Abstract
Antibiotic resistance among highly pathogenic strains of bacteria and fungi is a growing concern in the face of the ability to sustain life during critical illness with advancing medical interventions. The longer patients remain critically ill, the more likely they are to become colonized by multidrug-resistant (MDR) pathogens. The human gastrointestinal tract is the primary site of colonization of many MDR pathogens and is a major source of life-threatening infections due to these microorganisms. Eradication measures to sterilize the gut are difficult if not impossible and carry the risk of further antibiotic resistance. Here, we present a strategy to contain rather than eliminate MDR pathogens by using an agent that interferes with the ability of colonizing pathogens to express virulence in response to host-derived and local environmental factors. The antivirulence agent is a phosphorylated triblock high-molecular-weight polymer (here termed Pi-PEG 15–20) that exploits the known properties of phosphate (Pi) and polyethylene glycol 15-20 (PEG 15-20) to suppress microbial virulence and protect the integrity of the intestinal epithelium. The compound is nonmicrobiocidal and appears to be highly effective when tested both in vitro and in vivo. Structure functional analyses suggest that the hydrophobic bis-aromatic moiety at the polymer center is of particular importance to the biological function of Pi-PEG 15-20, beyond its phosphate content. Animal studies demonstrate that Pi-PEG prevents mortality in mice inoculated with multiple highly virulent pathogenic organisms from hospitalized patients in association with preservation of the core microbiome.
INTRODUCTION
During critical illness, the human gastrointestinal tract is subjected to multiple physiological perturbations due to the availability of modern life support measures. The ability to prolong life with extreme medical interventions results in a major disturbance in the gut microbial ecology, as the use of powerful broad-spectrum antibiotics becomes unavoidable. Such circumstances expose the intestinal microflora to an unprecedented degree of selective pressures whereby the normal “microbiome” (1) is rapidly replaced by a “pathobiome,” which consists of highly virulent and resistant health care-acquired pathogens (2–4). As such, the intestinal tract and its colonizing pathobiome become the “motor” of multiple organ failure and drive systemic inflammation, progressive organ damage, and ultimately the death of the patient.
To date, two approaches to prevent overgrowth of pathogens in the gut and their consequences have been tried in such circumstances that include replacing the lost microbiome with probiotic bacteria (5) or eliminating all potential pathogens by using oral antibiotics to decontaminate the gut (6). The former approach suffers from the false assumption that the core microbiome can be functionally replaced by a few known strains of probiotic bacteria, while the latter approach wrongly assumes that a more comprehensive antibiotic killing strategy can eliminate all harmful microbial agents for the long duration of the “at-risk” period of confinement. Here, we posit that a strategy toward containment rather than elimination is needed to prevent lethal sepsis from intestinal pathogens in order to avoid the risk of antibiotic resistance.
In this report, we critically examine a virulence-directed agent, phosphorylated high-molecular-weight polyethylene glycol 15-20 (PEG 15-20) with a hydrophobic bis-aromatic moiety at the polymer center. The rationale for delivering phosphate is based on the conserved response of a wide variety of microbes that express virulence and a lethal phenotype when the phosphate supply is limited (7–12). We covalently bonded phosphate (Pi) onto PEG 15-20, a high-molecular-weight polymer with an ABA structure (A is hydrophilic and B is hydrophobic) that we previously demonstrated distributes along the entire intestinal axis and remains functionally durable in mouse intestine (13, 14). As the data below demonstrate, Pi-PEG 15-20 combines the effect of inorganic phosphate and a specific ABA polymer, resulting in effective inhibition of the multidirectional signaling between microbes, pathogens, and the host response, such that lethal sepsis due to multidrug-resistant pathogens present in the gut is prevented.
MATERIALS AND METHODS
Microorganisms.
Microorganisms used in the experiments were isolated from stools of critically ill patients admitted to the intensive care units (ICU) at the University of Chicago. Isolates included Candida albicans ICU1-2, C. albicans ICU12, Pseudomonas aeruginosa ICU3-4, Enterococcus faecalis ICU1-2, Serratia marcescens ICU1-2, and Klebsiella oxytoca ICU1-2. The carbapenem-resistant pathogen Acinetobacter baumannii ATCC 19606 (KPC A. baumannii) was obtained from the American Type Culture Collection. The procedure of obtaining stool samples from critically ill patients was approved by the Institutional Review Board (IRB) at the University of Chicago (protocol IRB16494B).
Caenorhabditis elegans killing assay.
C. elegans N2 nematodes provided by the Caenorhabditis Genetic Center (CGC), University of Minnesota, were used in all experiments. For synchronization, nematodes were grown on Escherichia coli OP50 lawns until egg laying. Then, all nematodes were removed, and a new synchronized generation of nematodes was grown on E. coli OP50 lawns at 25°C up to the L4, young adult larval stage. Nematodes were then transferred onto new nonseeded plain agarized plates (diameter, 30 mm), and 1 ml of kanamycin (Km) solution (100 μg/ml), was poured on the plate. After 3 h, nematodes were transferred into 1.3 ml of 0.1× TY (tryptone/yeast extract) microbial cultures and incubated at room temperature with 7 to 10 worms per plate. Five plates per experiment were used for each group, with reiterative independent experiments performed. Worm mortality was tracked whereby worms were considered dead if they did not respond to the touch of a platinum picker.
Microbial cultures were prepared as follows. Cells from frozen stocks were plated on either tryptic soy broth (TSB) agarized medium in the case of bacteria or on yeast extract-peptone-dextrose (YPD; 1% [wt/vol] yeast extract, 2% [wt/vol] peptone, 2% [wt/vol] dextrose, 1.5% agar) agarized medium in the case of C. albicans. Plates were incubated overnight at 37°C, and a few colonies from the plates were picked to inoculate TY medium containing 10 g/liter tryptone (peptone from casein, pancreatic; EMD) and 5 g/liter yeast extract. The microbial cultures in TY were grown at 37°C under shaking conditions (200 rpm). For experiments, microbial cultures were diluted at a ratio of 1:100 in 0.1× TY (TY diluted 10-fold with water). Potassium phosphate buffer, pH 6.0, was included in the 0.1× TY to a final concentration of 0.1 to 25 mM, as indicated below. In selected groups, the kappa-opioid receptor agonist U-50,488 was added to a final concentration of 50 μM after 2 h of subgrowth in 0.1× TY medium. After a total of 4 h of growth in 0.1× TY medium, 1.3 ml of microbial culture adjusted to room temperature was poured in the 30-mm-diameter dishes into which nematodes were transferred. In the case of the whole assembled pathogen community, individual microbial cultures after 4 h of growth in 0.1× TY medium (with and without 50 μM U-50,488, as described above) were adjusted to an optical density (OD) of 0.07 and then mixed. C. elegans worms were transferred into the mixture as described above. For Pi-PEG 15-20-containing samples, Pi-PEG 15-20 was dissolved in 0.1× TY to a final concentration of 0.1 to 5% as indicated, and the pH was adjusted with KOH to an initial pH for 0.1× TY medium (pH 5.2). For PEG 15-20-containing samples, PEG 15-20 was dissolved in 0.1× TY to a final concentration of 0.1 to 5%. Microbial cultures from TY medium were diluted at a 1:100 ratio in Pi-PEG 15-20 and PEG 15-20 solutions and cultivated as described above. U-50,488 was added when needed, as described above.
Synthesis and characterization of Pi-PEG 15-20.
A flame-dried flask under an inert atmosphere was charged with PEG (18 g; ∼1.2 mmol). Tetrahydofuran (400 ml, distilled over lithium aluminum hydride [LAH]) was added via cannula. The solution was heated to 50°C, until the polymer was fully dissolved. Phosphorus oxychloride (2.24 ml; 12 mmol) was added at once via syringe. The solution was stirred under argon pressure for 3 h. The reaction was quenched by the addition of water (2 ml; 110 mmol). Tetrahydrofuran was removed under reduced pressure. The resulting solid was dissolved in 300 ml water and dialyzed against pure deionized water. The sample was lyophilized to give a white powder (12.1 g). 1H-nuclear magnetic resonance (NMR; 500 MHz, CDCl3): δ 7.08 ppm (b, 4H), 6.78 ppm (b, 4 H), 4.2 ppm (m, 7H), 3.62 ppm (b, 1000H), 1.59 (b, 6H). 31P-NMR (CDCl3): δ 1 ppm. FTIR (cm−1): 3450, 2945 2881, 1467, 1360, 1342, 1278. GPC (0.1M NaNO3 in H2O): Mn = 10,500 Da, PDI = 2.4. Mn (SEC) = 10,500; PDI = 2.4. Mw (SLS) = 26,000.
Mn and Mw/Mn were measured by size exclusion chromatography at 30°C in aqueous buffer (0.10 M NaNO3, 0.02% [wt/wt] NaN3) as eluent on three Waters ultrahydrogel columns (exclusion limit, 3 × 106; pore size, 5,000 Å; flow rate, 1 ml min−1) equipped with a Wyatt T-rEX RI detector, a Water 2998 PDA detector, and a Wyatt MiniDAWN Treos multiangle static light-scattering detector, with a dn/dc of 0.135 ml/g. The columns were calibrated against 13 PEG standards.
Infrared (IR) analyses were performed on a PerkinElmer Frontier Fourier transform infrared spectroscopy (FTIR) apparatus equipped with a single-bounce universal total attenuated reflection (ATR) accessory. A total of 16 scans were performed for each sample, with a resolution of 4 cm−1.
NMR was performed on a Bruker DMX 500 NMR apparatus. 1H-NMR data were recorded at 500 MHz, 13C data were recorded at 125 MHz, and 31P data were recorded at 202 MHz.
Synthesis of other phosphorylated polymers.
PEG-7K (Mw, 7,100); PEG-10K (Mw, 10,200), PEG-20K (Mw, 20,000), PEG-82K (Mw, 82,200), mPEG-5K (Mw, 5,200, monoadduct), P123 (Mw, 5,900, pluronic), and phosphorus oxychloride were purchased from Sigma-Aldrich. Tetrahydrofuran was dried overnight over lithium aluminum hydride and distilled under inert gas. Water was obtained from a Millipore Integral ultrapure water system dispensed at 18.3 MΩ-cm. Dialysis was performed using Thermo Scientific SnakeSkin dialysis tubing with a 35,000 molecular weight cutoff.
DLS of Pi-PEG 15-20.
Dynamic light scattering (DLS) was performed on a Brookhaven BI-200SM light-scattering apparatus equipped with a 632-nm laser. Measurements were performed at 45°, 90°, and 135° at concentrations ranging from 0.1 to 5 mg/ml. Solutions were prepared by filtering an aqueous solution of the polymer through a 0.2-μm filter, lyophilizing the solution, and dissolving the polymer in 0.2 μM in filtered water at the desired concentrations.
Transmission electron microscopy (TEM) of Pi-PEG 15-20.
A 5% Pi-PEG 15-20 water solution was prepared, and 10 μl of solution was dropped onto a glow-discharged 400-mesh Formvar/carbon-coated copper support grid. For 30 s, the grid was washed several times with Tris-EDTA buffer and stained briefly with 1% uranyl acetate. The extra drops of uranyl acetate were removed from the grid, and the samples were viewed with the FEI Tecnai F30 transmission electron microscope operated at 300 kV and equipped with a Gatan charge-coupled-device (CCD) digital micrograph.
Immunoelectron microscopy of the LPS interaction with Pi-PEG 15-20.
Lipopolysaccharide (LPS) isolated from E. coli 0111B4 was obtained from Sigma (catalog number L3024). A stock solution was prepared by reconstitution of LPS in dimethyl sulfoxide followed by the preparation of 1% LPS water solution or 1% LPS in 5% Pi-PEG water solution. A 10-μl aliquot of solution was dropped onto 200-mesh Formvar-coated nickel grids. Grids were washed with phosphate-buffered saline (PBS) and then treated with 1% bovine serum albumin (BSA) for 20 min, followed by transferring to a mixture of goat polyclonal anti-LPS antibodies (LS-C71709/37211; Life Span Biosciences) diluted 1:10 in 1% BSA. After incubation in a humidified chamber for 4 h at room temperature, extensive washing with PBS and blocking with 0.5% BSA for 25 min was performed, followed by 1 h of incubation in the humidified chamber with rabbit anti-goat IgG conjugated with 10-nm gold particles (Ted Pella) at a 1:10 dilution in 0.5% BSA. Finally, grids were washed with PBS three times, fixed with 1% glutaraldehyde in PBS for 10 min, washed with water three times, and stained briefly with saturated uranyl acetate and then with 2.5% lead citrate. After drying in air at room temperature, they were viewed with the FEI Tecnai F30 transmission electron microscope operated at 300 kV equipped with a Gatan CCD digital micrograph.
Scanning electron microscopy of the Pi-PEG 15-20 effect on microbial population structures.
P. aeruginosa MPAO1 was grown in TSB, TSB supplemented with 200 μM U-50,488, or TSB plus U-50,488 plus 5% Pi-PEG 15-20 for 8 h, 37°C, 150 rpm. Bacterial cultures were dropped onto glass coverslips coated with poly-l-lysine (catalog number P8920; Sigma-Aldrich). Cells were fixed in 3% glutaraldehyde buffered with 0.1 M phosphate buffer, pH 7.2, washed with 0.1 M phosphate buffer, and dehydrated in a graded ethanol solution in water (30% increased gradually to 100%; 20 min each). The samples were dried with a Leica CPD300 critical point dryer and coated with Pt(80)/Pd(20) of an 8- to 12-nm thickness by using a Cressington sputter coater, model 208HR. Samples were viewed with a FEI Nova NanoSEM230 scanning electron microscope.
In the case of pathogen community ICU1-2, the individual member stocks of C. albicans ICU1-2, E. faecalis ICU1-2, Serratia marcescens iCU1-2, and Klebsiella oxytoca ICU1-2 were prepared as a solution with an OD at 600 nm (OD600) of 1.0 in 0.1× TY and mixed in 0.1× TY or 0.1× TY plus 5% Pi-PEG 15-20 to a final density of 0.025 each. The mixture was grown for 8 h, followed by fixation on poly-l-lysine-coated coverslips with 3% glutaraldehyde in phosphate buffer and postfixation with 1% osmium tetroxide in phosphate buffer. The samples were dried with a Leica CPD300 critical point dryer and coated with Pt(80)/Pd(20) to an 8- 12-nm-thickness using a Cressington sputter coater model 208HR. Samples were viewed with an FEI Nova NanoSEM230 scanning electron microscope.
Imaging of P. aeruginosa and C. albicans in the intestinal tubes of C. elegans.
P. aeruginosa MPAO1 was grown in low-nutrient 0.1× TY medium in the presence of 50 μM U-50,488 with and without 5% Pi-PEG 15-20, as described for the C. elegans mortality assays. After 4 h of growth, microbial cells were collected by centrifugation at 6,000 rpm for 5 min and stained with PKH67 green fluorescent dye (Sigma), following the manufacturer's protocol. Fluorescent cells were resuspended in the initial supernatants, and prestarved worms were transferred in microbial cultures as previously described (10). Accumulation of microbes within the worm digestive tube was tracked by fluorescence microscopy.
Transmission electron microscopy of the intestinal tube of C. elegans.
Wild type N2 Bristol worms were synchronized by hypochlorite treatment. Eggs were incubated in M9 solution O/N, and then L1 were transferred in NGM liquid culture with E. coli OP50 (low-rotate shaker at room temperature). After ∼120 to 130 h (5 to 5.5 days), L4/adult worms were collected, washed with sterile distilled water, and allowed to fast for 4 h in Km solution (50 μg/ml). P. aeruginosa MPAO1 was grown in low-nutrient 0.1× TY medium in the presence of 50 μM U-50,488 with and without 5% Pi-PEG 15-20 as described for the C. elegans mortality assays. Then, bacterial cultures (7 ml) were poured into 25-ml flasks into which prestarved C. elegans (3,000 4,000 nematodes) were transferred and incubated for 5 to 6 h with slow shaking at room temperature. Following this procedure, nematodes were collected and transferred to paraformaldehyde buffer (2.0% glutaraldehyde, 4.0% paraformaldehyde in 0.1 M sodium cacodylate buffer, pH 7.4) for 2 h at a temperature equal to that of the nematodes (room temperature in the current experiments), followed by overnight incubation at 4°C. Buffer was then removed, and nematodes were incubated in 1.0% osmium tetroxide, 0.1 M cacodylate buffer for 60 min, followed by washing samples with cacodylate buffer. Samples were rinsed with maleate buffer, pH 5.1, and stained in 1% uranyl acetate in maleate buffer. Samples were then dehydrated through a graded series of ethanol washes to 100% and infiltrated with propylene oxide (2:1 propylene oxide, Spurr resin twice for 30 min; 1:1 propylene oxide, Spurr resin twice for 30 min and then overnight, and finally 100% Spurr resin 6 times for 60 min each). Spurr was polymerized with the embedded cells into 60°C oven for 1 to 2 days. Ninety-nanometer sections were cut using a Reichert Ultracut E ultramicrotome and collected on Formvar-coated gold grids. Sections were poststained with uranyl acetate and lead citrate and viewed using a 300 kV FEI Tecnai F30 electron microscope, and images were collected with a Gatan CCD digital micrograph. For each observation, whenever possible, at least 10 cross-sections were evaluated, and representative images were chosen.
Pyoverdin production.
Pyoverdin was measured by fluorescence as previously described (11).
Biofilm production.
Biofilm was measured by staining adherent cells with crystal violet as previously described (11).
QRT-PCR.
P. aeruginosa MPAO1 was grown in defined citrate medium (DCM) plus 0.1 mM potassium phosphate buffer, pH 6.0, supplemented with either 25 mM potassium phosphate buffer or 5% PEG 15-20, or 5% Pi-PEG 15-20 for 5 h, 37°C, 180 rpm (each group in triplicate). Cell cultures were then immediately diluted with 2 volumes of RNA Protect solution (Qiagen; 2 ml cell culture plus 4 ml RNA Protect). RNA was isolated by using an Ultraclean microbial RNA isolation kit (MoBio). DNA was removed with a Turbo DNA-free kit (Ambion). Ten nanograms of RNA (measured with a Qubit 2.0; Invitrogen) was transformed to cDNA by using a high-capacity RNA-to-cDNA kit (Applied Biosystems). One microliter of cDNA was used in the quantitative reverse transcriptase PCR (qRT-PCR) analysis in a total 10-μl reaction mixture containing SYBR green (Platinum SYBR green qPCR super mix-UDG with ROX; catalog number 11744-100; Invitrogen) and primers at a 0.2 μM final concentration. Each sample was run in triplicate with RT (+RT) or without RT (-RT, a negative control to ensure complete DNA removal). The -RT samples were obtained from 10 ng of RNA processed with the high-capacity RNA-to-cDNA kit without including the RT enzyme mix. Blank controls in which cDNA was replaced with water were run for each set of primers. The qPCR was performed with a 7900HT Fast real-time PCR system (Applied Biosystems). The program for amplification had an initial heat step at 50°C for 2 min, followed by a denaturation step at 95°C for 15 s, and then 40 cycles of 95°C for 15 s and 60°C for 1 min. The specificity of the reaction was monitored by melt-curve analysis following the real-time program. Gene expression was estimated relative to the expression of the housekeeping gene PA47480 as an example: 2−[Ct(pstS treatment − PstS control) − Ct(PA4748 treatment − PA4748 control)]. Average and standard deviation values (Microsoft Excel) of three biological replicates were calculated.
The primers were designed with Primer3 software: PstS-F, 5′-GGCGGCCCTGACGTTCGT-3′; PstS-R, 5′-CGACACACCGCTGGCTTTCTG-3′; PvdS-F, 5′-CAAGCAGGCGCTCGAACAGAA-3′; PvdS-R, 5′-CGCGTAGTTGATGTGCGAGGTTT-3′; PqsA-F, 5′-GGTGGACCGCGAAGGACACA-3′; PqsA-R, 5′-TTCGCTGGCCCGCCAGTA-3′; PhzA1-F, 5′-CAGGGCTATTGCGAGAACCACTACA-3′; PhzA1-R, 5′-CACGCAGTTTCTGTATCGGGTTCA-3′; PA4748-F, 5′-AACAAGCAAGGCGGCATCACA-3′; PA4748-R, 5′-TGCACGGTACGCATTCCAGTGT3-3′.
IL-8 assay.
The concentrations of interleukin-8 (IL-8) were determination in an enzyme-linked immunosorbent assay (ELISA; BD OptEIA; BD Bioscience, San Diego, CA) in accordance with the manufacturer's protocol as outlined in experiments using transformed human colonic HT-29 epithelial cells (ATCC HTB 38; American Type Culture Collection, Bethesda, MD). HT-29 cells were used between passages 19 and 34 and grown in Dulbecco's minimal essential medium (DMEM; Gibco, Long Island, NY) supplemented with 10% fetal bovine serum (Gibco), 2 mM l-glutamine (Gibco), and antibiotics (penicillin at 100 U/ml, streptomycin at 100 μg/ml). Cells were cultured in a water-saturated atmosphere of 95% air–5% CO2. Before stimulation, the monolayers were pretreated for 30 min with serum-free DMEM, containing when needed 5% Pi- PEG 15-20. As a positive control, LPS from Escherichia coli (150 ng/ml; strain 0111:B4; Sigma) was added for 1 h, then medium was replaced with serum-free DMEM and incubated for another 6 h. Supernatants of culture media were collected and frozen at −20°C until use in the ELISA. To prepare the assembled pathogen community, Enterococcus faecalis ICU1-2, Klebsiella oxytoca ICU1-2, and Serratia marcescens ICU1-2 were grown in TSB and Candida albicans ICU1-2 was grown in YPD medium overnight at 37°C. Microbial pellets were harvested by centrifugation (4,000 × g, 6 min, 20°C) and resuspended in serum-free DMEM to a final OD600 of 1.0, and the community was assembled by mixing microbial solutions at equal volumes. Four microliters of microbial mixture was dropped on the top of serum-free DMEM (400 μl volume) on HT-29 monolayers. After 1 h of coincubation of HT-29 with the microbial community, the medium was replaced with serum-free DMEM containing gentamicin (20 μg/ml) and then incubated for another 6 h. Supernatants of culture media were collected and frozen at −20°C until analysis. Experiments were carried out in triplicate, and averages are reported.
Mouse model of gut-derived sepsis.
C. albicans, E. faecalis, K. oxytoca, and S. marcescens were grown overnight in TSB before all experiments. Colonies were suspended in liquid TSB medium for 1 h and then adjusted to a final OD600 of 0.10. All four microbe species were then combined together in equal amounts. One milliliter of the bacterial suspension was centrifuged at 6,000 rpm for 5 min, the excess TSB was removed, and the remaining pellet was resuspended in the same volume of either sterile 10% glycerol or 5% glycerol plus 5% Pi-PEG 15-20. A 200-μl aliquot of the microbial suspension was injected into mouse cecum as described below.
All experiments were approved by the Institutional Animal Care and Use Committee at the University of Chicago (protocol 71744). Male C57B/L6 mice weighing 18 to 22 g and that were 6 to 8 weeks old were used for all experiments. Mice were routinely fed tap water and Harland Teklad feed (Madison, WI) under 12-h light/dark cycles and were allowed to acclimate for at least 48 h before surgery. Approximately 18 h prior to surgery, mice received a 1-ml enema of either tap water or 5% Pi-PEG 15-20. At that time, each of the mice also received subcutaneously a 1-ml bolus of normal saline. Animals were allowed only tap water, ad libitum, for the next 72 h. On the day of surgery, the mice were sterilely draped and first underwent a 30% hepatectomy. This was immediately followed by an inoculation of 200 μl of the bacterial community suspension into the cecum. For those mice that had received a water enema preoperatively, they were injected with the community suspended in 10% glycerol. For the mice that had received a Pi-PEG 15-20 enema, they were inoculated with the community suspended in 5% glycerol plus 5% Pi-PEG 15-20. These injections were performed with slight occlusion of the cecum so that there was reflux into the terminal ileum. The mice were then followed for 1 week postoperatively for mortality.
IL-6 and IL12p40 assays in dendritic cells exposed to cecal content lysates.
Dendritic cells (DCs) were isolated from mouse bones as previously described (15–17) and were stimulated for differentiation using granulocyte-macrophage colony-stimulating factor (GM-CSF). Cecal contents were isolated from mice and vortexed with zirconia beads for 10 min to prepare cecal content lysates. Lysates were centrifuged at 10,000 × g for 30 s to remove beads, and supernatants were sterilized using a 0.22-μm filter, aliquoted, and frozen until use. The protein concentration in cecal lysates was measured and normalized to upload 25 μg of total protein in one well of a 48-well plate, each well containing 500,000 bone marrow-derived DCs (BMDCs). Plates were incubated at 37°C, without shaking, for 24 h. For IL analysis, secreted fractions of BMDCs were used, for which conditioned medium was collected and centrifuged at 6,000 × g, 4°C, 5 min. The supernatant was used in an ELISA (BD Biosciences).
Proteomic analysis.
Samples of cecal contents were thawed on ice and were digested by using trypsin (Promega). Briefly, the protein concentration was determined by a bicinchoninic acid protein assay (Thermo Scientific, Rockford, IL). Samples were adjusted to equal volumes and combined with 7 M urea and 5 mM dithiothreitol (DTT), followed by incubation for 30 min at 60°C with shaking. Trypsin was resuspended into 150 mM ammonium bicarbonate and warmed to 37°C for 10 min before use. After 10-fold dilution of each sample with 150 mM ammonium bicarbonate, CaCl2 was added to 1 mM and trypsin was added to each sample at a ratio of 1 unit trypsin:50 units protein. Samples were incubated for 3 h at 37°C with gentle shaking, and then peptide samples underwent strong cation exchange solid-phase extraction.
Peptide samples were analyzed using a nanocapillary LC column (18, 19) coupled to an LTQ-Orbitrap Velos apparatus (Thermo Fisher Scientific, San Jose, CA). The heated capillary temperature and spray voltage were 350°C and 2.3 kV. For each cycle, the 10 most abundant ions from the MS analysis were selected for tandem MS (MS/MS) analysis. For all analyses, data acquisition occurred over an m/z range of 400 to 2,000. Raw spectra were searched with MS-GF+ (20) against a mouse gut commensal metagenome sequence (21), and peptide results were filtered using the MS-GF+ SpecProb value of ≤1 × 10−11 (false-discovery rate, 2.6%). Peptide observation counts and spectral counts were calculated using Microsoft Access, and principal component analysis (PCA) plots were generated using the software Inferno (http://code.google.com/p/inferno4proteomics/wiki/Introduction).
RESULTS
Synthesis of Pi-PEG 15-20.
The parent polymer in this study, PEG 15-20 (commercial name of a polymer purchased from Aldrich) was chosen because of previous work demonstrating its ability to protect the intestinal epithelium (13, 22). This polymer consists of ∼9-kDa PEG arms appended to a bis-phenol A moiety at the center of the polymer chain, giving an ABA structure wherein the B group is small but very hydrophobic and A groups are hydrophilic homopolymers (see Fig. S1 in the supplemental material). As a consequence of how the polymer is synthesized, there are primary alcohols at the chain ends as well as secondary alcohols near the hydrophobic core, both of which are potential sites for functionalization with phosphate. Faced with a variety of potential chemistries by which phosphate could be covalently attached to PEG 15-20, we chose the reaction with phosphorus oxychloride (POCl3) and subsequent hydrolysis with water to give phosphate groups at available alcoholic sites (23–25). This route is advantageous in that the high reactivity of phosphorus oxychloride helps to overcome problems associated with end-functionalization of higher-molecular-weight polymers and facilitates near-complete reaction of chain ends of the polymers synthesized in this manner. Furthermore, the by-products of the hydrolysis of polymer-bound phosphoryl chloride and excess phosphorus oxychloride, phosphoric acid and hydrochloric acid, are both water soluble and easily removable by dialysis. By limiting the reagents used to distilled tetrahydrofuran, phosphorus oxychloride, PEG 15-20, and water, we limited the possibility of deleterious by-products and avoided the use of additional organic solvents in purification by precipitation. The resultant polymers were analyzed by 31P-NMR to confirm the presence of phosphate (see Fig. S1b) and by 1H-NMR (see Fig. S1c) to determine the degree of functionalization by using end-group analysis. Using the number-average molecular weight determined by size exclusion chromatography, the average number of phosphate groups per polymer chain was determined to be 3.5 by integration of the alpha protons to phosphate groups. This corresponds to a degree of functionalization of the available alcohols of 85 to 90%. The aggregation behavior of Pi-PEG 15-20 was studied in water solution. Light-scattering analysis demonstrated the ability of Pi-PEG 15-20 to form aggregates of 100 to 200 nm (see Fig. S1d and e). This was confirmed by transmission electron microscopy when multiple-form aggregates of >200-nm diameter were visualized (see Fig. 2c, below).
FIG 2.
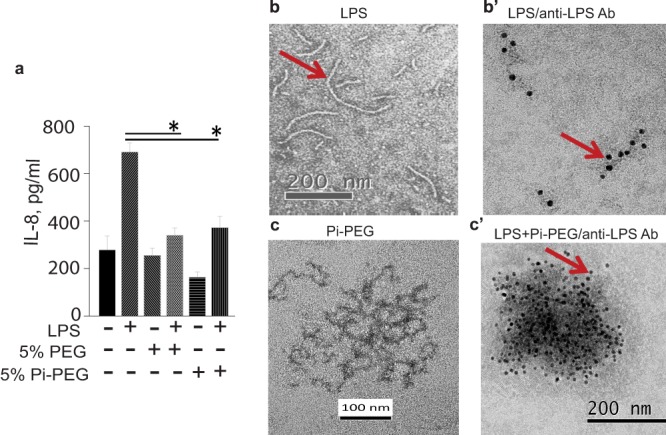
Pi-PEG 15-20 and PEG 15-20 inhibit LPS-induced IL-8 release from intestinal epithelial cells. (a) PEG 15-20 and Pi-PEG 15-20 attenuated LPS-mediated IL-8 release in HT-29 cells. n = 3. *, P < 0.05 (Student t test). (b and b′) TEM (b) and immunoelectron microscopy (IEM) (b) images of LPS. (c) TEM image of a Pi-PEG aqueous solution. (c′) IEM image of Pi-PEG plus LPS stained with anti-LPS antibodies.
Pi-PEG 15-20 suppresses environmental and cell-to-cell signaling. (i) Environmental signaling.
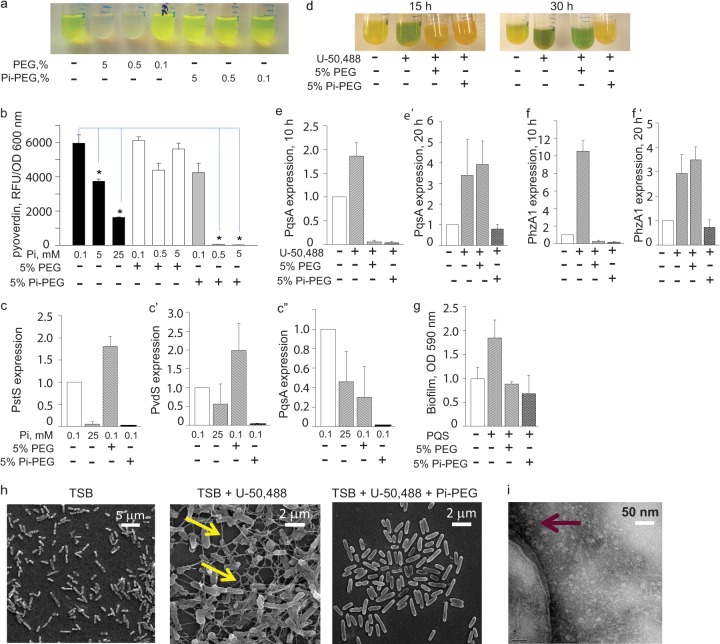
We used P. aeruginosa MPAO1 as a model microorganism and defined the role of the phosphate content in Pi-PEG 15-20 on signaling pathways involved in phosphate-, iron-, and quorum-sensing regulation, all of which are known to be altered in response to the extracellular phosphate concentration (10, 11). We first grew P. aeruginosa MPAO1 in low-phosphate/low-iron defined citrate medium supplemented with 0.1 mM Pi (DCM) and with varying doses of Pi-PEG 15-20 (0.1, 0.5, and 5%), or PEG 15-20. After overnight incubation, we visualized production of the yellow-green fluorescent pigment pyoverdin in MPAO1 in both DCM and DCM supplemented with two concentrations of PEG 15-20. In contrast to the nonphosphorylated polymer, 5% and 0.5% Pi-PEG 15-20 suppressed the production of the fluorescent pigment (Fig. 1a). Next, in reiterative experiments, we tested the inhibitory effect of inorganic phosphate (0.1, 5.0, and 25 mM potassium phosphate buffer, pH 6.0) compared to Pi-PEG 15-20 and to PEG 15-20. This was carried out by measuring pyoverdin production at a fluorescence of 400/460 nm (excitation/emission) normalized to cell density, as previously described (11). The results demonstrated a superior inhibitory effect of Pi-PEG 15-20 (Fig. 1b). Next, we isolated RNA from the cells and analyzed the expression of pstS, which encodes the high-affinity phosphate binding protein PstS (9, 26–28), pvdS, which encodes PvdS, the transcriptional regulator of pyoverdin biosynthesis, and pqsA, the first gene of the pqsABCDE operon, which is involved in quinolone biosynthesis. The results demonstrated that Pi-PEG 15-20 completely suppressed the phosphate- (PstS), iron- (PvdS), and quorum-sensing (PqsA) regulated systems, while PEG 15-20 only attenuated the expression of pqsA (Fig. 1c and c″).
FIG 1.
Pi-PEG 15-20 suppresses environmental and cell-to-cell signaling. (a) Gross images of the typical yellow-green fluorescent appearance of P. aeruginosa pyoverdin grown in DCM. (b) Pyoverdin production normalized to cell density in the presence of various concentrations of phosphate, PEG 15-20, and Pi-PEG 15-20 in DCM. n = 3/group. *, P < 0.01 (Student t test), from two independent experiments. (c to c″).QRT-PCR to determine the effect of Pi, PEG 15-20, and Pi-PEG 15-20 on pstS (c), pvdS (c′), and pqsA (c″) expression in MPAO1 grown in DCM. (d) Gross images of the typical green pigmentation appearance of P. aeruginosa grown in TSB spiked with opioids. (e and f) QRT-PCR to determine the effect of PEG 15-20 and Pi-PEG 15-20 on pqsA and phzA1 expression in MPAO1 exposed to the opioid U-50,488 (50 μM) for 10 h (e and f) or 20 h of growth (e′ and f′). (g) Biofilm production by MPAO1 grown in TSB spiked with PQS (40 μM) with or without PEG 15-20 or Pi-PEG 15-20. n = 5/group. *, P < 0.05 (Student t test), from two independent experiments. (h) SEM images demonstrating Pi-PEG 15-20-mediated inhibition of extracellular matrix (biofilm) formation produced in the presence of opioid U-50,488. (i) TEM image of P. aeruginosa cells incubated with Pi-PEG 15-20, demonstrating the high-density electron halo effect at the cell membrane (arrow).
(ii) Host-to-bacterium signaling.
We next grew MPAO1 in nutrient-rich TSB medium spiked with an opioid to mimic a host stress signal, using the kappa-specific receptor ligand U-50,488. This synthetic opioid, similar to the endogenous kappa-opioid dynorphin, which is known to be released during physiologic stress, is a potent inducer of pyocyanin, which can be easily visualized as green pigmentation in P. aeruginosa cells grown in TSB (11). Confirming our previously published results, U-50,488 (200 μM) induced bright green pigmentation that was seen at 15 h of growth (Fig. 1d). Both the nonphosphorylated and phosphorylated polymers suppressed the production of pyocyanin at this time point. However, later, at 30 h of incubation, only Pi-PEG 15-20 continued to prevent pyocyanin production (Fig. 1d), which lasted for the duration of bacterial growth (3 days). In agreement with our previously published data (10, 11), the expression of pqsA (a gene involved in quinolone synthesis) and phzA1 (a gene involved in pyocyanin biosynthesis) were induced by the opioid U-50,488 (Fig. 1e and f). Both PEG 15-20 and Pi-PEG 15-20 suppressed pqsA and phzA1 at 10 h (Fig. 1e and f), and only Pi-PEG 15-20 suppressed the expression of both genes at 20 h (Fig. 1e′ and f′), suggesting that Pi-PEG 15-20 is more resistant to bacterial degradation. This may be a result of the known ability of phosphate to suppress the expression of multiple bacterial proteases (11).
Bacterium-to-bacterium signaling.
The Pseudomonas quinolone signal (PQS) is among several quorum-sensing signaling molecules known to induce biofilm production (29, 30). We therefore determined if PEG polymers could inhibit PQS-mediated biofilm production. MPAO1 was grown in TSB spiked with 40 μM PQS, and biofilm production was measured after 8 h of growth. As seen in Fig. 1g, both PEG 15-20 and Pi-PEG 15-20 suppressed PQS-mediated biofilm production. The opioid U-50,488 also induced biofilm formation (see Fig. S2 in the supplemental material). This was not surprising, given that opioids induce PQS synthesis (11). Using scanning electron microscopy (SEM), we examined biofilm formation in MPAO1. As seen in Fig. 1h, an extracellular matrix developed in the presence of the synthetic opioid that was suppressed by PEG 15-20. Pi-PEG 15-20's effect did not appear to be due to alterations in bacterial growth. Although Pi-PEG 15-20 slowed the growth of MPAO1 in TSB at exponential phase, it allowed for equal cell density to be achieved at stationary phase (see Fig. S3 in the supplemental material). Finally, by using TEM we demonstrated high-density aggregates of Pi-PEG at the bacterial cell surface (Fig. 1i), suggesting that Pi-PEG may concentrate phosphate at the bacterial membrane.
Bacterium-to-host signaling.
Next, we determined if Pi-PEG 15-20 could interrupt bacterium-to-host signaling. To address this, we tested the ability of Pi-PEG 15-20 to inhibit bacterial LPS-induced IL-8 release in the human intestinal epithelial cell line HT-29. IL-8 is the main chemokine released by intestinal epithelial cells in response to pathogen-associated molecular pattern molecules that activate Toll-like receptors (31, 32). Therefore, HT-29 cells were apically exposed to LPS in the presence and absence of 5% Pi-PEG 15-20 or 5% PEG 15-20. Results demonstrated significant inhibition of LPS induced IL-8 secretion in HT-29 cells in the presence of both polymers (Fig. 2a). We then performed immunoelectron microscopy with anti-LPS antibodies to determine if Pi-PEG 15-20 could trap extracellular LPS. As seen in Fig. 2c′, LPS became trapped in Pi-PEG 15-20 aggregates. Thus, it appears that Pi-PEG may attenuate microbial pathogenicity at multiple points.
Pi-PEG 15-20 prevents mortality of C. elegans caused by P. aeruginosa, attenuates P. aeruginosa stasis within the intestinal tube of C. elegans, and preserves intestinal integrity.
Previous work from our laboratory demonstrated how nutrient depletion and opioid exposure, as might occur in the gut during critical illness, shift P. aeruginosa to express a lethal phenotype against C. elegans (10). Therefore, we used these conditions to determine the comparative protective effect of PEG 15-20, Pi-PEG 15-20, and free inorganic phosphate on C. elegans survival. Nematodes were fed P. aeruginosa MPAO1 in nutrient-limited medium (0.1× TY supplemented with 0.1 mM potassium phosphate buffer, pH 6.0) spiked with 50 μM U-50,488 as previously described (10). The results indicated that 5% Pi-PEG 15-20 conferred a significant and superior protective effect against P. aeruginosa compared to 5% PEG 15-20 or 5 mM potassium phosphate buffer, pH 6.0 (Fig. 3a). The protective effect of Pi-PEG 15-20 was not attributable to its effect on P. aeruginosa growth, since in nutrient-poor medium, Pi-PEG 15-20 actually enhanced the growth of P. aeruginosa (see Fig. S4 in the supplemental material), perhaps owing to its ability to deliver phosphate to bacterial cells. To track microbial stasis, we labeled P. aeruginosa with PKH67 and tracked its movement over time. Figure 3b demonstrates stasis of P. aeruginosa under conditions of nutrient depletion and opioid exposure, whereas the addition of Pi-PEG 15-20 prevented stasis of P. aeruginosa within the digestive tube of C. elegans (Fig. 3b′). We next examined the ability of Pi-PEG 15-20 to preserve epithelial surface ultrastructure and integrity of the C. elegans digestive tube during exposure to P. aeruginosa by using TEM analysis. First, we performed TEM of control nematodes feeding on E. coli OP50 lawns grown on nematode growth agarized medium. TEM demonstrated intact epithelial villi and an intact glycocalyx (Fig. 4a). When C. elegans fed on P. aeruginosa in liquid low-nutrient medium (0.1× TY) spiked with the kappa-specific opioid U-50,488, profound changes were observed, including a distended intestine (Fig. 4b), destroyed villi and direct contact of the epithelial surface with bacterial cells (Fig. 4d), and a disintegrated glycocalyx (Fig. 4e). The addition of 5% Pi-PEG 15-20 resulted in attenuated distension and formation of a visual barrier (Fig. 4c), intact and structured villi, and a well-formed glycocalyx (Fig. 4f).
FIG 3.
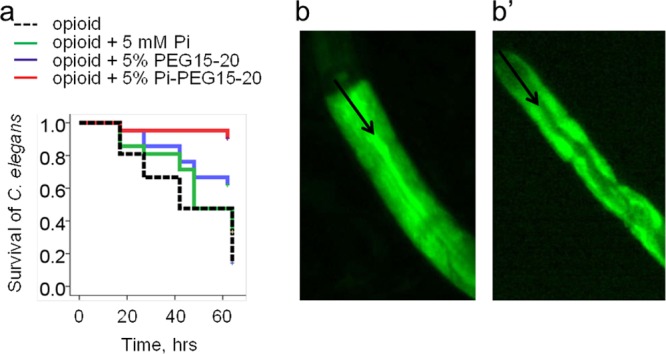
Pi-PEG 15-20 protects C. elegans against mortality due to P. aeruginosa and accumulation of P. aeruginosa in the intestinal tube of C. elegans. (a) Kaplan-Meier survival curves of C. elegans feeding on P. aeruginosa MPAO1. n = 63/group. P < 0.01 for the Pi-PEG 15-20 group compared to all other groups, based on 3 independent experiments. (b and b′) Microscopy images of intestinal tubes of C. elegans after overnight coincubation with P. aeruginosa MPAO1 stained with PKH67 without (b) or with (b′) 5% Pi-PEG 15-20. Black arrows indicate the intestinal tube lumen of C. elegans.
FIG 4.
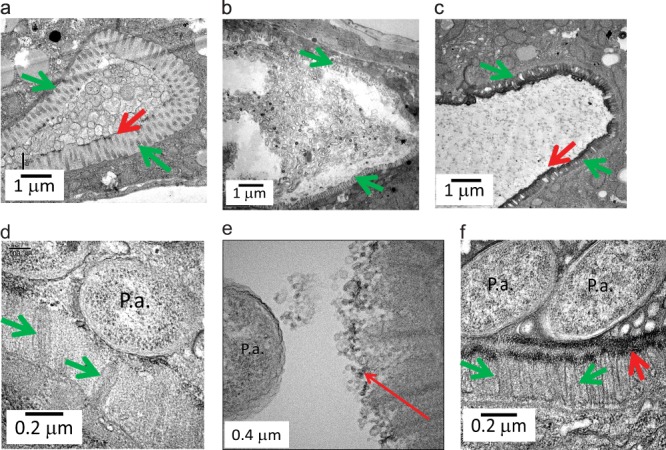
TEM images of the intestinal tube of C. elegans, demonstrating the protective effect of Pi-PEG 15-20 against disintegration caused by P. aeruginosa. (a) An intact glycocalyx (red arrow) and villi (green arrow) of the intestinal tube of C. elegans feeding on E. coli OP50 grown on nematode growth agarized medium. (b, d, and e) Dysregulated villi (green arrow) and disrupted glycocalyx (red arrow) in C. elegans feeding on P. aeruginosa MPAO1 in liquid 0.1× TY medium spiked with 50 μM U-50,488. (c and f) Formation of a protective layer (red arrows) and intact villi (green arrows) in C. elegans feeding on MPAO1 in 0.1× TY plus 50 μM U-50,488 plus 5% Pi-PEG 15-20. P.a., cells of P. aeruginosa.
The ABA structure and phosphate are critical determinants for the biologic function of Pi-PEG 15-20.
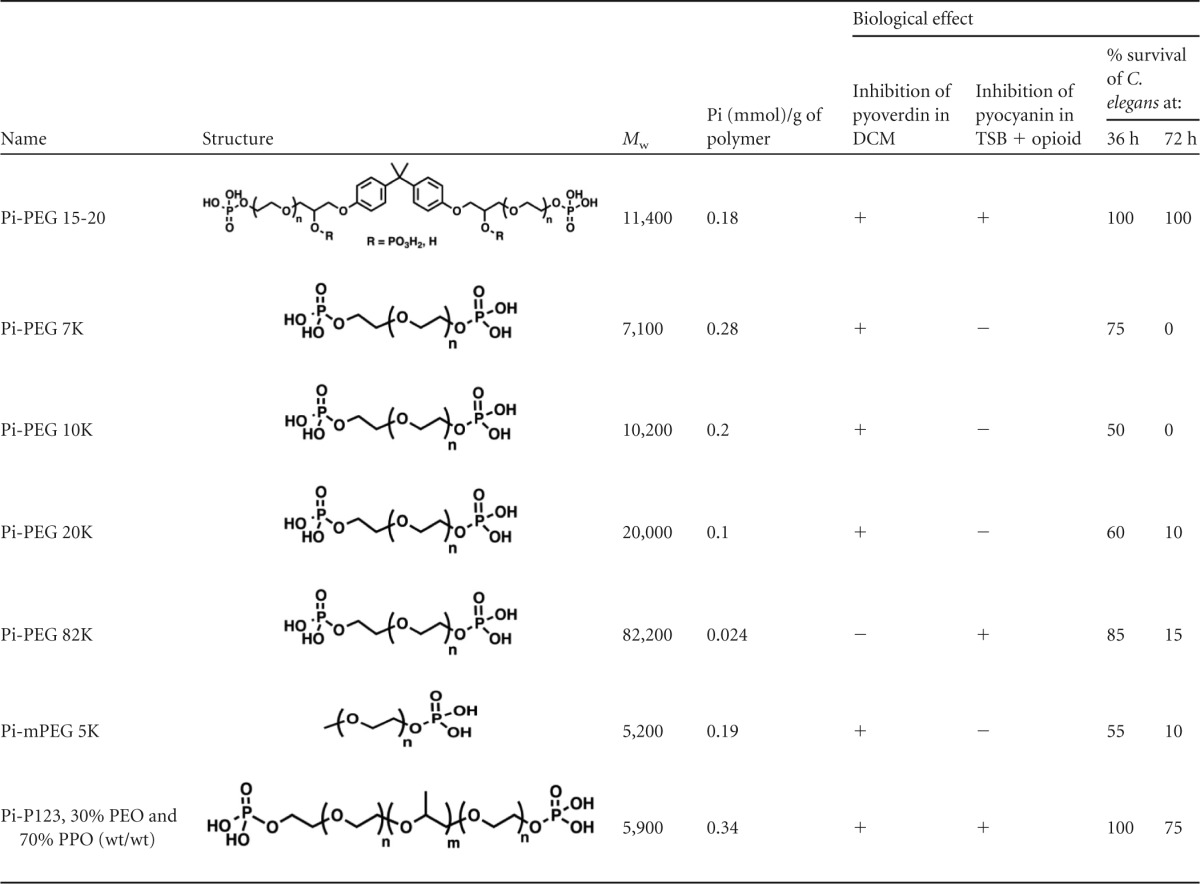
We next investigated if there are structural requirements of the PEG polymer that are critical for its protective effect when functionalized by phosphate. We synthesized a set of phosphorylated compounds with and without the hydrophobic core and with different lengths of homopolymers and tested them in biologic assays for pyoverdin and pyocyanin production. As outlined above, the pyoverdin assay reflects the response of P. aeruginosa to environmental physico-chemical signals, such as iron and phosphate depletion, whereas the pyocyanin assay, performed by exposing P. aeruginosa to the opioid U-50,488, reflects host-to-bacterium signaling. The results indicated that the presence of phosphate in the polymer was required to suppress pyoverdin, whereas polymer phosphate content was not as important to suppress pyocyanin as was the polymer structure itself (Table 1). For example, Pi-PEG 15-20 and Pi-P123, both of which conform to the ABA structure (hydrophilic/hydrophobic/hydrophilic) suppressed pyocyanin. Pi-PEG-82K, a long homopolymer, appeared to be an exception, as it suppressed pyocyanin but not pyoverdin. However, as seen in Table 1, its relative phosphate concentration was 5- to 10-fold lower than in all other tested phosphorylated polymers, which may explain its failure to prevent pyoverdin production. The ability to protect against pyocyanin (opioid signaling) may be explained by its interaction with bacterial membranes, as high-Mw homopolymers change their interaction with membranes (33). Importantly, the compounds that demonstrated the protective effect against both pyoverdin and pyocyanin production displayed the highest level of protection against P. aeruginosa-induced mortality of C. elegans nematodes, suggesting that suppressing the virulence in response to physico-chemical signals (low iron, low phosphate) and host-derived compensatory signals (opioids) confers a superior biological response in vivo. Supply of inorganic phosphate at 5 mM, corresponding to the concentration of phosphate bound to 5% Pi-PEG 15-20, only partially protected against C. elegans mortality (Fig. 3a) or pyoverdin production in DCM medium (Fig. 1b) and did not prevent pyocyanin production induced by opioid in nutrient-rich medium. Taken together, these findings further confirm the potential advantage of PEG 15-20 as a vehicle for phosphate delivery, while also providing protective functionality.
TABLE 1.
Correlation of phosphorylated polyethylene glycol-based polymer structures to their biological functions
Pi-PEG 15-20 suppresses virulence across various individual pathogens and in an assembled multipathogen community.
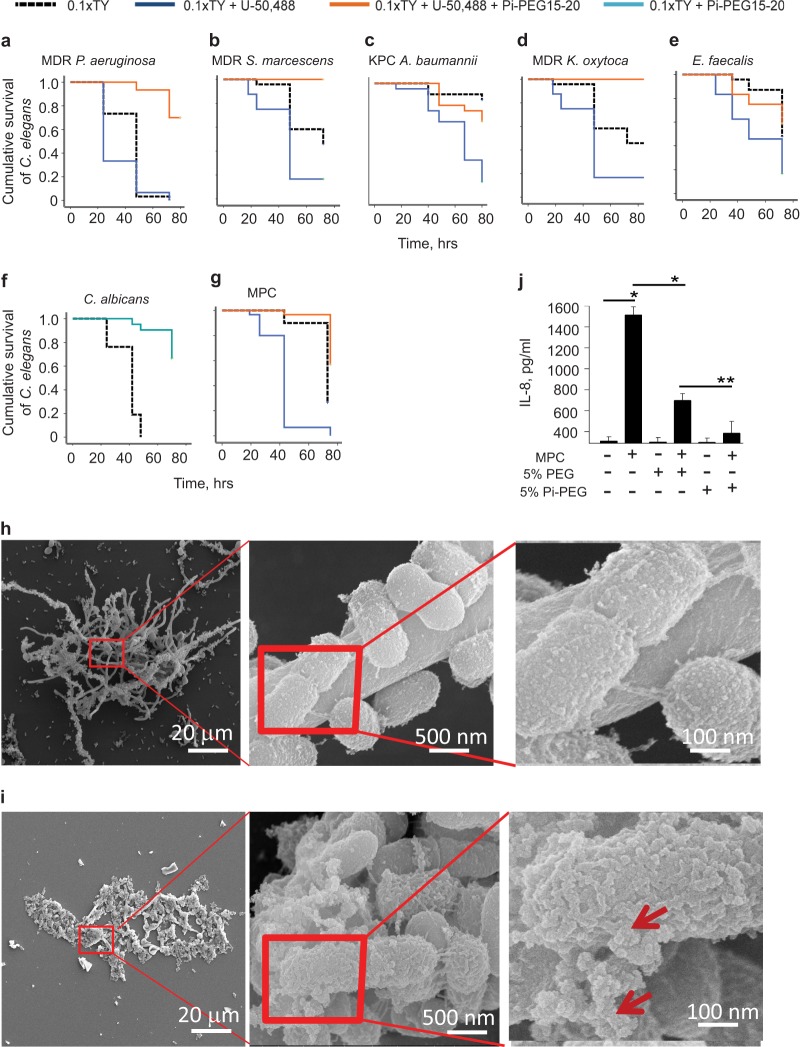
We have assembled a library of pathogens isolated from the stools of critically ill patients and characterized many of them as multidrug-resistant virulent pathogens (8). All of the pathogens in our library are known major causes of lethal sepsis that use the gut as their primary site of colonization and source of dissemination in critically ill patients. Therefore, we used C. elegans as a model of intestinal infection to screen the effect of Pi-PEG 15-20 against several of these pathogens, including Candida albicans, MDR P. aeruginosa, MDR Serratia marcescens, MDR Klebsiella oxytoca, and Enterococcus faecalis. We also included a carbapenem-resistant Acinetobacter baumannii 19606 isolate obtained from the ATCC (Fig. 5a to f). We initially observed that U-50,488 significantly enhanced C. elegans killing across strains that otherwise had a low/moderate killing effect in this model, such as A. baumannii and E. faecalis (Fig. 5c and e). Opioids did not enhance mortality of C. albicans and, therefore, we performed experiments in the absence of U-50,488 with this strain (Fig. 5f). Pi-PEG 15-20 significantly attenuated mortality in C. elegans in all our experiments, confirming proof of principle that Pi-PEG 15-20 can be an efficacious drug against a broad range of problematic pathogens. Similar to experiments with P. aeruginosa, we labeled C. albicans with PKH67 to track its movement. Pi-PEG 15-20 attenuated accumulation of C. albicans within the digestive tubes of nematodes (see Fig. S5 in the supplemental material), suggesting that a part of the mechanism of Pi-PEG 15-20 protection is related to its ability to alter the surface chemistry at the pathogen-epithelium interface. We then tested if Pi-PEG 15-20 could protect C. elegans against a multipathogen community that we previously isolated from the gut of a critically ill patient (i.e., MDR Serratia marcescens, MDR Klebsiella oxytoca, Enterococcus faecalis, and C. albicans; termed a multipathogen community [MPC]). Figure 5g demonstrates the lethal effect of the MPC under conditions of nutrient depletion (0.1× TY) spiked with an opioid (50 μM U-50,488) while 5% Pi-PEG 15-20 significantly attenuated worm mortality. Scanning electron microscopy demonstrated a structure of the MPC, with C. albicans expressing long hyphae to which rod- shaped and cocci-shaped bacteria attached (Fig. 5h). In the presence of Pi-PEG 15-20, the community was deconstructed, as C. albicans hyphae were no longer visible and the rod- and cocci-shaped bacteria changed their surface characteristics whereby they appeared to be covered by an external synthetic substance (Fig. 5i, red arrows). Finally, we determined if Pi-PEG 15-20 had a protective effect on intestinal epithelial cells exposed to the MPC. We observed that HT-29 cells coincubated with the MPC released a significant amount of the proinflammatory cytokine IL-8. Both Pi-PEG 15-20 and PEG 15-20 protected against IL-8 release; however, the effect of Pi-PEG 15-20 was superior to that of PEG 15-20 (Fig. 5j).
FIG 5.
Protective effect of Pi-PEG 15-20 against emergent pathogens and the MPC. (a to g) Kaplan-Meier survival curves, demonstrating the protective effect of Pi-PEG 15-20 on C. elegans when feeding on several individual pathogens isolated from the stools of critically ill patients (a to f) or MPC (g). n = 8/dish, 3 dishes/group. Results were reproduced in triplicate independent experiments. (h and i) SEM images of MPC grown in 0.1× TY (h) and 0.1× TY plus 5%Pi-PEG 15-20 (i). Red squares indicate the area of magnification shown in the right panels. Red arrows indicate an external synthetic- appearing covering of bacterial cells incubated with Pi-PEG 15-20. (j) IL-8 release by HT-29 cells induced by MPC with or without treatment with 5% PEG 15-20 or 5% Pi-PEG 15-20. n = 3/group. *, P < 0.001; **, P < 0.01 (Student t test).
Pi-PEG 15-20 prevents lethal sepsis in mice intestinally inoculated with a microbial community of resistant health care-associated pathogens while preserving the normal gut microflora.
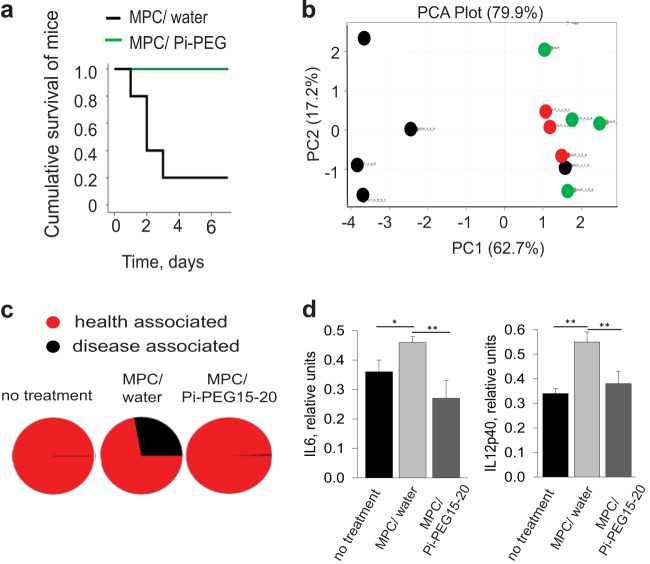
Finally, to confirm the efficacy of Pi-PEG 15-20 in vertebrate animals, we used a well-established mouse model of lethal gut-derived sepsis that was developed in our laboratory. Briefly, mice underwent a 30% partial hepatectomy with simultaneous intestinal inoculation via direct cecal puncture of the 4-pathogen community. One milliliter of 5% Pi-PEG 15-20 was administered via a rectal enema 18 h prior to intestinal pathogen inoculation. For comparison, a 1-ml H2O enema was administered as a control. The 1-ml volume was calibrated based on preliminary direct observations that this volume filled the colon to the cecum in mice. This approach ensured equal compound delivery between mice. Mice were sacrificed when septic/moribund or were followed if they remained healthy/recovered and were then sacrificed on day 7. Results demonstrated that Pi-PEG 15-20 completely protected mice, whereby they appeared perfectly healthy up to day 7 (Fig. 6a). Mice that received a water enema (MPC/water) developed severe sepsis (lethargy, chromodacctyrrhea, ruffled fur), except for one mouse that appeared perfectly healthy up to day 7. All mice were sacrificed when they appeared moribund. If they appeared healthy throughout the study, they were sacrificed on day 7. At sacrifice, cecal contents were harvested and lysed to analyze microbial protein composition and identify the taxa. Compared to untreated controls, microbial proteins from the MPC/water group demonstrated disappearance of multiple species of Clostridium, Eubacterium, Coprococcus, Bryantella, Parabacteroides, Ruminococcus, Blautia, Roseburia, Butyrivibrio, and other butyrate-producing bacteria and the appearance of Enterobacteriaceae-related proteins (see Table S1 in the supplemental material). The microbial protein composition in the Pi-PEG 15-20 enema-treated group aligned with those of the untreated control group, suggesting that Pi-PEG 15-20 maintains microbiota diversity. Of note, the one mouse in the water enema group that did not develop sepsis and that survived to day 7 while appearing perfectly healthy also demonstrated maintenance of a diverse microbiota, suggesting that microbiota diversity is a marker of survival. The PCA displayed in Fig. 6b confirmed a preservation effect of Pi-PEG 15-20 on the core microbiome. Analysis of microbial composition by taxa demonstrated the presence (∼25%) of pathogenic species in all septic moribund animals, but not in healthy-appearing animals independent of treatment (Fig. 6c; see also Table S2 in the supplemental material). The appearance of pathogenic species was not only related to those injected into the cecum (i.e., MPC) but also to other pathogenic species, like Chronobacter, Enterobacter, E. coli, Salmonella, and Shigella (see Table S2). This effect of the introduction of a pathogenic species uncovering the presence of otherwise-undetectable other pathogens has been recently described (21). As such, Pi-PEG 15-20 not only prevented colonization of the introduced pathogen community (MPC), but it also suppressed propagation of opportunistic pathogen members of the commensal microflora.
FIG 6.
Pi-PEG prevents mortality due to the MPC and preserves the intestinal microbiome. (a) Kaplan- Meier survival curves for MCP plus water enema versus MCP plus Pi-PEG enema. n = 5/group. P < 0.001. (b) PCA of microbial taxa based upon protein identification of filtered lysates from cecal contents. Red dots (n = 3) are the nontreated controls, black dots represent mice treated with MPC and a water enema, and green dots represent mice treated with MPC and an Pi-PEG 15–20 enema. The single black dot clustered with the red and green dots in the PCA represents the one mouse in the MPC/water enema group that did not develop sepsis and appeared perfectly healthy on day 7. (c) Taxa analysis, showing the relative distribution of commensal versus pathogenic species by treatment group. (d) IL-6 and IL-12p40 cytokine release by dendritic cells exposed to filtered lysates from mouse cecal contents. n = 5/group. *, P < 0.05l **, P < 0.01.
In order to define the proinflammatory potential of the cecal contents themselves across treatment groups, we prepared lysates of freshly obtained cecal contents during sacrifice, as previously described. Filtered lysates were then inoculated into BMDCs stimulated with transforming growth factor-β and retinoic acid to shift them toward an intestinal phenotype (15). We measured BMDC production of two key cytokines, IL-6 and IL12p40. The results in Fig. 6d demonstrate that the proinflammatory potential of the cecal contents was attenuated by Pi-PEG 15-20.
DISCUSSION
The environmentally harsh and nutrient-depleted gut during critical illness can be viewed as undergoing a form of ecological collapse, where antibiotic use is unavoidable, the core microbiome becomes depleted, and health care-acquired multidrug-resistant pathogens predominate. Based on our previous work and the work of others demonstrating the importance of local phosphate abundance for promoting mutualism and suppressing microbial virulence activation (7, 10, 11), Pi-PEG 15-20 appears to be a suitable agent to deliver phosphate to the site of the host-pathogen interaction. Results from the present study confirmed that Pi-PEG confers a particularly effective suppressive action on genes whose expression is increased during phosphate depletion. By using a single organism such as P. aeruginosa, we demonstrated that the phosphorylated polymer Pi-PEG 15-20 prevented the expression of the same virulence pathways as does inorganic phosphate, including those induced by phosphate limitation, iron limitation, and Pseudomonas quorum-sensing signaling (10, 11, 34–36). In this report, we have further demonstrated that Pi-PEG 15-20 appears to suppress these pathways in P. aeruginosa more effectively than an oversupply of inorganic phosphate. A possible explanation for this finding may be related to its ability to form high-density aggregates on the bacterial cell surfaces and thus concentrate local phosphate to a greater degree than free extracellular inorganic phosphate. We have previously observed an “electron halo effect” around P. aeruginosa cells in the presence of PEG 15-20 that correlated with an increased height of deflection of the bacterium-polymer surface, when analyzed by atomic force microscopy (22). Whether Pi-PEG interacts with bacterial membranes and affects bacterial membrane signal transduction to the cytosol is unknown and will require further inquiry. However, we have previously shown that PEG 15-20 interacts with epithelial membrane lipid rafts, key structures involved in signal transduction, preventing dysregulation of barrier function and apoptosis (13). Bacteria also have membrane lipid microdomains to which Pi-PEG might bind and affect signaling. Work is ongoing in this regard.
In this report, we tested Pi-PEG 15-20 for its protective effect across several cellular and animal models and across several pathogens and pathogen communities in order to predict how it might function as a clinically useful agent. It was particularly important to model lethal infection from intestinal microbes that are the actual pathogens that cause sepsis in critically ill patients. These patients suffer sepsis late (>1 week) in the course of their intensive treatment with cardiopulmonary support, dialysis, vasoactive pharmacologic agents, and exposure to multiple antibiotics. Pathogens that colonize the intestinal tract of these patients are exposed to unprecedented selective pressures and a virtual complete absence of the normal microbiota due to promiscuous and unavoidable antibiotic use. These pathogens are often multidrug resistant, and adding further antibiotic therapy often compounds the problem by further elimination of the colonization resistance and health-promoting effects of the normal microbiota. As such, an agent that is nonmicrobiocidal is preferred.
Traditionally, antivirulence strategies have been developed with the idea to target single molecules that interfere with quorum sensing. One limitation with this approach is that candidate molecules tend to be species specific. Another limitation is the problem of emergence of resistance, similar to that which occurs with antibiotics. Finally, it is difficult to predict how the microbiome will respond to either a specific or nonspecific quorum-sensing inhibitor when applied to the constantly changing microbial ecology of the gut during critical illness. When considering how to therapeutically intervene to prevent lethal sepsis from intestinal pathogens during critical illness, it is important to recognize that there is a multidirectional molecular dialogue that takes place between microbiota, pathobiota, the local microenvironment, and the host immune system. Therefore, an approach that can leverage the strength of normal microbiota to control and contain the virulence of the pathobiota and directly suppress virulence by embedding critical resources, such as phosphate, into the local biologic exchange market may be the best way to create molecular détente in this multidimensional molecular dialogue. By working with an ex vivo-assembled multipathogen community (i.e., S. marsescens, K. oxytoca, E. faecalis, C. albicans) and inoculating animals exposed to surgical injury, we sought to recapitulate the selective pressures under which these microbes may express enhanced virulence as a whole, interacting community. Under such conditions, Pi-PEG appeared to function in a durable and protective manner by attenuating the killing ability of this assembled interactive pathogenic community under provocative conditions that might exist in patients. The observation that Pi-PEG protected the normal microbiota while containing the virulence of the pathobiota offers an advantage that, to our knowledge, has not been previously described for any other anti-infective agent to date. Further preclinical exploration of this compound is warranted across various models of sepsis, especially those that mimic the complex clinical conditions of patients dying of sepsis due to gut-derived pathogens that are multidrug resistant, express a high degree of virulence, and are difficult to prevent and treat.
Supplementary Material
ACKNOWLEDGMENTS
We thank Yimei Chen, Transmission Electron Microscopy Core Facility, University of Chicago; Qiti Guo, Chicago Materials Research Center, Scanning Electron Microscopy Core Facility, University of Chicago; Alexander Polozov, Argonne National Laboratories; and Charles Pierce, University of Chicago, for technical assistance.
This study was funded by NIH RO1 5R01GMO62344-12 (J.C.A.) and P41GM103493-10 (R.D.S.). Portions of the experimental work described herein were performed in the Environmental Molecular Sciences Laboratory (EMSL), a U.S. Department of Energy (DOE) national scientific user facility located at PNNL in Richland, WA. PNNL is a national laboratory operated by Battelle Memorial Institute for the DOE under contract DE-AC05-76RL01830. M.K. and M.T. were supported by the Laboratory Directed Research and Development Program of the Argonne National Laboratory under U.S. Department of Energy contract number DE-AC02-06CH11357.
Footnotes
Published ahead of print 25 November 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.02183-13.
REFERENCES
- 1.Shimizu K, Ogura H, Asahara T, Nomoto K, Morotomi M, Tasaki O, Matsushima A, Kuwagata Y, Shimazu T, Sugimoto H. 2012. Probiotic/synbiotic therapy for treating critically ill patients from a gut microbiota perspective. Dig. Dis. Sci. 58:23–32. 10.1007/s10620-012-2334-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones RN. 2010. Microbial etiologies of hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia. Clin. Infect. Dis. 51(Suppl 1):S81–S87. 10.1086/653053 [DOI] [PubMed] [Google Scholar]
- 3.Fernandez-Guerrero ML, Verdejo C, Azofra J, de Gorgolas M. 1995. Hospital-acquired infectious endocarditis not associated with cardiac surgery: an emerging problem. Clin. Infect. Dis. 20:16–23 [DOI] [PubMed] [Google Scholar]
- 4.Hussein K, Raz-Pasteur A, Finkelstein R, Neuberger A, Shachor-Meyouhas Y, Oren I, Kassis I. 2013. Impact of carbapenem resistance on the outcome of patients' hospital-acquired bacteraemia caused by Klebsiella pneumoniae. J. Hosp. Infect. 83:307–313. 10.1016/j.jhin.2012.10.012 [DOI] [PubMed] [Google Scholar]
- 5.Drisko JA, Giles CK, Bischoff BJ. 2003. Probiotics in health -maintenance and disease prevention. Altern. Med. Rev. 8:143–155 http://www.altmedrev.com/publications/8/2/143.pdf [PubMed] [Google Scholar]
- 6.Carlet J. 2012. The gut is the epicentre of antibiotic resistance. Antimicrob. Resist. Infect. Control 1:39. 10.1186/2047-2994-1-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lamarche MG, Wanner BL, Crepin S, Harel J. 2008. The phosphate regulon and bacterial virulence: a regulatory network connecting phosphate homeostasis and pathogenesis. FEMS Microbiol. Rev. 32:461–473. 10.1111/j.1574-6976.2008.00101.x [DOI] [PubMed] [Google Scholar]
- 8.Romanowski K, Zaborin A, Valuckaite V, Rolfes RJ, Babrowski T, Bethel C, Olivas A, Zaborina O, Alverdy JC. 2012. Candida albicans isolates from the gut of critically ill patients respond to phosphate limitation by expressing filaments and a lethal phenotype. PLoS One 7(1):e30119. 10.1371/journal.pone.0030119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zaborina O, Holbrook C, Chen Y, Long J, Zaborin A, Morozova I, Fernandez H, Wang Y, Turner JR, Alverdy JC. 2008. Structure-function aspects of PstS in multi-drug-resistant Pseudomonas aeruginosa. PLoS Pathog. 4(2):e43. 10.1371/journal.ppat.0040043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zaborin A, Gerdes S, Holbrook C, Liu DC, Zaborina OY, Alverdy JC. 2012. Pseudomonas aeruginosa overrides the virulence inducing effect of opioids when it senses an abundance of phosphate. PLoS One 7(4):e34883. 10.1371/journal.pone.0034883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zaborin A, Romanowski K, Gerdes S, Holbrook C, Lepine F, Long J, Poroyko V, Diggle SP, Wilke A, Righetti K, Morozova I, Babrowski T, Liu DC, Zaborina O, Alverdy JC. 2009. Red death in Caenorhabditis elegans caused by Pseudomonas aeruginosa PAO1. Proc. Natl. Acad. Sci. U. S. A. 106:6327–6332. 10.1073/pnas.0813199106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Long J, Zaborina O, Holbrook C, Zaborin A, Alverdy J. 2008. Depletion of intestinal phosphate after operative injury activates the virulence of P aeruginosa causing lethal gut-derived sepsis. Surgery 144:189–197. 10.1016/j.surg.2008.03.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valuckaite V, Zaborina O, Long J, Hauer-Jensen M, Wang J, Holbrook C, Zaborin A, Drabik K, Katdare M, Mauceri H, Weichselbaum R, Firestone MA, Lee KY, Chang EB, Matthews J, Alverdy JC. 2009. Oral PEG 15–20 protects the intestine against radiation: role of lipid rafts. Am. J. Physiol. Gastrointest. Liver Physiol. 297:G1041–G1052. 10.1152/ajpgi.00328.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edelstein A, Fink D, Musch M, Valuckaite V, Zaborina O, Grubjesic S, Firestone MA, Matthews JB, Alverdy JC. 2011. Protective effects of nonionic triblock copolymers on bile acid-mediated epithelial barrier disruption. Shock 36:451–457. 10.1097/SHK.0b013e31822d8de1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Depaolo RW, Tang F, Kim I, Han M, Levin N, Ciletti N, Lin A, Anderson D, Schneewind O, Jabri B. 2008. Toll-like receptor 6 drives differentiation of tolerogenic dendritic cells and contributes to LcrV-mediated plague pathogenesis. Cell Host Microbe 4:350–361. 10.1016/j.chom.2008.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DePaolo RW, Abadie V, Tang F, Fehlner-Peach H, Hall JA, Wang W, Marietta EV, Kasarda DD, Waldmann TA, Murray JA, Semrad C, Kupfer SS, Belkaid Y, Guandalini S, Jabri B. 2011. Co-adjuvant effects of retinoic acid and IL-15 induce inflammatory immunity to dietary antigens. Nature 471:220–224. 10.1038/nature09849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marketon MM, DePaolo RW, DeBord KL, Jabri B, Schneewind O. 2005. Plague bacteria target immune cells during infection. Science 309:1739–1741. 10.1126/science.1114580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adkins JN, Mottaz HM, Norbeck AD, Gustin JK, Rue J, Clauss TR, Purvine SO, Rodland KD, Heffron F, Smith RD. 2006. Analysis of the Salmonella typhimurium proteome through environmental response toward infectious conditions. Mol. Cell. Proteomics 5:1450–1461. 10.1074/mcp.M600139-MCP200 [DOI] [PubMed] [Google Scholar]
- 19.Shen Y, Tolic N, Zhao R, Pasa-Tolic L, Li L, Berger SJ, Harkewicz R, Anderson GA, Belov ME, Smith RD. 2001. High-throughput proteomics using high-efficiency multiple-capillary liquid chromatography with on-line high-performance ESI FTICR mass spectrometry. Anal. Chem. 73:3011–3021. 10.1021/ac001393n [DOI] [PubMed] [Google Scholar]
- 20.Kim S, Gupta N, Pevzner PA. 2008. Spectral probabilities and generating functions of tandem mass spectra: a strike against decoy databases. J. Proteome Res. 7:3354–3363. 10.1021/pr8001244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deatherage Kaiser BL, Li J, Sanford JA, Kim YM, Kronewitter SR, Jones MB, Peterson CT, Peterson SN, Frank BC, Purvine SO, Brown JN, Metz TO, Smith RD, Heffron F, Adkins JN. 2013. A multi-omic view of host-pathogen-commensal interplay in Salmonella-mediated intestinal infection. PLoS One 8(6):e67155. 10.1371/journal.pone.0067155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu L, Zaborina O, Zaborin A, Chang EB, Musch M, Holbrook C, Shapiro J, Turner JR, Wu G, Lee KY, Alverdy JC. 2004. High-molecular-weight polyethylene glycol prevents lethal sepsis due to intestinal Pseudomonas aeruginosa. Gastroenterology 126:488–498. 10.1053/j.gastro.2003.11.011 [DOI] [PubMed] [Google Scholar]
- 23.Boyer JC, Manseau MP, Murray JI, van Veggel FC. 2010. Surface modification of upconverting NaYF4 nanoparticles with PEG-phosphate ligands for NIR (800 nm) biolabeling within the biological window. Langmuir 26:1157–1164. 10.1021/la902260j [DOI] [PubMed] [Google Scholar]
- 24.Diamente PR, van Veggel F. 2005. Water-soluble Ln(3+)-doped LaF3 nanoparticles: retention of strong luminescence and potential as biolabels. J. Fluoresc. 15:543–551. 10.1007/s10895-005-2827-5 [DOI] [PubMed] [Google Scholar]
- 25.Imokawa G, Tsutsumi H, Kurosaki T. 1978. Surface activity and cutaneous effects of monoalkyl phosphate surfactants. J. Am. Oil Chem. Soc. 55:839–844 [DOI] [PubMed] [Google Scholar]
- 26.Orihuela CJ, Mills J, Robb CW, Wilson CJ, Watson DA, Niesel DW. 2001. Streptococcus pneumoniae PstS production is phosphate responsive and enhanced during growth in the murine peritoneal cavity. Infect. Immun. 69:7565–7571. 10.1128/IAI.69.12.7565-7571.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsuzaki M, Abe M, Hara S, Iwasaki Y, Yamamoto I, Satoh T. 2003. An abundant periplasmic protein of the denitrifying phototroph Rhodobacter sphaeroides f. sp. denitrificans is PstS, a component of an ABC phosphate transport system. Plant Cell Physiol. 44:212–216. 10.1093/pcp/pcg021 [DOI] [PubMed] [Google Scholar]
- 28.Makino K, Shinagawa H, Amemura M, Kimura S, Nakata A, Ishihama A. 1988. Regulation of the phosphate regulon of Escherichia coli. activation of pstS transcription by PhoB protein in vitro. J. Mol. Biol. 203:85–95 [DOI] [PubMed] [Google Scholar]
- 29.Dubern JF, Diggle SP. 2008. Quorum sensing by 2-alkyl-4-quinolones in Pseudomonas aeruginosa and other bacterial species. Mol. Biosyst. 4:882–888. 10.1039/b803796p [DOI] [PubMed] [Google Scholar]
- 30.Diggle SP, Cornelis P, Williams P, Camara M. 2006. 4-Quinolone signalling in Pseudomonas aeruginosa: old molecules, new perspectives. Int. J. Med. Microbiol. 296:83–91 http://dx.doi.org/10.1016/j.ijmm.2006.01.038 [DOI] [PubMed] [Google Scholar]
- 31.He W, Qu T, Yu Q, Wang Z, Lv H, Zhang J, Zhao X, Wang P. 2012. LPS induces IL-8 expression through TLR4, MyD88, NF-κB and MAPK pathways in human dental pulp stem cells. Int. Endod. J. 10.1111/j.1365-2591.2012.02096.x [DOI] [PubMed] [Google Scholar]
- 32.Angrisano T, Pero R, Peluso S, Keller S, Sacchetti S, Bruni CB, Chiariotti L, Lembo F. 2010. LPS-induced IL-8 activation in human intestinal epithelial cells is accompanied by specific histone H3 acetylation and methylation changes. BMC Microbiol. 10:172. 10.1186/1471-2180-10-172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Winterhalter M, Burner H, Marzinka S, Benz R, Kasianowicz JJ. 1995. Interaction of poly(ethylene-glycols) with air-water interfaces and lipid monolayers: investigations on surface pressure and surface potential. Biophys. J. 69:1372–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Romanowski K, Zaborin A, Fernandez H, Poroyko V, Valuckaite V, Gerdes S, Liu DC, Zaborina OY, Alverdy JC. 2011. Prevention of siderophore- mediated gut-derived sepsis due to P. aeruginosa can be achieved without iron provision by maintaining local phosphate abundance: role of pH. BMC Microbiol. 11:212. 10.1186/1471-2180-11-212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jensen V, Lons D, Zaoui C, Bredenbruch F, Meissner A, Dieterich G, Munch R, Haussler S. 2006. RhlR expression in Pseudomonas aeruginosa is modulated by the Pseudomonas quinolone signal via PhoB-dependent and -independent pathways. J. Bacteriol. 188:8601–8606. 10.1128/JB.01378-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Faure LM, Llamas MA, Bastiaansen KC, de Bentzmann S, Bigot S. 2013. Phosphate starvation relayed by PhoB activates the expression of the Pseudomonas aeruginosa sigmavreI ECF factor and its target genes. Microbiology 159:1315–1327. 10.1099/mic.0.067645-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.