Abstract
We evaluated in vitro activity of ceftolozane-tazobactam (TOL-TAZ), formerly CXA-201, against recent clinical anaerobic isolates with emphasis on the Bacteroides fragilis group. Ceftolozane-tazobactam showed good activity against B. fragilis species and intermediate to limited activity against other species of Bacteroides. Ceftolozane-tazobactam showed very good activity against Prevotella spp., Fusobacterium spp., and Propionibacterium spp., varying activities against Gram-positive cocci, and limited activity against Clostridium spp.
TEXT
Ceftolozane-tazobactam is a novel antibacterial with activity against Pseudomonas aeruginosa, including drug-resistant strains, and other common Gram-negative pathogens, including most extended-spectrum beta-lactamase (ESBL)-producing enterobacteriaceae (1–5; D. R. Snydman, N. V. Jacobus, and L. A. McDermott presented at the 22nd European Congress of Clinical Microbiology and Infectious Diseases, London, England, abstr P1445, 2012). Ceftozolane exerts its bactericidal activity by inhibiting essential penicillin-binding proteins (PBP), resulting in inhibition of cell wall synthesis and subsequent cell death. Tazobactam is a potent β-lactamase inhibitor of most common class A and C β-lactamases. It binds covalently to chromosomally and plasmid-mediated bacterial β-lactamases to broaden the coverage of ceftolozane-tazobactam to include β-lactamase-producing Gram-negative organisms.
Tazobactam, a well-established β-lactamase inhibitor that has been combined with piperacillin, has proven to have excellent activity against most of the β-lactamases produced by the Bacteroides fragilis group of anaerobes (6–8). The purpose of this study was to evaluate the in vitro activities of ceftolozane-tazobactam against a large number of anaerobic pathogens, with emphasis on its activities against B. fragilis and related species.
(This study was presented in part at the European Congress on Clinical Microbiology and Infectious Diseases, London, England, 31 March to 3 April 2012.)
Included in the study were 605 recent clinical Gram-positive and Gram-negative anaerobic pathogens. Among the Gram-negative pathogens, 466 were Bacteroides fragilis group isolates, including strains with known resistance against some of the comparator agents tested. Also among the Gram-negative pathogens were 33 Prevotella spp. and 12 Fusobacterium spp. The 94 anaerobic Gram-positive pathogens included 54 Clostridium spp., 31 anaerobic cocci, and 9 Propionibacterium spp. All isolates were collected between 2010 and 2012. The B. fragilis group isolates were referred by 8 medical centers throughout the United States (6, 7). All other isolates were from patients from Tufts Medical Center, Boston, MA.
The MICs of the isolates were determined using agar dilution following Clinical and Laboratory Standards Institute (CLSI) recommendations (9). The susceptibilities of the isolates to ceftolozane, ceftolozane-tazobactam, ampicillin-sulbactam, piperacillin-tazobactam, imipenem, meropenem, tigecycline, moxifloxacin, clindamycin, linezolid, and metronidazole were tested. Vancomycin was added to the test panel when anaerobic Gram-positive isolates were tested, and ertapenem was added to the test panel when B. fragilis group isolates were tested. The following ATCC reference organisms were included in quality control (QC) testing: Bacteroides fragilis ATCC 25285, Bacteroides thetaiotaomicron ATCC 29741, Clostridium difficile ATCC 700057, Eubacterium lentum ATCC 43055, and Staphylococcus aureus ATCC 25923 (used for QC testing for vancomycin). CLSI QC ranges were applied to the results of the QC testing (2).
Table 1 shows the cumulative percentages and MIC distributions of the B. fragilis group isolates. Ceftozolane alone exhibited very poor activities against all the species of the B. fragilis group: the highest concentration of ceftolozane used in the test, 256 μg/ml, failed to inhibit most of the B. fragilis group isolates. The addition of 4 μg/ml of tazobactam to ceftolozane resulted in a significant reduction in the MIC90 for all the species of the B. fragilis group. The greatest reduction was observed against B. fragilis. The MIC90 for ceftolozane alone was ≥256 μg/ml, compared to 4 μg/ml for ceftolozane-tazobactam. For most of the other species, the MIC90 for ceftolozane-tazobactam was 32 μg/ml. Resistant isolates from all species also showed elevated MICs to ceftolozane-tazobactam. Approximately 10% of the B. fragilis group isolates showed no synergistic effect of the addition of tazobactam. This lack of effect did not appear to be species associated (data not shown).
TABLE 1.
Cumulative percentages and MIC distributions for ceftolozane and ceftolozane-tazobactam against species of the B. fragilis group
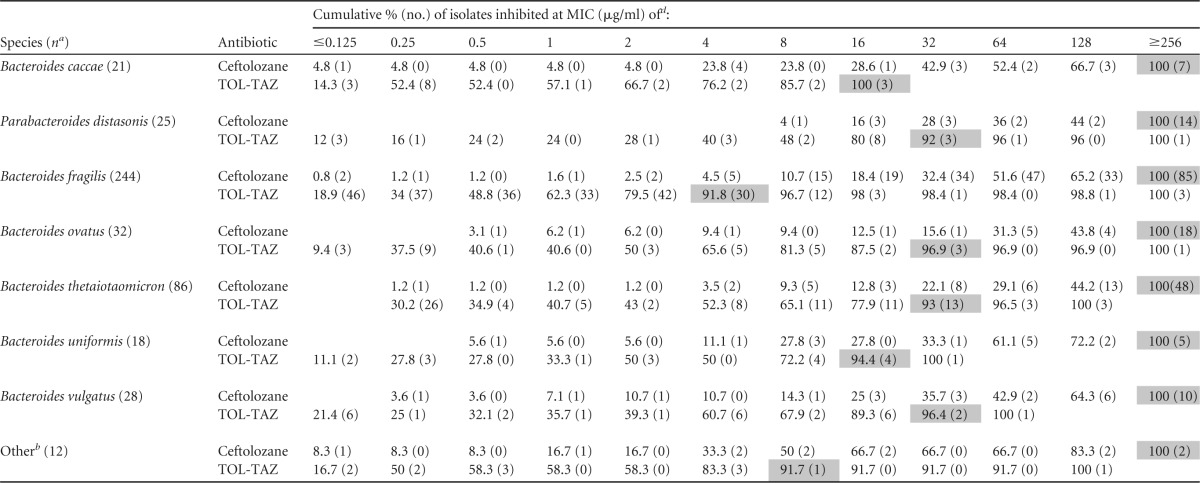
n, no. of isolates. Bacteroides thetaiota., Bacteroides thetaiotaomicron.
Included in this group are isolates of the following species: 2 of Bacteroides dorei, 4 of Parabacteroides goldsteinii, 2 of Bacteroides intestinalis, 1 of Parabacteroides johnsonii, 1 of Parabacteroides merdae, 1 of Bacteroides stercoris, and 1 not identified to the species level.
TOL-TAZ, ceftolozane-tazobactam.
Shaded areas indicate MIC90s.
Table 2 shows the comparative activities of ceftolozane-tazobactam and the other agents against the 605 anaerobic isolates tested. The combination was very active against other Gram-negative anaerobes included in the study, Fusobacterium spp. and Prevotella spp. All 12 isolates of Fusobacterium spp. were inhibited at a concentration of 0.25 μg/ml or less of ceftolozane-tazobactam, while all 33 Prevotella spp. isolates were inhibited at a concentration of 4 μg/ml or less of ceftolozane-tazobactam.
TABLE 2.
Activities of ceftolozane, ceftozolane-tazobactam, and comparative agents against anaerobic isolates
| Species or group (na) | Antibiotic | MIC (μg/ml) |
% Resb | ||
|---|---|---|---|---|---|
| Range | 50% | 90% | |||
| Bacteroides caccae (21) | Ceftolozane | ≤0.125–≥256 | 64 | ≥256 | NA |
| Ceftolozane-tazobactam | ≤0.125–16 | 0.25 | 16 | NA | |
| Ampicillin-sulbactam | ≤0.5–8 | 1 | 4 | 0 | |
| Piperacillin-tazobactam | ≤0.5–16 | ≤0.5 | 16 | 0 | |
| Moxifloxacin | ≤0.5–>16 | 2 | >16 | 33.3 | |
| Linezolid | ≤0.5–8 | 1 | 2 | 4.8 | |
| Tigecycline | ≤0.06–16 | 0.5 | 4 | 4.8 | |
| Imipenem | ≤0.125–0.5 | ≤0.125 | 0.25 | 0 | |
| Meropenem | ≤0.125–8 | 0.25 | 2 | 0 | |
| Ertapenem | ≤0.125–2 | 0.25 | 2 | 0 | |
| Clindamycin | ≤0.5–>128 | ≤0.5 | >128 | 19.0 | |
| Metronidazole | 1–2 | 1 | 2 | 0 | |
| Parabacteroides distasonis (25) | Ceftolozane | 8–≥256 | ≥256 | ≥256 | NA |
| Ceftolozane-tazobactam | ≤0.125–16 | 16 | 32 | NA | |
| Ampicillin-sulbactam | 1–128 | 8 | 32 | 16 | |
| Piperacillin-tazobactam | ≤0.5–64 | 2 | 8 | 0 | |
| Moxifloxacin | ≤0.5–>16 | 2 | 8 | 20 | |
| Linezolid | ≤0.5–8 | 2 | 4 | 4 | |
| Tigecycline | 0.5–16 | 2 | 4 | 4 | |
| Imipenem | ≤0.125–8 | 0.25 | 1 | 0 | |
| Meropenem | ≤0.125–2 | 0.25 | 1 | 0 | |
| Ertapenem | ≤0.125–8 | 0.5 | 4 | 0 | |
| Clindamycin | ≤0.5–>128 | 16 | >128 | 44 | |
| Metronidazole | 1–2 | 1 | 2 | 0 | |
| Bacteroides fragilis (244) | Ceftolozane | ≤0.125–≥256 | 64 | ≥256 | NA |
| Ceftolozane-tazobactam | ≤0.125–≥256 | 1 | 4 | NA | |
| Ampicillin-sulbactam | ≤0.5–≥256 | 2 | 8 | 1.1 | |
| Piperacillin-tazobactam | ≤0.5–≥256 | ≤0.5 | 2 | 0.6 | |
| Moxifloxacin | ≤0.5–>16 | 1 | 8 | 8.8 | |
| Linezolid | ≤0.5–4 | 1 | 2 | 0 | |
| Tigecycline | ≤0.06–16 | ≤0.5 | 2 | 0.2 | |
| Imipenem | ≤0.125–16 | ≤0.125 | 0.5 | 0.6 | |
| Meropenem | ≤0.125–16 | ≤0.125 | 0.5 | 1.3 | |
| Ertapenem | ≤0.125–16 | 0.25 | 1 | 1.3 | |
| Clindamycin | ≤0.5–>128 | ≤0.5 | >128 | 10.5 | |
| Metronidazole | 1–2 | 1 | 2 | 0 | |
| Bacteroides ovatus (32) | Ceftolozane | 1–≥256 | ≥256 | ≥256 | NA |
| Ceftolozane-tazobactam | ≤0.125–≥256 | 4 | 32 | NA | |
| Ampicillin-sulbactam | ≤0.5–8 | 1 | 8 | 0 | |
| Piperacillin-tazobactam | ≤0.5–32 | 1 | 8 | 0 | |
| Moxifloxacin | ≤0.5–>16 | 4 | 16 | 43.8 | |
| Linezolid | ≤0.5–2 | 1 | 2 | 0 | |
| Tigecycline | ≤0.06–4 | 0.25 | 2 | 0 | |
| Imipenem | ≤0.125–2 | ≤0.125 | 0.25 | 0 | |
| Meropenem | ≤0.125–8 | ≤0.125 | 0.5 | 0 | |
| Ertapenem | ≤0.125–8 | 0.5 | 2 | 0 | |
| Clindamycin | ≤0.5–128 | 1 | 128 | 15.6 | |
| Metronidazole | 1–2 | 1 | 2 | 0 | |
| Bacteroides thetaiotaomicron (86) | Ceftolozane | 0.25–≥256 | ≥256 | ≥256 | NA |
| Ceftolozane-tazobactam | ≤0.125–128 | 4 | 32 | NA | |
| Ampicillin-sulbactam | ≤0.5–64 | 1 | 8 | 2.3 | |
| Piperacillin-tazobactam | ≤0.5–32 | 1 | 8 | 0 | |
| Moxifloxacin | ≤0.5–>16 | 2 | >16 | 32.6 | |
| Linezolid | ≤0.5–16 | 1 | 2 | 2.3 | |
| Tigecycline | ≤0.06–16 | 0.25 | 2 | 1.2 | |
| Imipenem | ≤0.125–4 | ≤0.125 | 0.5 | 0 | |
| Meropenem | ≤0.125–4 | ≤0.125 | 1 | 0 | |
| Ertapenem | ≤0.125–8 | 0.25 | 2 | 0 | |
| Clindamycin | ≤0.5–>128 | 1 | >128 | 29.1 | |
| Metronidazole | 1–2 | 1 | 2 | 0 | |
| Bacteroides uniformis (18) | Ceftolozane | 0.5–≥256 | 64 | ≥256 | NA |
| Ceftolozane-tazobactam | ≤0.125–32 | 2 | 16 | NA | |
| Ampicillin-sulbactam | ≤0.5–8 | 1 | 2 | 0 | |
| Piperacillin-tazobactam | ≤0.5–16 | 1 | 2 | 0 | |
| Moxifloxacin | ≤0.5–>16 | 1 | 4 | 11.1 | |
| Linezolid | ≤0.5–16 | 1 | 2 | 11.1 | |
| Tigecycline | ≤0.06–4 | 0.125 | 0.5 | 0 | |
| Imipenem | ≤0.125–0.25 | ≤0.125 | 0.25 | 0 | |
| Meropenem | ≤0.125–0.5 | ≤0.125 | 0.25 | 0 | |
| Ertapenem | ≤0.125–2 | 0.25 | 0.5 | 0 | |
| Clindamycin | ≤0.5–128 | 1 | >128 | 27.8 | |
| Metronidazole | 1–2 | 1 | 2 | 0 | |
| Bacteroides vulgatus (28) | Ceftolozane | 0.25–≥256 | 128 | ≥256 | NA |
| Ceftolozane-tazobactam | <0.125–≥256 | 4 | 32 | NA | |
| Ampicillin-sulbactam | ≤0.5–16 | 4 | 16 | 0 | |
| Piperacillin-tazobactam | ≤0.5–16 | 2 | 8 | 0 | |
| Moxifloxacin | ≤0.5–>16 | >16 | 32 | 75 | |
| Linezolid | <0.5–4 | 1 | 2 | 0 | |
| Tigecycline | ≤0.06–4 | 0.5 | 2 | 0 | |
| Imipenem | ≤0.125–0.5 | 0.25 | 0.5 | 0 | |
| Meropenem | ≤0.125–1 | 0.25 | 0.5 | 0 | |
| Ertapenem | ≤0.125–2 | 0.5 | 1 | 0 | |
| Clindamycin | ≤0.5–>128 | ≤0.5 | >128 | 39.3 | |
| Metronidazole | 1–2 | 1 | 2 | 0 | |
| Other Bacteroidesc (12) | Ceftolozane | 0.25–≥256 | 8 | ≥256 | NA |
| Ceftolozane-tazobactam | ≤0.125–128 | 0.25 | 8 | NA | |
| Ampicillin-sulbactam | ≤0.5–8 | 0.5 | 8 | 0 | |
| Piperacillin-tazobactam | ≤0.5–4 | 0.5 | 2 | 0 | |
| Moxifloxacin | ≤0.5–>16 | ≤0.5 | 8 | 25 | |
| Linezolid | ≤0.5–4 | 1 | 2 | 0 | |
| Tigecycline | ≤0.06–8 | 0.25 | 2 | 0 | |
| Imipenem | ≤0.125–0.5 | ≤0.125 | 0.25 | 0 | |
| Meropenem | ≤0.125–8 | 0.5 | 8 | 0 | |
| Ertapenem | ≤0.125–8 | 0.5 | 4 | 0 | |
| Clindamycin | ≤0.5–>128 | ≤0.5 | 1 | 0 | |
| Metronidazole | 1–2 | 1 | 2 | 0 | |
| Prevotella spp. (33) | Ceftolozane | ≤0.125–≥256 | 16 | ≥256 | NA |
| Ceftolozane-tazobactam | ≤0.125–4 | ≤0.125 | 1 | NA | |
| Ampicillin-sulbactam | ≤0.5–4 | 1 | 4 | 0 | |
| Piperacillin-tazobactam | ≤0.5–4 | 0.5 | 2 | 0 | |
| Moxifloxacin | ≤0.5–16 | 2 | 8 | 9.1 | |
| Linezolid | ≤0.5–8 | 2 | 4 | 3 | |
| Tigecycline | ≤0.06–4 | ≤0.5 | 1 | 0 | |
| Imipenem | All ≤0.125 | ≤0.125 | ≤0.125 | 0 | |
| Meropenem | ≤0.125–0.5 | ≤0.125 | 0.25 | 0 | |
| Clindamycin | ≤0.5–>128 | ≤0.5 | >128 | 21.2 | |
| Metronidazole | 1–2 | 1 | 2 | 0 | |
| Fusobacterium spp. (12) | Ceftolozane | ≤0.125–16 | ≤0.125 | 16 | NA |
| Ceftolozane-tazobactam | ≤0.125–0.25 | ≤0.125 | 0.25 | NA | |
| Ampicillin-sulbactam | ≤0.5–1 | ≤0.5 | 1 | 0 | |
| Piperacillin-tazobactam | ≤0.5–1 | ≤0.5 | 8 | 0 | |
| Moxifloxacin | ≤0.5–4 | ≤0.5 | 4 | 0 | |
| Linezolid | ≤0.5–2 | ≤0.5 | 2 | 0 | |
| Tigecycline | ≤0.06–0.125 | ≤0.06 | 0.125 | 0 | |
| Imipenem | All ≤0.125 | ≤0.125 | ≤0.125 | 0 | |
| Meropenem | All ≤0.125 | ≤0.125 | ≤0.125 | 0 | |
| Clindamycin | ≤0.5–2 | ≤0.5 | 2 | 0 | |
| Metronidazole | All 1 | 1 | 1 | 0 | |
| Clostridium difficile (30) | Ceftolozane | 32–≥256 | ≥256 | ≥256 | NA |
| Ceftolozane-tazobactam | 0.25–≥256 | ≥256 | ≥256 | NA | |
| Ampicillin-sulbactam | ≤0.5–16 | 1 | 4 | 0 | |
| Piperacillin-tazobactam | ≤0.5–128 | 8 | 16 | 3.3 | |
| Moxifloxacin | ≤0.5–>16 | 2 | >16 | 43.3 | |
| Linezolid | ≤0.5–4 | 1 | 2 | 0 | |
| Tigecycline | ≤0.06–4 | ≤0.06 | 1 | 0 | |
| Imipenem | ≤0.125–16 | 4 | 8 | 3.3 | |
| Meropenem | ≤0.125–8 | 2 | 2 | 0 | |
| Vancomycin | ≤0.125–8 | 2 | 4 | NA | |
| Clindamycin | 1–16 | 2 | 16 | 23.3 | |
| Metronidazole | 1–4 | 2 | 4 | 0 | |
| Clostridium perfringens (11) | Ceftolozane | 0.5–64 | 1 | 64 | NA |
| Ceftolozane-tazobactam | ≤0.125–32 | 0.25 | 32 | NA | |
| Ampicillin-sulbactam | ≤0.5–1 | ≤0.5 | 1 | 0 | |
| Piperacillin-tazobactam | ≤0.5–2 | ≤0.5 | 2 | 0 | |
| Moxifloxacin | ≤0.5–4 | 1 | 4 | 0 | |
| Linezolid | 1–4 | 2 | 4 | 0 | |
| Tigecycline | 0.125–8 | 0.5 | 8 | 0 | |
| Imipenem | ≤0.125–0.25 | ≤0.125 | 0.25 | 0 | |
| Meropenem | All ≤0.125 | ≤0.125 | ≤0.125 | 0 | |
| Vancomycin | 1–2 | 1 | 2 | NA | |
| Clindamycin | ≤0.5–4 | 2 | 4 | 0 | |
| Metronidazole | 1–2 | 1 | 2 | 0 | |
| Clostridium spp.d (13) | Ceftolozane | 0.5–≥256 | ≥ 256 | ≥256 | NA |
| Ceftolozane-tazobactam | ≤0.125–≥256 | 16 | ≥256 | NA | |
| Ampicillin-sulbactam | ≤0.5–4 | ≤0.5 | 1 | 0 | |
| Piperacillin-tazobactam | ≤0.5–4 | 2 | 4 | 0 | |
| Moxifloxacin | ≤0.5–4 | 1 | 2 | 0 | |
| Linezolid | ≤0.5–4 | 2 | 4 | 0 | |
| Tigecycline | 0.125–4 | 2 | 4 | 0 | |
| Imipenem | ≤0.125–4 | 1 | 4 | 0 | |
| Meropenem | ≤0.125–2 | 0.25 | 2 | 0 | |
| Vancomycin | ≤0.125–4 | 1 | 4 | NA | |
| Clindamycin | 1–16 | 2 | 4 | 7.7 | |
| Metronidazole | 1–4 | 2 | 4 | 0 | |
| Anaerobic Gram+ cocci (31) | Ceftolozane | ≤0.125–≥256 | 4 | 16 | NA |
| Ceftolozane-tazobactam | ≤0.125–64 | 2 | 8 | NA | |
| Ampicillin-sulbactam | ≤0.5–2 | ≤0.5 | 1 | 0 | |
| Piperacillin-tazobactam | ≤0.5–16 | ≤0.5 | 2 | 0 | |
| Moxifloxacin | ≤0.5–16 | ≤0.5 | 16 | 9.7 | |
| Linezolid | ≤0.5–4 | 1 | 2 | 0 | |
| Tigecycline | ≤0.06–1 | 0.125 | 0.5 | 0 | |
| Imipenem | ≤0.125–4 | ≤0.125 | 0.25 | 0 | |
| Meropenem | ≤0.125–0.25 | ≤0.125 | ≤0.125 | 0 | |
| Vancomycin | ≤0.125–2 | 0.5 | 2 | NA | |
| Clindamycin | ≤0.5–16 | ≤0.5 | 2 | 3.2 | |
| Metronidazole | 1–16 | 1 | 2 | 0 | |
| Propionibacteriume spp. (9) | Ceftolozane | ≤0.125–16 | 0.5 | NA | |
| Ceftolozane-tazobactam | All ≤0.125 | ≤0.125 | NA | ||
| Ampicillin-sulbactam | All ≤0.5 | ≤0.5 | 0 | ||
| Piperacillin-tazobactam | All ≤0.5 | ≤0.5 | 0 | ||
| Moxifloxacin | All 1 | 1 | 0 | ||
| Linezolid | All 1 | 1 | 0 | ||
| Tigecycline | ≤0.06–0.5 | ≤0.06 | 0 | ||
| Imipenem | All ≤0.125 | ≤0.125 | 0 | ||
| Meropenem | ≤0.125–0.25 | ≤0.125 | 0 | ||
| Vancomycin | 0.25–1 | 0.5 | 0 | ||
| Clindamycin | ≤0.5–16 | ≤0.5 | 22.2 | ||
| Metronidazole | All >32 | >32 | 100 | ||
n, no. of isolates.
Percent resistance calculated using CLSI recommendations for most antimicrobial agents. For resistance breakpoints, refer to reference 9, CLSI document M11-A7. For tigecycline, the breakpoint for resistance of 16 μg/ml is the FDA recommendation. Please see reference 10, tigecycline package insert. NA, not applicable; there are no established breakpoints for these agents.
Includes isolates of the following Bacteroides species: 2 of Bacteroides dorei, 4 of Parabacteroides goldsteinii, 2 of Bacteroides intestinalis, 1 of Parabacteroides johnsonii, 1 of Parabacteroides merdae, 1 of Bacteroides stercoris, and 1 not identified to the species level.
Includes isolates of the following species: 3 of C. septicum, 1 of C. subterminale, 1 of C. tertium, 1 of C. cadaveris, 1 of C. clostridioforme, and 6 of Clostridium spp.
The number of Propionibacterium species isolates is less than 10; therefore, the MIC90 was not calculated.
The activity of ceftolozane-tazobactam varied against the Gram-positive anaerobic species. The combination showed excellent activity against the 9 Propionibacterium spp. (all isolates inhibited by ≤0.125 μg/ml), intermediate activity against anaerobic Gram-positive cocci (MIC90, 8 μg/ml), and very limited activity against Clostridium spp. (MIC90 range, 64 to >256 μg/ml).
This study demonstrates that the addition of tazobactam to ceftolozane enhances its activity against Bacteroides species. However, the enhanced activity is mostly limited to B. fragilis and to non-Bacteroides Gram-negative anaerobes. This might have clinical significance, since B. fragilis is the most commonly isolated species in intra-abdominal infections (6, 7). Ceftolozane-tazobactam will likely need to be used in combination with more potent antianaerobic agents for the management of intra-abdominal infections. For head and neck infections or aspiration pneumonia, the combination of ceftolozane and tazobactam may be sufficient, pending results from clinical trials.
ACKNOWLEDGMENT
This study was sponsored by a grant from Cubist Pharmaceutical, Lexington, MA.
Footnotes
Published ahead of print 25 November 2013
REFERENCES
- 1.Bulik CC, Christensen H, Nicolau DM. 2010. In vitro potency of CXA-101, a novel cephalosporin against Pseudomonas aeruginosa displaying various resistance phenotypes, including multidrug resistance. Antimicrob. Agents Chemother. 54:557–559. 10.1128/AAC.00912-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moya B, Zamorano L, Juan C, Perez JL, Ge Y, Oliver A. 2010. Activity of a new cephalosporin, CXA-101 (FR264205), against β-lactam-resistant Pseudomonas aeruginosa mutants selected in vitro and after antipseudomonal treatment of intensive care unit patients. Antimicrob. Agents Chemother. 54:1213–1217. 10.1128/AAC.01104-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sader HS, Rhomberg PR, Farell DJ, Jones RN. 2011. Antimicrobial activity of CXA-101, a novel cephalosporin tested in combination with tazobactam against Enterobacteriaceae, Pseudomonas aeruginosa, and Bacteroides fragilis strains having various resistance phenotypes. Antimicrob. Agents Chemother. 55:2390–2394. 10.1128/AAC.01737-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sader HS, Flamm RK, Farell DJ, Jones RN. 2012. Activity of the novel antimicrobial ceftolozane/tazobactam (CXA-201) tested against contemporary clinical strains from European hospitals, abstr P1443. 22nd European Congress of Clinical Microbiology and Infectious Diseases (ECCMID), London, England [Google Scholar]
- 5.Titelman EI, Karlsson M, Ge Y, Giske CG. 2011. In vitro activity of CXA-101 plus tazobactam (CXA-201) against CTX-M-14 and CTX-M-15-producing Escherichia coli and Klebsiella pneumoniae. Diagn. Microbiol. Infect. Dis. 70:137–141. 10.1016/j.diagmicrobio.2011.02.004 [DOI] [PubMed] [Google Scholar]
- 6.Snydman DR, Jacobus NV, McDermott LA, R. Ruthazer R., Golan Y, Goldstein EJC, Finegold SM, Harrell LJ, Hecht DW, Jenkins SG, Pierson C, Venezia R, Yu V, Rihs J, Gorbach SL. 2007. National survey on the susceptibility of B. fragilis group: report and analysis of trends from 1997–2004 in the United States. Antimicrob. Agents Chemother. 51:1649–1655. 10.1128/AAC.01435-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Snydman DR, Jacobus NV, McDermott LA, Golan Y, Hecht DW, Goldstein EJC, Harrell L, Jenkins SG, Newton D, Pierson C, Rihs J, Yu VL, Venezia R, Iannini P, Finegold SM, Gorbach SL. 2010. Lessons learned from the anaerobe survey: historical perspectives and review of the most recent data 2005-2007. Clin. Infect. Dis. 50(Suppl 1):S26–S33. 10.1086/647940 [DOI] [PubMed] [Google Scholar]
- 8.Snydman DR, Jacobus NV, McDermott LA, Golan Y, Goldstein EJC, Harrell LJ, Jenkins SG, Newton D, Pierson C, Rosenblatt J, Venezia R, Gorbach SL, Queenan AM, Hecht DW. 2011. Update on resistance of Bacteroides fragilis group and related species with special attention to carbapenems 2006-2009. Anaerobe 17:147–151. 10.1016/j.anaerobe.2011.05.014 [DOI] [PubMed] [Google Scholar]
- 9.Clinical and Laboratory Standards Institute 2007. Methods for antimicrobial susceptibility testing of anaerobic bacteria. Approved standard, CLSI document M11-A7. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 10.Pfizer 2012. Tygacil monograph: interpretative criteria for tygacil. Pfizer, New York, NY [Google Scholar]


