Abstract
Bacterial resistance to antibiotics and biocides is an increasing public health problem. Genes encoding integral membrane proteins belonging to the DedA family are present in most bacterial genomes, including Escherichia coli. An E. coli strain lacking partially redundant DedA family genes yqjA and yghB (strain BC202) displays temperature sensitivity and cell division defects. These phenotypes can be corrected by overexpression of mdfA, an Na+-K+/H+ antiporter of the major facilitator superfamily. We show that BC202 is hypersensitive to several biocides and cationic compounds that are known substrates of several multidrug resistance transporters, including MdfA, EmrE, and AcrB. The introduction of deletions of genes encoding these drug transporters into BC202 results in additional sensitivity. Expression of wild-type yghB or yqjA can restore drug resistance, but this is eliminated upon mutation of two membrane-embedded acidic amino acids (E39 or D51 in either protein). This dependence upon membrane-embedded acidic amino acids is a hallmark of proton-dependent antiporters. Overexpression of mdfA in BC202 or artificially restoring proton motive force (PMF) restores wild-type resistance to substrates of MdfA as well as other drug resistance transporters such as EmrE and AcrAB. These results suggest that YqjA and YghB may be membrane transporters required for PMF-dependent drug efflux in E. coli.
INTRODUCTION
Bacterial resistance to biocides and antibiotics is an increasing problem. Resistance is often conferred by expression of efflux pumps that can accommodate numerous unrelated drugs (1, 2). Efflux pumps belonging to the major facilitator superfamily (MFS), the small multidrug resistance (SMR) family, and ABC transporter families are found widespread throughout evolution. While the functions of these protein families have been studied in detail, genome sequencing projects have revealed the existence of many families of membrane proteins whose functions are not well understood (3).
DedA is a large superfamily of membrane proteins that are found within all three domains of life and are present in nearly all sequenced bacterial genomes (4). They are sometimes annotated “SNARE-associated family” based on homology to yeast Tvp38 (5). Protein databases do not classify members of the DedA family as membrane transporters. They bear no amino acid similarity to the MFS, SMR family, ABC transporters, ion channels, or other types of transporters. They bear limited similarity to the LeuT family of transporters when analyzed using a novel type of evolutionary analysis called AlignMe (6). The Escherichia coli genome encodes eight members of the DedA family displaying ∼25 to 60% amino acid identity (annotated dedA, yqjA, yghB, yabI, yohD, yqaA, ydjX, and ydjZ) (7). YqjA and YghB are DedA family proteins of 220 and 219 amino acids, respectively, with 61% amino acid identity and partially redundant functions. Simultaneous in-frame deletion of these two DedA family genes in strain BC202 (W3110 ΔyghB, ΔyqjA) results in numerous phenotypes, including temperature sensitivity (8), cell division defects due to inefficient export of periplasmic amidases by the twin-arginine pathway (9), and activation of envelope stress response pathways and compromised membrane proton motive force (PMF) (10). Viability at elevated temperatures and normal cell division can be restored to BC202 by growth at pH 6.0 (10) or overexpression of a subset of DedA family genes (8, 11) or mdfA (10), encoding an Na+-K+/H+ antiporter belonging to the MFS (12). These results suggest roles for YqjA/YghB in maintenance of the pH gradient (ΔpH) component of the PMF, composed of both the electrical potential (ΔΨ) and the ΔpH (12). The ΔyqjA mutant is sensitive to alkaline pH (13), and yqjA expression is repressed at low pH (14), suggesting that one function of YqjA may be to promote alkaline tolerance. Similar functions have been shown for E. coli MdfA and Bacillus subtilis Tet(L) (1, 15, 16). A role for YqjA/YghB in PMF maintenance is also supported by our own direct measurements of ΔΨ in BC202 using the JC-1 dye-based assay (10).
In addition to functioning as an ion transporter, MdfA exports numerous cationic and zwitterionic lipophilic compounds (17). Overexpression of MdfA increases resistance of E. coli to a wide variety of structurally diverse drugs and biocides (18), and ΔmdfA mutants are sensitive to exposure to such compounds (19). Given the ability of MdfA to correct the observed phenotypes of BC202, we measured the growth of BC202 and its parent strain W3110 in the presence of biocidal drugs and dyes belonging to different classes. We found that BC202 is hypersensitive to a number of structurally diverse compounds. Resistance can be restored to BC202 by expression of DedA family gene yghB or yqjA. Mutation of membrane-embedded acidic amino acids compromised the ability of YghB and YqjA to restore resistance to BC202, suggesting that these E. coli membrane proteins are possibly a new class of membrane proton-dependent transporters.
MATERIALS AND METHODS
Materials.
All chemicals were reagent grade and purchased from Sigma-Aldrich or VWR. Restriction enzymes, DNA polymerases, and T4 DNA ligase were purchased from New England BioLabs. The following compounds were used for the susceptibility testing, and their classes/modes of action and sources are given in parentheses: chloramphenicol (Sigma-Aldrich), erythromycin (macrolide; Sigma-Aldrich), ciprofloxacin (fluoroquinolone; Sigma-Aldrich), norfloxacin (fluoroquinolone; Sigma-Aldrich), nalidixic acid (quinolone precursor; Sigma-Aldrich), sodium dodecyl sulfate (detergent; Bio-Rad), acriflavine hydrochloride (intercalator; Acros Organics), ethidium bromide (EthBr) (intercalator; Amresco), cetyltrimethyl ammonium bromide (CTAB) (quaternary ammonium compound; Amresco), benzalkonium chloride (quaternary amino compound; Alfa Aesar), methyl viologen (redox cycling drug; Acros Organics), oxacillin (penicillin; Sigma-Aldrich), cloxacillin (penicillin; Sigma-Aldrich), and piperacillin (penicillin; TCI America).
Bacterial growth conditions.
Bacterial cultures were grown in Luria-Bertani broth (LB) (1% tryptone, 0.5% yeast extract, and 1% NaCl) unless otherwise stated. LB medium of pH 6.0 was buffered with 100 mM 2-(N-morpholino)-ethanesulfonic acid (MES) (10). Solid media were obtained by addition of 1.5% agar (wt/vol). When required, the medium was supplemented with ampicillin (Amp) at 100 μg/ml, kanamycin (Kan) at 30 μg/ml, tetracycline (Tet) at 12.5 μg/ml, chloramphenicol (Cam) at 30 μg/ml, arabinose (0.002% or 0.1%, wt/vol), or isopropyl-1-thio-β-d-galactopyranoside (IPTG) (0.5 mM). All cultures were grown at 30°C unless otherwise indicated.
Strain construction.
The multiple-deletion mutants were generated in E. coli W3110, and correct configuration was verified through PCR with primers flanking the appropriate gene. In order to eliminate the kanamycin resistance (Kanr) gene from BC202, plasmid pCP20 expressing FLP recombinase was used as described previously (20). Since this protocol requires overnight incubation at 42°C to cure pCP20 (nonpermissive for BC202), the yghB::Kanr strain was first made markerless (W3110 background). yqjA::Tetr (8) was then introduced by P1 transduction. Additional mutations (i.e., ΔemrE::Kanr, ΔmdfA::Kanr, or ΔacrB::Kanr) were introduced by P1 transduction with P1vir lysate prepared from the indicated E. coli strains (see Table S1 in the supplemental material) obtained from the Keio collection (21). P1 transductions were carried out as described previously (22). All strains and genotypes are listed in Table S1 in the supplemental material.
Microscopy.
Overnight cultures of E. coli strains were diluted 1:100 in fresh LB medium with suitable antibiotics and additives, and grown to an optical density at 600 nm (OD600) of ∼ 0.6 at 30°C in a shaking incubator. Cells were resuspended to a final OD600 of 1.0 in LB, and 10 μl of cells was applied to a 1% agarose coated glass slide for imaging. A Leica DM-RXA2 deconvolution microscope was used for all of the differential interference contrast (DIC) micrographs. Observations were made with a 100×, 1.30-numerical-aperture oil immersion objective lens. The images were captured through a DIC filter by a cooled Cooke SensiCamQE 12-bit, 1,3-megapixel charge-coupled device digital camera and recorded using Slidebook software (Intelligent Imaging Innovation, Denver, CO).
Susceptibility to toxic compounds.
For testing the susceptibility on solid medium, overnight cultures of E. coli strains were freshly diluted 1:100 in LB medium with appropriate antibiotics and additives and grown to an OD600 of ∼0.6 at 30°C in a shaking incubator. Five microliters of serially log10-diluted cells was spotted on LB agar plates containing various concentrations of corresponding biocides. Growth was analyzed after incubation for 20 to 24 h at 30°C. All experiments were repeated at least three times.
MIC determination.
The MICs of biocides were carried out using a 2-fold dilution technique either in 1.5- by 15-cm glass tubes or in 96-well microtiter plates in liquid medium (23). Overnight cultures were freshly diluted 1:100 into LB medium with suitable antibiotics and additives. Exponentially growing cultures at an OD600 of ∼1.0 were inoculated at a density of 105 cells per ml into LB medium supplemented with a series of 2-fold dilutions of indicated biocide. Cell growth was determined visually after incubation at 30°C for 24 h. All experiments were repeated at least three times.
Site-directed mutagenesis.
Site-specific mutants were created by using a previously published protocol (24). The primers carrying the site-specific mutations (see Table S2 in the supplemental material) were used in a PCR to amplify a vector containing the specified wild-type gene. The PCR product was then digested with DpnI and used to transform competent XL1-Blue cells. Colonies obtained after transformation were screened by colony PCR using gene-specific primers. The mutations were confirmed by DNA sequencing conducted at the LSU College of Science Genomics Facility.
Membrane preparation and Western blotting.
Cell membranes was prepared from exponential-phase cultures of BC202 containing appropriate plasmid DNA grown under inducing condition (0.1% arabinose or 0.5 mM IPTG) using a previously published protocol (25). Equal amounts of protein were resolved by 12% SDS-PAGE and transfer to polyvinylidene difluoride (PVDF). Western blotting was done using penta-His (Qiagen) or green fluorescent protein (GFP) (JL-8; Clontech) primary antibody at a 1:5,000 dilution and goat anti-mouse horseradish peroxidase (HRP)-conjugated secondary antibody (Pierce) at 1:5,000. Detection was carried out using the ImmunStar HRP kit (Bio-Rad).
RESULTS
A DedA family mutant is highly sensitive to a number of biocides.
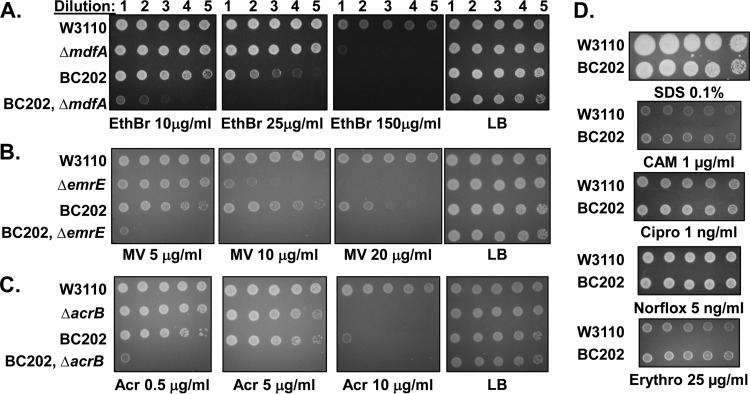
Ethidium bromide (EthBr) efflux is a hallmark of several bacterial organic cation transporters, including MdfA (16, 17), EmrE (26), and QacA (27). We tested for sensitivity to EthBr using a standard growth assay and found that BC202 is significantly more sensitive than the parent strain W3110 to this cationic dye (Fig. 1A). We also observed increased sensitivity of BC202 to biocides known to be exported by other drug efflux proteins (19, 28, 29), including methyl viologen (resistance conferred by EmrE), benzalkonium chloride (mainly MdfA), cetyltrimethyl ammonium bromide (mainly AcrAB), nalidixic acid (AcrAB), acriflavine (EmrE and AcrAB), and β-lactam antibiotics (AcrAB) on solid (Fig. 1B and C; see Fig. 3B) and/or liquid (Table 1) media. We have tested BC202 for sensitivity to many antibiotics and other compounds (8) and have found that BC202 is not generally hypersensitive to all biocides. For example, BC202 is not sensitive to SDS, indicating that the outer membrane is intact in this strain, or, intriguingly, to chloramphenicol, a known substrate of the MdfA efflux pump (Fig. 1D; Table 1). Remarkably, BC202 is sensitive to concentrations of EthBr that are roughly 10-fold lower than what is needed to kill an ΔmdfA mutant (Fig. 1A; Table 1). Expression of either yqjA or yghB individually was capable of restoring resistance to BC202, indicating that these proteins have redundant functions (see Fig. S1 in the supplemental material). These results indicate that YqjA and YghB either are directly involved in drug efflux or are required for proper function of other membrane transporters.
FIG 1.
Sensitivity of BC202, multidrug resistance mutants, and combination mutants to selected biocides. (A to C) BC202 (ΔyghB ΔyqjA) is sensitive to ethidium bromide (EthBr) (A), methyl viologen (MV) (B), and acriflavine (Acr) (C), while the corresponding multidrug resistance mutants display sensitivity approximately equal to or less than that of BC202. MdfA promotes EthBr resistance, EmrE promotes MV resistance, and AcrB is required for acriflavine resistance (19). Combination mutants (with multidrug resistance gene deletions in the BC202 genetic background) display additive or synergistic sensitivity (see also Table 1). (D) BC202 is not appreciably sensitive to SDS, chloramphenicol (CAM), ciprofloxacin (Cipro), norfloxacin (Norflox), or erythromycin (Erythro) on solid LB plates. Dilutions of log-phase cells of the indicated genotype were spotted onto LB plates containing the indicated concentrations of biocide. All strains grew equally well on LB plates with no added biocide.
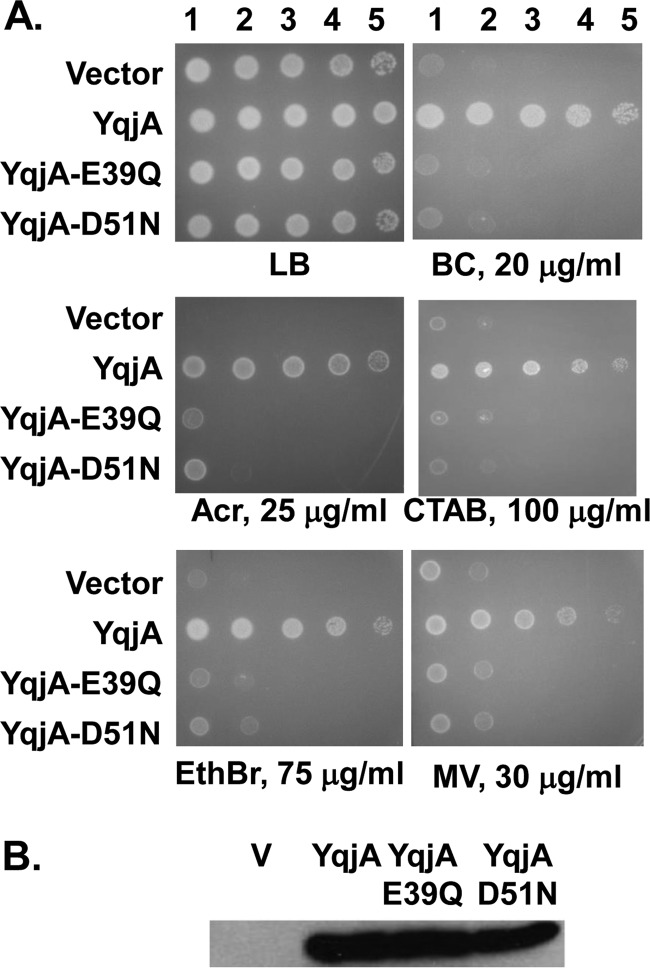
FIG 3.
Isosteric substitutions of YqjA E39 or D51 abolish the ability to restore drug resistance to BC202. (A) Sensitivity of BC202 transformed with control vector pBAD, cloned yqjA, yqjA(E39Q), or yqjA(D51N) to benzalkonium chloride (20 μg/ml), acriflavine (25 μg/ml), CTAB (100 μg/ml), EthBr (75 μg/ml), and methyl viologen (30 μg/ml). All strains grew on LB plates without biocides at 30°C. Wild-type YqjA, but not point mutants, restores drug resistance to BC202 at 30°C. (B) Expression of yqjA, yqjA(E39Q), and yqjA(D51N) in membrane fractions as determined by Western blotting with antihexahistidine antibody. All growth was carried out in LB-Amp supplemented with 0.002% (A) or 0.1% (B) arabinose.
TABLE 1.
MICs for Escherichia coli strainsa
| Compound (MIC unit) | MIC for strainb: |
|||||||
|---|---|---|---|---|---|---|---|---|
| W3110 | BC202 | ΔmdfA mutant | BC202 M | ΔemrE mutant | BC202E | ΔacrB mutant | BC202A | |
| SDS (%) | >1 | >1 | >1 | >1 | >1 | >1 | 0.5 | 0.06 |
| CAM (μg/ml) | 6.0 | 6.0 | 3.0 | 3.0 | 6.0 | 6.0 | 0.4 | 0.4 |
| BC (μg/ml) | 25 | 6.3 | 6.3 | 6.3 | 12.5 | 3.1 | 0.8 | 0.2 |
| NA (μg/ml) | 6.0 | 3.0 | 6.0 | 3.0 | 6.0 | 1.6 | 3.0 | 0.4 |
| CTAB (μg/ml) | 12.5 | 6.3 | 6.3 | 3.1 | 6.3 | 1.6 | 1.6 | 1.6 |
| MV (μg/ml) | 100 | 25 | 100 | 25 | 25 | 12.5 | 100 | 25 |
| Acr (μg/ml) | 100 | 12.5 | 50 | 6.3 | 25 | 6.3 | 6.3 | 0.8 |
| EthBr (μg/ml) | 400 | 25 | 200 | 12.5 | 200 | 12.5 | 3.1 | 0.8 |
| Erythro (μg/ml)c | 100 | 50 | 100 | 50 | 100 | 50 | 6.3 | 3.1 |
| Cipro (ng/ml)c | 5.0 | 2.5 | 5.0 | 2.5 | 5.0 | 2.5 | 1.25 | 0.3 |
| Norflox (ng/ml)c | 40 | 20 | 40 | 20 | 40 | 20 | 20 | 5.0 |
| Ox (μg/ml) | 400 | 200 | ND | ND | ND | ND | 25 | 3.1 |
| Clox (μg/ml) | 400 | 200 | ND | ND | ND | ND | 25 | 3.1 |
| Pip (μg/ml) | 2 | 0.5 | ND | ND | ND | ND | 0.25 | 0.03 |
W3110 (parent), BC202, and mutants with efflux pump mutations in either the W3110 (ΔmdfA, ΔemrE, and ΔacrB) or BC202 (BC202M, BC202E, and BC202A) background strain were grown in liquid culture in the presence of a series of dilutions of the indicated compounds. Abbreviations: SDS, sodium dodecyl sulfate; CAM, chloramphenicol; BC, benzalkonium chloride; NA, nalidixic acid; CTAB, cetyl trimethyl ammonium bromide; MV, methyl viologen; Acr, acriflavine hydrochloride; EthBr, ethidium bromide; Erythro, erythromycin; Cipro, ciprofloxacin; Norflox, norfloxacin; Ox, oxacillin; Clox, cloxacillin; Pip, piperacillin; ND, not determined.
In strain BC202, increased sensitivity to the indicated compounds is shown in italic. In strains BC202M, BC202E, and BC202A, additive or synergistic effects are shown in bold.
BC202 was not sensitive to erythromycin, ciprofloxacin, and norfloxacin on plates (Fig. 1 and data not shown) but was slightly sensitive in liquid culture.
Deletion of known efflux pumps causes an increase in BC202 sensitivity.
Introducing deletions of known transporters into BC202 consistently resulted in a marked increase in drug sensitivity on both solid and liquid media (Fig. 1A to C; Table 1). MdfA and EmrE have been proposed to be the major contributors to EthBr resistance of E. coli (30). The presence of either the ΔmdfA or ΔemrE mutation in the BC202 background strain results in additional sensitivity, suggesting independent resistance mechanisms by these protein families. We see the same pattern with other biocides and the mutants of the corresponding export proteins involved in their efflux, including EthBr, acriflavine, and methyl viologen (Fig. 1A to C; Table 1). Strikingly, the presence of the ΔacrB deletion in BC202 results in a nearly synergistic increase in sensitivity to a number of compounds, including SDS, many-fold higher than what is observed in either background individually (Table 1). These results suggest that the effect on drug sensitivity of these DedA family deletions is independent of other known drug transporters.
Membrane-embedded acidic amino acids are essential for function of DedA family members.
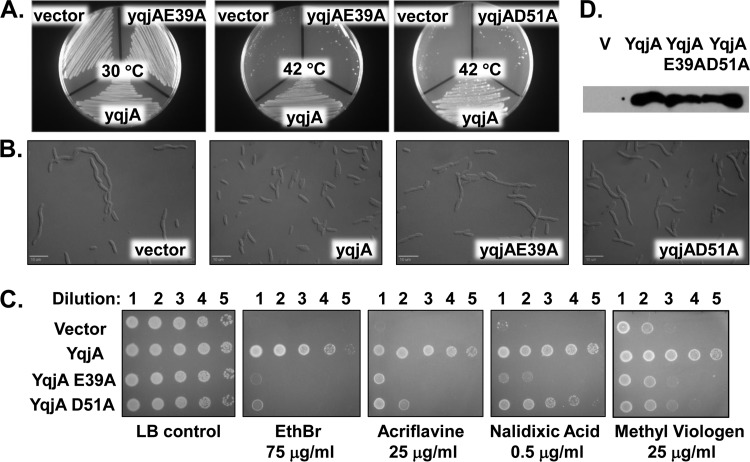
In many multidrug resistance (MDR) transporters, including MdfA, TetL, and EmrE, it has been demonstrated that membrane-embedded acidic residues such as glutamic acid (E) and aspartic acid (D) play a role in PMF-dependent drug transport (Table 2) (31–35). E26 and D34 both play roles in proton translocation in exchange of substrate by MdfA (35). Many DedA proteins contain a predicted membrane-embedded or membrane-proximal glutamic acid and/or aspartic acid in the first transmembrane-spanning (TMS) region (E39 and D51 in YqjA and YghB [Table 2]). We performed site-directed mutagenesis on these residues and found the YqjA point mutants (YqjA-E39A and YqjA-D51A) no longer restored growth at 42°C (Fig. 2A), cell division (Fig. 2B), or drug resistance (Fig. 2C) when expressed in BC202. Mutant protein expression levels were equivalent to wild-type levels in purified membranes (Fig. 2D), demonstrating that the mutant proteins were correctly folded. Wild-type yqjA completely restored growth and cell division (Fig. 2A and B), in agreement with previous reports (8, 11), as well as drug resistance to BC202 (Fig. 2C). To further support these data, we created isosteric substitutions at positions E39 and D51 in YqjA, converting these residues to glutamine and asparagine, respectively. YqjA-E39Q and YqjA-D51N were unable to restore drug resistance (Fig. 3A), growth at 42°C (see Fig. S2A in the supplemental material), or normal cell division (see Fig. S2B in the supplemental material) to BC202. Again, levels of mutant protein found in the membrane were similar to those of the wild-type YqjA (Fig. 3B). This suggests that the charge carried by amino acids E39 and D51 is essential for the function of YqjA. Similar observations were made with YghB and its corresponding point mutants YghB-E39A and YghB-D51A (see Fig. S3 in the supplemental material). The presence of membrane-embedded acidic amino acids that are essential for activity suggests that YqjA and YghB may be proton-dependent transporters.
TABLE 2.
Membrane-embedded acidic amino acids in TM1 of DedA family proteins and selected drug transport proteinsa
| Protein name | TM1 amino acid sequenceb | Accession no. | Family |
|---|---|---|---|
| YqjA | FVLFVILFLENGLLPAAFLPGDS | NP417566 | DedA |
| YghB | IVSVVYFVMFATLFLENGLLPAS(FLPGD)c | NP417482 | DedA |
| BB0250 | VFFSLLILAGLNVPISEDAIVLMd | NP212384 | DedA |
| MdfA | RLGRQALLFPLCLVLYEFSTYIG(ND)e | P0AEZ0 | 12-TMS family of the MFS |
| TetL | LIWLCILSFFSVLNEMVLNVSLP | AAB09024 | 14-TMS family of the MFS |
| EmrE | NPYIYLGGAILAEVIGTTLMKFS | P23895 | 4-TMS family of the SMR family |
Database sequences were analyzed for predicted membrane domains using SOSUI (45). This table is for illustration purposes only and is not a complete list of transport proteins. Abbreviations: TM1, transmembrane region 1; MFS, major facilitator superfamily; SMR, small multidrug resistance.
Conserved acidic amino acids are shown in bold.
YghB D51 is predicted to lie just outside TM1 but is also required for function (see Fig. S3 in the supplemental material).
BB0250 is an essential DedA family protein of Borrelia burgdorferi (36). E39 and D40 are each required for BB0250 to complement growth and cell division defects of BC202 (see Fig. S3 in the supplemental material).
E26 and D34 both play roles in transport by MdfA (35).
FIG 2.
Mutation of YqjA E39 or D51 abolishes the ability to restore growth, cell division, or drug resistance to BC202. (A) BC202 transformed with pBAD, pBAD-yqjA, pBAD-yqjA(E39A), or pBAD-yqjA(D51A) was grown at 30°C (left) or 42°C (center and right). Wild-type YqjA, but not point mutants, restores growth to BC202 at 42°C. BC202/pBAD-yqjA(D51A) also grew at 30°C (not shown). (B) Micrographs of BC202 transformed with pBAD, pBAD-yqjA, pBAD-yqjA(E39A), or pBAD-yqjA(D51A) and grown at 30°C. Wild-type YqjA, but not point mutants, restores normal cell division to BC202. Bar, 10 μm. (C) Sensitivity of BC202 transformed with control vector, cloned yqjA, yqjA(E39A), or yqjA(D51A) to EthBr (75 μg/ml), acriflavine (25 μg/ml), nalidixic acid (0.5 μg/ml), and methyl viologen (25 μg/ml). All strains grew on LB plates without biocides at 30°C. Wild-type YqjA, but not point mutants, restores drug resistance to BC202 at 30°C. (D) Expression of yqjA, yqjA(E39A), and yqjA(D51A) in membrane fractions as determined by Western blotting with antihexahistidine antibody. All growth was carried out in LB-Amp supplemented with 0.002% (A and C) or 0.1% (B and D) arabinose.
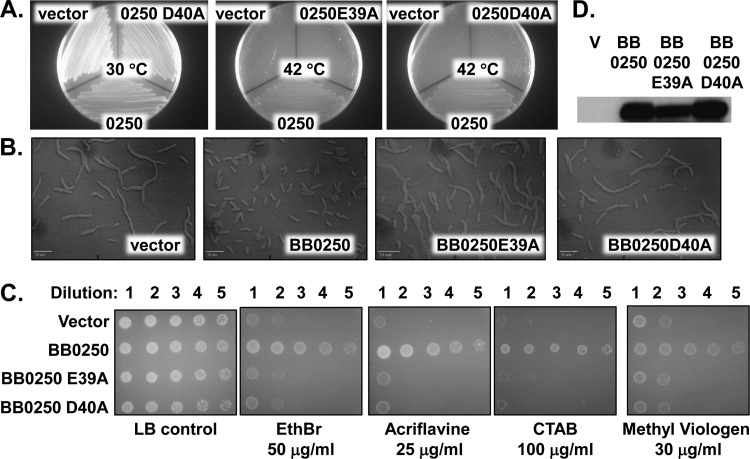
We were interested in determining whether these properties applied to DedA family members found in other bacterial species. BB0250 is the sole DedA family protein of the Lyme disease pathogen Borrelia burgdorferi and is essential for viability (36). Depletion of BB0250 in B. burgdorferi results in cell death preceded by defects in cell division. In other words, the Borrelia mutant phenotypes resemble those of BC202, with the exception being that bb0250 is essential at all temperatures. In addition, cloned bb0250 can fully complement the growth and cell division phenotypes of E. coli BC202 even though BB0250 displays only ∼19% amino acid identity to E. coli YqjA. BB0250 also possesses acidic amino acids within its first predicted transmembrane domain (Table 2). We performed site-directed mutagenesis on residues E39 and D40 in TM1 of BB0250, replacing each with alanine, and showed that each point mutant is unable to restore cell division, temperature sensitivity, and drug resistance to BC202 (Fig. 4A to C) while also being expressed in the membrane at wild-type levels (Fig. 4D). These data collectively suggest that members of the larger DedA family are proton-dependent membrane transporters necessary for resistance to a number of structurally diverse compounds in E. coli and likely other species of bacteria.
FIG 4.
Inability of Borrelia burgdorferi BB0250 E39A and D40A point mutants to restore growth, cell division, or drug resistance to BC202. (A) BC202 was transformed with empty vector pBB0, pBB0250-GFP, pBB0250(E39A)-GFP, or pBB0250(D40A)-GFP and grown at 30 or 42°C. BC202/pBB0250(D40A)-GFP also grew at 30°C (not shown). Expression of wild-type but not mutant BB0250 restored growth to BC202 at 42°C. (B) BC202 was transformed with empty vector, pBB0250-GFP, pBB0250(E39A)-GFP, or pBB0250(D40A)-GFP and grown at 30°C in liquid medium. Cells were visualized using a Leica DM-RXA2 deconvolution microscope. Bar, 10 μm. Expression of wild-type but not mutant BB0250 restored normal cell division to BC202. The ability of wild-type BB0250, included as a positive control, to restore both growth and cell division to BC202 is in agreement with previously published results (36). (C) Sensitivity of BC202 transformed with control vector, pBB0250-GFP, pBB0250(E39A)-GFP, or pBB0250(D40A) to EthBr (50 μg/ml), acriflavine (25 μg/ml), cetyltrimethyl ammonium bromide (CTAB) (100 μg/ml), and methyl viologen (30 μg/ml). All strains grew on LB plates without biocides at 30°C. Wild-type BB0250, but not point mutants, restores drug resistance to BC202 at 30°C. (D) Expression of bb0250, bb0250(E39A), and bb0250(D40A) in membrane fractions as determined by Western blotting with anti-GFP antibody. All growth was carried out in LB-Amp supplemented with 0.5 mM IPTG.
Artificially increasing PMF restores drug resistance to BC202.
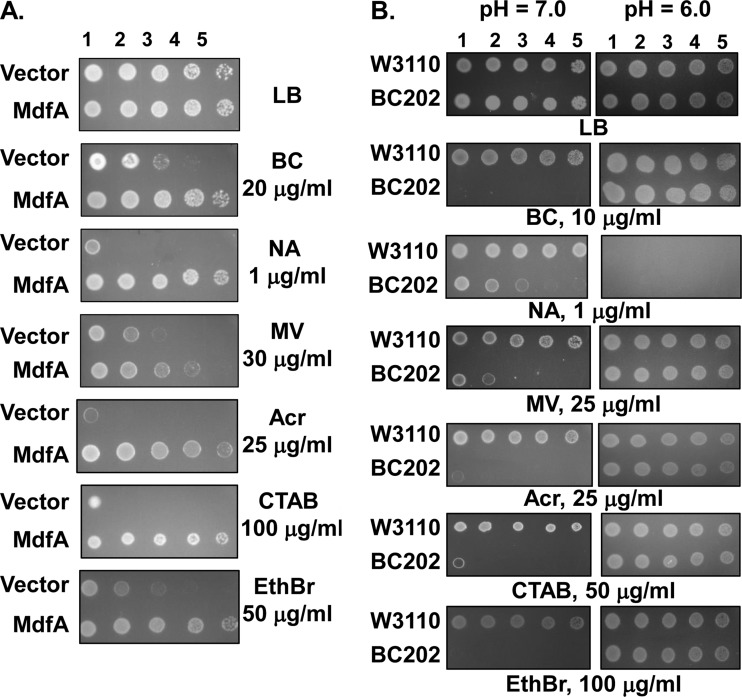
One potential explanation for these results is that YqjA/YghB are themselves drug efflux pumps. Alternatively, these DedA family proteins may indirectly affect the ability of other efflux pumps to function by compromising the proton motive force (PMF). A role for YqjA/YghB in PMF maintenance is supported by our own published data showing correction of BC202 growth and cell division by growth at acidic pH or by overexpression of the Na+-K+/H+ antiporter MdfA (10). PMF is also required for proper functioning of the twin-arginine transport pathway (37), which functions inefficiently in BC202, leading to the observed cell division defect (9). To directly test this question, we expressed mdfA from a plasmid in BC202 and tested its sensitivity to the collection of biocides to which it is sensitive. If mdfA overexpression corrects sensitivity to all drugs, this would support a role for YqjA/YghB in PMF maintenance, which is indirectly affecting the drug export mechanism. We found that expression of mdfA corrects sensitivity of BC202 to all drugs, including those that are not transported directly by MdfA (19, 28, 35) (i.e., acriflavine, methyl viologen, and nalidixic acid) (Fig. 5A). Growth at pH 6.0 (increasing the ΔpH component of the PMF) also restores drug resistance to BC202 on both solid and liquid media (Fig. 5B and Table 3), indicating that the sensitivity is likely due to loss of PMF. Growth in the presence of 400 mM NaCl or 10 mM Mg2+, both conditions that restore growth and cell division to BC202 (8, 10), also restores BC202 drug resistance to most compounds tested (see Fig. S4 in the supplemental material). Each of these conditions that restore resistance (mdfA expression or growth at pH 6.0 or in the presence of 400 mM NaCl or 10 mM Mg2+) likely assists in restoring pH homeostasis to BC202 (see Discussion) (10).
FIG 5.
mdfA overexpression or growth at pH 6.0 restores drug resistance to BC202. (A) BC202 harboring vector alone or pBAD-MdfA was spotted on plates containing LB (Amp, 0.1% arabinose) alone or this medium supplemented with 20 μg/ml benzalkonium chloride (BC) (exported by MdfA), 1 μg/ml nalidixic acid (NA) (exported by AcrAB), 30 μg/ml methyl viologen (MV) (exported by EmrE), 25 μg/ml acriflavine (Acr) (exported by AcrAB), 100 μg/ml cetyl trimethyl ammonium bromide (CTAB) (mainly exported by AcrAB), or 50 μg/ml ethidium bromide (EthBr) (exported by MdfA, EmrE, and others). (B) BC202 and the parent W3110 was spotted on LB plates buffered to pH 6.0 or 7.0 and containing no additive, 10 μg/ml BC, 1 μg/ml NA, 25 μg/ml MV, 25 μg/ml Acr, 50 μg/ml CTAB, or 100 μg/ml EthBr. LB medium was buffered at pH 6.0 or 7.0 as described previously (10). Nalidixic acid was toxic to both strains at pH 6.0 for unknown reasons.
TABLE 3.
MICs for W3110 and BC202 in LB medium adjusted to pH 6.0a
| Compound | MIC (μg/ml) for strain: |
|
|---|---|---|
| W3110 | BC202 | |
| BC | 12.5 | 12.5 |
| NA | 6.0 | 6.0 |
| CTAB | 12.5 | 12.5 |
| MV | 100 | 100 |
| Acr | 100 | 100 |
| EthBr | 400 | 400 |
The pH of LB medium was adjusted to 6 as described in Materials and Methods. Abbreviations are as for Table 1.
DISCUSSION
Members of the DedA family of membrane proteins are found in the vast majority of completed bacterial genomes (4), and there are virtually thousands of DedA members in the protein databases. While it has been reported that members of this family are evolutionarily related to the LeuT superfamily of transporters (6), they have never been defined as true membrane transporters. Deletion of functionally redundant genes yqjA and yghB in strain BC202 causes a number of pleiotropic effects in E. coli. BC202 is unable to grow at elevated temperatures or to complete cell division (8, 9), and it activates numerous envelope stress responses (10). In this work, we show that BC202 is highly sensitive to several cationic biocides that are known substrates of PMF-dependent efflux pumps. It appears that loss of YqjA and YghB may indirectly cause massive drug sensitivity due to impairment of the PMF in BC202. This is likely the reason that Salmonella ΔyqjA mutants are sensitive to magainin (38), a cationic antimicrobial peptide that likely disrupts the membrane PMF (39). In addition, PMF-disrupting lipopeptides have recently been reported to sensitize E. coli to a number of antibiotics (40).
Overexpression of DedA family genes in BC202 does not by itself increase resistance to any of the biocides tested here beyond the level of resistance seen in parent strain W3110 (Fig. 2 and 4; see Fig. S3 in the supplemental material). However, the essentiality of membrane-embedded amino acids strongly suggests that DedA proteins themselves are proton-dependent transporters, as similar acidic amino acids are found in many other classes of such transporters. In the case of MdfA, is has been shown that acidic amino acids E26 and D34 play roles in proton translocation during substrate transport (35). It is possible that such amino acids serve a similar function for DedA family proteins. Whether this family also functions as antiporters and the nature of the transported substrate(s) is currently unknown.
Growth at 42°C, normal cell division, and wild-type drug resistance can be restored to BC202 by overexpression of mdfA (Fig. 5A) or growth at pH 6.0 (Fig. 5B) or in the presence of 400 mM NaCl or 10 mM Mg2+ (see Fig. S4 in the supplemental material) (400 mM NaCl did not restore resistance to 50 μg/ml CTAB, for reasons that are unclear [see Fig. S4 in the supplemental material]). It is likely that each of these conditions helps to restore an altered cytoplasmic pH. For example, MdfA is an antiporter that can provide proton influx in exchange for Na+ or K+ (16). In a similar manner, medium buffered to pH 6.0 results in an increased ΔpH component of the PMF. An increase in extracellular Mg2+ can enhance membrane integrity and can also inhibit the activity of the Fo/F1 proton-transporting ATPase (41), which is also upregulated in response to alkaline growth conditions (12). Finally, high levels of monovalent cations such as Na+ or K+ can stimulate a number of proton-dependent antiporters, such as NhaA and MdfA, which play critical roles in cellular pH homeostasis (16, 42). Interestingly, the ability of 400 mM Na or 10 mM Mg to restore growth and cell division to BC202 is dependent upon an intact Cpx envelope stress response pathway, suggesting that some Cpx-induced gene may be required for this effect (10).
One of the more interesting properties of the DedA family that sets it apart from many membrane transporter families is its apparent essentiality. This is supported by several lines of evidence. First, the DedA family is collectively essential in E. coli (11), and essential DedA genes are found in Borrelia burgdorferi (BB0250) (36) and Caulobacter crescentus (CCNA_01607) (43). Furthermore, expression of a DedA family gene from Mycobacterium bovis BCG (BCG2664) confers resistance to and may be the target of the antituberculosis drug halicyclamine A (44). In this work, we show that members of the DedA family may be membrane transporters and that they are required for the normal function of several families of drug efflux pumps, possibly due to their requirement for PMF maintenance. DedA family proteins are thus an intriguing class of membrane proteins and potential targets for antibacterial drug design.
Supplementary Material
ACKNOWLEDGMENTS
Financial support has been provided by the National Science Foundation (MCB-0841853 to W.T.D.).
We thank Rakesh Sikdar and Lisa Boughner for valuable discussions.
Footnotes
Published ahead of print 25 November 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.02238-13.
REFERENCES
- 1.Krulwich TA, Lewinson O, Padan E, Bibi E. 2005. Do physiological roles foster persistence of drug/multidrug-efflux transporters? A case study. Nat. Rev. Microbiol. 3:566–572. 10.1038/nrmicro1181 [DOI] [PubMed] [Google Scholar]
- 2.Piddock LJ. 2006. Multidrug-resistance efflux pumps—not just for resistance. Nat. Rev. Microbiol. 4:629–636. 10.1038/nrmicro1464 [DOI] [PubMed] [Google Scholar]
- 3.Bernsel A, Daley DO. 2009. Exploring the inner membrane proteome of Escherichia coli: which proteins are eluding detection and why? Trends Microbiol. 17:444–449. 10.1016/j.tim.2009.07.005 [DOI] [PubMed] [Google Scholar]
- 4.Doerrler WT, Sikdar R, Kumar S, Boughner LA. 2013. New functions for the ancient DedA membrane protein family. J. Bacteriol. 195:3–11. 10.1128/JB.01006-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inadome H, Noda Y, Kamimura Y, Adachi H, Yoda K. 2007. Tvp38, Tvp23, Tvp18 and Tvp15: novel membrane proteins in the Tlg2-containing Golgi/endosome compartments of Saccharomyces cerevisiae. Exp. Cell Res. 313:688–697. 10.1016/j.yexcr.2006.11.008 [DOI] [PubMed] [Google Scholar]
- 6.Khafizov K, Staritzbichler R, Stamm M, Forrest LR. 2010. A study of the evolution of inverted-topology repeats from LeuT-fold transporters using AlignMe. Biochemistry 49:10702–10713. 10.1021/bi101256x [DOI] [PubMed] [Google Scholar]
- 7.Blattner FR, Plunkett G, Bloch CA, III, Perna NT, Burland V, Riley M, Collado-Vides J, Glasner JD, Rode CK, Mayhew GF, Gregor J, Davis NW, Kirkpatrick HA, Goeden MA, Rose DJ, Mau B, Shao Y. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453–1474. 10.1126/science.277.5331.1453 [DOI] [PubMed] [Google Scholar]
- 8.Thompkins K, Chattopadhyay B, Xiao Y, Henk MC, Doerrler WT. 2008. Temperature sensitivity and cell division defects in an Escherichia coli strain with mutations in yghB and yqjA, encoding related and conserved inner membrane proteins. J. Bacteriol. 190:4489–4500. 10.1128/JB.00414-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sikdar R, Doerrler WT. 2010. Inefficient Tat-dependent export of periplasmic amidases in an Escherichia coli strain with mutations in two DedA family genes. J. Bacteriol. 192:807–818. 10.1128/JB.00716-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sikdar R, Simmons AR, Doerrler WT. 2013. Multiple envelope stress response pathways are activated in an Escherichia coli strain with mutations in two members of the DedA membrane protein family. J. Bacteriol. 195:12–24. 10.1128/JB.00762-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boughner LA, Doerrler WT. 2012. Multiple deletions reveal the essentiality of the DedA membrane protein family in Escherichia coli. Microbiology 158:1162–1171. 10.1099/mic.0.056325-0 [DOI] [PubMed] [Google Scholar]
- 12.Krulwich TA, Sachs G, Padan E. 2011. Molecular aspects of bacterial pH sensing and homeostasis. Nat. Rev. Microbiol. 9:330–343. 10.1038/nrmicro2549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Price NL, Raivio TL. 2009. Characterization of the Cpx regulon in Escherichia coli strain MC4100. J. Bacteriol. 191:1798–1815. 10.1128/JB.00798-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maurer LM, Yohannes E, Bondurant SS, Radmacher M, Slonczewski JL. 2005. pH regulates genes for flagellar motility, catabolism, and oxidative stress in Escherichia coli K-12. J. Bacteriol. 187:304–319. 10.1128/JB.187.1.304-319.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng J, Guffanti AA, Wang W, Krulwich TA, Bechhofer DH. 1996. Chromosomal tetA(L) gene of Bacillus subtilis: regulation of expression and physiology of a tetA(L) deletion strain. J. Bacteriol. 178:2853–2860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewinson O, Padan E, Bibi E. 2004. Alkalitolerance: a biological function for a multidrug transporter in pH homeostasis. Proc. Natl. Acad. Sci. U. S. A. 101:14073–14078. 10.1073/pnas.0405375101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lewinson O, Adler J, Poelarends GJ, Mazurkiewicz P, Driessen AJ, Bibi E. 2003. The Escherichia coli multidrug transporter MdfA catalyzes both electrogenic and electroneutral transport reactions. Proc. Natl. Acad. Sci. U. S. A. 100:1667–1672. 10.1073/pnas.0435544100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edgar R, Bibi E. 1997. MdfA, an Escherichia coli multidrug resistance protein with an extraordinarily broad spectrum of drug recognition. J. Bacteriol. 179:2274–2280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tal N, Schuldiner S. 2009. A coordinated network of transporters with overlapping specificities provides a robust survival strategy. Proc. Natl. Acad. Sci. U. S. A. 106:9051–9056. 10.1073/pnas.0902400106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cherepanov PP, Wackernagel W. 1995. Gene disruption in Escherichia coli—Tc(R) and Km(R) cassettes with the option of FLP-catalyzed excision of the antibiotic-resistance determinant. Gene 158:9–14. 10.1016/0378-1119(95)00193-A [DOI] [PubMed] [Google Scholar]
- 21.Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. 2:2006.0008. 10.1038/msb4100050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silhavy TJ, Berman ML, Enquist LW. 1984. Experiments with gene fusions. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 23.Wiegand I, Hilpert K, Hancock RE. 2008. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 3:163–175. 10.1038/nprot.2007.521 [DOI] [PubMed] [Google Scholar]
- 24.Zheng L, Baumann U, Reymond JL. 2004. An efficient one-step site-directed and site-saturation mutagenesis protocol. Nucleic Acids Res. 32:e115. 10.1093/nar/gnh110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doerrler WT, Raetz CRH. 2002. ATPase activity of the MsbA lipid flippase of Escherichia coli. J. Biol. Chem. 277:36697–36705. 10.1074/jbc.M205857200 [DOI] [PubMed] [Google Scholar]
- 26.Purewal AS. 1991. Nucleotide sequence of the ethidium efflux gene from Escherichia coli. FEMS Microbiol. Lett. 66:229–231 [DOI] [PubMed] [Google Scholar]
- 27.Brown MH, Skurray RA. 2001. Staphylococcal multidrug efflux protein QacA. J. Mol. Microbiol. Biotechnol. 3:163–170 [PubMed] [Google Scholar]
- 28.Sulavik MC, Houseweart C, Cramer C, Jiwani N, Murgolo N, Greene J, DiDomenico B, Shaw KJ, Miller GH, Hare R, Shimer G. 2001. Antibiotic susceptibility profiles of Escherichia coli strains lacking multidrug efflux pump genes. Antimicrob. Agents Chemother. 45:1126–1136. 10.1128/AAC.45.4.1126-1136.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lim SP, Nikaido H. 2010. Kinetic parameters of efflux of penicillins by the multidrug efflux transporter AcrAB-TolC of Escherichia coli. Antimicrob. Agents Chemother. 54:1800–1806. 10.1128/AAC.01714-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishino K, Yamaguchi A. 2001. Analysis of a complete library of putative drug transporter genes in Escherichia coli. J. Bacteriol. 183:5803–5812. 10.1128/JB.183.20.5803-5812.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edgar R, Bibi E. 1999. A single membrane-embedded negative charge is critical for recognizing positively charged drugs by the Escherichia coli multidrug resistance protein MdfA. EMBO J. 18:822–832. 10.1093/emboj/18.4.822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seeger MA, von Ballmoos C, Verrey F, Pos KM. 2009. Crucial role of Asp408 in the proton translocation pathway of multidrug transporter AcrB: evidence from site-directed mutagenesis and carbodiimide labeling. Biochemistry 48:5801–5812. 10.1021/bi900446j [DOI] [PubMed] [Google Scholar]
- 33.Sigal N, Fluman N, Siemion S, Bibi E. 2009. The secondary multidrug/proton antiporter MdfA tolerates displacements of an essential negatively charged side chain. J. Biol. Chem. 284:6966–6971. 10.1074/jbc.M808877200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soskine M, Adam Y, Schuldiner S. 2004. Direct evidence for substrate-induced proton release in detergent-solubilized EmrE, a multidrug transporter. J. Biol. Chem. 279:9951–9955. 10.1074/jbc.M312853200 [DOI] [PubMed] [Google Scholar]
- 35.Fluman N, Ryan CM, Whitelegge JP, Bibi E. 2012. Dissection of mechanistic principles of a secondary multidrug efflux protein. Mol. Cell 47:777–787. 10.1016/j.molcel.2012.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liang FT, Xu Q, Sikdar R, Xiao Y, Cox JS, Doerrler WT. 2010. BB0250 of Borrelia burgdorferi is a conserved and essential inner membrane protein required for cell division. J. Bacteriol. 192:6105–6115. 10.1128/JB.00571-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palmer T, Berks BC. 2012. The twin-arginine translocation (Tat) protein export pathway. Nat. Rev. Microbiol. 10:483–496. 10.1038/nrmicro2814 [DOI] [PubMed] [Google Scholar]
- 38.Shi Y, Cromie MJ, Hsu FF, Turk J, Groisman EA. 2004. PhoP-regulated Salmonella resistance to the antimicrobial peptides magainin 2 and polymyxin B. Mol. Microbiol. 53:229–241. 10.1111/j.1365-2958.2004.04107.x [DOI] [PubMed] [Google Scholar]
- 39.Westerhoff HV, Juretic D, Hendler RW, Zasloff M. 1989. Magainins and the disruption of membrane-linked free-energy transduction. Proc. Natl. Acad. Sci. U. S. A. 86:6597–6601. 10.1073/pnas.86.17.6597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goldberg K, Sarig H, Zaknoon F, Epand RF, Epand RM, Mor A. 2013. Sensitization of gram-negative bacteria by targeting the membrane potential. FASEB J. 27:3818–3826. 10.1096/fj.13-227942 [DOI] [PubMed] [Google Scholar]
- 41.Bulygin VV, Vinogradov AD. 1991. Interaction of Mg2+ with F0.F1 mitochondrial ATPase as related to its slow active/inactive transition. Biochem. J. 276:149–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mager T, Rimon A, Padan E, Fendler K. 2011. Transport mechanism and pH regulation of the Na+/H+ antiporter NhaA from Escherichia coli: an electrophysiological study. J. Biol. Chem. 286:23570–23581. 10.1074/jbc.M111.230235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Christen B, Abeliuk E, Collier JM, Kalogeraki VS, Passarelli B, Coller JA, Fero MJ, McAdams HH, Shapiro L. 2011. The essential genome of a bacterium. Mol. Syst. Biol. 7:528. 10.1038/msb.2011.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arai M, Liu L, Fujimoto T, Setiawan A, Kobayashi M. 2011. DedA protein relates to action-mechanism of halicyclamine A, a marine spongean macrocyclic alkaloid, as an anti-dormant mycobacterial substance. Mar. Drugs 9:984–993. 10.3390/md9060984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hirokawa T, Boon-Chieng S, Mitaku S. 1998. SOSUI: classification and secondary structure prediction system for membrane proteins. Bioinformatics 14:378–379. 10.1093/bioinformatics/14.4.378 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.