 3.06 | 17.7 | 3.16 (2.09, 4.20) |
| CLlin-CLCR power | 0.343 | 17.5 | 0.333 (0.206, 0.424) |
| Vc (liters)e | 8.67 | 4.93 | 8.64 (8.11, 9.25) |
| Vc shift for POPj | 7.02 | 22.1 | 6.97 (5.01, 8.69) |
| CLd (liters/h)f | 8.59 | 6.07 | 8.59 (7.39, 10.0) |
| CLd shift for males | 4.88 | 17.1 | 5.04 (3.42, 6.90) |
| Vp (liters)g | 11.7 | 5.02 | 11.6 (10.5, 12.7) |
| Vp shift for males | 2.87 | 16.7 | 2.97 (2.05, 3.90) |
| ω2CLi | 30.2% CVh | 21.5 | 29.6% CV (23.9, 35.5) |
| Covariance (CLi, Km) | −0.0927 (r2 = 0.196) | 55.6 | −0.0780 (−0.160, 0.0001) |
| ω2Km | 67.0% CV | 28.6 | 65.0% CV (50.5, 77.4) |
| ω2Vc | 43.5 | 25.2 | 43.4% CV (34.7, 52.2) |
| ω2CLd | 31.8% CV | 25.8 | 31.8% CV (24.8, 39.1) |
| Covariance (CLd, Vp) | 0.0643 (r2 = 0.959) | 25.5 | 0.0645 (0.0412, 0.0929) |
| ω2Vp | 20.6% CV | 26.7 | 21.2% CV (17.2, 25.4) |
| σ2Additive | 0.0392 | 8.74 | 0.0387 (0.0331, 0.0440) |
| σ2CCV | 0.00180 | 53.1 | 0.0019 (0.0011, 0.0031) |
Data were obtained from reference 8.
CCV, constant coefficient of variation; CLd, distribution clearance for the peripheral compartment for ceftaroline; CLi, intrinsic ceftaroline clearance for the saturable elimination pathway; CLlin, linear ceftaroline clearance; Km, ceftaroline concentration producing 50% of CLi; ω2, interindividual variability; σ2, residual variability; Vc, ceftaroline central volume of distribution; Vp, = volume of distribution for the peripheral compartment for ceftaroline.
Population mean CLi (liters/h) = 11.6 · (CLCRj/102)0.441 −0.0883 · (agej − 36) + 4.11 · POPj, where POPj is an indicator variable in the jth subject with a value of 1 for phase 2/3 patients and 0 otherwise.
Population mean CLlin (liters/h) = 3.06 · (CLCRj/102)0.343.
Population mean Vc (liters) = 8.67 + 7.02 · POPj.
Population mean CLd (liters/h) = 8.59 + 4.88 · MALEj, where MALEj is an indicator variable in the jth subject with a value of 1 for males and 0 for females.
Population mean Vp (liters) = 11.7 + 2.87 · MALEj.
CV, coefficient of variation.
Monte Carlo simulation.
A total of 2,000 patients, of whom 50% were males, were simulated in each of four renal function categories using SAS version 9.2 software (11). For this simulation exercise, renal function was defined by CLCR calculated using the Cockcroft-Gault equation and normalized by body surface area (BSA). Renal function categories were defined as follows: normal renal function, 80 ≤ CLCR ≤ 170 ml/min/1.73 m2; mild renal impairment, 50 ≤ CLCR < 80 ml/min/1.73 m2; moderate renal impairment, 30 ≤ CLCR < 50 ml/min/1.73 m2; and severe renal impairment, 15 ≤ CLCR < 30 ml/min/1.73 m2. In the group with normal renal function, CLCR was assigned using a truncated normal distribution with a mean (standard deviation [SD]) of 118 (30.8) ml/min/1.73 m2 based on the observed data from 624 patients with ABSSSI who had a CLCR of ≥80 ml/min/1.73 m2 (12, 13, 14). For all other renal function categories, CLCR was uniformly distributed. Age was assigned according to a normal distribution with a mean (SD) of 46.2 (16.6) years of age based on the data from 839 patients with ABSSSI studied (12, 13, 14); the simulated age distribution was truncated to include only individuals between 18 and 90 years of age.
Steady-state ceftaroline concentrations in plasma were simulated every 0.1 h during the dosing interval for patients in each renal function group after i.v. administration of the following ceftaroline fosamil dosing regimens infused over 1 h: normal renal function and mild renal impairment, 600 mg q12h; moderate renal impairment, 300 mg q12h, 400 mg q12h, 600 mg q12h, and 600 mg q24h; and severe renal impairment, 300 mg q12h, 400 mg q12h, 600 mg q12, 600 mg q24h, and 800 mg q24h. These dosing regimens were previously evaluated to determine which regimen(s) provides comparable steady-state ceftaroline maximum concentration (Cmax) and area under the concentration-time curve (AUC) values among simulated patients in each of the above-described renal function groups (8). To confirm the reliability of the simulations, a visual predictive check was performed in which percentiles (5th, 50th, and 95th) of the simulated data following administration of 600 mg ceftaroline fosamil q12h were overlaid upon the observed plasma ceftaroline concentration-time data in patients with ABSSSI or CABP with either normal renal function or mild renal impairment receiving this same dose. Similarly, a visual predictive check was carried out for the ceftaroline fosamil dosing regimen of 400 mg q12h administered to phase 2/3 ABSSSI and CABP patients with moderate renal impairment, a dosing regimen which provided exposures similar to those for patients with normal renal function administered ceftaroline fosamil at 600 mg q12h (12, 13, 14, 15, 16).
Calculation of ceftaroline exposures.
Free-drug (f) ceftaroline concentrations in plasma were calculated by multiplying the total-drug concentration by 0.8 based on a concentration-independent plasma protein binding estimate of 20% (6). For each simulated profile, f %T>MIC was calculated by MIC values ranging from 0.008 to 32 mg/liter.
PK-PD target attainment analyses.
For each dosing regimen and renal function group, the percentage of simulated patients achieving PK-PD targets was assessed by MIC value. PK-PD targets were based on f %T>MIC, the PK-PD index associated with efficacy for ceftaroline (9). Specific f %T>MIC targets associated with various levels of bacterial reduction from baseline for S. aureus and S. pneumoniae based on data from a neutropenic murine thigh infection model were assessed. For bacterial reduction endpoints of net bacterial stasis and 1- and 2-log10 CFU reductions from baseline, f %T>MIC targets for S. aureus were 26, 36, and 51, respectively. For S. pneumoniae, f %T>MIC targets associated with these endpoints were 35, 44, and 51, respectively. These studies evaluated four S. aureus isolates (including one MRSA isolate) and five S. pneumoniae isolates (one penicillin susceptible, one with intermediate sensitivity, and three penicillin resistant) (9). Nonclinical PK-PD targets associated with net bacterial stasis and 90% animal survival have been shown to be correlated with a high percentage of successful outcomes in patients with ABSSSI and pneumonia, respectively (17). Given these data, focus was given to results based on achieving an f %T>MIC target of 26 for S. aureus, which was associated with net bacterial stasis. An f %T>MIC target of ≥44 for S. pneumoniae, which was associated with a 1-log10 CFU reduction from baseline, was chosen for evaluation rather than the target for net bacterial stasis given the higher bacterial burden typically associated with pneumonia compared to ABSSSI.
The percentage of simulated patients among each renal function group achieving the above-described f %T>MIC targets was assessed over an MIC range of 0.008 to 32 mg/liter for each dosing regimen evaluated. Percentages of PK-PD target attainment were also assessed in the context of MIC distributions for ceftaroline against S. aureus and S. pneumoniae based on contemporary surveillance data (18, 19). These data, which included ceftaroline MIC values for 3,965 and 894 S. aureus and S. pneumoniae isolates, respectively, were collected from medical centers in the United States. MIC values for the 3,965 S. aureus isolates were stratified by MRSA and methicillin-susceptible S. aureus (MSSA) isolates. The minimum MIC, the MIC inhibiting 50% of isolates (MIC50), the MIC inhibiting 90% of isolates (MIC90), and the maximum MIC were 0.12, 1, 1, and 2 mg/liter, respectively, for the 2,254 MRSA isolates collected and ≤0.008, 0.25, 0.25, and 0.5 mg/liter, respectively, for the 1,711 MSSA isolates collected. For the 894 S. pneumoniae isolates collected, these values were ≤0.008, 0.015, 0.12, and 0.5 mg/liter, respectively.
RESULTS
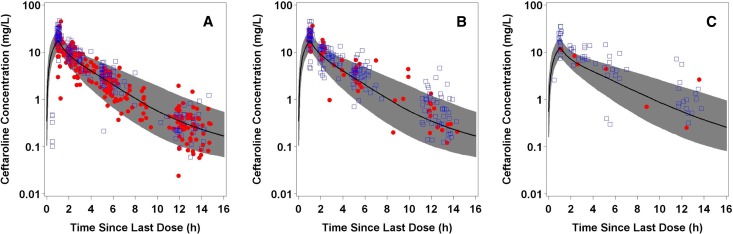
As shown in Fig. 1, steady-state plasma ceftaroline concentrations over time for simulated patients with either normal renal function or mild renal impairment following administration of 600 mg ceftaroline fosamil q12h, as well as for simulated patients with moderate renal impairment following administration of 400 mg ceftaroline fosamil q12h, agreed well with the observed data collected from patients with ABSSSI and CABP in each of these renal function groups. This evaluation demonstrated that the population PK model used in PK-PD target attainment simulations was adequate.
FIG 1.
Steady-state ceftaroline concentrations in plasma over time for patients with ABSSSI (●) or CABP (□) with normal renal function following administration of ceftaroline fosamil at 600 mg q12h (n = 129) (A), mild renal impairment following administration of ceftaroline fosamil at 600 mg q12h (n = 68) (B), and moderate renal impairment following administration of ceftaroline fosamil at 400 mg q12h (n = 19) (C).The solid black line represents the 50th percentile and the gray shaded area represents the 5th and 95th percentiles of the simulated data. The symbols represent the observed data used to develop and validate the population PK model.
The percentages of simulated patients with normal renal function and mild renal impairment following administration of ceftaroline fosamil at 600 mg q12h who achieved f %T>MIC targets by MIC for S. aureus and S. pneumoniae are shown in Table S1 in the supplemental material. The percentages of simulated patients with moderate and severe renal impairment following administration of the various dosing regimens evaluated who achieved each of these f %T>MIC targets by MIC are shown in Tables S2 and S3 in the supplemental material, respectively. The percentages of simulated patients with normal renal function and mild renal impairment following administration of ceftaroline fosamil at 600 mg q12h who achieved f %T>MIC targets by MIC, overlaid on MIC distributions for S. aureus or S. pneumoniae, are shown in Fig. 2 and 3, respectively. Similar data are presented for simulated patients with moderate renal impairment following administration of ceftaroline fosamil at 400 mg q12h in Fig. 4 and for those with severe renal impairment following administration of ceftaroline fosamil at 300 mg q12h in Fig. 5.
FIG 2.
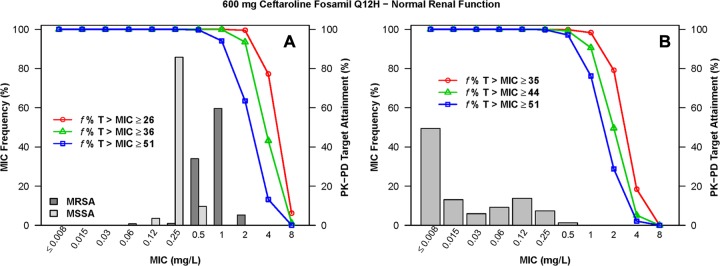
Percentages of simulated patients with normal renal function (80 ≤ CLCR ≤ 170 ml/min/1.73 m2) achieving f %T>MIC targets by MIC following administration of ceftaroline fosamil at 600 mg q12h, overlaid on a histogram showing the MIC distribution for S. aureus (A) or S. pneumoniae (B).
FIG 3.
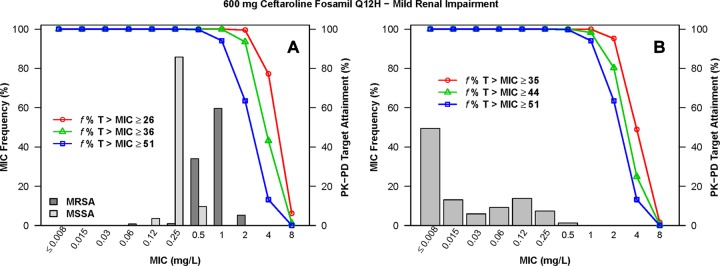
Percentages of simulated patients with mild renal impairment (50 ≤ CLCR < 80 ml/min/1.73 m2) achieving f %T>MIC targets by MIC following administration of ceftaroline fosamil at 600 mg q12h, overlaid on a histogram showing the MIC distribution for S. aureus (A) or S. pneumoniae (B).
FIG 4.
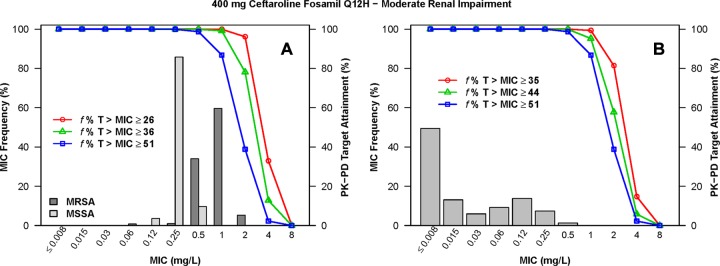
Percentages of simulated patients with moderate renal impairment (30 ≤ CLCR < 50 ml/min/1.73 m2) achieving f %T>MIC targets by MIC following administration of ceftaroline fosamil at 400 mg q12h, overlaid on a histogram showing the MIC distribution for S. aureus (A) or S. pneumoniae (B).
FIG 5.
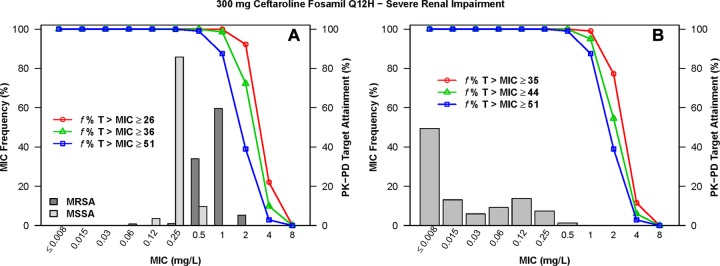
Percentages of simulated patients with severe renal impairment (15 ≤ CLCR < 30 ml/min/1.73 m2) achieving f %T>MIC targets by MIC following administration of ceftaroline fosamil at 300 mg q12h, overlaid on a histogram showing the MIC distribution for S. aureus (A) and S. pneumoniae (B).
When examining PK-PD target attainment for S. aureus, the percentages of simulated patients achieving f %T>MIC targets of ≥26 (net bacterial stasis) and ≥36 (1-log10 CFU reduction from baseline) were ≥99.4% and ≥92.4%, respectively, at an MIC of 1 mg/liter across all renal function groups for all dosing regimens evaluated, with the exception of the dosing regimen of ceftaroline fosamil at 600 mg q24h in simulated patients with moderate renal impairment. For this population and dosing regimen, 96.5% and 74.1% of simulated patients achieved an f %T>MIC of ≥26 and ≥36, respectively (see Table S2 in the supplemental material). At an MIC value of 2 mg/liter, the percentages of simulated patients with normal renal function and mild renal impairment following administration of 600 mg ceftaroline fosamil q12h achieving an f %T>MIC of ≥26 were 95.9% and 99.5%, respectively (see Table S1 in the supplemental material). For simulated patients with moderate renal impairment following administration of ceftaroline fosamil at 400 mg q12h and with severe renal impairment following ceftaroline fosamil at 300 mg q12h, these percentages were 96.1% and 92.2%, respectively (see Tables S2 and S3 in the supplemental material, respectively).
When examining PK-PD target attainment for S. pneumoniae, the percentages of simulated patients achieving f %T>MIC targets of ≥35 (net bacterial stasis) and ≥44 (1-log10 CFU reduction from baseline) were ≥99.6% and ≥95.8%, respectively, at an MIC of 0.25 mg/liter across all renal function groups for all dosing regimens evaluated. At an MIC value of 0.5 mg/liter, the percentages of simulated patients achieving an f %T>MIC of ≥35 and ≥44 were ≥99.3% and ≥94.3%, respectively, across renal function groups for all dosing regimens, with the exception of the dosing regimen of ceftaroline fosamil at 600 mg q24h in simulated patients with moderate renal impairment. The percentages of simulated patients achieving an f %T>MIC of ≥35 and ≥44 for this population and dosing regimen were 95.6% and 80.7%, respectively (see Table S2 in the supplemental material). At an MIC value of 1 mg/liter, the percentages of simulated patients with normal renal function and mild renal impairment following administration of 600 mg q12h achieving an f %T>MIC of ≥44 were 90.6% and 98.3%, respectively (see Table S1 in the supplemental material). For simulated patients with moderate renal impairment following administration of 400 mg q12h and with severe renal impairment following administration of 300 mg q12h, these percentages were 95.2% and 95.0%, respectively (see Tables S2 and S3 in the supplemental material, respectively).
DISCUSSION
The objective of the analyses described herein was to evaluate PK-PD target attainment by MIC in simulated patients following administration of ceftaroline fosamil at 600 mg q12h, with the goal of providing support for in vitro susceptibility test interpretive criteria decisions for S. aureus and S. pneumoniae. As part of this evaluation, PK-PD target attainment was assessed for various ceftaroline fosamil dosing regimens within various renal function categories to support recommendations for dose adjustments in patients with renal impairment.
The results of the PK-PD target attainment analyses support susceptibility test interpretive criteria which define susceptible as MICs of ≤2 and ≤1 mg/liter for ceftaroline against S. aureus and S. pneumoniae, respectively. At these MIC values, the percentage of simulated patients who achieved f%T>MIC targets associated with bacterial reduction endpoints linked with a high percentage of successful outcomes in patients with ABSSSI and pneumonia (17) exceeded 90% for the following ceftaroline fosamil dosing regimens: 600 mg q12h in patients with normal renal function and mild renal impairment, 400 mg q12h in patients with moderate renal impairment, and 300 mg q12h in patients with severe renal impairment.
Although PK-PD analyses have been conducted based on clinical data from ceftaroline fosamil-treated patients with ABSSSI and S. aureus at baseline or CABP and S. pneumoniae at baseline (20, 21), the results of the analyses described herein were based on nonclinical f %T>MIC targets for each of these organisms. While PK-PD analyses of patients with ABSSSI and S. aureus isolated at baseline, which were conducted using data from two phase 2 and two phase 3 studies, revealed significant relationships between microbiological response and f %T>MIC evaluated continuously or categorically, there was a high degree of uncertainty around the identified PK-PD relationships (20). This was likely due to the high percentage of patients with microbiological success (and hence, the low number of failures) in this analysis population. Although these data did provide support for the adequacy of the dosing regimen of 600 mg q12h, the uncertainty around the PK-PD relationships for microbiological response precluded reliable identification of f %T>MIC targets for efficacy. Thus, nonclinical f %T>MIC targets were chosen for evaluation in the analyses described herein.
For the PK-PD analyses of patients with CABP (21), which were based on data from two phase 3 studies, 124 microbiologically evaluable (ME) patients were assessed (35 of whom had S. pneumoniae isolated at baseline). The percentages of clinical and microbiological success in these two populations were ≥82.9%. Among the 124 patients, the majority of patients (91.1%) had f %T>MIC values ranging from 91.7 to 100; 98.4% of patients had f %T>MIC values of ≥63.3. All 35 patients with S. pneumoniae had an f %T>MIC equal to 100. Thus, given the high percentages of patients achieving successful clinical or microbiological responses and the large proportion of patients with high f %T>MIC values, it was anticipated that there would be some difficulty in identifying reliable PK-PD relationships for efficacy. Indeed, such relationships could not be identified based on these data as evidenced by the fact that f %T>MIC values were well above nonclinical f %T>MIC targets, thus suggesting that the majority of patients with CABP receiving the dosing regimen of ceftaroline fosamil at 600 mg q12h achieved exposures associated with the upper plateau of the PK-PD relationship for efficacy. Given the limitations of the PK-PD analyses based on the data from patients with CABP, nonclinical f %T>MIC targets for S. pneumoniae were chosen for evaluation in the analyses described herein.
While the PK-PD target attainment analyses undertaken did consider two of the three data sources typically required to establish in vitro susceptibility test interpretive criteria (i.e., organism drug susceptibility population statistics and PK-PD data), clinical response evaluations represent an important third data source. Although the results of PK-PD target attainment analyses in the context of surveillance data allow for predictions of efficacy by MIC beyond the MIC range observed in the clinical studies, examination of clinical response by MIC remains an important evaluation which is used to balance predictions relative to current clinical experience. Given these considerations, an MIC value at which there are an insufficient number of clinical outcomes is not usually selected to define in vitro susceptibility test interpretive criteria. Clinical and microbiological response data by MIC, summarized in Tables 3 and 4, were considered for ceftaroline during the U.S. Food and Drug Administration (FDA) Advisory Committee Meeting held on 7 September 2010 (data on file at Forest Research Institute, Inc.).
TABLE 3.
Percentages of clinical and microbiological success by MIC for ceftaroline fosamil-treated patients against S. aureus in the ME population for the pooled phase 3 ABSSSI studies
| Ceftaroline MIC (mg/liter) | N | Clinical success, n/N (%) | Microbiological success (eradicated/presumed eradicated), n/N (%) |
|---|---|---|---|
| 0.06 | 3 | 3/3 (100) | 3/3 (100) |
| 0.12 | 79 | 72/79 (91.1) | 73/79 (92.4) |
| 0.25 | 156 | 148/156 (94.9) | 149/156 (95.5) |
| 0.5 | 109 | 102/109 (93.6) | 102/109 (93.6) |
| 1 | 11 | 11/11 (100) | 11/11 (100) |
| 2 | 4 | 2/4 (50) | 2/4 (50) |
| Total | 362 | 338/362 (93.4) | 340/362 (93.9) |
TABLE 4.
Percentages of clinical and microbiological success by MIC for ceftaroline fosamil-treated patients against S. pneumoniae in the ME population for the pooled phase 3 CABP studies
| Ceftaroline MIC (mg/liter) | N | Clinical success, n/N (%) | Microbiological success (eradicated/presumed eradicated), n/N (%) |
|---|---|---|---|
| ≤0.004 | 4 | 4/4 (100) | 4/4 (100) |
| 0.008 | 20 | 16/20 (80) | 16/20 (80) |
| 0.015 | 8 | 6/8 (75) | 6/8 (75) |
| 0.03 | 2 | 2/2 (100) | 2/2 (100) |
| 0.06 | 1 | 1/1 (100) | 1/1 (100) |
| 0.25 | 1 | 1/1 (100) | 1/1 (100) |
| Total | 36 | 30/36 (83.3) | 30/36 (83.3) |
As shown in Table 3, the number of ceftaroline fosamil-treated ABSSSI patients with S. aureus at baseline with an MIC of 2 mg/liter based on the two pivotal phase 3 studies pooled was very low (n = 4). While the number of cases was also low for an MIC of 1 mg/liter (n = 11), the percentage of clinical and microbiological success was not different than for that for patients with an MIC of ≤0.5 mg/liter. Thus, the definition of susceptible based on an MIC of 1 mg/liter for ceftaroline against S. aureus was supported by both the results of PK-PD target attainment analyses and the observed clinical data.
As shown in Table 4, the number of ceftaroline fosamil-treated CABP patients with S. pneumoniae at baseline was low based on the two pivotal phase 3 studies. However, given the high percentages of clinical and microbiological success at each observed MIC value and the fact that there were patients with S. pneumoniae isolates at baseline with MIC values of <0.25 mg/liter, these data were supportive of a definition of susceptible of 0.25 mg/liter for ceftaroline against S. pneumoniae. As described herein, the results of PK-PD target attainment analyses provided support for a susceptibility breakpoint that was two dilutions higher (1.0 mg/liter).
Although there are differences in the assessment approach and perhaps the weighting of data sources, in vitro susceptibility test interpretive criteria determinations for S. aureus and S. pneumoniae made by the U.S. FDA (22), the Clinical and Laboratory Standards Institute (CLSI) (23), and the European Committee on Antimicrobial Susceptibility Testing (EUCAST) (24) were similar. Each defined susceptible for ceftaroline against S. aureus as an MIC of ≤1 mg/liter. While the U.S. FDA and EUCAST defined susceptible for ceftaroline against S. pneumoniae as an MIC of ≤0.25 mg/liter, the CLSI definition was one dilution higher (MIC ≤of 0.5 mg/liter). Recently, the FDA definition for susceptible for ceftaroline against S. pneumoniae was revised, and it is now an MIC of ≤0.5 mg/liter (6).
The PK-PD target attainment analyses carried out were used not only to provide support for in vitro susceptibility test interpretive criteria decisions for S. aureus and S. pneumoniae but also to evaluate the adequacy of ceftaroline fosamil dosing recommendations for patients with renal impairment. PK-PD data have been increasingly used to support dose selection during early and late stages of drug development. Metrics based on these principles provide a useful approach to assess both the dose and frequency of administration with regard to predicted probabilities of efficacy. The results of the analyses described herein, in conjunction with those conducted to identify matching exposures for patients with renal impairment to those with normal renal function, provide further support for ceftaroline fosamil dose adjustment recommendations for patients with renal impairment described in the U.S. product label (6).
In conclusion, the results of PK-PD target attainment analyses carried out for ceftaroline are consistent with FDA, CLSI, and EUCAST in vitro susceptibility test interpretive criteria for S. aureus and S. pneumoniae. These data also provide support for the U.S. FDA labeled ceftaroline fosamil dosing regimens of 600 mg q12h for patients with normal renal function and mild renal impairment, 400 mg q12h for patients with moderate renal impairment, and 300 mg q12h for patients with severe renal impairment.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by Forest Laboratories, Inc. Scientific Therapeutics Information, Inc., provided editorial coordination, which was funded by Forest Research Institute, Inc.
Forest Laboratories, Inc., was involved in the design, collection, analysis, interpretation of data, and decision to present these results.
We thank Kim Charpentier at the Institute for Clinical Pharmacodynamics, Latham, NY, for her assistance in the preparation of the manuscript.
Footnotes
Published ahead of print 25 November 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.01680-13.
REFERENCES
- 1.Clinical and Laboratory Standards Institute 2008. Development of in vitro susceptibility testing criteria and quality control parameters; approved guideline, 3rd ed. CLSI document M23-A3. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 2.Dudley MN, Ambrose PG, Bhavnani SM, Craig WA, Ferraro MJ, Jones RN. 2013. Background and rationale for revised Clinical Laboratory Standards Institute (CLSI) interpretive criteria (breakpoints) for Enterobacteriaceae and Pseudomonas aeruginosa. I. Cephalosporins and aztreonam. Clin. Infect. Dis. 56:1301–1309. 10.1093/cid/cit017 [DOI] [PubMed] [Google Scholar]
- 3.Ambrose PG, Meagher AK, Passarell JA, Van Wart SA, Cirincione BB, Bhavnani SM, Ellis-Grosse E. 2009. Application of patient population-derived pharmacokinetic-pharmacodynamic relationships to tigecycline breakpoint determination for staphylococci and streptococci. Diagn. Microbiol. Infect. Dis. 63:155–159. 10.1016/j.diagmicrobio.2008.10.011 [DOI] [PubMed] [Google Scholar]
- 4.Bhavnani SM, Rubino CM, Forrest A, Okusanya OO, Craig WA, Moeck G, Lehoux D, Parr TR, Jr, Ambrose PG. 2008. Pharmacokinetic-pharmacodynamic target attainment as decision support for oritavancin susceptibility breakpoints for Staphylococcus aureus, abstr A-994 Abstr. 48th Intersci Conf. Antimicrob. Agents Chemother. (ICAAC)-Infect. Dis. Soc. Am. (IDSA) 46th Annu. Meet. American Society for Microbiology and Infectious Diseases Society of America, Washington, DC [Google Scholar]
- 5.Laudano JB. 2011. Ceftaroline fosamil: a new broad-spectrum cephalosporin. J. Antimicrob. Chemother. 66(Suppl 3):11–18. 10.1093/jac/dkr095 [DOI] [PubMed] [Google Scholar]
- 6.Forest Pharmaceuticals, Inc 2013. Teflaro package insert. Forest Pharmaceuticals, Inc., St Louis, MO [Google Scholar]
- 7.AstraZeneca AB. 2012. Zinforo, summary of product characteristics. AstraZeneca AB, Sodertalje, Sweden [Google Scholar]
- 8.Van Wart SA, Forrest A, Khariton T, Rubino CM, Bhavnani SM, Riccobene TA, Ambrose PG. 2013. Population pharmacokinetics of ceftaroline in patients with complicated skin and skin structure infections or community-acquired pneumonia. J. Clin. Pharmacol. 53:1155–1167 [DOI] [PubMed] [Google Scholar]
- 9.Andes D, Craig WA. 2006. Pharmacodyamics of a new cephalosporin, PPI-0903 (TAK-599), active against methicillin-resistant Staphylococcus aureus in murine thigh and lung infection models: identification of an in vivo pharmacokinetic-pharmacodynamic target. Antimicrob. Agents Chemother. 50:1376–1383. 10.1128/AAC.50.4.1376-1383.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beal SL, Sheiner LB, Boeckmann AJ. 2006. NONMEM users guides. ICON Development Solutions, Ellicott City, MD [Google Scholar]
- 11.SAS Institute Inc 2010. SAS OnlineDoc9.2. SAS Institute Inc., Cary, NC [Google Scholar]
- 12.Talbot GH, Thye D, Das A, Ge Yigong. 2007. Phase 2 study of ceftaroline versus standard therapy in treatment of complicated skin and skin structure infections. Antimicrob. Agents Chemother. 51:3612–3616. 10.1128/AAC.00590-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corey GR, Wilcox MH, Talbot GH, Thye D, Friedland D, Baculik T, CANVAS 1 investigators 2010. CANVAS 1: the first phase III, randomized, double-blind study evaluating ceftaroline fosamil for the treatment of patients with complicated skin and skin structure infections. J. Antimicrob. Chemother. 65(Suppl 4):41–51. 10.1093/jac/dkq254 [DOI] [PubMed] [Google Scholar]
- 14.Wilcox MH, Corey GR, Talbot GH, Thye D, Friedland D, Baculik T, CANVAS 2 investigators 2010. CANVAS 2: the second phase III, randomized, double-blind study evaluating ceftaroline fosamil for the treatment of patients with complicated skin and skin structure infections. J. Antimicrob. Chemother. 65(Suppl 4):53–65. 10.1093/jac/dkq255 [DOI] [PubMed] [Google Scholar]
- 15.File TM, Jr, Low DE, Eckburg PB, Talbot GH, Friedland HD, Lee J, Llorens L, Critchely IA, Thye DA. 2011. FOCUS 1: a randomized, double-blinded, multicentre, phase III trial of the efficacy and safety of ceftaroline fosamil versus ceftriaxone in community-acquired pneumonia. J. Antimicrob. Chemother. 66(Suppl 3):19–32. 10.1093/jac/dkr096 [DOI] [PubMed] [Google Scholar]
- 16.Low DE, File TM, Jr, Eckburg PB, Talbot GH, Friedland HD, Lee J, Llorens L, Critchley IA, Thye DA. 2011. FOCUS 2: a randomized, double-blinded, multicentre, phase III trial of the efficacy and safety of ceftaroline fosamil versus ceftriaxone in community-acquired pneumonia. J. Antimicrob. Chemother. 66(Suppl 3):33–44. 10.1093/jac/dkr097 [DOI] [PubMed] [Google Scholar]
- 17.Ambrose PG, Bhavnani SM, Rubino CM, Louie A, Gumbo T, Forrest A, Drusano GL. 2007. Pharmacokinetics-pharmacodynamics of antimicrobial therapy: it's not just for mice anymore. Clin. Infect. Dis. 44:79–86. 10.1086/510079 [DOI] [PubMed] [Google Scholar]
- 18.Jones RN, Mendes RE, Sader HS. 2010. Ceftaroline activity against pathogens associated with complicated skin and skin structure infections: results from an international surveillance study. J. Antimicrob. Chemother. 65(Suppl 4):17–31. 10.1093/jac/dkq252 [DOI] [PubMed] [Google Scholar]
- 19.Jones RN, Farrell DJ, Mendes RE, Sader HS. 2011. Comparative ceftaroline activity tested against pathogens associated with community-acquired pneumonia: results from an international surveillance study. J. Antimicrob. Chemother. 66(Suppl 3):69–80. 10.1093/jac/dkr101 [DOI] [PubMed] [Google Scholar]
- 20.Bhavnani SM, Hammel JP, Van Wart SA, Rubino CR, Reynolds DK, Forrest A, Drusano DL, Khariton T, Riccobene TA, Ambrose PG. 2011. Pharmacokinetic-pharmacodynamic analysis for efficacy of ceftaroline fosamil in patients with complicated skin and skin structure infections, abstr A2-552 Abstr. 51st Intersci. Conf. Antimicrob. Agents Chemother [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhavnani SM, Hammel JP, Van Wart SA, Rubino CR, Reynolds DK, Forrest A, Khariton T, Friedland HD, Riccobene TA, Ambrose PG. 2013. Pharmacokinetic-pharmacodynamic analyses for efficacy of ceftaroline fosamil in patients with community-acquired bacterial pneumonia. Antimicrob. Agents Chemother. 57:6348–6350. 10.1128/AAC.01748-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forest Pharmaceuticals, Inc 2012. Teflaro package insert. Forest Pharmaceuticals, Inc., St Louis, MO [Google Scholar]
- 23.Clinical and Laboratory Standards Institute 2013. Performance standards for antimicrobial susceptibility testing; 23rd information supplement. CLSI document M100-S23. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 24.European Committee on Antimicrobial Susceptibility Testing 2013. Clinical breakpoint table v. 3.1 9 (valid from 02-11). http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/Breakpoint_table_v_3.1.pdf Accessed 22 February 2013 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.