Abstract
Recent reports raised concerns about the role that farm stock may play in the dissemination of extended-spectrum β-lactamase (ESBL)-producing bacteria. This study characterized the ESBLs in two Escherichia coli and three Klebsiella pneumoniae subsp. pneumoniae isolates from cases of clinical bovine mastitis in the United Kingdom. Bacterial culture and sensitivity testing of bovine mastitic milk samples identified Gram-negative cefpodoxime-resistant isolates, which were assessed for their ESBL phenotypes. Conjugation experiments and PCR-based replicon typing (PBRT) were used for characterization of transferable plasmids. E. coli isolates belonged to sequence type 88 (ST88; determined by multilocus sequence typing) and carried blaCTX-M-15 and blaTEM-1, while K. pneumoniae subsp. pneumoniae isolates carried blaSHV-12 and blaTEM-1. Conjugation experiments demonstrated that blaCTX-M-15 and blaTEM-1 were carried on a conjugative plasmid in E. coli, and PBRT identified this to be an IncI1 plasmid. The resistance genes were nontransferable in K. pneumoniae subsp. pneumoniae isolates. Moreover, in the E. coli isolates, an association of ISEcp1 and IS26 with blaCTX-M-15 was found where the IS26 element was inserted upstream of both ISEcp1 and the blaCTX-M promoter, a genetic arrangement highly similar to that described in some United Kingdom human isolates. We report the first cases in Europe of bovine mastitis due to E. coli CTX-M-15 and also of bovine mastitis due to K. pneumoniae subsp. pneumoniae SHV-12 β-lactamases in the United Kingdom. We also describe the genetic environment of blaCTX-M-15 and highlight the role that IncI1 plasmids may play in the spread and dissemination of ESBL genes, which have been described in both human and cattle isolates.
INTRODUCTION
Worldwide, mastitis is one of the most important and costly infectious diseases of the dairy industry, affecting animal welfare and having potential public health implications if untreated or if inadequately treated milk is consumed. In the etiology of bovine mastitis, Gram-negative organisms such as Escherichia coli and Klebsiella pneumoniae are regarded as significant agents of environment-associated bovine mastitis. In the United Kingdom, the incidence of clinical mastitis in dairy herds varies from 45 to 65 cases per 100 cows per year, with E. coli being the second most frequently isolated pathogen after Streptococcus uberis, while K. pneumoniae is also a frequently isolated Gram-negative organism (1). Significantly, E. coli is the most common cause of toxic mastitis, an acute to peracute form of the disease that results in a higher incidence of death or culling of cows. Although less prevalent, Klebsiella infections appear to be particularly problematic due to their relatively long period of infection, leading to significant milk production losses and increased mortality of affected cows (2).
E. coli producing CTX-M-15 extended-spectrum β-lactamases (ESBLs) is a significant cause of both nosocomial and community infections in people and represents the most prevalent genotype of ESBL in the United Kingdom. Of 1,500 E. coli isolates producing CTX-M enzymes analyzed by the Health Protection Agency in the United Kingdom, 91% produced group 1 enzymes, with the majority of them being of the CTX-M-15 type (3). Farm animals are well recognized as a potential reservoir of ESBL-producing E. coli, and therefore, it has been considered that the spread of such resistant bacteria or determinants may occur via the food chain (4). In the United Kingdom, cattle, chicken, and turkey fecal samples have been analyzed for carriage of CTX-M-producing E. coli, and CTX-M-14 has been shown to be the predominant type found in cattle. However, CTX-M-15-producing strains have recently been isolated from a small number of bovine fecal samples (5). CTX-M-1-producing E. coli has been found to predominate in chickens and CTX-M-14 has been found to predominate in turkeys, although CTX-M-15-producing E. coli has also been recovered from both chickens and turkeys (6). Significantly, both serotyping and multilocus sequence typing (MLST) analyses have shown that the CTX-M-15-producing E. coli isolates found in cattle and chicken fecal samples are different from those found in humans in the United Kingdom, where the pandemic clone of sequence type 131 (ST131) predominates (6).
Reports of clinical infections due to ESBL-producing strains in animals and particularly in food-producing species are few in number. Locatelli et al. reported the isolation of a CTX-M-1-producing K. pneumoniae subsp. pneumoniae strain and a CTX-M-1-producing E. coli strain from cases of bovine mastitis in Italy (7, 8). Recently, Geser et al. also reported the isolation of an E. coli strain producing CTX-M-14/TEM-1 following analysis of 67 E. coli isolates from the milk of cattle with E. coli mastitis in Switzerland (4). Dahmen et al. also found CTX-M-14 to be the most prevalent ESBL type in clinical cattle mastitis in France (9), while CTX-M-15 was the predominant type of β-lactamase found in E. coli mastitis isolates in Japan (10).
Here, we report the first isolation, from bovine mastitic milk, in the United Kingdom and Europe of E. coli producing CTX-M-15 β-lactamase, the most prevalent type of ESBL in human infections in the United Kingdom and worldwide. We also report the first isolation and characterization of a Klebsiella pneumoniae subsp. pneumoniae strain harboring an SHV-12-type β-lactamase from bovine mastitic milk in the United Kingdom.
MATERIALS AND METHODS
Clinical isolates.
Seventeen bovine mastitic milk samples from one dairy farm from the northwest of England were submitted to the Liverpool Veterinary Diagnostic Laboratory for culture and sensitivity testing; the herd consisted of approximately 300 home-bred Brown Swiss × Holstein Friesian milking cows, plus dry cows and young stock. Due to ongoing problems with clinical mastitis in this herd, which had worsened in the month prior to the sampling, six milk samples were submitted for diagnostic investigation during June 2010. In each case, milk samples were collected aseptically from the affected quarter before any treatment was administered. Repeated samples were submitted from one cow, where the second sample was collected after therapy with a course of an intramammary preparation containing 75 mg cefquinome. This is a “fourth generation” cephalosporin which is used for the treatment of clinical mastitis in lactating dairy cows.
The bovine mastitic milk specimens were plated out on media specific for isolation and differentiation of the most common bovine pathogens associated with this condition; these were sheep blood agar, MacConkey agar, and Edwards's medium with sheep blood (all media were from Oxoid, Basingstoke, United Kingdom). The identification of isolates was performed using API 20E identification kits (bioMérieux, France) and GNID Sensititre identification plates (Trek Diagnostic Systems, West Sussex, United Kingdom).
Antimicrobial sensitivity testing.
The initial sensitivity testing of clinical isolates was performed by disc diffusion on Iso-Sensitest agar supplemented with horse blood according to the British Society of Antimicrobial Chemotherapy (BSAC) methodology using a farm animal antimicrobial sensitivity panel composed of penicillin G (P; 1.5 units), amoxicillin-clavulanic acid (AMC; 30 μg), sulfamethoxazole-trimethoprim (SXT; 25 μg), framycetin (FY; 100 μg), neomycin (N; 10 μg), streptomycin (S; 10 μg), tylosin (TY; 30 μg), ceftiofur (EFT; 30 μg), and cefquinome (CEQ; 30 μg) (all discs were from Oxoid, Basingstoke, United Kingdom). Interpretation of results was performed according to BSAC criteria (where available) or by the Clinical and Laboratory Standards Institute (CLSI) criteria for veterinary antimicrobials (11, 12). Current protocols applied in the Liverpool Veterinary Diagnostic Laboratory include routine screening and detection of organisms producing ESBLs in all clinical specimens. Cefpodoxime (10 μg) was used as the indicator cephalosporin for initial screening, and subsequently, all cefpodoxime-resistant isolates were tested for ESBL production by the double disc diffusion test (DDST) (13). For the later characterization of isolates, the MIC was performed by broth microdilution using Sensititre ESBL 96-well plates (ESB1F; Trek Diagnostic Systems, United Kingdom); interpretation of the results was performed according to CLSI criteria.
Molecular detection of genes for bacterial species and plasmids.
To identify the ESBL genes carried by the E. coli and Klebsiella pneumoniae subsp. pneumoniae isolates, cell lysates were subjected to PCR for the presence of blaCTX-M, blaSHV, blaTEM, and blaOXA, and multiplex PCR was used for the detection of family-specific plasmid-mediated AmpC β-lactamase genes (14–18). In addition, PCR for the uidA gene was performed to confirm that the isolates were E. coli (19). For detection of blaCTX-M, universal as well as group-specific (CTX-M-1, CTX-M-9) primers were used (20). Furthermore, the isolates were tested for plasmid-mediated quinolone resistance genes (qnrA, qnrB, and qnrS) (21). Specific PCR assays were performed with primers PROM+/PRECTX-M-3B as previously described, to identify the possible association of CTX-M-15 with the ISEcp1 or with the IS26 insertion element (22, 23). Subsequent to the conjugation experiments, PCR was also used to detect blaCTX-M, blaTEM, and IS26 and ISEcp1 elements in the transconjugants. O25b typing of E. coli isolates, based on allele-specific PCR, was performed as previously described with primers rfb1bis.f and rfbO25b.r to determine if isolates belonged to the pandemic human clone (24). All PCR products obtained for the blaCTX-M, blaSHV, and blaTEM genes and the ISEcp and IS26 elements were subject to DNA sequencing on both strands using the same sets of primers (Eurofins MWG Operon, United Kingdom), and the resulting DNA sequences from the isolates were compared (BLAST) against those in GenBank.
Typing of isolates by MLST and phylogroup typing.
To assign the E. coli isolates to a phylogenetic group, a triplex PCR for the chuA, yjaA, and tspE4C2 genes was performed as described by Clermont et al. (25). Molecular typing was performed for one representative E. coli isolate by MLST (http://mlst.ucc.ie/mlst/dbs/Ecoli) (26).
Resistance transfer and PCR-based replicon typing (PBRT).
To determine whether the ESBL phenotypes were transferable, two of the E. coli isolates [isolates 26(2104A) and 32(2188)] and one of the K. pneumoniae subsp. pneumoniae isolates [isolate 28(2104C)] were used for conjugation experiments with nalidixic acid-resistant E. coli K-12 as the recipient strain. The transconjugants were selected on nutrient agar (Oxoid) supplemented with nalidixic acid (30 μg/ml) and cefotaxime (1 μg/ml). The resistance phenotype of the parental strains and transconjugants was determined by disc diffusion using nalidixic acid (NA; 30 μg), gentamicin (GEN; 10 μg), ciprofloxacin (CIP; 1 μg), ampicillin (AMP; 10 μg), amoxicillin-clavulanic acid (AMC; 30 μg), tetracycline (T; 30 μg), cefpodoxime (CPD; 30 μg), cefotaxime (CTX; 30 μg), ceftazidime (CAZ; 30 μg), streptomycin (S; 10 μg), sulfamethoxazole-trimethoprim (SXT; 25 μg), and ceftiofur (EFT; 30 μg). The resistance profiles of the transconjugants were also determined from the MICs and compared with the resistance profiles of the donor; in addition, DDST was used to confirm the transfer of the ESBL phenotype.
Plasmid replicon typing was performed as described by Carattoli et al. (27) on the two E. coli isolates and one selected Klebsiella pneumoniae subsp. pneumoniae parental isolate 28(2104C). Furthermore, in order to identify the mobile genetic elements involved in the transfer of the resistance genes, both multiplex and simplex plasmid replicon typing assays were performed on the transconjugants and also on the E. coli K-12 recipient strain.
Nucleotide sequence accession number.
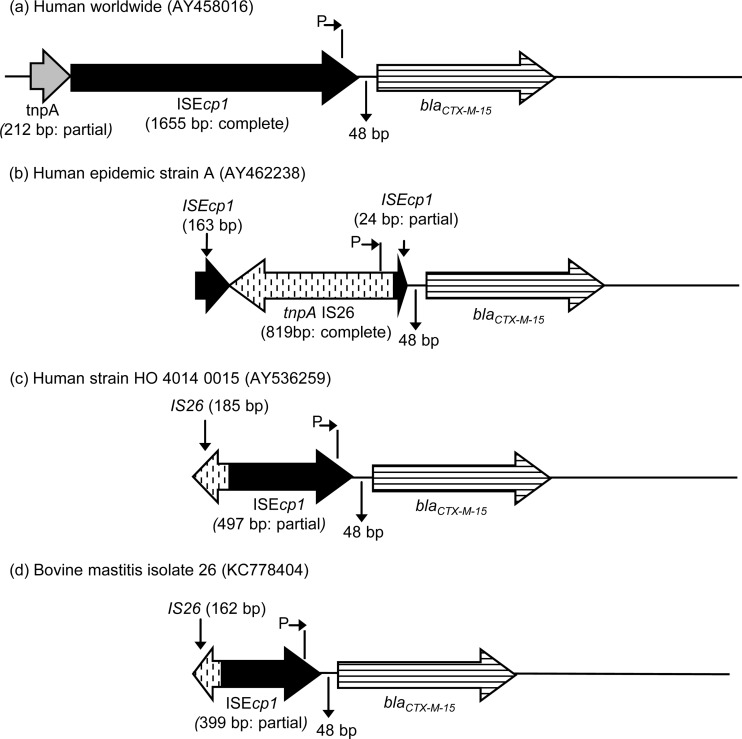
The IS26 nucleotide sequence of E. coli isolate 26(2104A) was deposited in GenBank under accession number KC778404 (see Fig. 1d).
FIG 1.
Schematic representations of environments surrounding blaCTX-M-15 in common human isolates (a to c) and bovine mastitis isolate 26 reported here (d). Where known, elements are qualified as partial or complete (due to primer locations, in some cases elements cannot be classified as either partial or complete).
RESULTS
Clinical isolates.
From the 17 mastitic milk samples submitted to the Liverpool Veterinary Diagnostic Laboratory for culture and sensitivity testing in 2010, two E. coli and three K. pneumoniae subsp. pneumoniae cefpodoxime-resistant isolates were obtained, and all were obtained from the same dairy farm from the northwest of England. Both E. coli isolates were obtained from the same cow [isolates 26(2104A) and 32(2188)], but from two separate samples submitted 10 days apart, where the first isolate was obtained prior to antimicrobial therapy, while the second one was obtained after treatment with cefquinome. Also, three isolates of K. pneumoniae subsp. pneumoniae were obtained from three other clinical mastitis cases [isolates 25(2053), 27(2104B), and 28(2104C)].
Antimicrobial sensitivity testing.
When tested by disc diffusion with the farm animal antibiotic panel, all five isolates were resistant to penicillin G, amoxicillin-clavulanic acid, co-trimoxazole, neomycin, streptomycin, tylosin, ceftiofur, and cefquinome and were sensitive only to framycetin. They were also resistant to cefpodoxime and gave a positive confirmatory result on the DDST, demonstrating the presence of the ESBL phenotype. The MIC values of the parental isolates and three of the transconjugants are summarized in Table 1. Determination of the MICs showed that all E. coli and Klebsiella isolates were resistant to ampicillin, cefazolin, cephalothin, cefotaxime, cefpodoxime, ceftazidime, and ceftriaxone. In addition, they were also resistant to gentamicin, and one Klebsiella pneumoniae subsp. pneumoniae isolate [27(2104B)] was also resistant to piperacillin-tazobactam. The presence of clavulanic acid reduced the MICs of cefotaxime and ceftazidime by 3 or more 2-fold concentrations in all 5 isolates and also in E. coli transconjugants, further confirming the presence of the ESBL phenotype.
TABLE 1.
Antimicrobial susceptibility profiles of bovine mastitis E. coli and Klebsiella pneumoniae subsp. pneumoniae isolates and selected transconjugantsa
| Antimicrobial | MIC (μg/ml)b |
|||||||
|---|---|---|---|---|---|---|---|---|
| Parental isolates |
Conjugants |
|||||||
| 25(2053) | 26(2104A) | 27(2104B) | 28(2104C) | 32(2188) | 26C(2104A) | 28C(2104C) | 32C(2188) | |
| AMP | 32 | 32 | 32 | 32 | 32 | 32 | 4 | 32 |
| CFZ | 32 | 32 | 32 | 32 | 32 | 32 | 4 | 32 |
| FEP | 2 | 4 | 8 | 4 | 8 | 8 | 2 | 8 |
| CTX | 4 | 32 | 32 | 16 | 64 | 32 | 0.12 | 32 |
| CTX-CLA | 0.25 | 0.06 | 0.5 | 0.5 | 0.25 | 4 | 0.06 | 4 |
| FOX | 128 | 2 | 128 | 128 | 2 | 128 | 16 | 128 |
| CPD | 64 | 64 | 64 | 64 | 64 | 64 | 1 | 64 |
| CAZ | 64 | 64 | 256 | 128 | 64 | 8 | 2 | 8 |
| CAZ-CLA | 0.5 | 0.06 | 0.5 | 0.5 | 0.25 | 2 | 0.25 | 2 |
| CRO | 16 | 64 | 64 | 32 | 128 | 64 | 0.5 | 64 |
| CEF | 32 | 32 | 32 | 32 | 32 | 32 | NM | 32 |
| CIP | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 4 | 0.5 | NM |
| GEN | 32 | 32 | 16 | 16 | 32 | 16 | 2 | 16 |
| IPM | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| MEM | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| PIP-TZB | 2 | 2 | 128 | 8 | 2 | 2 | 2 | 2 |
Abbreviations: AMP, ampicillin; CFZ, cefazolin; FEP, cefepime; CTX, cefotaxime; CTX-CLA, cefotaxime-clavulanic acid; FOX, cefoxitin; CPD, cefpodoxime; CAZ, ceftazidime; CAZ-CLA, ceftazidime-clavulanic acid; CRO, ceftriaxone; CEF, cephalothin; CIP, ciprofloxacin; GEN, gentamicin; IPM, imipenem; MEM, meropenem; PIP-TZB, piperacillin-tazobactam; NM, not measured.
Bold represents resistance by CLSI interpretation rules.
Molecular characterization.
The amplification of CTX-M class-encoding genes showed that both E. coli isolates carried genes of the CTX-M-1 group, while all three Klebsiella pneumoniae subsp. pneumoniae isolates carried blaSHV genes. Further sequence analysis of blaCTX-M identified that the two E. coli isolates produced CTX-M-15 enzymes and all three Klebsiella pneumoniae subsp. pneumoniae isolates produced SHV-12 β-lactamases. Together, all five isolates were found to be positive for the blaTEM gene, which was identified to be blaTEM-1, but were negative for the blaOXA and blaAmpC genes. None of the E. coli or Klebsiella isolates carried any of the qnrA or qnrB genes, but the latter were positive for qnrS (Table 2). The presence of the uidA gene confirmed the E. coli identification, but no amplicons were obtained in the O25b PCR.
TABLE 2.
Characteristics of β-lactamase and ESBL genes in two E. coli and three Klebsiella pneumoniae subsp. pneumoniae clinical mastitis isolates
| Isolatea | ESBL/fluoroquinolone genes in the isolates |
Presence of replicon type: |
ST by MLST | Phylogenetic group | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| blaCTX-M | blaSHV | blaTEM | blaQnrS | B/O | P1 | K/B | FIB | I1 | F | |||
| 25 (K. pneumoniae) | SHV-12 | TEM-1 | + | − | − | + | − | − | ||||
| 26 (E. coli) | CTX-M-15 | TEM-1 | − | + | − | − | + | + | + | ST88 | A | |
| 27(K. pneumoniae) | SHV-12 | TEM-1 | + | − | − | + | − | − | − | |||
| 28 (K. pneumoniae) | SHV-12 | TEM-1 | + | − | − | + | − | − | − | |||
| 32 (E. coli) | CTX-M-15 | TEM-1 | − | + | − | − | + | + | + | A | ||
| 26C | CTX-M-15 | TEM-1 | − | + | − | − | + | − | ||||
| 28C | − | + | − | − | − | − | ||||||
| 32C | CTX-M-15 | TEM-1 | − | + | − | − | + | − | ||||
| K-12 | − | + | − | − | − | − | ||||||
25, 26, 27, 28, and 32 correspond to 25(2053), 26(2104A), 27(2104B), 28(2104C), and 32(2188), respectively. Isolates 25, 26, 27, 28, and 32 are original isolates; isolates 26C, 28C, and 32C are transconjugants; and K-12 is the E. coli recipient strain.
When tested with primers PROM+/PRECTX-M-3B, both E. coli isolates produced PCR amplicons of the expected size (900 bp), which identified the link between CTX-M-15 and the ISEcp1 element. Moreover, they also produced amplicons with the IS26-specific primers, but these were not of the expected size of 400 bp but instead were 800 bp. Sequencing of the 800-bp product found IS26 placed upstream of the blaCTX-M promoter (Fig. 1d), showing a sequence nearly identical to the sequence deposited in GenBank from the study of Woodford et al. (22) (accession number AY536259; Fig. 1c), except for a 98-base indel (deletion) at the start of the ISEcp1 and a base substitution within the spacer region between ISEcp1 and blaCTX-M-15.
Following conjugation experiments, blaCTX-M, blaTEM, and IS26 and ISEcp1-like elements were detected by PCR in the E. coli transconjugants, while no blaSHV or blaTEM genes were found in the Klebsiella transconjugant [28(2104C)].
MLST of isolates.
Both E. coli isolates belonged to phylogenetic group A, and MLST analysis of one representative E. coli isolate showed that this was ST88 (clonal complex 23).
Resistance transfer and PCR-based replicon typing (PBRT).
The resistance profiles of the E. coli transconjugants were similar to those of the donor strains, demonstrating the transfer of antimicrobial resistance, including the ESBL phenotype. In addition, resistance to gentamicin cotransferred, along with the β-lactam resistance, while resistance to tetracycline or co-trimoxazole was not transferred. In contrast, no β-lactam, gentamicin, tetracycline, or co-trimoxazole resistance was transferred from the Klebsiella isolate.
Plasmid replicon typing showed that in the E. coli isolates the blaCTX-M-15-containing plasmids had multiple replicons positive for replicons repF, B/O, FIB, and I1, while the three Klebsiella pneumoniae subsp. pneumoniae parental isolates were positive for K/B replicons only (Table 2). Furthermore, PCR replicon typing of transconjugants and also of the recipient E. coli K-12 strain identified only 2 replicons, P1 and I1, in these cells. Replicon P1 was not found in any of the E. coli or Klebsiella donor strains but was present in all transconjugants and in the recipient strain (E. coli K-12), suggesting that it originated in the recipient strain. No other replicons were identified in the Klebsiella transconjugant, thus explaining the failure of transfer of the ESBL phenotype and resistance genes from the donor to the receptor strain. The replicons repF, B/O, and FIB which were found in the E. coli donors were absent in the transconjugants, with I1 being the only replicon found in both E. coli donors and transconjugants and associated with transfer of the ESBL phenotype.
DISCUSSION
In this study, we have characterized the ESBL genes and the conjugative plasmids in E. coli and Klebsiella pneumoniae subsp. pneumoniae isolates obtained from cases of bovine clinical mastitis in the United Kingdom. The two E. coli isolates were obtained from the same cow, and although these may represent duplicate isolates, both isolates were analyzed, as their repeated isolation (before and after cefquinome treatment) underlines the clinical significance of E. coli CTX-M-15 in this case of bovine mastitis. Determination of MICs showed that all E. coli and Klebsiella isolates were resistant to extended-spectrum cephalosporins and gentamicin. In addition, both E. coli isolates were sensitive to cefoxitin, while all three Klebsiella isolates were resistant to cefoxitin. Although no plasmid-mediated blaAmpC genes were detected in these isolates, the concurrent combination of different mechanisms, such as the production of SHV-12 enzymes and possible porin deficiency, may lead to this phenotype (28).
The insertion sequence ISEcp1 has previously been shown to play an important role in the mobilization and expression of genes encoding ESBLs (23), and therefore, linkage of blaCTX-M-15 with ISEcp1 was assessed and shown to be present in the two E. coli isolates but was absent in all three Klebsiella pneumoniae subsp. pneumoniae isolates. In the United Kingdom, most community and hospital-acquired ESBL E. coli isolates produce CTX-M-15 β-lactamases and have been assigned to 5 major clonally related epidemic strains (strains A to E) on the basis of their macrorestriction pulsed-field gel electrophoresis pattern. Strains B to E belong to serotype O25 and have an uninterrupted ISEcp–blaCTX-M-15, while strain A, the most widespread epidemic strain in humans in the United Kingdom, has an IS26 element inserted between the blaCTX-M-15 gene and its promoter found in ISEcp1 (22, 29). In addition, Woodford et al. developed a PCR assay to amplify a 400-bp fragment identifying an IS26–blaCTX-M-15 sequence and have shown that members of strain A produced amplicons of the expected size, while most isolates from the others strains did not (22). Screening of our E. coli isolates for the presence of such an IS26–blaCTX-M link generated PCR amplicons, but surprisingly, they were not of the expected size (400 bp) but, instead, were approximately 800 bp. Furthermore, Dhanji et al. showed that in E. coli isolates from the feces of travelers returning to the United Kingdom, seven different genetic arrangements can be found surrounding blaCTX-M-15, and most of these isolates harbored the international genetic arrangement, which is also found in the United Kingdom strain D (30). Sequencing showed a high degree of similarity between our sequence with GenBank accession number KC778404 and the sequence deposited in GenBank from the study of Woodford et al. (22) (accession number AY536259), and comparison of both of these with worldwide and United Kingdom epidemic strain A (30) sequences demonstrated that this genetic environment for the blaCTX-M-15 gene has local arrangements that are more similar to the arrangement of the worldwide strain than that of epidemic strain A. Moreover, besides the presence of a similar uninterrupted ISEcp1 element linked to blaCTX-M-15, our E. coli isolates were gentamicin resistant and were highly resistant to extended-spectrum cephalosporins, which are characteristics similar to those of United Kingdom E. coli human epidemic strains B to E (29). However, the United Kingdom human epidemic strains A to E belong to the international O25b:H4-ST131 clone (31), while MLST identified our E. coli isolate to be ST88 and phylogenetic group A. Lau et al. (31) characterized 88 human uropathogenic E. coli isolates from the northwest of England and showed that the isolates from the community were more diverse than those from hospitals specimens and that they belonged to 12 different STs, including 1 isolate that was ST88. Furthermore, the dissemination and potential interspecies transfer of E. coli ST88 are demonstrated by its recovery from cattle in France and Japan (10, 32), as well as from clinical hospital isolates in France and Spain (33, 34).
In our study, PBRT showed that in E. coli, IncI1 was the only replicon found in the transconjugants and that the blaCTX-M-15 gene was cotransferred with the blaTEM gene and IS26 and ISEcp1-like elements. In 2012, Madec et al. (32) showed that the ESBL gene was carried equally on IncI1 and IncFII plasmids in E. coli isolates from cattle and that these were highly related to plasmids in human E. coli isolates. Kirchner et al. (35) confirmed these data in England and Wales and showed that IncI1 plasmids were more likely to be transferred by conjugation than IncF plasmids. Our findings provide further evidence for the exchange of ESBL-carrying plasmids that may occur between human and animal E. coli isolates through the shared environment. The highly similar CTX-M-15 genetic environment between our E. coli isolates and those described in the study of Woodford et al. in 2004 (22) and the fact that, in our isolates, the ESBL genes are placed on IncI1 replicons described in both human and cattle populations in the United Kingdom suggest that plasmids may have a key role in disseminating blaCTX-M genes between animals and humans.
To our knowledge, this is the first report in the United Kingdom and Europe of E. coli isolates from cases of clinical bovine mastitis producing CTX-M-15, the most prevalent type of ESBL in human clinical specimens in the United Kingdom. K. pneumoniae subsp. pneumoniae harboring blaSHV-12 and blaTEM-1 β-lactamases has previously been reported from cases of bovine mastitis in Egypt (36), while this is the first report in the United Kingdom. These are important findings which emphasize the role which food-producing animals may play as a reservoir of resistant bacteria or resistance genes.
Our study shows that E. coli and Klebsiella isolates can harbor, respectively, CTX-M-15 and SHV-12 β-lactamases, conferring resistance to both ceftiofur and cefquinome. That this occurs among bovine clinical isolates emphasizes the clinical importance of detecting ESBL-producing bacteria in food production animals and the need for routine screening in veterinary diagnostic laboratories. Furthermore, to preserve the efficacy of extended-spectrum cephalosporins for the treatment of problematic cases of bovine mastitis or other clinical conditions, it is essential that culture and sensitivity testing always be performed, especially for recurrent infections, and therefore, empirical therapy should be avoided in such cases.
Footnotes
Published ahead of print 18 November 2013
REFERENCES
- 1.Bradley AJ, Leach KA, Breen JE, Green LE, Green MJ. 2007. Survey of the incidence and aetiology of mastitis on dairy farms in England and Wales. Vet. Rec. 160:253–258. 10.1136/vr.160.8.253 [DOI] [PubMed] [Google Scholar]
- 2.Grohn YT, Gonzalez RN, Wilson D, Hertl JA, Bennett G, Schulte H, Schukken YH. 2005. Effect of pathogen-specific clinical mastitis on herd life in two New York State dairy herds. Prev. Vet. Med. 71:105–125. 10.1016/j.prevetmed.2005.06.002 [DOI] [PubMed] [Google Scholar]
- 3.Hunter PA, Dawson S, French GL, Goossens H, Hawkey PM, Kuijper EJ, Nathwani D, Taylor DJ, Teale CJ, Warren RE, Wilcox MH, Woodford N, Wulf MW, Piddock LJV. 2010. Antimicrobial-resistant pathogens in animals and man: prescribing, practices and policies. J. Antimicrob. Chemother. 65(Suppl 1):i3–i7. 10.1093/jac/dkp433 [DOI] [PubMed] [Google Scholar]
- 4.Geser N, Stephan R, Haechler H. 2012. Occurrence and characteristics of extended-spectrum beta-lactamase (ESBL) producing Enterobacteriaceae in food producing animals, minced meat and raw milk. BMC Vet. Res. 8:21. 10.1186/1746-6148-8-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horton RA, Randall LP, Snary EL, Cockrem H, Lotz S, Wearing H, Duncan D, Rabie A, McLaren I, Watson E, La Ragione RM, Coldham NG. 2011. Fecal carriage and shedding density of CTX-M extended-spectrum beta-lactamase-producing Escherichia coli in cattle, chickens, and pigs: implications for environmental contamination and food production. Appl. Environ. Microbiol. 77:3715–3719. 10.1128/AEM.02831-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Randall LP, Clouting C, Horton RA, Coldham NG, Wu G, Clifton-Hadley FA, Davies RH, Teale CJ. 2011. Prevalence of Escherichia coli carrying extended-spectrum beta-lactamases (CTX-M and TEM-52) from broiler chickens and turkeys in Great Britain between 2006 and 2009. J. Antimicrob. Chemother. 66:86–95. 10.1093/jac/dkq396 [DOI] [PubMed] [Google Scholar]
- 7.Locatelli C, Caronte I, Scaccabarozzi L, Migliavacca R, Pagani L, Moroni P. 2009. Extended-spectrum beta-lactamase production in E. coli strains isolated from clinical bovine mastitis. Vet. Res. Commun. 33(Suppl 1):S141–S144. 10.1007/s11259-009-9263-y [DOI] [PubMed] [Google Scholar]
- 8.Locatelli C, Scaccabarozzi L, Pisoni G, Moroni P. 2010. CTX-M1 ESBL-producing Klebsiella pneumoniae subsp. pneumoniae isolated from cases of bovine mastitis. J. Clin. Microbiol. 48:3822–3823. 10.1128/JCM.00941-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dahmen S, Metayer V, Gay E, Madec J-Y, Haenni M. 2013. Characterization of extended-spectrum beta-lactamase (ESBL)-carrying plasmids and clones of Enterobacteriaceae causing cattle mastitis in France. Vet. Microbiol. 162:793–799. 10.1016/j.vetmic.2012.10.015 [DOI] [PubMed] [Google Scholar]
- 10.Ohnishi M, Okatani AT, Harada K, Sawada T, Marumo K, Murakami M, Sato R, Esaki H, Shimura K, Kato H, Uchida N, Takahashi T. 2013. Genetic characteristics of CTX-M-type extended-spectrum-beta-lactamase (ESBL)-producing Enterobacteriaceae involved in mastitis cases on Japanese dairy farms, 2007 to 2011. J. Clin. Microbiol. 51:3117–3122. 10.1128/JCM.00920-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clinical and Laboratory Standards Institute 2008. Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals; approved standard, 3rd ed. CLSI document M31-A3. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 12.British Society for Antimicrobial Chemotherapy 2011. Methods for antimicrobial susceptibility testing, version 10.2, May. British Society for Antimicrobial Chemotherapy, Birmingham, United Kingdom [Google Scholar]
- 13.M'Zali FH, Chanawong A, Kerr KG, Birkenhead D, Hawkey PM. 2000. Detection of extended-spectrum beta-lactamases in members of the family Enterobacteriaceae: comparison of the MAST DD test, the double disc and the Etest ESBL. J. Antimicrob. Chemother. 45:881–885. 10.1093/jac/45.6.881 [DOI] [PubMed] [Google Scholar]
- 14.Boyd DA, Tyler S, Christianson S, McGeer A, Muller MP, Willey BM, Bryce E, Gardam M, Nordmann P, Mulvey MR, Canadian Nosocomial Infection Surveillance Program. Health Canada 2004. Complete nucleotide sequence of a 92-kilobase plasmid harboring the CTX-M-15 extended-spectrum beta-lactamase involved in an outbreak in long-term-care facilities in Toronto, Canada. Antimicrob. Agents Chemother. 48:3758–3764. 10.1128/AAC.48.10.3758-3764.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liebana E, Gibbs M, Clouting C, Barker L, Clifton-Hadley FA, Pleydell E, Abdalhamid B, Hanson ND, Martin L, Poppe C, Davies RH. 2004. Characterization of beta-lactamases responsible for resistance to extended-spectrum cephalosporins in Escherichia coli and Salmonella enterica strains from food-producing animals in the United Kingdom. Microb. Drug Resist. 10:1–9. 10.1089/107662904323047745 [DOI] [PubMed] [Google Scholar]
- 16.Essack SY, Hall LMC, Pillay DG, McFadyen ML, Livermore DM. 2001. Complexity and diversity of Klebsiella pneumoniae strains with extended-spectrum beta-lactamases isolated in 1994 and 1996 at a teaching hospital in Durban, South Africa. Antimicrob. Agents Chemother. 45:88–95. 10.1128/AAC.45.1.88-95.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Costa D, Poeta P, Saenz Y, Vinue L, Rojo-Bezares B, Jouini A, Zarazaga M, Rodrigues J, Torres C. 2006. Detection of Escherichia coli harbouring extended-spectrum beta-lactamases of the CTX-M, TEM and SHV classes in faecal samples of wild animals in Portugal. J. Antimicrob. Chemother. 58:1311–1312. 10.1093/jac/dkl415 [DOI] [PubMed] [Google Scholar]
- 18.Perez-Perez FJ, Hanson ND. 2002. Detection of plasmid-mediated AmpC beta-lactamase genes in clinical isolates by using multiplex PCR. J. Clin. Microbiol. 40:2153–2162. 10.1128/JCM.40.6.2153-2162.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDaniels AE, Rice EW, Reyes AL, Johnson CH, Haugland RA, Stelma GN. 1996. Confirmational identification of Escherichia coli, a comparison of genotypic and phenotypic assays for glutamate decarboxylase and beta-d-glucuronidase. Appl. Environ. Microbiol. 62:3350–3354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Batchelor M, Hopkins K, Threlfall EJ, Clifton-Hadley FA, Stallwood AD, Davies RH, Liebana E. 2005. bla(CTX-M) genes in clinical Salmonella isolates recovered from humans in England and Wales from 1992 to 2003. Antimicrob. Agents Chemother. 49:1319–1322. 10.1128/AAC.49.4.1319-1322.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robicsek A, Strahilevitz J, Sahm DF, Jacoby GA, Hooper DC. 2006. qnr prevalence in ceftazidime-resistant Enterobacteriaceae isolates from the United States. Antimicrob. Agents Chemother. 50:2872–2874. 10.1128/AAC.01647-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woodford N, Ward ME, Kaufmann ME, Turton J, Fagan EJ, James D, Johnson AP, Pike R, Warner M, Cheasty T, Pearson A, Harry S, Leach JB, Loughrey A, Lowes JA, Warren RE, Livermore DM. 2004. Community and hospital spread of Escherichia coli producing CTX-M extended-spectrum beta-lactamases in the UK. J. Antimicrob. Chemother. 54:735–743. 10.1093/jac/dkh424 [DOI] [PubMed] [Google Scholar]
- 23.Poirel L, Gniadkowski M, Nordmann P. 2002. Biochemical analysis of the ceftazidime-hydrolysing extended-spectrum beta-lactamase CTX-M-15 and of its structurally related beta-lactamase CTX-M-3. J. Antimicrob. Chemother. 50:1031–1034. 10.1093/jac/dkf240 [DOI] [PubMed] [Google Scholar]
- 24.Clermont O, Lavollay M, Vimont S, Deschamps C, Forestier C, Branger C, Denamur E, Arlet G. 2008. The CTX-M-15-producing Escherichia coli diffusing clone belongs to a highly virulent B2 phylogenetic subgroup. J. Antimicrob. Chemother. 61:1024–1028. 10.1093/jac/dkn084 [DOI] [PubMed] [Google Scholar]
- 25.Clermont O, Bonacorsi S, Bingen E. 2000. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl. Environ. Microbiol. 66:4555–4558. 10.1128/AEM.66.10.4555-4558.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wirth T, Falush D, Lan RT, Colles F, Mensa P, Wieler LH, Karch H, Reeves PR, Maiden MCJ, Ochman H, Achtman M. 2006. Sex and virulence in Escherichia coli: an evolutionary perspective. Mol. Microbiol. 60:1136–1151. 10.1111/j.1365-2958.2006.05172.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carattoli A, Bertini A, Villa L, Falbo V, Hopkins KL, Threlfall EJ. 2005. Identification of plasmids by PCR-based replicon typing. J. Microbiol. Methods 63:219–228. 10.1016/j.mimet.2005.03.018 [DOI] [PubMed] [Google Scholar]
- 28.Garcia-Sureda L, Juan C, Domenech-Sanchez A, Alberti S. 2011. Role of Klebsiella pneumoniae LamB porin in antimicrobial resistance. Antimicrob. Agents Chemother. 55:1803–1805. 10.1128/AAC.01441-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Livermore DM, Warner M, Ward E, James D, Warren R, Woodford N. 2004. Inconsistent cephalexin resistance of CTX-M-15-producing Escherichia coli strains prevalent in the UK: implications for detection, p 146. Abstr. 44th Intersci. Conf. Antimicrob. Agents Chemother American Society for Microbiology, Washington, DC [Google Scholar]
- 30.Dhanji H, Patel R, Wall R, Doumith M, Patel B, Hope R, Livermore DM, Woodford N. 2011. Variation in the genetic environments of bla(CTX-M-15) in Escherichia coli from the faeces of travellers returning to the United Kingdom. J. Antimicrob. Chemother. 66:1005–1012. 10.1093/jac/dkr041 [DOI] [PubMed] [Google Scholar]
- 31.Lau SH, Kaufmann ME, Livermore DM, Woodford N, Willshaw GA, Cheasty T, Stamper K, Reddy S, Cheesbrough J, Bolton FJ, Fox AJ, Upton M. 2008. UK epidemic Escherichia coli strains A-E, with CTX-M-15 beta-lactamase, all belong to the international O25:H4-ST131 clone. J. Antimicrob. Chemother. 62:1241–1244. 10.1093/jac/dkn380 [DOI] [PubMed] [Google Scholar]
- 32.Madec J-Y, Poirel L, Saras E, Gourguechon A, Girlich D, Nordmann P, Haenni M. 2012. Non-ST131 Escherichia coli from cattle harbouring human-like bla(CTX-M-15)-carrying plasmids. J. Antimicrob. Chemother. 67:578–581. 10.1093/jac/dkr542 [DOI] [PubMed] [Google Scholar]
- 33.Cremet L, Caroff N, Giraudeau C, Dauvergne S, Lepelletier D, Reynaud A, Corvec S. 2010. Occurrence of ST23 complex phylogroup A Escherichia coli isolates producing extended-spectrum AmpC beta-lactamase in a French hospital. Antimicrob. Agents Chemother. 54:2216–2218. 10.1128/AAC.01580-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ortega A, Oteo J, Aranzamendi-Zaldumbide M, Bartolome RM, Bou G, Cercenado E, Carmen Conejo M, Jose Gonzalez-Lopez J, Marin M, Martinez-Martinez L, Merino M, Navarro F, Oliver A, Pascual A, Rivera A, Rodriguez-Bano J, Weber I, Aracil B, Campos J. 2012. Spanish multicenter study of the epidemiology and mechanisms of amoxicillin-clavulanate resistance in Escherichia coli. Antimicrob. Agents Chemother. 56:3576–3581. 10.1128/AAC.06393-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kirchner M, Wearing H, Hopkins KL, Teale C. 2011. Characterization of plasmids encoding cefotaximases group 1 enzymes in Escherichia coli recovered from cattle in England and Wales. Microb. Drug Resist. 17:463–470. 10.1089/mdr.2010.0151 [DOI] [PubMed] [Google Scholar]
- 36.Ahmed AM, Shimamoto T. 2011. Molecular characterization of antimicrobial resistance in Gram-negative bacteria isolated from bovine mastitis in Egypt. Microbiol. Immunol. 55:318–327. 10.1111/j.1348-0421.2011.00323.x [DOI] [PubMed] [Google Scholar]