Abstract
Resistant Gram-negative bacteria are increasing central-line-associated bloodstream infection threats. To better combat this, chlorhexidine (CHX) was added to minocycline-rifampin (M/R) catheters. The in vitro antimicrobial activity of CHX-M/R catheters against multidrug resistant, Gram-negative Acinetobacter baumannii, Enterobacter cloacae, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Stenotrophomonas maltophilia was tested. M/R and CHX-silver sulfadiazine (CHX/SS) catheters were used as comparators. The novel CHX-M/R catheters were significantly more effective (P < 0.0001) than CHX/SS or M/R catheters in preventing biofilm colonization and showed better antimicrobial durability.
TEXT
Central venous catheters (CVCs) are essential medical devices in the care of critically ill and cancer patients. Despite their significant usefulness, CVCs are also the source of 87% of the bloodstream infections that occur in intensive care units (1), with attributable mortality rates ranging from 13 to 25% and increases in hospital stays ranging from 7 to 12 days (2, 3). In the United States, more than 5 million CVCs are inserted annually (4, 5) and are responsible for 250,000 to 400,000 annual cases of health care-associated bloodstream infections (6, 7). Hence, central-line-associated bloodstream infections (CLABSIs) are the most common and serious complications associated with indwelling CVCs (5).
CVCs coated with antimicrobial agents have been proven to considerably reduce the risk of CLABSIs, and the use of antimicrobial CVCs has become a standard of care (8, 9). Two antimicrobial CVCs, minocycline-rifampin (M/R) and chlorhexidine-silver sulfadiazine (CHX/SS), were clinically proven to reduce CLABSIs at a time when skin-derived, Gram-positive bacteria were the most significant pathogens and were given CDC category 1A recommendations in the most recent guidelines for the prevention of intravascular-catheter-related infections (8–10). However, significant advances in skin antisepsis and sterile barrier precautions have shifted the epidemiologic threat increasingly to Gram-negative bacteria (11). The in vitro efficacy of M/R and CHX/SS against emerging Gram-negative pathogens is limited. In this report, we describe the development of a chlorhexidine-minocycline-rifampin (CHX-M/R) CVC. We compared the antimicrobial adherence activity and durability of M/R, CHX/SS, and CHX-M/R CVCs in a well-established in vitro model for the preventing biofilm colonization by multidrug-resistant clinical isolates of A. baumannii, E. cloacae, E. coli, K. pneumoniae, P. aeruginosa, and S. maltophilia. These organisms were found to contribute to the majority of Gram-negative CLABSIs in the United States from 2009 to 2010 (12). Developing an antimicrobial catheter with broad-spectrum antifungal and antibacterial activity that includes resistant Gram-negative bacteria is of paramount importance because of the mortality rates associated with Gram-negative bacteremia, which may exceed 40% (13, 14).
CHX-M/R was prepared by a proprietary sequential coating method. Briefly, polyurethane catheter material was first impregnated with a mixture of minocycline and rifampin. After drying, catheters were treated on the lumen and exterior surfaces with a CHX-polymer coating. A previous study with CHX-M/R (15) employed the reverse sequence. The updated method provided a smoother surface finish. Commercially available triple lumen 7Fr. M/R (Cook Medical, Bloomington, IN) and CHX/SS (Arrowgard BluePlus; Arrow International, Inc., Reading, PA) catheters were tested as comparators. An uncoated polyurethane CVC was used as a positive control.
The antimicrobial activity of catheters was tested against A. baumannii strain 2021, E. cloacae strain 2265, E. coli strain 2131, K. pneumoniae strain 2856, P. aeruginosa strain 4698, and S. maltophilia strain 5572 in a well-established biofilm colonization model (11, 15, 16). Briefly, uncoated and coated catheter segments were incubated at 37°C for 24 h in donor plasma in triplicate. The donor plasma was then replaced with 5.0 × 105 cells in Muller-Hinton broth containing Gram-negative bacteria and incubated for an additional 24 h. After washing, the segments were sonicated in 5 ml of 0.9% sterile saline for 15 min and 100 μl of liquid from each segment was serially diluted and spread onto Trypticase soy agar–5% sheep blood agar plates for quantitative culture. Plates were then incubated at 37°C inverted for 24 h and then examined for colony growth.
To test the durability of inhibition of biofilm colonization on the CVCs, uncoated and coated catheters were incubated for up to 3 weeks in serum at 37°C and then challenged with bacteria for 24 h to assess retained antimicrobial activity in the biofilm colonization model (11, 15, 16). The shelf stability of the CHX-M/R catheters was tested by storing them at 25°C for 1 year and then retesting their baseline antimicrobial activity in the biofilm colonization model (11, 15, 16). Statistical analyses were performed by using SAS version 9.1 (SAS Institute Inc., Cary, NC) for the Kruskal-Wallis test, the Wilcoxon rank sum test, and two-way nonparametric analysis of variance.
Biofilm colonization on coated CVCs.
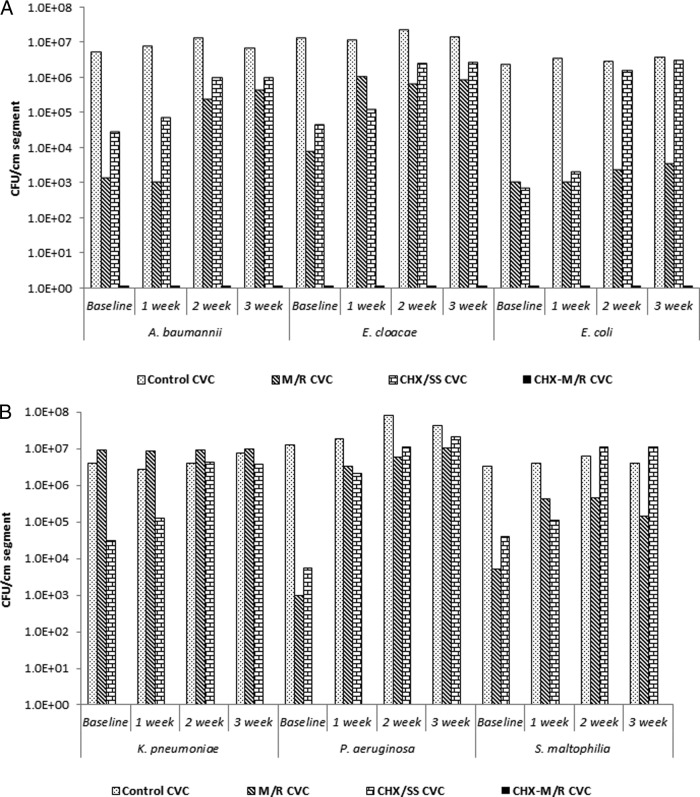
CHX-M/R CVC segments completely prevented biofilm colonization of all pathogens tested at 24 h (baseline). The inhibition of CHX-M/R CVCs was significantly greater than that obtained for M/R, CHX/SS, and uncoated control CVCs (P < 0.0001) (Fig. 1A&B).
FIG 1.
In vitro antimicrobial activity for 24 h (baseline) and durability for up to 3 weeks of different antimicrobial-coated catheters against A. baumannii, E. cloacae, and E. coli (A) and K. pneumoniae, P. aeruginosa, and S. maltophilia (B).
M/R-coated CVC segments exhibited a 5-log reduction of P. aeruginosa (median [range], 9.5 × 102 [2.6 × 101 to 5.1 × 103] versus 1.3 × 107 [1.2 × 107 to 2.3 × 108]) (Fig. 1B), a 4-log reduction of E. cloacae (median [range], 8.0 × 103 [3.2 × 103 to 3.1 × 104] versus 1.3 × 107 [1.2 × 107 to 2.8 × 107]) (Fig. 1A), and a 3-log reduction relative to uncoated controls in viable biofilm colony counts of A. baumannii (median [range], 1.4 × 103 [0 to 2.8 × 103] versus 5.6 × 106 [3.9 × 106 to 7.5 × 106]), E. coli (median [range], 1.1 × 103 [0 to 2.4 × 103] versus 2.4 × 106 [8.5 × 105 to 5.1 × 106]) (Fig. 1A), and S. maltophilia (median [range], 5.2 × 103 [2.9 × 103 to 8.2 × 103] versus 3.2 × 106 [1.5 × 105 to 7.4 × 106]) (Fig. 1B).
CHX/SS catheter segments exhibited a 4-log reduction in viable biofilm colony counts of E. coli (median [range], 7.3 × 102 [0 to 1.3 × 103] versus 2.4 × 106 [8.5 × 105 to 5.1 × 106]) (Fig. 1A), P. aeruginosa (median [range], 5.4 × 103 [2.8 × 103 to 8.8 × 103] versus 1.3 × 107 [1.2 × 107 to 2.3 × 108]) (Fig. 1B) and a 3-log reduction in viable biofilm colony counts of E. cloacae (median [range], 4.5 × 104 [1.8 × 104 to 5.8 × 104] versus 1.3 × 107 [1.2 × 107 to 2.8 × 107]) (Fig. 1B) compared to uncoated control CVCs. The difference between the antimicrobial activities of M/R and CHX/SS CVCs against A. baumannii, E. cloacae (Fig. 1A), P. aeruginosa, and S. maltophilia (Fig. 1B) was significant (P = 0.01).
Durability of antimicrobial-coated CVCs.
CHX-M/R catheter segments retained antimicrobial durability against A. baumannii, E. cloacae, E. coli, K. pneumoniae, P. aeruginosa, and S. maltophilia compared to M/R, CHX/SS, and uncoated control CVCs over 3 weeks. Weekly results were significant (P < 0.0001) (Fig. 1A and B) throughout the 3-week incubation of catheters in serum.
Shelf life of CHX-M/R CVCs.
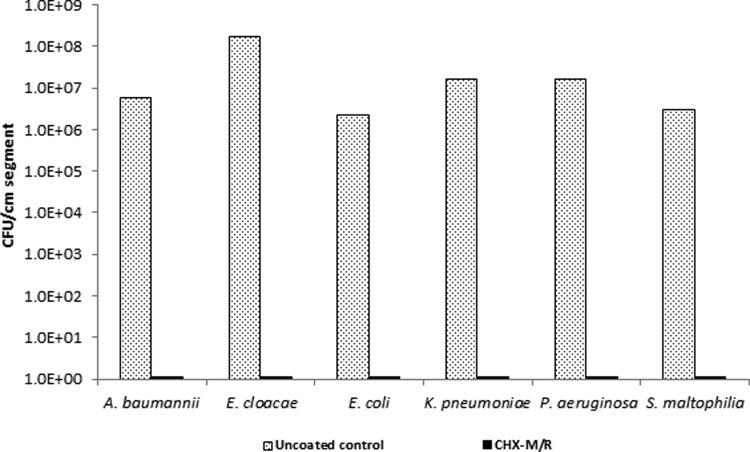
After 1 year of real-time storage of CHX-M/R catheters at 25°C, antimicrobial activity was fully retained. Biofilm colonization of Gram-negative bacteria was fully inhibited compared to uncoated control catheters (P < 0.0001); indicating that the baseline antimicrobial activity of CHX-M/R was stable (Fig. 2).
FIG 2.
Antimicrobial activities of CHX-M/R catheters against A. baumannii, E. cloacae, E. coli, K. pneumoniae, P. aeruginosa, and S. maltophilia after 1 year of storage at 25°C.
CHX has been reported to increase outer membrane permeability in Gram-negative organisms by binding to anionic moieties on the cell membrane, thus disrupting the transmembrane transport system (17). We demonstrated here a synergistic augmentation of the activity of M/R against Gram-negative bacterial biofilm colonization through complete prevention of the formation of biofilms of Gram-negative pathogens. Our recent study showed that CHX-M/R CVCs had prolonged antimicrobial durability against Gram-positive bacteria, fungi, and P. aeruginosa (15). Thus, the broad-spectrum activity of the CHX-M/R catheter has the potential to bring the rate of CLABSIs close to zero.
A large multicenter prospective randomized trial showed that CHX/SS-coated CVCs have a short antimicrobial durability and are not as effective in preventing infection as M/R-coated CVCs (18). The CVC impregnated with M/R has been associated with prolonged antimicrobial durability in situ for around 50 days (4) and has excellent activity against resistant staphylococci (19). However, in large prospective randomized trials the antibiotic M/R catheter, which performed well in completely preventing CLABSI caused by staphylococci (4, 20, 21), failed to completely prevent CLABSI caused by Gram-negative pathogens such as K. pneumoniae, E. cloacae, and Pseudomonas species (20).
Among 217 bacteremias caused by S. maltophilia, 73% were diagnosed as catheter-related infections in cancer patients (22). Furthermore, the prevalence of Gram-negative bacillus CLABSIs has increased from 14 to 19% of the CLABSI cases reported from 1986 to 1999 (23, 24) to 28.2% of the CLABSI cases reported in the last decade (25). Therefore, the novel antimicrobial CVC impregnated and coated with CHX-M/R should be highly useful in preventing these rapidly emerging Gram-negative CLABSIs, as well as catheter infections caused by Gram-positive bacteria and fungi (15).
Conclusions.
A novel CHX-M/R CVC was superior to M/R and CHX/SS CVCs in inhibiting biofilm colonization by various resistant Gram-negative bacteria. The CHX-M/R CVC also displayed superior antimicrobial durability against the same Gram-negative bacteria. Clinical testing is warranted and necessary to prove that this treatment significantly reduces bloodstream infections caused by resistant Gram-negative bacteria.
ACKNOWLEDGMENTS
Issam Raad is a coinventor of the approved catheters coated with minocycline-rifampin. This technology is the property of the University of Texas M. D. Anderson Cancer Center and Baylor College of Medicine and is licensed to Cook, Inc.
Footnotes
Published ahead of print 28 October 2013
REFERENCES
- 1.Richards MJ, Edwards JR, Culver DH, Gaynes RP. 1999. Nosocomial infections in medical intensive care units in the United States. National Nosocomial Infections Surveillance System. Crit. Care Med. 27:887–892 [DOI] [PubMed] [Google Scholar]
- 2.Pittet D, Tarara D, Wenzel RP. 1994. Nosocomial bloodstream infection in critically ill patients. Excess length of stay, extra costs, and attributable mortality. JAMA 271:1598–1601 [DOI] [PubMed] [Google Scholar]
- 3.Raad I, Hanna H, Maki D. 2007. Intravascular catheter-related infections: advances in diagnosis, prevention, and management. Lancet Infect. Dis. 7:645–657. 10.1016/S1473-3099(07)70235-9 [DOI] [PubMed] [Google Scholar]
- 4.Darouiche RO, Berger DH, Khardori N, Robertson CS, Wall MJ, Jr, Metzler MH, Shah S, Mansouri MD, Cerra-Stewart C, Versalovic J, Reardon MJ, Raad II. 2005. Comparison of antimicrobial impregnation with tunneling of long-term central venous catheters: a randomized controlled trial. Ann. Surg. 242:193–200. 10.1097/01.sla.0000171874.29934.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mermel LA, Farr BM, Sherertz RJ, Raad II, O'Grady N, Harris JS, Craven DE. 2001. Guidelines for the management of intravascular catheter-related infections. Clin. Infect. Dis. 32:1249–1272. 10.1086/320001 [DOI] [PubMed] [Google Scholar]
- 6.Maki DG, Stolz SM, Wheeler S, Mermel LA. 1997. Prevention of central venous catheter-related bloodstream infection by use of an antiseptic-impregnated catheter. A randomized, controlled trial. Ann. Intern. Med. 127:257–266 [DOI] [PubMed] [Google Scholar]
- 7.Raad I. 1998. Intravascular-catheter-related infections. Lancet 351:893–898. 10.1016/S0140-6736(97)10006-X [DOI] [PubMed] [Google Scholar]
- 8.Hockenhull JC, Dwan KM, Smith GW, Gamble CL, Boland A, Walley TJ, Dickson RC. 2009. The clinical effectiveness of central venous catheters treated with anti-infective agents in preventing catheter-related bloodstream infections: a systematic review. Crit. Care Med. 37:702–712. 10.1097/CCM.0b013e3181958915 [DOI] [PubMed] [Google Scholar]
- 9.Ramritu P, Halton K, Collignon P, Cook D, Fraenkel D, Battistutta D, Whitby M, Graves N. 2008. A systematic review comparing the relative effectiveness of antimicrobial-coated catheters in intensive care units. Am. J. Infect. Control 36:104–117. 10.1016/j.ajic.2007.02.012 [DOI] [PubMed] [Google Scholar]
- 10.O'Grady NP, Alexander M, Dellinger EP, Gerberding JL, Heard SO, Maki DG, Masur H, McCormick RD, Mermel LA, Pearson ML, Raad II, Randolph A, Weinstein RA. 2002. Guidelines for the prevention of intravascular catheter-related infections. Centers for Disease Control and Prevention. MMWR Recommend. Rep. 51(RR-10):1–29 http://www.cdc.gov/mmwr/PDF/rr/rr5110.pdf [PubMed] [Google Scholar]
- 11.Hanna H, Bahna P, Reitzel R, Dvorak T, Chaiban G, Hachem R, Raad I. 2006. Comparative in vitro efficacies and antimicrobial durabilities of novel antimicrobial central venous catheters. Antimicrob. Agents Chemother. 50:3283–3288. 10.1128/AAC.01622-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sievert DM, Ricks P, Edwards JR, Schneider A, Patel J, Srinivasan A, Kallen A, Limbago B, Fridkin S. 2013. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009-2010. Infect. Control Hosp. Epidemiol. 34:1–14. 10.1086/668770 [DOI] [PubMed] [Google Scholar]
- 13.Gudiol C, Tubau F, Calatayud L, Garcia-Vidal C, Cisnal M, Sanchez-Ortega I, Duarte R, Calvo M, Carratala J. 2011. Bacteraemia due to multidrug-resistant Gram-negative bacilli in cancer patients: risk factors, antibiotic therapy and outcomes. J. Antimicrob. Chemother. 66:657–663. 10.1093/jac/dkq494 [DOI] [PubMed] [Google Scholar]
- 14.Michalopoulos A, Falagas ME, Karatza DC, Alexandropoulou P, Papadakis E, Gregorakos L, Chalevelakis G, Pappas G. 2011. Epidemiologic, clinical characteristics, and risk factors for adverse outcome in multiresistant gram-negative primary bacteremia of critically ill patients. Am. J. Infect. Control 39:396–400. 10.1016/j.ajic.2010.06.017 [DOI] [PubMed] [Google Scholar]
- 15.Raad I, Mohamed JA, Reitzel RA, Jiang Y, Raad S, Al Shuaibi M, Chaftari AM, Hachem RY. 2012. Improved antibiotic-impregnated catheters with extended-spectrum activity against resistant bacteria and fungi. Antimicrob. Agents Chemother. 56:935–941. 10.1128/AAC.05836-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raad I, Hanna H, Jiang Y, Dvorak T, Reitzel R, Chaiban G, Sherertz R, Hachem R. 2007. Comparative activities of daptomycin, linezolid, and tigecycline against catheter-related methicillin-resistant Staphylococcus bacteremic isolates embedded in biofilm. Antimicrob. Agents Chemother. 51:1656–1660. 10.1128/AAC.00350-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Franklin TJ, Snow GA. 2005. Antimicrobial agents and cell membranes. Springer Science+Business Media LLC, Philadelphia, PA [Google Scholar]
- 18.Darouiche RO, Raad II, Heard SO, Thornby JI, Wenker OC, Gabrielli A, Berg J, Khardori N, Hanna H, Hachem R, Harris RL, Mayhall G. 1999. A comparison of two antimicrobial-impregnated central venous catheters. N. Engl. J. Med. 340:1–8. 10.1056/NEJM199901073400101 [DOI] [PubMed] [Google Scholar]
- 19.Raad I, Reitzel R, Jiang Y, Chemaly RF, Dvorak T, Hachem R. 2008. Anti-adherence activity and antimicrobial durability of anti-infective-coated catheters against multidrug-resistant bacteria. J. Antimicrob. Chemother. 62:746–750. 10.1093/jac/dkn281 [DOI] [PubMed] [Google Scholar]
- 20.Fraenkel D, Rickard C, Thomas P, Faoagali J, George N, Ware R. 2006. A prospective, randomized trial of rifampicin-minocycline-coated and silver-platinum-carbon-impregnated central venous catheters. Crit. Care Med. 34:668–675. 10.1097/01.CCM.0000201404.05523.34 [DOI] [PubMed] [Google Scholar]
- 21.Raad I, Darouiche R, Dupuis J, Abi-Said D, Gabrielli A, Hachem R, Wall M, Harris R, Jones J, Buzaid A, Robertson C, Shenaq S, Curling P, Burke T, Ericsson C. 1997. Central venous catheters coated with minocycline and rifampin for the prevention of catheter-related colonization and bloodstream infections. A randomized, double-blind trial. Ann. Intern. Med. 127:267–274 [DOI] [PubMed] [Google Scholar]
- 22.Boktour M, Hanna H, Ansari S, Bahna B, Hachem R, Tarrand J, Rolston K, Safdar A, Raad I. 2006. Central venous catheter and Stenotrophomonas maltophilia bacteremia in cancer patients. Cancer 106:1967–1973. 10.1002/cncr.21846 [DOI] [PubMed] [Google Scholar]
- 23.Crump JA, Collignon PJ. 2000. Intravascular catheter-associated infections. Eur. J. Clin. Microbiol. Infect. Dis. 19:1–8. 10.1007/s100960050001 [DOI] [PubMed] [Google Scholar]
- 24.Schaberg D. R., Culver D. H., Gaynes R. P. 1991. Major trends in the microbial etiology of nosocomial infection. Am. J. Med. 91:72S–75S. 10.1016/0002-9343(91)90346-Y [DOI] [PubMed] [Google Scholar]
- 25.Hidron AI, Edwards JR, Patel J, Horan TC, Sievert DM, Pollock DA, Fridkin SK. 2008. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006-2007. Infect. Control Hosp. Epidemiol. 29:996–1011. 10.1086/591861 [DOI] [PubMed] [Google Scholar]