Abstract
Limited therapeutic options exist for the treatment of vancomycin-resistant Enterococcus (VRE) bacteremia; the most commonly used are daptomycin and linezolid. We attempted a systematic review and meta-analysis of the comparative efficacy of those two agents. Studies comparing daptomycin to linezolid treatment for VRE bacteremia, published until August 2012, were identified from the MEDLINE, EMBASE, CENTRAL, ISI Web of Science, and SCOPUS databases. All comparative studies on patients older than 18 years of age that provided mortality data were considered eligible for this systematic review and meta-analysis. Τhe primary outcome of the meta-analysis was 30-day all-cause mortality. Ten retrospective studies including 967 patients were identified. Patients treated with daptomycin had significantly higher 30-day all-cause mortality (odds ratio [OR], 1.61; 95% confidence interval [CI], 1.08 to 2.40) and infection-related mortality (OR, 3.61; 95% CI, 1.42 to 9.20) rates than patients treated with linezolid. When data from all 10 studies were combined, overall mortality was also significantly increased among patients treated with daptomycin (OR, 1.41; 95% CI, 1.06 to 1.89). These findings were confirmed when odds ratios adjusted for potential confounders were pooled. Relapse rates among patients treated with daptomycin were also higher (OR, 2.51; 95% CI, 0.94 to 6.72), although this difference did not reach statistical significance. Adverse event rates were not significantly different between the two groups. Notwithstanding the absence of randomized prospective data, available evidence suggests that mortality rates may be higher with daptomycin than with linezolid among patients treated for VRE bacteremia.
INTRODUCTION
Enterococci are the third most common cause of health care-associated bloodstream infections (BSIs) (1). Vancomycin is the first-line treatment of BSIs caused by ampicillin-resistant enterococci; however vancomycin-resistant enterococci (VRE) nowadays account for approximately one-third of the enterococcal health care-associated infections in the United States (2) and for more than 20% of such infections in some European countries (3). Mortality rates in patients with VRE BSIs range between 20 and 46% (4–6). Patients with BSI due to VRE are 2.5 times more likely to die than patients with BSI due to vancomycin-susceptible strains (7).
Treatment of VRE BSIs is particularly challenging. Strains causing such infections are usually resistant to ampicillin (8), and therapeutic options include linezolid, daptomycin, quinupristin-dalfopristin, tigecycline, teicoplanin, and telavancin (for which limited clinical data are available). Teicoplanin is not available in the United States and can only be used for some VRE infections (i.e., strains with the VanB [vancomycin-resistant, teicoplanin-susceptible] phenotype and the rare species Enterococcus gallinarum and E. casseliflavus). Tigecycline does not achieve high serum concentrations and has not been approved for treatment of bacteremias (9). Use of quinupristin-dalfopristin (effective only against E. faecium) is limited by the need of central venous access for administration, frequent side effects, and drug interactions (10).
Clinical experience and data for the treatment of VRE BSIs are available mainly for linezolid and daptomycin. Linezolid has been approved by the U.S. Food and Drug Administration (FDA) for the treatment of vancomycin-resistant E. faecium infections, including those with concurrent bacteremia. Although daptomycin is not FDA approved for the treatment of VRE bacteremia, its rapid bactericidal activity (11, 12) offers an off-label alternative (13, 14). According to the relevant clinical practice guidelines of the Infectious Diseases Society of America, linezolid or daptomycin is recommended for the treatment of catheter-related BSIs caused by ampicillin- and vancomycin-resistant enterococci (15). Limited data exist on the comparative efficacy of daptomycin versus linezolid for enterococcal bacteremias (5, 6). Herein we summarize the available evidence and provide estimates of the clinical effectiveness of linezolid versus daptomycin for the treatment of VRE bacteremia by using meta-analytic methodology.
MATERIALS AND METHODS
Search strategy.
A computerized literature search in the MEDLINE, EMBASE, CENTRAL, ISI Web of Science, and SCOPUS electronic databases covering the period until 31 August 2012 was performed independently by two individuals. The strategy employed for this study is presented in detail in the supplemental material.
Selection of studies.
In order for the studies to be eligible for this systematic review, the following inclusion criteria were established prior to literature search: (i) studies should compare the outcomes of treatment between daptomycin and linezolid for VRE bacteremia in two groups of patients, (ii) patients should be older than 18 years, and (iii) the study should provide data on patient mortality outcomes.
All studies identified to address the research question were initially considered for the present systematic review, regardless of the direction of study (retrospective or prospective) and the sample size. Case reports and case series of patients treated with either one of the two agents were not included.
Studies identified.
The electronic search resulted in the retrieval of 2,365 publications (see Fig. S1 in the supplemental material). Their titles were screened to exclude irrelevant studies, resulting in 46 potentially eligible studies. A search of meeting abstracts resulted in the retrieval of eight additional studies. Of the total of 54 studies, 39 were excluded after examination of their abstracts (8 retrospective, noncomparative studies, 26 reviews and opinion papers, and 5 irrelevant studies), while 4 further studies published in meeting proceedings were excluded because they provided data already included in the identified published full texts (overlapping publications) (16–19). Eventually, 11 studies were considered for further evaluation. One study was excluded at this stage because daptomycin was not included in the comparator agents (20).
The full reference lists of the studies whose full text was examined were hand searched, which did not result in the identification of any additional studies, nor did a search of the clinical trial registries. Eventually, 10 studies comparing the efficacy of daptomycin and linezolid for the treatment of VRE bacteremia were included in this systematic review and meta-analysis (5, 6, 21–28).
Data extraction.
The methodology that was followed for extracting the data is described in the supplemental material.
Outcomes.
The primary outcome examined in the meta-analysis was mortality, expressed as 30-day all-cause mortality (defined as death from any reason within 30 days from the first culture positive for VRE). Ιnfection-related mortality (defined as death attributed to VRE bacteremia) and in-hospital mortality (defined as death from any reason during hospital stay) were also evaluated. Since mortality endpoints were different across studies, a composite outcome—defined as overall mortality—was also calculated by including any relevant comparison on mortality rates between daptomycin and linezolid, irrespective of the definition used (i.e., all-cause, infection-related, in-hospital, 30-day, etc.). When some data on the outcomes of interest were not provided in the full-text papers or abstracts, the authors were contacted for further information.
Secondary outcome measures included (i) clinical cure (defined as a resolution of signs and/or symptoms of infection after treatment for VRE was discontinued), (ii) microbiological cure (with the last blood culture drawn after initiation of VRE treatment being negative), (iii) recurrence of VRE bacteremia (with a posttreatment blood culture positive for VRE following at least one negative blood culture), and (iv) adverse events (defined as the development of an adverse event proven or suspected to be related to the agent used for VRE treatment or to the route of administration).
Quantitative data synthesis.
Information on quantitative data synthesis is presented in detail in the supplemental material.
RESULTS
Systematic review.
The 10 studies identified as fulfilling the inclusion criteria for the systematic review included 967 patients in total. The characteristics of those studies are listed in Tables S2 to S4 in the supplemental material.
All studies were published between 2005 and 2012 and were of a retrospective cohort nature. Two were multicenter studies (22, 28), seven reported the experience of single centers, and in one case this information was not provided (27). The primary outcome measure was microbiological cure in two studies (21, 28), 30-day all-cause mortality in one study (5), and clinical and microbiological cure in one study (6), while in five studies the primary outcome among those examined was not stated.
The sample sizes of the included studies ranged from 31 to 201 patients (median, 82 patients). With two exceptions (26, 27), the studies included mixed populations, with various percentages of immunocompromised and nonimmunocompromised patients (see Table S2 in the supplemental material).
Definitions of VRE BSIs differed slightly across studies. The Centers for Disease Control and Prevention (CDC) definition for enterococcal bacteremia was used in four studies (5, 21, 26, 28). Two or more positive blood cultures or one positive blood culture with an identifiable source in a clinical scenario consistent with bacteremia defined VRE bacteremia in one study (22). The presence of one or more blood cultures positive for VRE (without further clarifications) was used in three studies (6, 23, 25). In the remaining two studies, an explicit definition of VRE BSI was not provided (24, 27).
Statistically significant differences in potential confounders between groups of patients treated with daptomycin or linezolid are listed in Table S3 in the supplemental material. Adjustments for potential confounders were performed by the authors in six studies, using multivariable logistic regression analysis (5, 21–23, 25, 28).
The median daily daptomycin dose was 6 mg/kg of body weight in six studies (6, 21–23, 27, 28), 5.5 mg/kg in one study (26), and not reported in three studies (5, 24, 25). The median duration of treatment ranged from 13 to 15 days in the daptomycin group and from 11 to 15 days in the linezolid group (21–23, 28). Combination with aminoglycosides was reported in two studies (22, 28). Patients simultaneously treated with more than one anti-VRE agent were excluded in two studies (6, 21) (see Table S4 in the supplemental material).
Prior vancomycin use was reported in two studies (22, 28) and was significantly different across groups in one of them (22). Four studies reported inclusion of patients with endocarditis (6, 21, 22, 28). Outcomes of these patients were reported separately from those for nonendocarditis bacteremia in one study only (28). Patients were switched from linezolid to daptomycin during treatment of bacteremia in two studies (due to failure, intolerance, or clinical preference [22] or to resistance or intolerance [25]), and one patient was switched from daptomycin to linezolid due to adverse events (26). Linezolid susceptibility was tested in three studies (6, 21, 26), and daptomycin susceptibility was tested in two studies (6, 21).
Meta-analysis. (i) Thirty-day all-cause mortality.
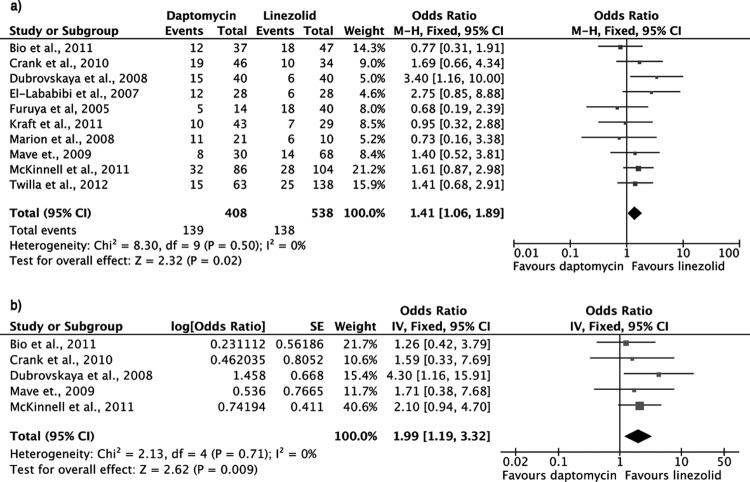
All-cause mortality at 30 days (our prespecified primary endpoint) was significantly increased in patients treated with daptomycin compared to those treated with linezolid (odds ratio [OR], 1.61; 95% confidence interval [CI], 1.08 to 2.40; fixed-effects model; heterogeneity P = 0.42) (Fig. 1a). No publication bias was detected (Egger's test P = 0.84). Four studies offered data for this outcome (5, 6, 23, 26).
FIG 1.
Forest plots (using Mantel-Haenszel [M-H] analysis) of unadjusted (a) and adjusted (b) odds ratios for 30-day all-cause mortality among patients treated with linezolid or daptomycin for VRE bacteremia. CI, confidence interval; SE, standard error; IV, Inverse variance.
In two studies, odds ratios were adjusted for potential confounders in multivariate logistic regression models (5, 23). When these were combined, a statistically significant increase in mortality rate was still present for patients of the daptomycin group compared to those in the linezolid group (adjusted OR, 2.56; 95% CI, 1.29 to 5.08; fixed-effects model; heterogeneity P = 0.36) (Fig. 1b).
(ii) Infection-related mortality.
Infection-related mortality was significantly higher in patients who received daptomycin than in those who received linezolid (OR, 3.61; 95% CI, 1.42 to 9.20; fixed-effects model; heterogeneity P = 0.49) (Fig. 2a). Adjusted odds ratios for infection-related mortality were not available.
FIG 2.
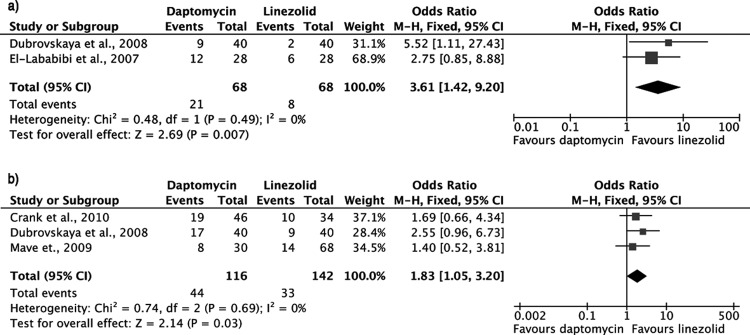
Forest plots (using Mantel-Haenszel [M-H] analysis) of odds ratios for infection-related mortality (a) and in-hospital mortality (b) among patients treated with linezolid or daptomycin for VRE bacteremia.
(iii) In-hospital mortality.
The in-hospital mortality rate was significantly higher with daptomycin than with linezolid (OR, 1.83; 95% CI, 1.05 to 3.20; fixed-effects model; heterogeneity P = 0.69) (Fig. 2b). Two studies estimated adjusted odds ratios for in-hospital mortality after controlling for potential confounders in multivariate logistic regression models (22, 28). When these data were combined, a higher mortality with daptomycin was observed; however, the difference did not reach statistical significance (OR, 1.65; 95% CI, 0.56 to 4.90; fixed-effects model; heterogeneity P = 0.95).
In the study by Crank et al., 21 patients were switched to daptomycin after linezolid failure, intolerance, or other reason as determined by the treating physicians (22). The odds ratio for mortality in this case was calculated after excluding these 21 patients, while the adjusted odds ratios provided by the authors of the study were statistically controlled for prior linezolid use.
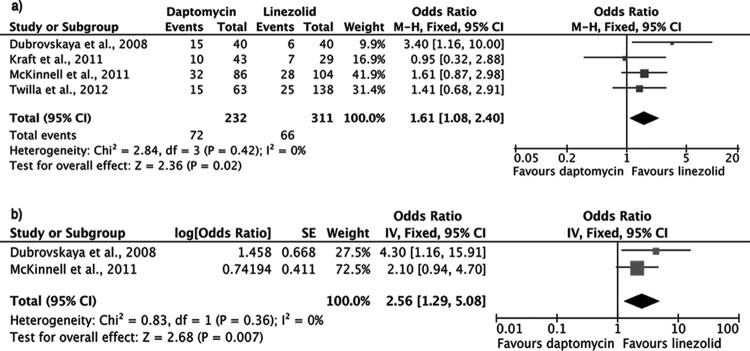
(iv) Overall mortality.
The overall mortality rate, as defined for the purposes of this meta-analysis, was significantly increased in patients treated with daptomycin compared to those treated with linezolid for VRE bacteremia (OR, 1.41; 95% CI, 1.06 to 1.89; fixed-effects model; heterogeneity P = 0.50). No publication bias was detected (Egger's test P = 0.58) (Fig. 3a).
FIG 3.
Forest plots (using Mantel-Haenszel [M-H] analysis) of unadjusted (a) and adjusted (b) odds ratios for overall mortality among patients treated with linezolid or daptomycin for VRE bacteremia.
In the study by Furuya et al., a significant proportion of patients were switched to daptomycin following linezolid failure or intolerance (25). Since this could have potentially resulted in bias, we performed a sensitivity analysis excluding this study, which did not substantially alter the findings (OR, 1.48; 95% CI, 1.09 to 2.00; fixed-effects model; heterogeneity P = 0.54).
Five studies provided adjusted odds ratios after controlling for potential confounders (5, 21–23, 28). When these data were pooled, overall mortality was still significantly increased in the daptomycin group compared to the linezolid group (OR, 1.99; 95% CI, 1.19 to 3.32; fixed-effects model; heterogeneity P = 0.71) (Fig. 3b).
(v) Clinical cure.
A significant difference in clinical cure rate was not detected in patients treated with daptomycin compared to those treated with linezolid (OR, 1.04; 95% CI, 0.63 to 1.72; fixed-effects model; heterogeneity P = 0.12). Three studies provided data for this outcome (6, 21, 24).
(vi) Microbiological cure.
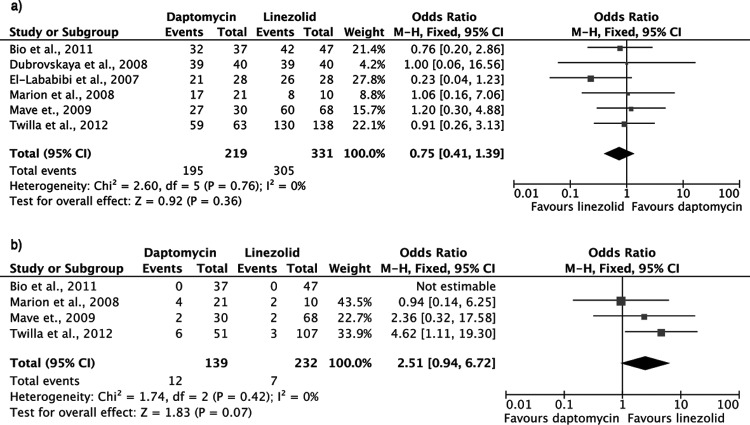
Microbiological cure rates did not differ significantly between the two groups (OR, 0.75; 95% CI, 0.41 to 1.39; fixed-effects model; heterogeneity P = 0.76) (Fig. 4a). Six studies offered data on this outcome (6, 21, 23, 24, 27, 28).
FIG 4.
Forest plots (using Mantel-Haenszel [M-H] analysis) of odds ratios for microbiological cure (a) and bacteremia recurrence (b) in patients treated with daptomycin or linezolid for VRE bacteremia.
(vii) Recurrence of VRE bacteremia.
There was a trend toward higher relapse rates among patients treated with daptomycin than among those treated with linezolid, with the difference marginally failing to reach statistical significance (OR, 2.51; 95% CI, 0.94 to 6.72; fixed-effects model; heterogeneity P = 0.42) (Fig. 4b). Data for this outcome were provided by four studies (6, 21, 27, 28).
(viii) Adverse events.
Notwithstanding the study of Kraft et al., which reported a significant difference in increased liver function tests among patients treated with daptomycin (26), no significant differences in adverse event rates between the two groups were detected when data from individual studies were combined (see Table S5 in the supplemental material).
DISCUSSION
The present systematic review and meta-analysis summarizes the available data regarding the efficacy of linezolid versus daptomycin for the treatment of VRE bacteremia. To the best of our knowledge, this is the first study that attempts to critically appraise the existing evidence on this controversial issue. Based on the meta-analysis results, 30-day all-cause mortality was significantly higher among patients with VRE bacteremia who were treated with daptomycin than among those treated with linezolid. Notably, the in-hospital mortality and infection-related mortality rates were also increased in the daptomycin group compared to the linezolid group. These findings were not materially altered in the sensitivity analyses (performed by pooling the adjusted odds ratios for mortality that were provided by the authors of individual studies). Administration of both drugs was relatively safe in high-risk patient cohorts, and the frequency of adverse events did not seem to differ between the two treatment options.
An important strength of meta-analysis is its inherent ability to increase the statistical power of individual studies. Notably, most of the studies included in this analysis showed a trend toward increased mortality rates among patients treated with daptomycin. With the exception, however, of one study (23), the difference from linezolid did not reach statistical significance. When the results of individual studies were combined, a significant increase in all mortality outcomes in the daptomycin group surfaced, coupled with negligible (I2 = 0%) heterogeneity across studies. We acknowledge, however, that despite the absence of statistical heterogeneity, significant clinical heterogeneity was present across the studies analyzed (i.e., in terms of patients included, other antibiotics used, doses, etc. [summarized in Tables S2 to S4 in the supplemental material]). For this reason, the results of the studies were also combined with the use of a random-effects model, and the pooled estimates for all mortality outcomes remained unaltered (data not shown). Hence, the results obtained in this meta-analysis are stable and thus seem to accurately reflect the underlying effect present in the available comparative studies.
In order to further increase the statistical power of this meta-analysis, the composite outcome of overall mortality rate was calculated. This outcome combined data on mortality from individual studies, whether this was expressed as 30-day all-cause mortality (n = 4) (5, 6, 23, 26), in-hospital mortality (n = 2) (22, 28), infection-related mortality (n = 1) (24), mortality at the end of therapy (n = 1) (25), mortality 7 days after the end of therapy (n = 1) (21), or overall mortality (n = 1) (27). The pooled overall mortality rate confirmed the findings of primary analysis.
Certain limitations apply for the interpretation of our results. All available studies were retrospective and observational. The possibility of significant confounders therefore exists (e.g., selection bias, with patients with worse prognoses being treated with daptomycin, patients able to swallow being treated with linezolid, etc.). A proportion of patients treated with either agent were later changed to the other (usually due to failure), had previously received another antibiotic (typically vancomycin), or had additional organisms recovered in blood cultures. Characteristics such as the presence of endocarditis (6, 21, 22, 28), source of any secondary bacteremias (21, 22) (including the rare possibility of enterococcal pneumonias, where daptomycin would not be indicated), treating physicians and ID consultations (5), daptomycin dosing (6, 21–23, 26–28), and combination therapies (22, 28) were not available for all patients. Although such biases cannot be eliminated outside the context of a randomized prospective trial, we note that results from adjustment that took into account known confounders (listed in Table S3 in the supplemental material) were all in agreement with those of the primary analysis. We also note that pooling such patients (i.e., patients with and without endocarditis, with and without additional therapies, etc.) in itself risks introducing bias. Even so, consistent results in favor of linezolid were obtained when authors of individual studies adjusted for known confounders (5, 21–23, 28). Notably, similar characteristics between the two patient groups were recorded in most studies; in fact, factors associated with unfavorable prognosis were overrepresented among the linezolid patient group in some studies (i.e., patients who were older [5, 6, 28], in the intensive care unit [ICU] [21], or had higher APACHE scores [28]). On the other hand, whether daptomycin or linezolid is advantageous in specific patient populations (e.g., hemodialysis, transplant recipients, etc.) could not be evaluated in the present study due to the limited number of data available.
A potential explanation for the observed inferior outcomes for patients treated for VRE bacteremia with a bactericidal agent (daptomycin) compared to those treated with a bacteriostatic (linezolid) should perhaps be sought in the context of recent reports on daptomycin failures during treatment of enterococcal infections and emergence of resistance, especially among VRE strains (29–31). In regards to this, we note the higher (although marginally failing statistical significance tests) relapse rates of VRE bacteremia following daptomycin treatment than following linezolid treatment in our analysis (Fig. 4b). In contrast with mortality and tendency toward relapses, clinical and microbiological cure rates did not differ between the two agents. Given that neither mortality cause nor clinical/microbiological cure data were available for all studies, a definite conclusion on any relationship between those outcomes cannot be drawn with certainty.
Optimal daptomycin dosing for treatment of severe infections remains a challenge, as higher doses have been proposed (30, 32) and recently supported by in vitro data on VRE (33). Inferences regarding the optimal dose of daptomycin for treating VRE bacteremia could not be made from this review, since six of seven studies used a median dose of 6 mg/kg (6, 21–23, 27, 28), while one study used a median dose of 5.5 mg/kg (26). It is possible that some of the suboptimal outcomes were associated with daptomycin underdosing (i.e., <6 mg/kg). Whether even higher, off-label daptomycin doses would increase efficacy in the treatment of VRE bacteremia, without increasing toxicity, also remains to be explored. Similarly, the effect of proposed strategies of combination treatment with daptomycin and ampicillin (31) or rifampin (34) could not be assessed adequately from these data.
Based on the evidence summarized herein, daptomycin may be associated with worse outcomes in patients treated for VRE bacteremia than linezolid. Given, however, the methodologic limitations of the existing studies, a properly designed randomized controlled multicenter trial to evaluate therapeutic options for VRE bacteremia is required, although this would be a challenging task.
Supplementary Material
ACKNOWLEDGMENTS
S. Miyakis has received research support, travel grants, and honoraria from Pfizer and Novartis. C. A. Venetis and E. P. Balli report no conflicts of interest.
No funding was received for this study.
E.P.B. and C.A.V. performed the computerized literature search in the MEDLINE, EMBASE, CENTRAL, ISI Web of Science, and SCOPUS electronic databases.
Footnotes
Published ahead of print 18 November 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.01289-13.
REFERENCES
- 1.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. 2004. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 39:309–317. 10.1086/421946 [DOI] [PubMed] [Google Scholar]
- 2.Hidron AI, Edwards JR, Patel J, Horan TC, Sievert DM, Pollock DA, Fridkin SK. 2008. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect. Control Hosp. Epidemiol. 29:996–1011. 10.1086/591861 [DOI] [PubMed] [Google Scholar]
- 3.European Centre for Disease Prevention and Control 2010. Antimicrobial resistance surveillance in Europe 2009. Annual report of the European Antimicrobial Resistance Surveillance Network (EARS-Net). ECDC, Stockholm, Sweden: http://ecdc.europa.eu/en/publications/Publications/1011_SUR_annual_EARS_Net_2009.pdf [Google Scholar]
- 4.Han SH, Chin BS, Lee HS, Jeong SJ, Choi HK, Kim CO, Yong D, Choi JY, Song YG, Lee K, Kim JM. 2009. Vancomycin-resistant enterococci bacteremia: risk factors for mortality and influence of antimicrobial therapy on clinical outcome. J. Infect. 58:182–190. 10.1016/j.jinf.2009.01.013 [DOI] [PubMed] [Google Scholar]
- 5.McKinnell JA, Patel M, Shirley RM, Kunz DF, Moser SA, Baddley JW. 2011. Observational study of the epidemiology and outcomes of vancomycin-resistant Enterococcus bacteraemia treated with newer antimicrobial agents. Epidemiol. Infect. 139:1342–1350. 10.1017/S0950268810002475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Twilla JD, Finch CK, Usery JB, Gelfand MS, Hudson JQ, Broyles JE. 2012. Vancomycin-resistant Enterococcus bacteremia: an evaluation of treatment with linezolid or daptomycin. J. Hosp. Med. 7:243–248. 10.1002/jhm.994 [DOI] [PubMed] [Google Scholar]
- 7.DiazGranados CA, Zimmer SM, Klein M, Jernigan JA. 2005. Comparison of mortality associated with vancomycin-resistant and vancomycin-susceptible enterococcal bloodstream infections: a meta-analysis. Clin. Infect. Dis. 41:327–333. 10.1086/430909 [DOI] [PubMed] [Google Scholar]
- 8.Deshpande LM, Fritsche TR, Moet GJ, Biedenbach DJ, Jones RN. 2007. Antimicrobial resistance and molecular epidemiology of vancomycin-resistant enterococci from North America and Europe: a report from the SENTRY antimicrobial surveillance program. Diagn. Microbiol. Infect. Dis. 58:163–170. 10.1016/j.diagmicrobio.2006.12.022 [DOI] [PubMed] [Google Scholar]
- 9.Arias CA, Contreras GA, Murray BE. 2010. Management of multidrug-resistant enterococcal infections. Clin. Microbiol. Infect. 16:555–562. 10.1111/j.1469-0691.2010.03214.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rubinstein E, Prokocimer P, Talbot GH. 1999. Safety and tolerability of quinupristin/dalfopristin: administration guidelines. J. Antimicrob. Chemother. 44(Suppl A):37–46. 10.1093/jac/44.suppl_1.37 [DOI] [PubMed] [Google Scholar]
- 11.Akins RL, Rybak MJ. 2001. Bactericidal activities of two daptomycin regimens against clinical strains of glycopeptide intermediate-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus faecium, and methicillin-resistant Staphylococcus aureus isolates in an in vitro pharmacodynamic model with simulated endocardial vegetations. Antimicrob. Agents Chemother. 45:454–459. 10.1128/AAC.45.2.454-459.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jorgensen JH, Crawford SA, Kelly CC, Patterson JE. 2003. In vitro activity of daptomycin against vancomycin-resistant enterococci of various Van types and comparison of susceptibility testing methods. Antimicrob. Agents Chemother. 47:3760–3763. 10.1128/AAC.47.12.3760-3763.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kvirikadze N, Suseno M, Vescio T, Kaminer L, Singh K. 2006. Daptomycin for the treatment of vancomycin resistant Enterococcus faecium bacteremia. Scand. J. Infect. Dis. 38:290–292. 10.1080/00365540500434687 [DOI] [PubMed] [Google Scholar]
- 14.Poutsiaka DD, Skiffington S, Miller KB, Hadley S, Snydman DR. 2007. Daptomycin in the treatment of vancomycin-resistant Enterococcus faecium bacteremia in neutropenic patients. J. Infect. 54:567–571. 10.1016/j.jinf.2006.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O'Grady NP, Raad II, Rijnders BJ, Sherertz RJ, Warren DK. 2009. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 49:1–45. 10.1086/599376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crank CW, Scheetz M, Brielmaier B, Rose W, Patel G, Ritchie D, Segreti J. 2008. Comparison of daptomycin (D) vs. linezolid (L) for vancomycin-resistant enterococcal bacteremia (VRE), abstr K-3442 Abstr. Joint 48th Intersci. Conf. Antimicrob. Agents Chemother. 46th Infect. Dis. Soc. Am. Annu. Meet [Google Scholar]
- 17.Locastro LG, Perez ME, Marino EA, Abrardo LA, Gallagher JC. 2008. Comparison of linezolid (LZD) and daptomycin (DAP) for vancomycin-resistant Enterococcus bacteremia (VREB), abstr K-3440 Abstr. Joint 48th Intersci. Conf. Antimicrob. Agents Chemother. 46th Infect. Dis. Soc. Am. Annu. Meet [Google Scholar]
- 18.Mave V, Hasbun R, Garcia-Diaz J. 2007. Vancomycin-resistant enterococcal bacteraemia: is daptomycin as effective as linezolid?, abstr 1090 Abstr. 45th Annu. Meet. Infect. Dis. Soc. Am [DOI] [PubMed] [Google Scholar]
- 19.McKinnell JA, Patel M, Shirley RM, Kunz DF, Baddley JW. 2009. Clinical outcomes with linezolid and daptomycin for the treatment of VRE bacteremia, abstr K-1603 Abstr. 49th Intersci. Conf. Antimicrob. Agents Chemother [Google Scholar]
- 20.Erlandson KM, Sun J, Iwen PC, Rupp ME. 2008. Impact of the more-potent antibiotics quinupristin-dalfopristin and linezolid on outcome measure of patients with vancomycin-resistant Enterococcus bacteremia. Clin. Infect. Dis. 46:30–36. 10.1086/523588 [DOI] [PubMed] [Google Scholar]
- 21.Bio LL, Perez ME, MacDougall C, Gallagher JC. 2011. Comparison of linezolid and daptomycin in the treatment of vancomycin-resistant enterococcal bacteremia. Infect. Dis. Clin. Pract. 19:343–347. 10.1097/IPC.0b013e31822b7f6e [DOI] [Google Scholar]
- 22.Crank CW, Scheetz MH, Brielmaier B, Rose WE, Patel GP, Ritchie DJ, Segreti J. 2010. Comparison of outcomes from daptomycin or linezolid treatment for vancomycin-resistant enterococcal bloodstream infection: a retrospective, multicenter, cohort study. Clin. Ther. 32:1713–1719. 10.1016/j.clinthera.2010.09.008 [DOI] [PubMed] [Google Scholar]
- 23.Dubrovskaya Y, Kubin CJ, Furuya EY. 2008. Daptomycin (D) compared to linezolid (L) for primary treatment of vancomycin-resistant enterococcal bacteremia (VREB), abstr K-3443 Abstr. Joint 48th Intersci. Conf. Antimicrob. Agents Chemother. 46th Infect. Dis. Soc. Am. Annu. Meet [Google Scholar]
- 24.El-Lababidi RM, Topal J, Tsukerman M. 2007. Daptomycin and linezolid in the treatment of vancomycin-resistant enterococcal bacteremia: a retrospective analysis of treatment outcomes, abstr 1095 Abstr. 45th Annu. Meet. Infect. Dis. Soc. Am [Google Scholar]
- 25.Furuya EY, Kubin C, Yin M, Lowy FD, Della-Latta P, Hammer S. 2005. Daptomycin experience and comparison with linezolid for the treatment of vancomycin-resistant enterococcal bacteremia, abstr K-2116 bstr. 45th Intersci. Conf. Antimicrob. Agents Chemother [Google Scholar]
- 26.Kraft S, MacKler E, Schlickman P, Welch K, Depestel DD. 2011. Outcomes of therapy: vancomycin-resistant enterococcal bacteremia in hematology and bone marrow transplant patients. Support Care Cancer 19:1969–1974. 10.1007/s00520-010-1038-z [DOI] [PubMed] [Google Scholar]
- 27.Marion C, Kennedy L, High K. 2008. Daptomycin or linezolid in the treatment of vancomycin-resistant enterococcal bacteremia in neutropenic cancer patients, abstr L-2120 Abstr. Joint 48th Intersci. Conf. Antimicrob. Agents Chemother. 46th Infect. Dis. Soc. Am. Annu. Meet [Google Scholar]
- 28.Mave V, Garcia-Diaz J, Islam T, Hasbun R. 2009. Vancomycin-resistant enterococcal bacteraemia: is daptomycin as effective as linezolid? J. Antimicrob. Chemother. 64:175–180. 10.1093/jac/dkp154 [DOI] [PubMed] [Google Scholar]
- 29.Kelesidis T, Humphries R, Uslan DZ, Pegues DA. 2011. Daptomycin nonsusceptible enterococci: an emerging challenge for clinicians. Clin. Infect. Dis. 52:228–234. 10.1093/cid/ciq113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.King ST, Usery JB, Holloway K, Koeth L, Cleveland KO, Gelfand MS. 2011. Successful therapy of treatment-emergent, non-clonal daptomycin-non-susceptible Enterococcus faecium infections. J. Antimicrob. Chemother. 66:2673–2675. 10.1093/jac/dkr343 [DOI] [PubMed] [Google Scholar]
- 31.Sakoulas G, Bayer AS, Pogliano J, Tsuji BT, Yang SJ, Mishra NN, Nizet V, Yeaman MR, Moise PA. 2012. Ampicillin enhances daptomycin- and cationic host defense peptide-mediated killing of ampicillin- and vancomycin-resistant Enterococcus faecium. Antimicrob. Agents Chemother. 56:838–844. 10.1128/AAC.05551-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crompton JA, North DS, McConnell SA, Lamp KC. 2009. Safety and efficacy of daptomycin in the treatment of osteomyelitis: results from the CORE Registry. J. Chemother. 21:414–420. 10.1179/joc.2009.21.4.414 [DOI] [PubMed] [Google Scholar]
- 33.Hall AD, Steed ME, Arias CA, Murray BE, Rybak MJ. 2012. Evaluation of standard- and high-dose daptomycin versus linezolid against vancomycin-resistant Enterococcus isolates in an in vitro pharmacokinetic/pharmacodynamic model with simulated endocardial vegetations. Antimicrob. Agents Chemother. 56:3174–3180. 10.1128/AAC.06439-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steenbergen JN, Mohr JF, Thorne GM. 2009. Effects of daptomycin in combination with other antimicrobial agents: a review of in vitro and animal model studies. J. Antimicrob. Chemother. 64:1130–1138. 10.1093/jac/dkp346 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.